Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chernyaev, A.P. Radiation Technologies. Science. National Economy Medicine; Moscow University Press: Moscow, Russia, 2019; p. 234. [Google Scholar]
- European Food Safety Authority. Statement Summarizing the Conclusions and Recommendations from the Opinions on the Safety of Irradiation of Food adopted by the BIOHAZ and CEF Panels. EFSA J. 2011, 9, 2107. [Google Scholar] [CrossRef]
- CAC, 2003, CODEX STAN 106-1983, Rev.1-2003,Codex Alimentarius Commission. In General Standard for Irradiated Foods; Codex Alimentarius, FAO/WHO: Rome, Italy, 2003.
- Sanzharova, N.I.; Kozmin, G.V.; Bondarenko, V.S. Radiation technologies in agriculture: Science and technological development strategy. Innov. Ekspertiza 2016, 16, 197–206. [Google Scholar]
- Jayathilakan, K.; Sultana, K.; Reddy, K.J.; Pandey, M.C. Radiation Processing of Meat and Meat Products—An Overview. Int. J. New Technol. Res. 2015, 1, 5–12. [Google Scholar]
- Akram, K.; Kwon, J.H. Food irradiation for mushrooms: A review. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 257–265. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A. Applications of Food Irradiation Technology. Def. Life Sci. J. 2020, 5, 54–62. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.H.; Lee, H.Y.; Ahn, D.U. Effect of irradiation on the parameters that influence quality characteristics of uncured and cured cooked turkey meat products. Poult. Sci. 2016, 95, 2986–2992. [Google Scholar] [CrossRef]
- Chernyaev, A.P.; Avdyukhina, V.M.; Bliznyuk, U.A.; Borshchegovskaya, P.Y.; Gordonova, I.K.; Ipatova, V.S.; Zolotov, S.V.; Leontiev, V.A.; Nikitina, Z.K.; Rozanov, V.V.; et al. Using Low-Energy Electron Beams for Processing Chilled Turkey Meat. Optimization of Exposure Parameters. Naukoem. Tekhnol. 2020, 21, 40–49. [Google Scholar] [CrossRef]
- Manual of Good Practice in Food Irradiation: Sanitary, Phytosanitary and Other Applications; International Atomic Energy Agency: Vienna, Austria, 2015; p. 104. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/trs481web-98290059.pdf (accessed on 26 July 2022).
- Kim, J.; Moreira, R.G.; Castell-Perez, M.E. Validation of irradiation of broccoli with a 10 MeV electron beam accelerator. J. Food Eng. 2008, 86, 595–603. [Google Scholar] [CrossRef]
- Cárcel, J.A.; Benedito, J.; Cambero, M.I. Modeling and optimization of the E-beam treatment of chicken steaks and hamburgers, considering food safety, shelf-life, and sensory quality. Food Bioprod. Processing 2015, 96, 133–144. [Google Scholar] [CrossRef]
- Qin, H.; Yang, G.; Kuang, S.; Wang, Q.; Liu, J.; Zhang, X.; Li, C.; Han, Z.; Li, Y. Concept development of X-ray mass thickness detection for irradiated items upon electron beam irradiation processing. Radiat. Phys. Chem. 2018, 143, 8–13. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Kumar, R.; Nadanasabapathy, S.; Bawa, A.S. Detection Methods for Irradiated Foods. Compr. Rev. Food Sci. Food Saf. 2008, 8, 4–16. [Google Scholar] [CrossRef]
- Timakova, R.T.; Tikhonov, S.L.; Tararkov, A.N.; Vakhnin, D.O. EPR spectroscopy of spices. Proc. Voronezh State Univ. Eng. Technol. 2016, 4, 187–193. [Google Scholar] [CrossRef][Green Version]
- Drouza, C.; Spanou, S.; Keramidas, A.D. EPR Methods Applied on Food Analysis. In Topics From EPR Research, Chapter 4; IntechOpen: London, UK, 2018; pp. 45–64. [Google Scholar] [CrossRef]
- Aleksieva, K.; Yordanov, N.D. Various approaches in EPR-identification of gamma-irradiated plant foodstuffs: A review. Food Res. Int. 2018, 105, 1019–1028. [Google Scholar] [CrossRef]
- Song, B.-S.; Kim, B.-K.; Yoon, Y.-M.; Jung, K.; Park, J.H.; Kim, J.K.; Kim, C.-T.; Lee, Y.; Kim, D.H.; Ryu, S.-R. Identification of red pepper powder irradiated with different types of radiation using luminescence methods: A comparative study. Food Chem. 2016, 200, 293–300. [Google Scholar] [CrossRef] [PubMed]
- EN13751-2009; Foodstuffs—Detection of Irradiated Food Using Photostimulated Luminescence. CEN: Brussels, Belgium, 2009.
- Rababah, T.; Hettiarachchy, N.S.; Horax, R. Thiobarbituric Acid Reactive Substances and Volatile Compounds in Chicken Breast Meat Infused with Plant Extracts and Subjected to Electron Beam Irradiation. Poult. Sci. 2016, 85, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Mársico, E.T.; Jesus, E.F.; Guimarães, C.F.; Cortez, M.A. Effect of gamma radiation on lipids by the TBARS and NMR. Brazilian. Arch. Biol. Technol. 2011, 54, 1343–1348. [Google Scholar] [CrossRef]
- de Azevedo Gomes, H.; Da Silva, E.N.; do Nascimento, M.R.; Fukuma, H.T. Evaluation of the 2-thiobarbituric acid method for the measurement of lipid oxidation in mechanically deboned gamma irradiated chicken meat. Food Chem. 2003, 80, 433–437. [Google Scholar] [CrossRef]
- Gladilovich, V.D.; Podolskaya, E.P. Possibilities of application of the GC-MS method (Review). Sci. Instrum. 2010, 20, 36–49. (In Russian) [Google Scholar]
- Campaniello, M.; Marchesani, G.; Zianni, R.; Tarallo, M.; Mangiacotti, M.; Chiaravalle, A.E. Validation of an alternative method for the identification of 2-Dodecylcyclebutanone (2-DCB) of irradiated meats by Solid-Phase Microextraction (SPME) Gas Chromatography-Mass Spectrometry (GC-MS). Int. J. Food Sci. Technol. 2019, 55, 961–969. [Google Scholar] [CrossRef]
- D’Oca, M.C.; Bartolotta, A.; Cammilleri, M.C.; Giuffrida, S.A.; Parlato, A.; Di Noto, A.M.; Caracappa, S. The gas chromatography/mass spectrometry can be used for dose estimation in irradiated pork. Radiat. Phys. Chem. 2009, 78, 687–689. [Google Scholar] [CrossRef]
- Bliznyuk, U.A.; Avdyukhina, V.M.; Borshchegovskaya, P.; Bolotnik, T.; Ipatova, V.; Nikitina, Z.; Nikitchenko, A.; Rodin, I.; Studenikin, F.; Chernyaev, A.; et al. Effect of electron and X-ray irradiation on microbiological and chemical parameters of chilled turkey. Sci. Rep. 2022, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Bliznyuk, U.A.; Avdyukhina, V.M.; Borshchegovskaya, P.; Bolotnik, T.A.; Ipatova, V.S.; Rodin, I.A.; Ikhalainen, Y.A.; Studenikin, F.R.; Chernyaev, A.P.; Shinkarev, O.V.; et al. Determination of chemical and microbiological characteristics of meat products treated by radiation. Ind. Laboratory. Diagn. Mater. 2021, 87, 5–13. [Google Scholar] [CrossRef]
- Kudryashov, Y.B. Radiation Biophysics (Ionizing Radiations); Mazurik, V.K., Lomanov, M.F., Eds.; FIZMATLIT: Moscow, Russia, 2004; p. 448. (In Russian) [Google Scholar]
- Tanino, T.; Shibuki, K.; Kubota, K.; Kannari, N.; Matsui, M.; Ohshima, T. Removal of volatile organic compounds in distillation steam by DBD decomposition treatment for water recycling in fermentation industry. Int. J. Plasma Environ. Sci. Technol. 2020, 14, e02003. [Google Scholar] [CrossRef]
- Hee Seo, S.; Park, J.-H.; Kim, K.; Kim, T.H.; Kim, H.W.; Son, Y.S. Decomposition of volatile fatty acids using electron beam irradiation. Chem. Eng. J. 2019, 15, 494–500. [Google Scholar] [CrossRef]
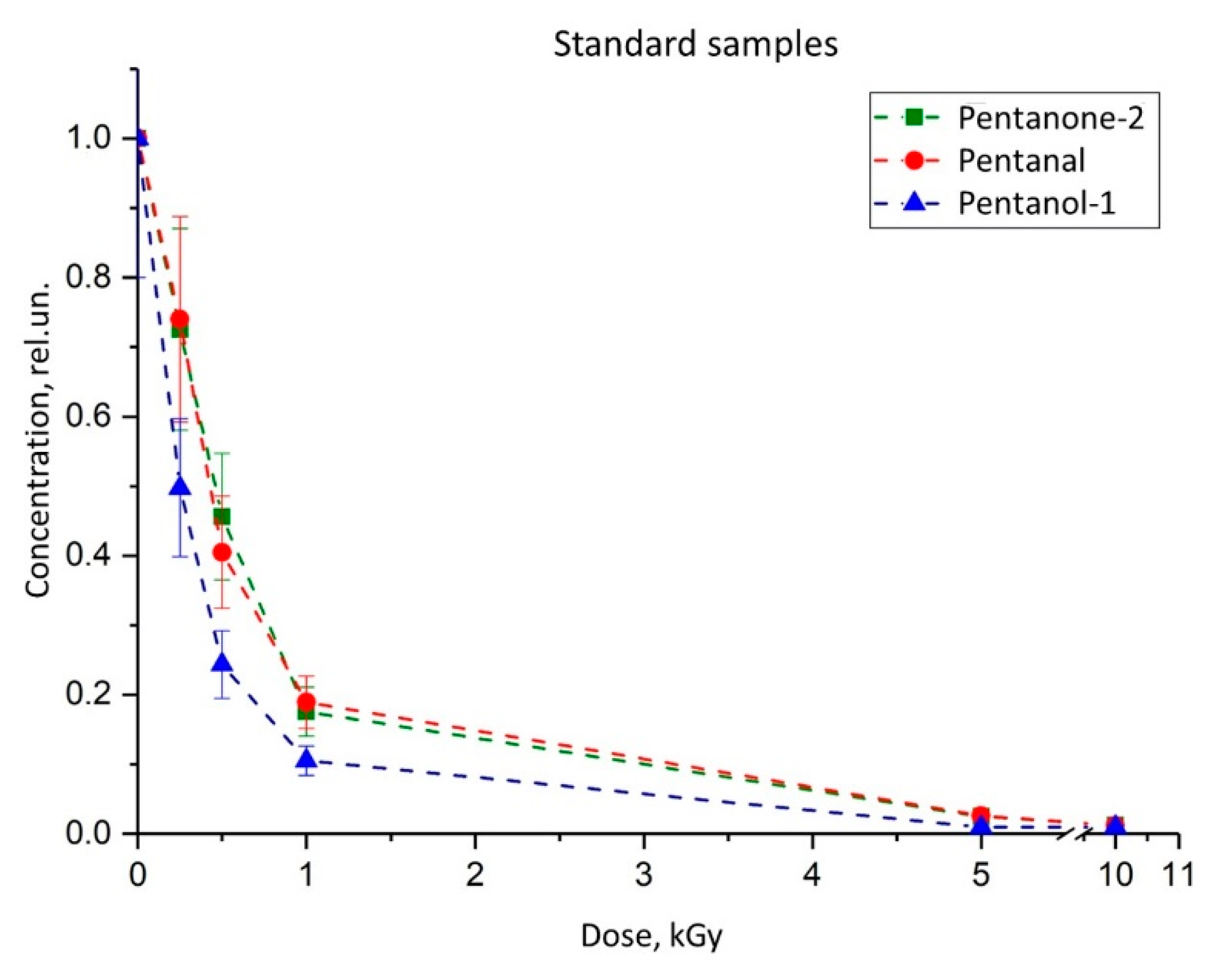
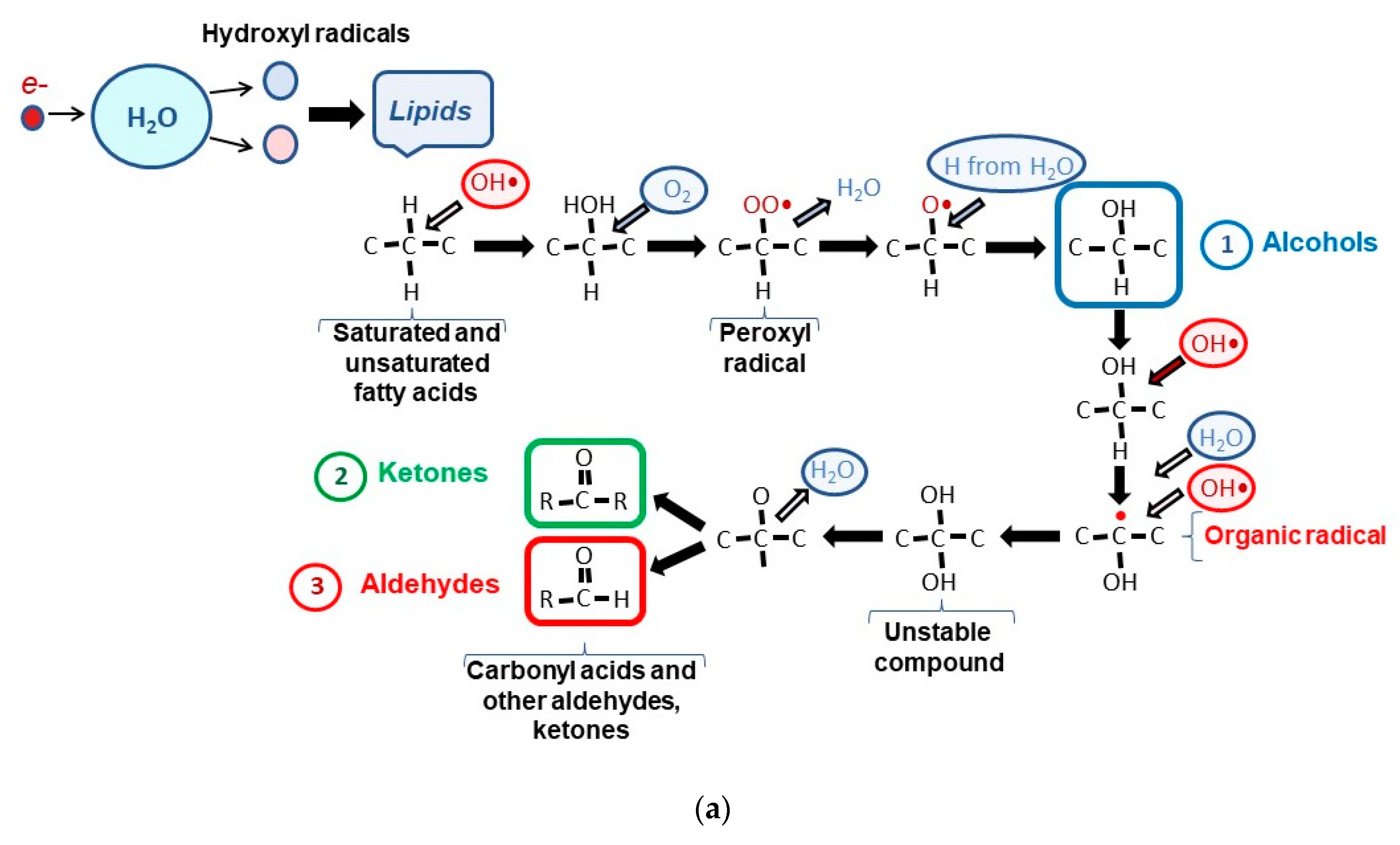

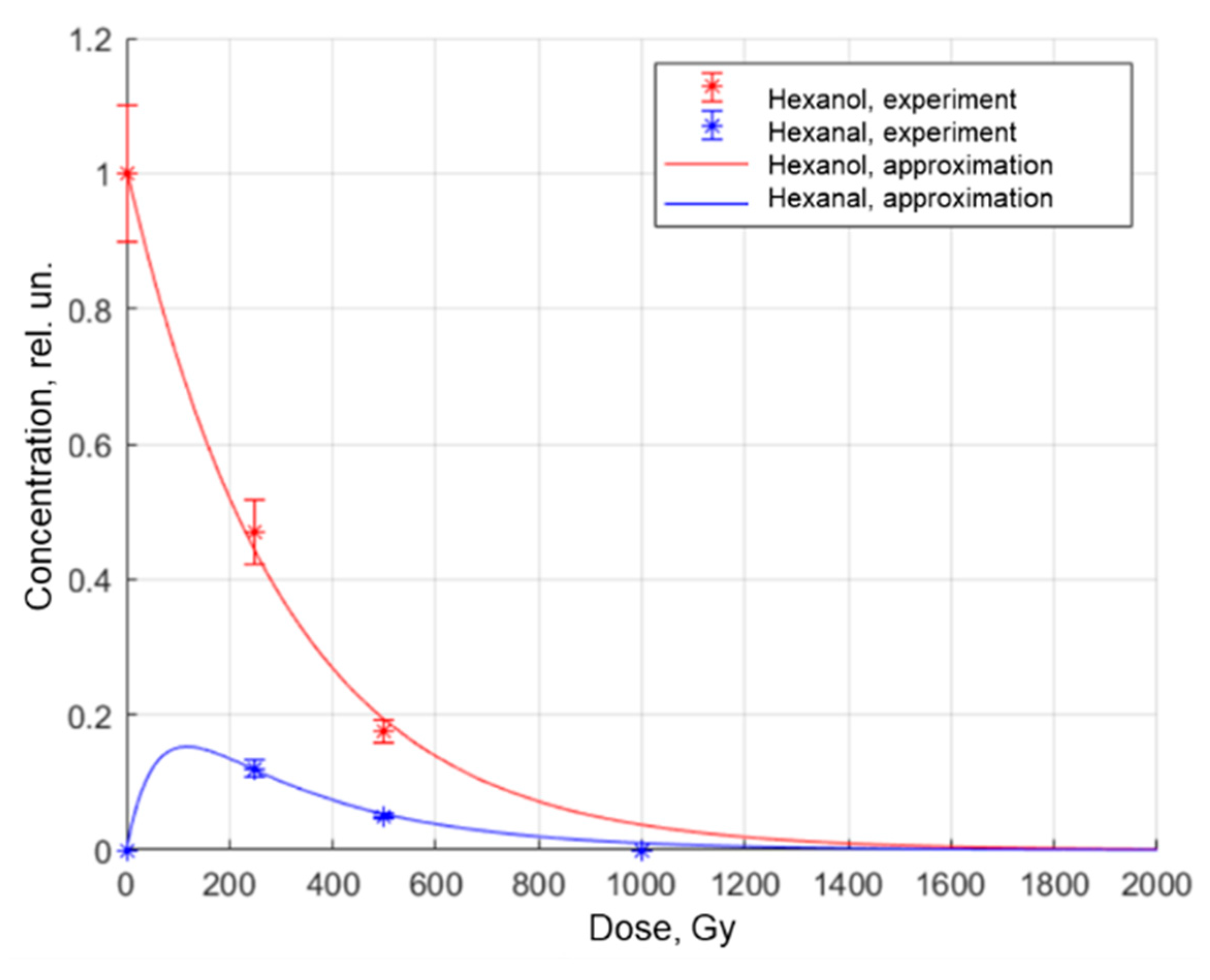

| № | Time of Exposure tirrad, s | The Charge Absorbed by Duraluminium Plate Q, nC | Transmittance τ, rel.un. | Absorbed Dose D, Gy |
|---|---|---|---|---|
| 1 | 34.6 ± 1 | 2390 ± 47 | 0.15 ± 0.01 | 40.8 ± 2.8 |
| 2 | 58.5 ± 1 | 4790 ± 95 | 0.28 ± 0.02 | 79.2 ± 5.5 |
| 3 | 78 ± 1 | 7380 ± 147 | 0.43 ± 0.03 | 120.3 ± 8.4 |
| 4 | 104 ± 1 | 9834 ± 197 | 0.57 ± 0.04 | 159.1 ± 11.1 |
| 5 | 130 ± 1 | 12,410 ± 248 | 0.71 ± 0.05 | 198.6 ± 13.9 |
| 6 | 201 ± 1 | 20,020 ± 400 | 1.16 ± 0.08 | 325.5 ± 22.8 |
| Organic Compound | The Function Parameters | |||||
|---|---|---|---|---|---|---|
| a, mg/L | d, Gy−1 | b, mg/L | c, Gy−1 | k, mg/(L × Gy) | R | |
| Hexanal | 0.99 ± 0.04 | 0.45 ± 0.01 | 0.34 ± 0.01 | 470,317 ± 235 | 0.090 ± 0.001 | 0.97 |
| Heptanal | 1.01 ± 0.05 | 0.87 ± 0.01 | 0.49 ± 0.01 | 5485 ± 25 | 0.041 ± 0.002 | 0.97 |
| Octanal | 1.05 ± 0.05 | 1.05 ± 0.03 | 0.32 ± 0.01 | 593 ± 12 | 0.032 ± 0.005 | 0.95 |
| Nonanal | 0.99 ± 0.04 | 1.45 ± 0.07 | 0.49 ± 0.01 | 3.8 × 106 ± 257 | 0.034 ± 0.002 | 0.97 |
| Pentanal | 1.01 ± 0.04 | 1.35 ± 0.05 | 0.49 ± 0.02 | 8.12 ± 0.92 | 0.045 ± 0.001 | 0.93 |
| Hexanol-1 | 17.07 ± 0.85 | 0.070 ± 0.015 | 1.08 ± 0.05 | 1230 ± 37 | 0.81 ± 0.01 | 0.95 |
| Pentanol-1 | 2.23 ± 0.07 | 0.27 ± 0.01 | 0.83 ± 0.03 | 1264 ± 57 | 0.25 ± 0.01 | 0.97 |
| 3-Methylbutanone-2 | 43.72 ± 0.99 | 7.81 ± 0.85 | 42.27 ± 0.89 | 77,683 ± 210 | −0.051 ± 0.002 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bliznyuk, U.; Borshchegovskaya, P.; Bolotnik, T.; Chernyaev, A.; Ipatova, V.; Nikitchenko, A.; Shinkarev, O.; Yurov, D.; Khmelevskiy, O.; Rodin, I. Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat. Separations 2022, 9, 227. https://doi.org/10.3390/separations9080227
Bliznyuk U, Borshchegovskaya P, Bolotnik T, Chernyaev A, Ipatova V, Nikitchenko A, Shinkarev O, Yurov D, Khmelevskiy O, Rodin I. Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat. Separations. 2022; 9(8):227. https://doi.org/10.3390/separations9080227
Chicago/Turabian StyleBliznyuk, Ulyana, Polina Borshchegovskaya, Timofey Bolotnik, Alexander Chernyaev, Victoria Ipatova, Alexander Nikitchenko, Oleg Shinkarev, Dmitry Yurov, Oleg Khmelevskiy, and Igor Rodin. 2022. "Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat" Separations 9, no. 8: 227. https://doi.org/10.3390/separations9080227
APA StyleBliznyuk, U., Borshchegovskaya, P., Bolotnik, T., Chernyaev, A., Ipatova, V., Nikitchenko, A., Shinkarev, O., Yurov, D., Khmelevskiy, O., & Rodin, I. (2022). Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat. Separations, 9(8), 227. https://doi.org/10.3390/separations9080227







