Iridescence and Luminescence from Opal Matrices for Show Business
Abstract
1. Introduction
2. Materials and Methods
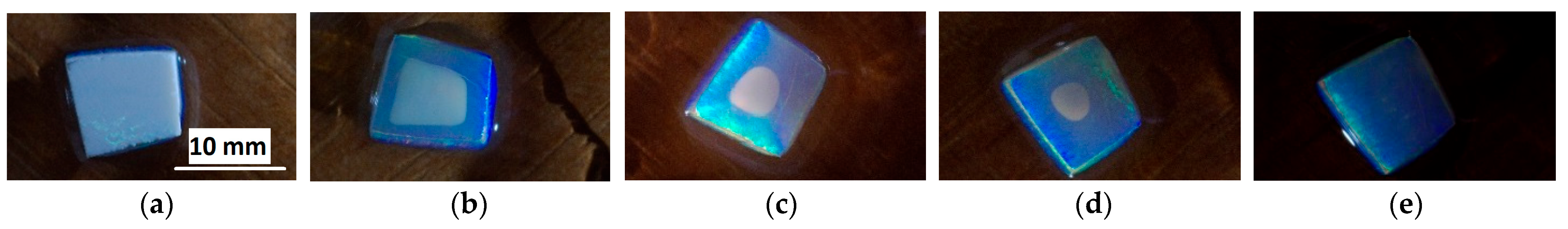
- Opal matrices without xerogel inside (as-grown) immersed in ethanol (samples #1 and #2).
- Opal matrix immersed once in sol corresponding to Er- and Yb-doped BaTiO3 xerogel (BAT-sol) and annealed at 450 °C for 30 min (sample #3) and immersed in ethanol.
- Opal matrix with BaTiO3 xerogel doped with Er and Yb (BaTiO3:(Er,Yb) xerogel) inside and annealed at 600 °C (sample #4). This matrix was immersed in BAT-sol several times, followed by drying and annealing at 450 °C for 30 min after each immersion, then the matrix was finally annealed at 600 °C for 30 min.
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, J.V. Diffraction of light by opals. Acta. Cryst. 1968, A24, 427–434. [Google Scholar] [CrossRef]
- Astratov, V.N.; Bogomolov, V.N.; Kaplyanskii, A.A.; Prokofiev, A.V.; Samoilovich, L.A.; Samoilovich, S.M.; Vlasov, Y.A. Optical spectroscopy of opal matrices with CdS embedded in its pores: Quantum confinement and photonic band gap effects. Il Nuovo Cimento D 1995, 17, 1349–1354. [Google Scholar] [CrossRef]
- Bogomolov, V.N.; Gaponenko, S.V.; Germanenko, I.N.; Kapitonov, A.M.; Petrov, E.P.; Gaponenko, N.V.; Prokofiev, A.V.; Ponyavina, A.N.; Silvanovich, N.I.; Samoilovich, S.M. Photonic band gap phenomenon and optical properties of artificial opals. Phys. Rev. E 1997, 55, 7619–7625. [Google Scholar] [CrossRef]
- Gaponenko, N.V.; Unuchak, D.M.; Mudryi, A.V.; Malyarevich, G.K.; Gusev, O.B.; Stepikhova, M.V.; Krasilnikova, L.V.; Stupak, A.P.; Kleshcheva, S.M.; Samoilovich, M.I.; et al. Modification of erbium photoluminescence excitation spectra for the emission wavelength 1.54 µm in mesoscopic structures. J. Lumin. 2006, 121, 217–221. [Google Scholar] [CrossRef]
- Lonergan, A.; Hu, C.; O’Dwyer, C. Filling in the gaps: The nature of light transmission through solvent-filled inverse opal photonic crystals. Phys. Rev. Mater. 2020, 4, 065201. [Google Scholar] [CrossRef]
- Lodahl, P.; van Driel, A.F.; Nikolaev, I.S.; Irman, A.; Overgaag, K.; Vanmaekelbergh, D.; Vos, W.L. Controlling the dynamics of spontaneous emission from quantum dots by photonic crystals. Nature 2004, 430, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Sun, X.; Wu, S.; Zhang, S. Manipulating the emission intensity and lifetime of NaYF4:Yb3+,Er3+ simultaneously by embedding it into CdS photonic crystals. Nanoscale 2017, 9, 7666–7673. [Google Scholar] [CrossRef] [PubMed]
- Gaponenko, N.V.; Labunov, V.A.; Lashkovskaya, E.I.; Zaitsev, V.A.; Kleshcheva, S.M.; Kargin, N.I. Device for Forming a Color Image on a Plane. Russian Federation Patent No. RU2837366C1, 8 May 2024. [Google Scholar]
- Samoilovich, M.I.; Samoilovich, S.M. Method of Preparing Synthetic Material with Noble Opal Structure. Russian Federation Patent No. RU2162456C1, 17 March 2000. [Google Scholar]
- Li, H.; Wang, X.; Huang, D.; Chen, G. Recent advances of lanthanide-doped upconversion nanoparticles for biological applications. Nanotechnology 2020, 31, 072001. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Lashkovskaya, E.I.; Gaponenko, N.V.; Stepikhova, M.V.; Yablonskiy, A.N.; Andreev, B.A.; Zhivulko, V.D.; Mudryi, A.V.; Martynov, I.L.; Chistyakov, A.A.; Kargin, N.I.; et al. Optical properties and upconversion luminescence of BaTiO3 xerogel structures doped with erbium and ytterbium. Gels 2022, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, J.E.G.J.; Vos, W.L. Preparation of photonic crystals made of air spheres in titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Golubev, V.G.; Davydov, V.Y.; Kartenko, N.F.; Kurdyukov, D.A.; Medvedev, A.V.; Pevtsov, A.B.; Scherbakov, A.V.; Shadrin, E.B. Phase transition-governed opal-VO2 photonic crystal. Appl. Phys. Lett. 2001, 79, 2127–2129. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, C.Q.; Pita, K.; Lam, Y.L.; Zhou, Y.; Ng, S.L.; Kam, C.H.; Li, L.T.; Gui, Z.L. Thermally tuning of the photonic band gap of SiO2 colloid-crystal infilled with ferroelectric BaTiO3. Appl. Phys. Lett. 2001, 78, 661–663. [Google Scholar] [CrossRef]
- Shrike Zhang, Y.; Zhu, C.; Xia, Y. Inverse Opal Scaffolds and Their Biomedical Applications. Adv. Mater. 2017, 29, 1701115. [Google Scholar] [CrossRef]
- Busch, K.; John, S. Liquid-Crystal Photonic-Band-Gap Materials: The Tunable Electromagnetic Vacuum. Phys. Rev. Lett. 1999, 83, 967–970. [Google Scholar] [CrossRef]
- Wu, Y.; Nan, J.; Ren, J.; Meng, Z.; Zhang, S.; Wu, S. Polarization-dependent structural colors in ZnS Nanosphere-Based Photonic Crystals for Anticounterfeiting Applications. ACS Appl. Nano Mater. 2021, 5, 423–429. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaponenko, N.V.; Kleshcheva, S.M.; Lashkovskaya, E.I.; Zaitsau, U.A.; Labunov, V.A.; Hamadneh, B.Z.S.; Zhivulko, V.D.; Mudryi, A.V.; Radyush, Y.V.; Kargin, N.I.; et al. Iridescence and Luminescence from Opal Matrices for Show Business. Photonics 2025, 12, 908. https://doi.org/10.3390/photonics12090908
Gaponenko NV, Kleshcheva SM, Lashkovskaya EI, Zaitsau UA, Labunov VA, Hamadneh BZS, Zhivulko VD, Mudryi AV, Radyush YV, Kargin NI, et al. Iridescence and Luminescence from Opal Matrices for Show Business. Photonics. 2025; 12(9):908. https://doi.org/10.3390/photonics12090908
Chicago/Turabian StyleGaponenko, Nikolai V., Svetlana M. Kleshcheva, Ekaterina I. Lashkovskaya, Uladzimir A. Zaitsau, Vladimir A. Labunov, Bashar Z. S. Hamadneh, Vadim D. Zhivulko, Alexander V. Mudryi, Yuriy V. Radyush, Nikolai I. Kargin, and et al. 2025. "Iridescence and Luminescence from Opal Matrices for Show Business" Photonics 12, no. 9: 908. https://doi.org/10.3390/photonics12090908
APA StyleGaponenko, N. V., Kleshcheva, S. M., Lashkovskaya, E. I., Zaitsau, U. A., Labunov, V. A., Hamadneh, B. Z. S., Zhivulko, V. D., Mudryi, A. V., Radyush, Y. V., Kargin, N. I., & Raichenok, T. F. (2025). Iridescence and Luminescence from Opal Matrices for Show Business. Photonics, 12(9), 908. https://doi.org/10.3390/photonics12090908





