Chitosan/Polyacrylic Acid Functionalized Side-Polish Polymer Optical Fiber-Based SPR Sensor for Cu2+ Ion Detection
Abstract
1. Introduction
2. Preparation and Working Principle
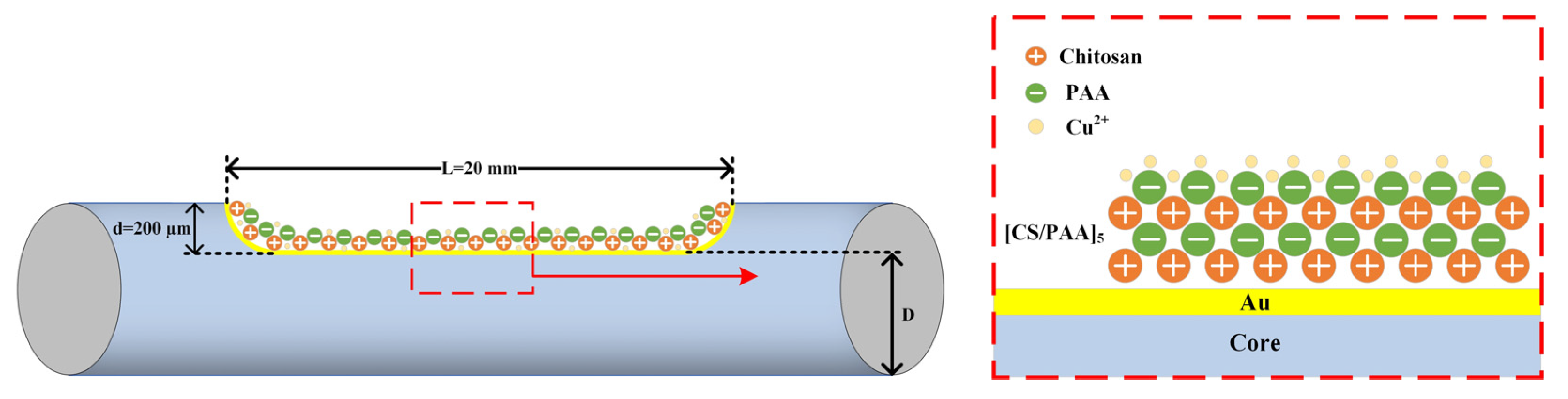
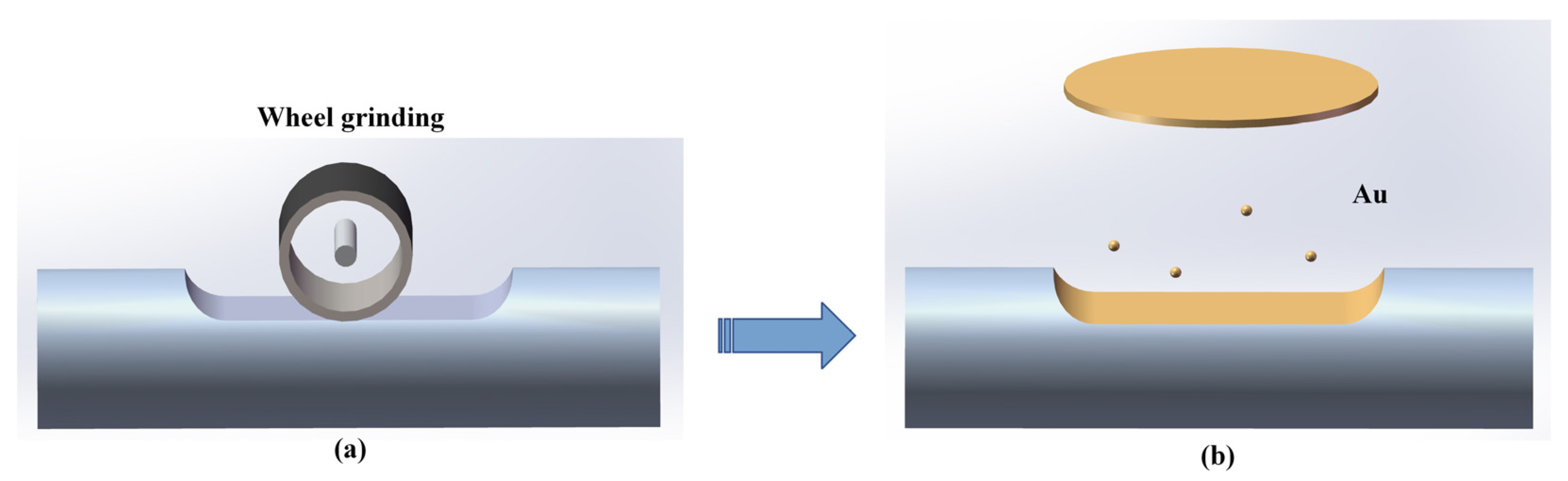
2.1. Structure and Preparation
2.2. Working Principle
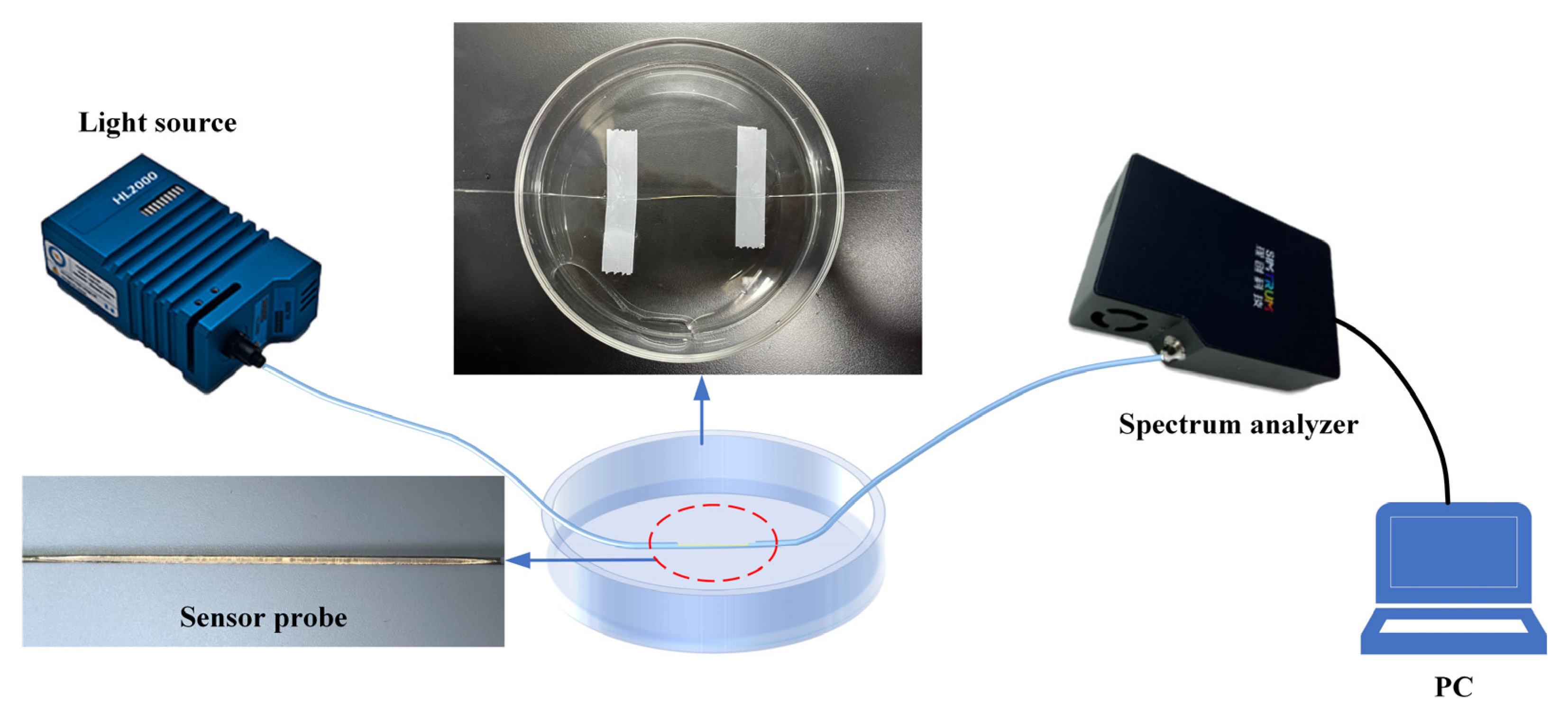
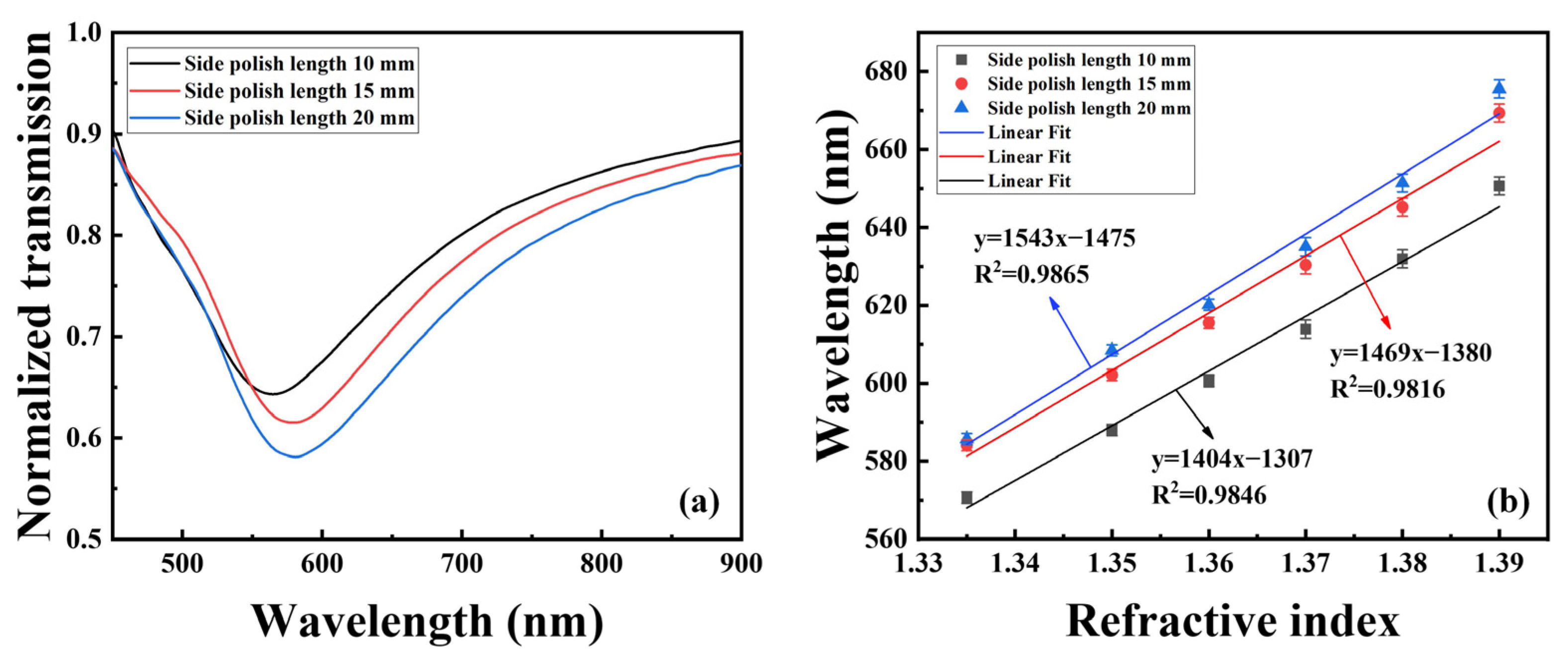
3. Experiment Setup and Structure Optimization
4. Results and Discussions
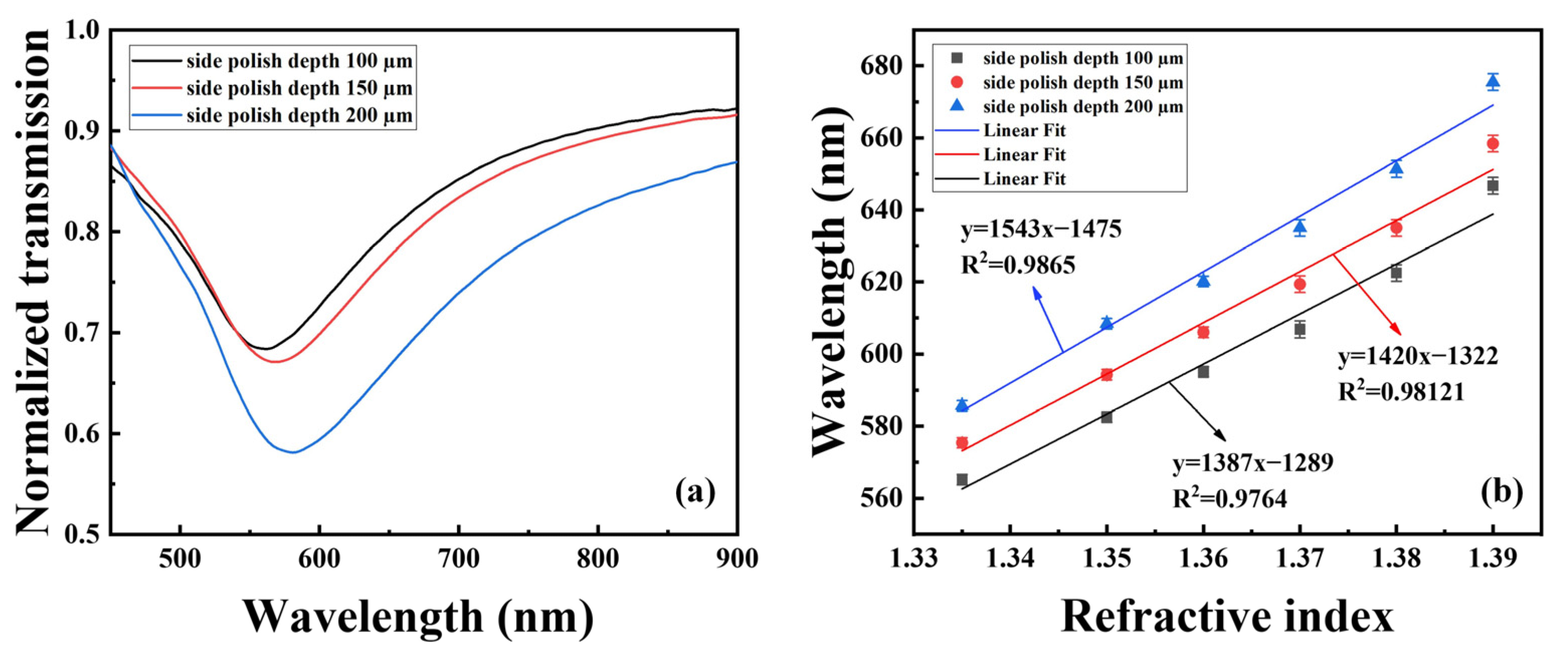
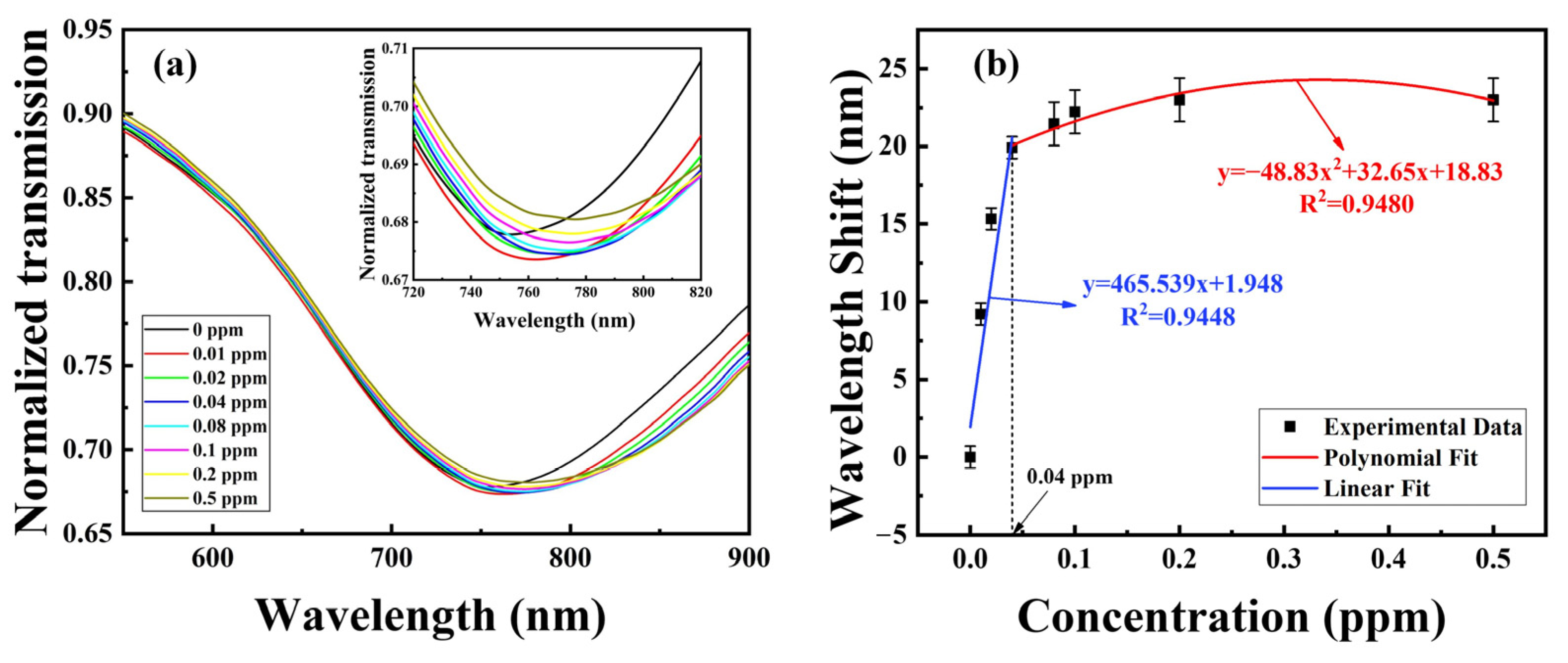
4.1. Analysis of the Spectral Response to Cu2+ Ion
4.2. Effect of the Number of Sensitive Film Layers
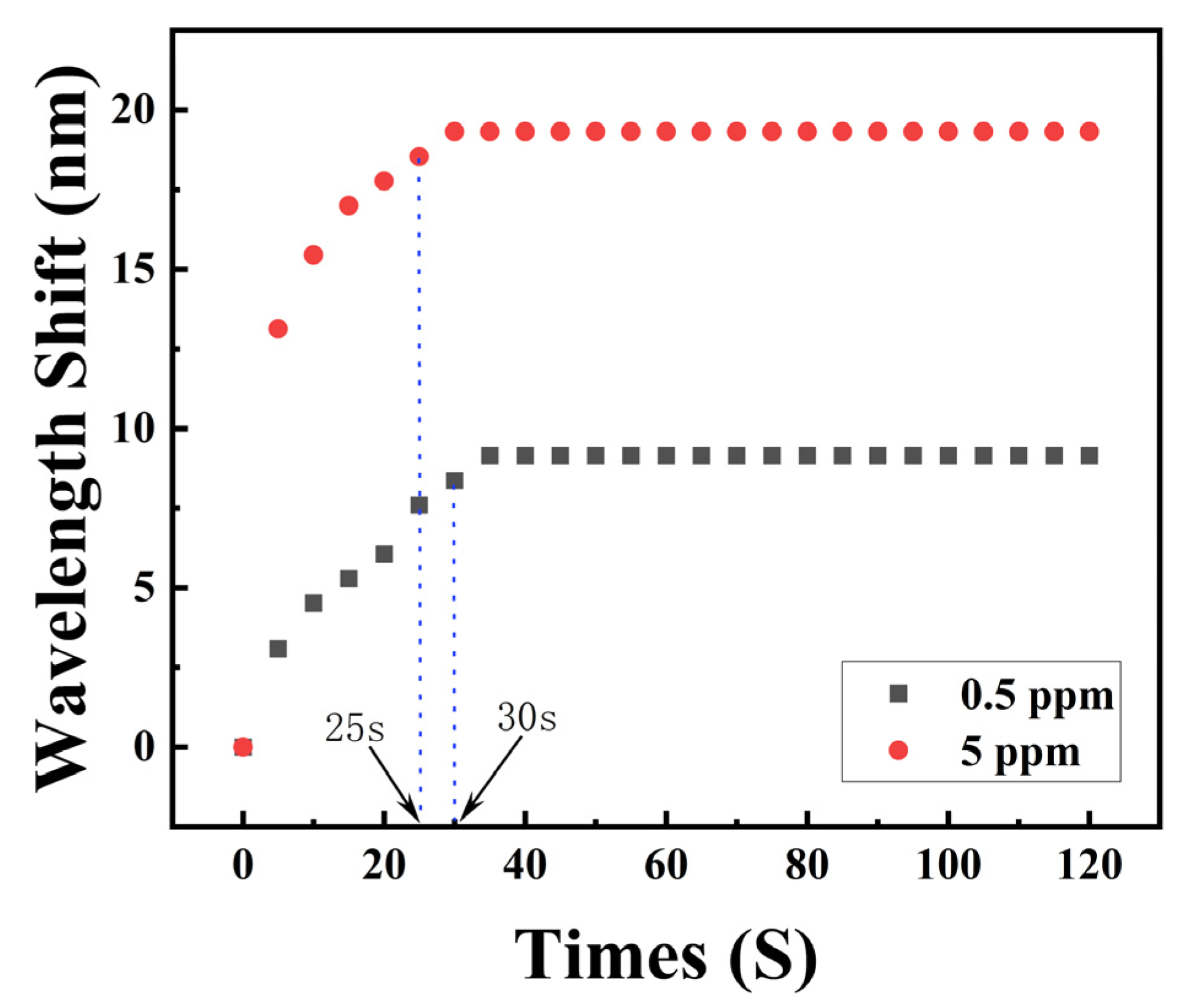
4.3. Response Time
4.4. Ion Selectivity
4.5. Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roli, V.; Banshi, D.G. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fiber sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar]
- Wan Ngah, W.S.; Fatinathan, S. Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ranjith, K.S.; Vilian, A.E.; gil Lee, S.; Won, J.; Huh, Y.S.; Han, Y.-K. Synergistic on engineering layered N-doped carbon/MXene heterostructure: A potential scaffold for simultaneous electrochemical detection of Cu2+ and Hg2+ ions. Sens. Actuators B Chem. 2025, 422, 136661. [Google Scholar] [CrossRef]
- Li, Z.; Che, T.; Yang, M.; Hu, X. Flame atomic absorption spectrometry combined with surface-modified magnetic mesoporous silica microspheres by polyethyleneimine for enrichment, isolation and determination of Cu2+ in preserved eggs after high-temperature digestion. Food Addit. Contam. Part A 2022, 39, 1828–1842. [Google Scholar] [CrossRef]
- You, Y.; Li, D.; Chen, Z.; Zhang, X.; Hu, Y.; Ouyang, S.; Li, N. Fluorescent and colorimetric dual-mode detection of Cu2+ based on carbon dots. Microchim. Acta 2024, 191, 563. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, L.-N.; Sun, Y.-Z.; Dong, S.-W.; Xue, Q.-W.; Xue, N.; Ding, T. Highly stable Zn (II)-based metal-organic framework as a bifunctional fluorescence sensor for efficient detection of Cu2+ and CrO42− ions. J. Mol. Struct. 2025, 1326, 141094. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Sun, T. Fiber optic sensor technology: An overview. Sens. Actuators A Phys. 2000, 82, 40–61. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.H.; Zheng, Y.; Dong, X.; Raghunandhan, R.; So, P.L.; Chan, C.C. Heavy metal ions probe with relative measurement of fiber Bragg grating. Sens. Actuators B Chem. 2016, 230, 353–358. [Google Scholar] [CrossRef]
- Ravikumar, R.; Chen, L.H.; Chan, C.C.; So, P.L.; Tou, Z.Q.; Peng, Z. Chitosan-hydrogel-based fiber optic sensor for heavy metal ion detection. In Proceedings of the 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015. [Google Scholar]
- Raikar, U.S.; Kulkarni, V.K.; Lalasangi, A.S.; Pattanashetti, I.I.; Akki, J.F. Evanescent field absorption sensor for detection of copper (II) in water using multimode optical fiber. Optoelectron. Lett. 2009, 5, 224–226. [Google Scholar] [CrossRef]
- Ding, Z.W.; Ravikumar, R.; Zhao, C.L.; Chen, L.H.; Chan, C.C. Chitosan/Poly (Acrylic Acid) Based Fiber-Optic Surface Plasmon Resonance Sensor for Cu2+ Ions Detection. J. Light. Technol. 2019, 37, 2246–2252. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, G.; Li, T.; Lou, X.; Zhu, L. A dual-channel optical fiber sensor based on surface plasmon resonance for heavy metal ions detection in contaminated water. Opt. Commun. 2020, 462, 124750. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, W.; Wang, Y.; Deng, Z.; Li, Z.; Peng, J.; Lyu, D.; Lewis, E.; Yang, M. Optical fiber plasmonic sensor for the ultrasensitive detection of copper (II) ion based on trimetallic Au@ AgPt core-shell nanospheres. Sens. Actuators B Chem. 2020, 321, 128480. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Gupta, B.D. Ion-imprinted nanoparticles for the concurrent estimation of Pb (II) and Cu (II) ions over a two channel surface plasmon resonance-based fiber optic platform. J. Biomed. Opt. 2018, 23, 017001. [Google Scholar]
- Maleki, M.J.; Soroosh, M.; Akbarizadeh, G. A compact low-loss 2-to-4 plasmonic decoder based on suspended graphene for surface plasmon polariton transmission. Diam. Relat. Mater. 2024, 144, 110983. [Google Scholar] [CrossRef]
- Brolo, A. Plasmonics for future biosensors. Nat. Photon 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Homola, J.; Slavik, R. Fiber-optic sensor based on surface plasmon resonance. Electron. Lett. 1996, 32, 480. [Google Scholar] [CrossRef]
- Coelho, L.C.C.; de Almeida, J.M.M.M.; Moayyed, H.; Santos, J.L.; Viegas, D. Multiplexing of Surface Plasmon Resonance Sensing Devices on Etched Single-Mode Fiber. J. Light. Technol. 2015, 33, 432–438. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhao, C.L.; Wang, Y.R.; Shen, C.Y.; Dong, X.Y. A Highly Sensitive Fibre-Optic Nano-Displacement Sensor Based on Surface Plasmon Resonance. J. Light. Technol. 2016, 34, 2324–2330. [Google Scholar] [CrossRef]
- Patnaik, A.; Senthilnathan, K.; Jha, R. Graphene-Based Conducting Metal Oxide Coated D-Shaped Optical Fiber SPR Sensor. IEEE Photonics Technol. Lett. 2015, 27, 2437–2440. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, L.; Hu, H.F. Fiber-Optic Refractive Index Sensor Based on Multi-Tapered SMS Fiber Structure. IEEE Sens. J. 2015, 15, 6348–6353. [Google Scholar] [CrossRef]
- Falah, A.A.S.; Wong, W.R.; Adikan, F.R.M. Single-mode eccentric-core D-shaped photonic crystal fiber surface plasmon resonance sensor. Opt. Laser Technol. 2022, 145, 107474. [Google Scholar] [CrossRef]
- Gonzalez-Valencia, E.; Reyes-Vera, E.; Del Villar, I.; Torres, P. Side-polished photonic crystal fiber sensor with ultra-high figure of merit based on Bloch-like surface wave resonance. Opt. Laser Technol. 2024, 169, 110129. [Google Scholar] [CrossRef]
- Teng, C.; Min, R.; Zheng, J.; Deng, S.; Li, M.; Hou, L.; Yuan, L. Intensity-modulated Polymer Optical Fiber-based Refractive Index Sensor: A Review. Sensors 2021, 22, 81. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. Localized surface plasmon resonance based U-shaped optical fiber probe for the detection of Pb2+ in aqueous medium. Sens. Actuators B Chem. 2018, 276, 89–94. [Google Scholar] [CrossRef]
- Bhavsar, K.; Prabhu, R.; Pollard, P. Development of dithizone based fibre optic evanescent wave sensor for heavy metal ion detection in aqueous environments. J. Phys. Conf. Series. IOP Publ. 2013, 450, 012011. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Liu, Z. The polishing detection method of side-polished fiber. In Proceedings of the 2011 International Conference on Optical Instruments and Technology, Beijing, China, 23 November 2011. [Google Scholar]
- Liu, L.; Zheng, J.; Deng, S.; Yuan, L.; Teng, C. Teng. Parallel Polished Plastic Optical Fiber-Based SPR Sensor for Simultaneous Measurement of RI and Temperature. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar]
- Raether, H. Surface plasmons on smooth and rough surfaces and on gratings. Springer 2006, 111, 4–39. [Google Scholar]
- Yan, H.; Yang, L.; Yang, Z.; Yang, H.; Li, A.; Cheng, R. Preparation of chitosan/poly (acrylic acid) magnetic composite microspheres and applications in the removal of copper (II) ions from aqueous solutions. J. Hazard. Mater 2012, 229, 371–380. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, J.S.; Monzon-Hernandez, D.; Martinez-Pinon, F.; May-Arrioja, D.A.; Hernandez-Romano, I. Surface plasmon resonance-based optical fiber embedded in PDMS for temperature sensing. IEEE J. Sel. Top. Quantum Electron. 2016, 23, 126–131. [Google Scholar] [CrossRef]
- Raghunandhan, R.; Chen, L.H.; Long, H.Y.; Leam, L.L.; So, P.L.; Ning, X.; Chan, C.C. Chitosan/PAA based fiber-optic interferometric sensor for heavy metal ions detection. Sens. Actuators B Chem. 2016, 233, 31–38. [Google Scholar] [CrossRef]







| Structure | Sensitive Materials | Sensitivity (nm/ppm) | Measurement Range (ppm) | Ref. |
|---|---|---|---|---|
| Side-polish polymer optical fiber | CS/PAA | 465.539 | 0.01–0.5 | This work |
| MMF-NCF-MMF pull taper | CS/PAA | 1.863 | 12.71–635.5 | [11] |
| PCS optical fiber stripping | CS/PAA | 3.919 | 1.27–635.5 | [12] |
| MMF-SMF-MMF | PEI-Au@AgPt NS | - | 6.355 × 10−12–6.355 × 10−7 | [13] |
| PCS optical fiber stripping | Ion-imprinted nanoparticles | 40,700 | 0–1000 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.; Yang, R.; Ying, S.; Xia, H.; Zhang, Y.; Shi, L.; Deng, S.; Chen, Z.; Qiao, H.; Yuan, L. Chitosan/Polyacrylic Acid Functionalized Side-Polish Polymer Optical Fiber-Based SPR Sensor for Cu2+ Ion Detection. Photonics 2025, 12, 461. https://doi.org/10.3390/photonics12050461
Teng C, Yang R, Ying S, Xia H, Zhang Y, Shi L, Deng S, Chen Z, Qiao H, Yuan L. Chitosan/Polyacrylic Acid Functionalized Side-Polish Polymer Optical Fiber-Based SPR Sensor for Cu2+ Ion Detection. Photonics. 2025; 12(5):461. https://doi.org/10.3390/photonics12050461
Chicago/Turabian StyleTeng, Chuanxin, Rongping Yang, Shiyuan Ying, Hongyun Xia, Yuting Zhang, Liying Shi, Shijie Deng, Zining Chen, Hanli Qiao, and Libo Yuan. 2025. "Chitosan/Polyacrylic Acid Functionalized Side-Polish Polymer Optical Fiber-Based SPR Sensor for Cu2+ Ion Detection" Photonics 12, no. 5: 461. https://doi.org/10.3390/photonics12050461
APA StyleTeng, C., Yang, R., Ying, S., Xia, H., Zhang, Y., Shi, L., Deng, S., Chen, Z., Qiao, H., & Yuan, L. (2025). Chitosan/Polyacrylic Acid Functionalized Side-Polish Polymer Optical Fiber-Based SPR Sensor for Cu2+ Ion Detection. Photonics, 12(5), 461. https://doi.org/10.3390/photonics12050461





