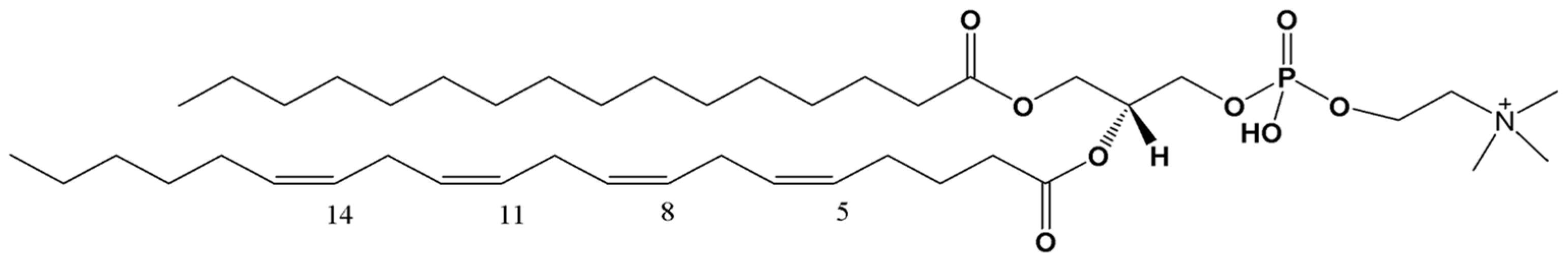
Analysis of Oxidized 1-Palmitoyl-2-Arachidonoyl-Sn-Glycero-3 Phosphocholine Products in Uremic Patients by LC-ESI/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Oxidation of PAPC Standard
2.3. Collection of Human Blood and Separation of Lipoproteins by Ultracentrifugation
2.4. Extraction of Phospholipids from Human Lipoproteins
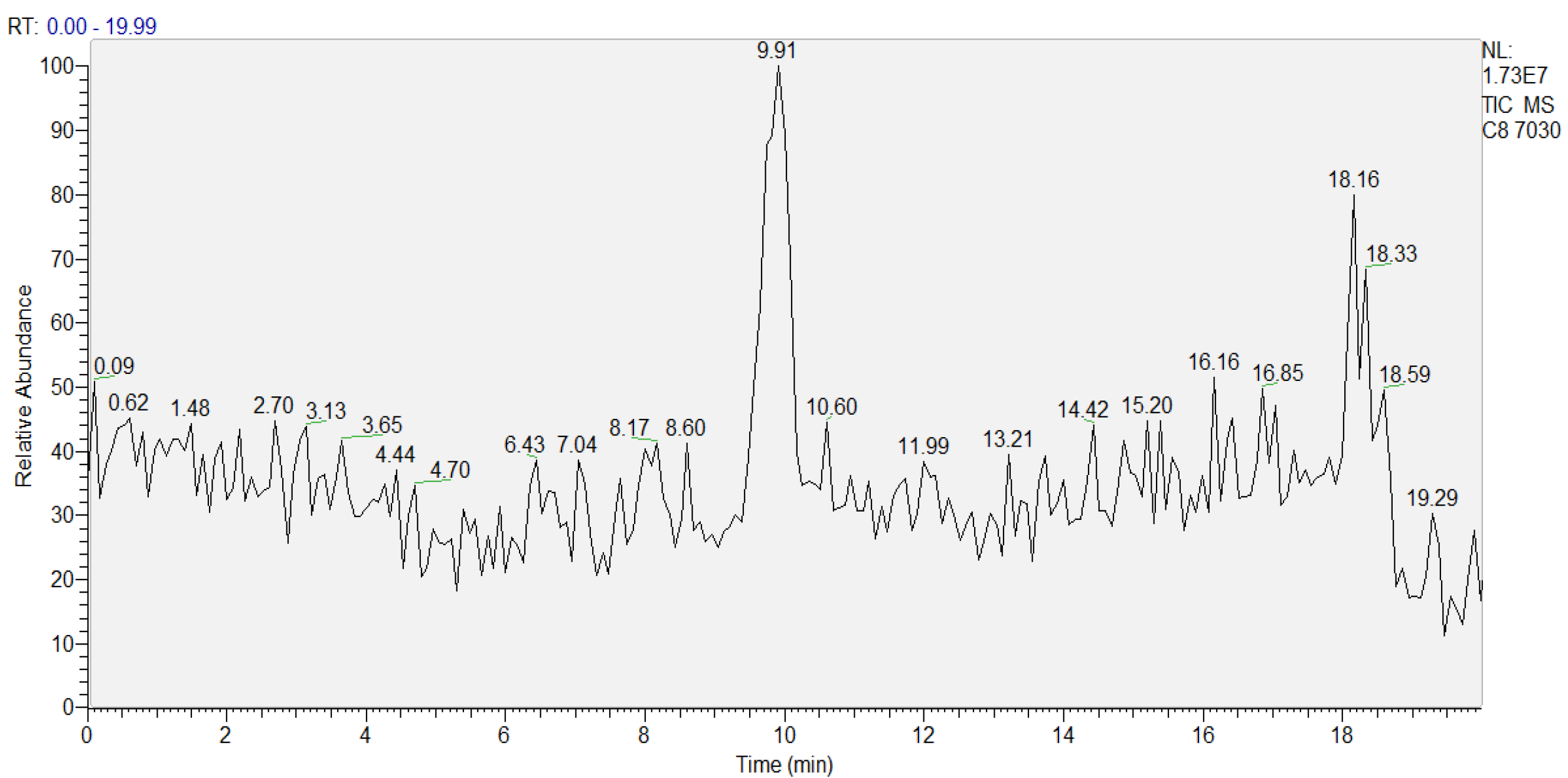
2.5. LC-ESI/MS Analysis of Ox-PAPC Products
3. Results and Discussion
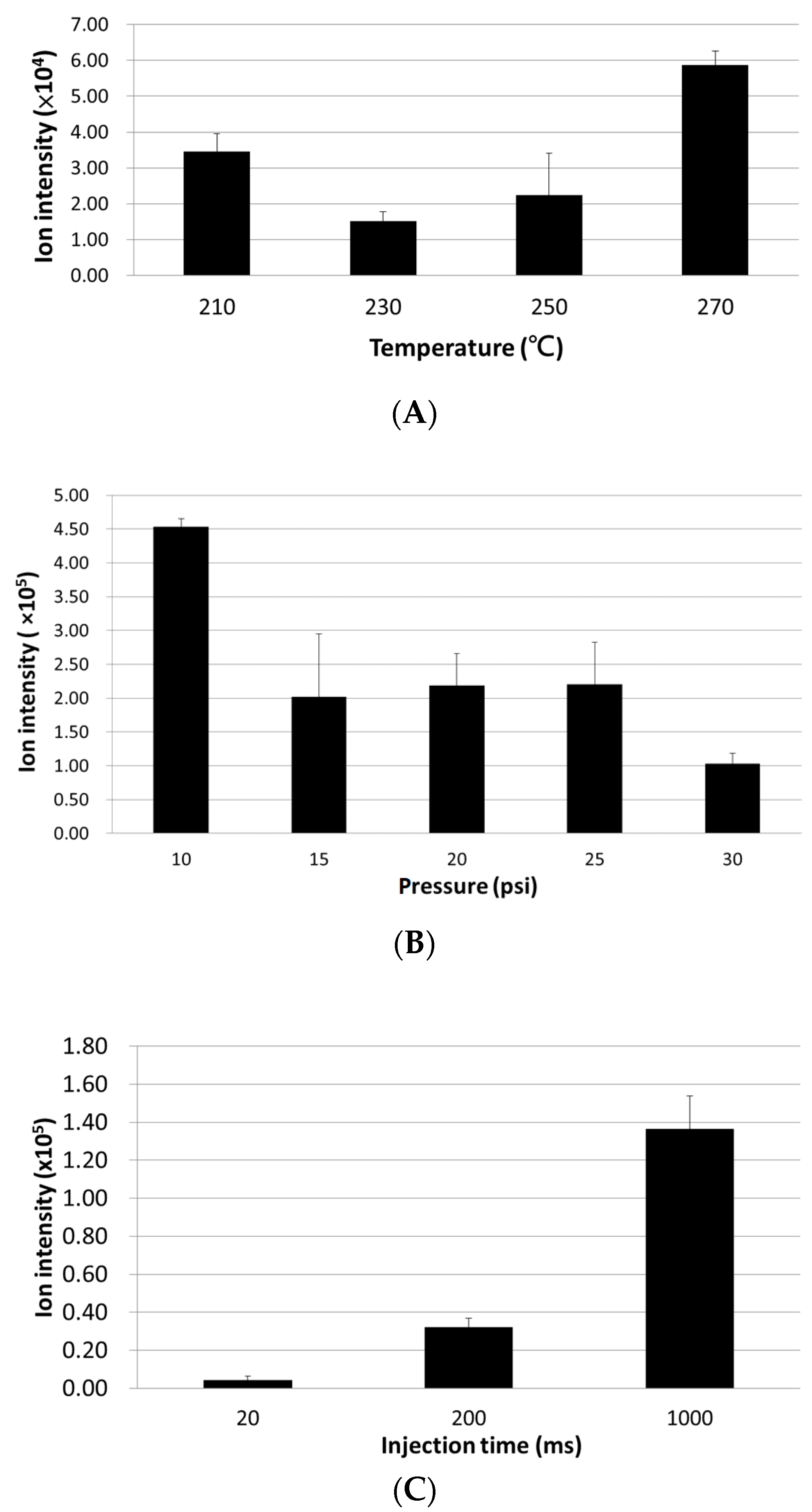
3.1. Optimization of LC ESI-MS Conditions for Analyzing In Vitro Oxidized PAPC Products
3.2. Linear Analysis of the Phospholipid Standard, PC(O-16:0/O-16:0) by LC ESI-MS
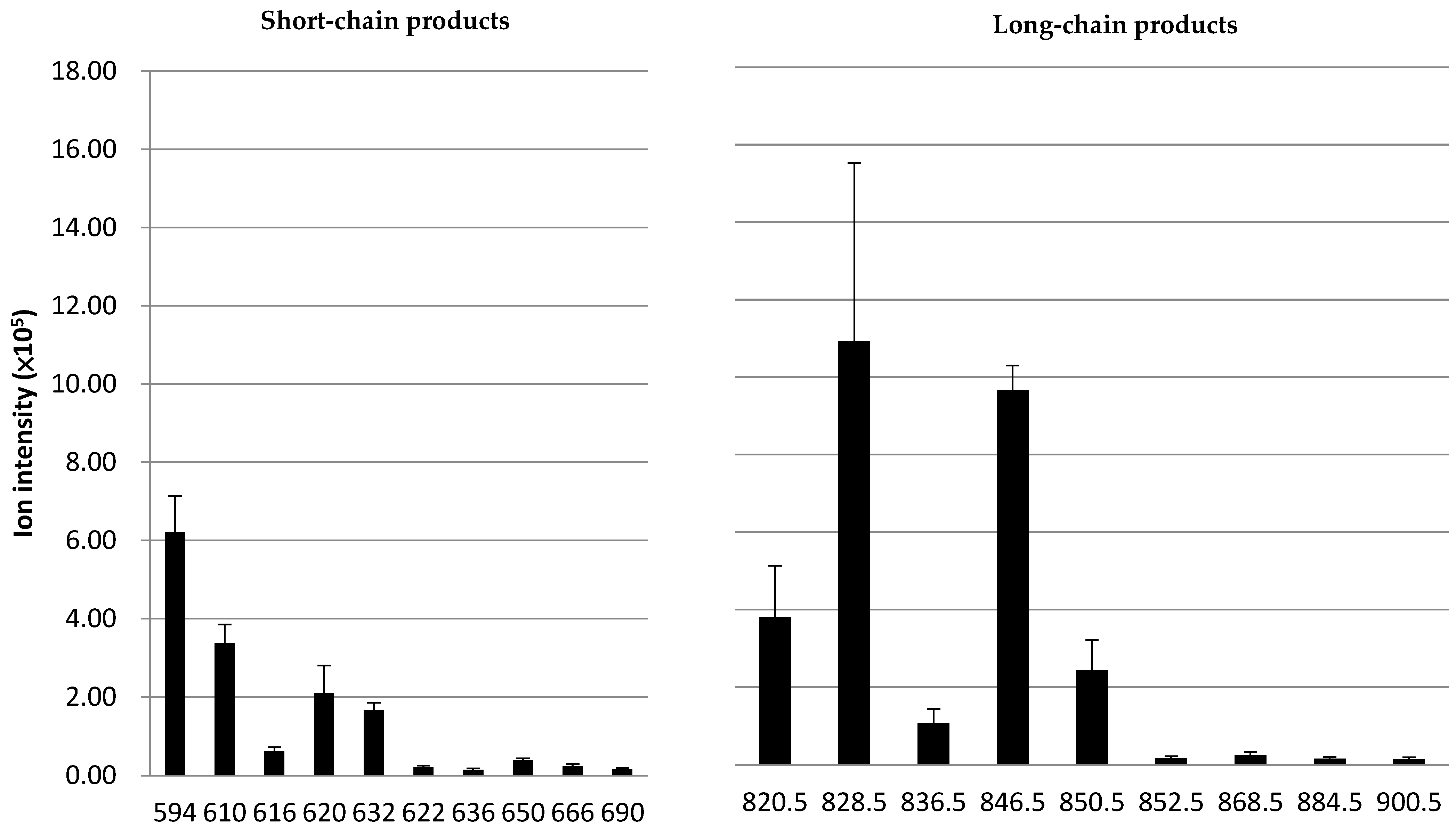
3.3. Analysis of ox-PAPC Products for Healthy and Uremic Subjects by LC ESI-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berliner, J.A.; Territo, M.C.; Sevanian, A. Minimally Modified Low Density Lipoprotein Stimulates Monocyte Endothelial Interactions. J. Clin. Invest. 1990, 85, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.D.; Leitinger, N.; Navab, M. Structural Identification by Mass Spectrometry of Oxidized Phospholipids in Minimally Oxidized Low Density Lipoprotein That Induce Monocyte/Endothelial Interactions and Evidence for Their Presence In Vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.D.; Subbanagounder, G.; Welsbie, D.S. Structural Identification of a Novel Pro-Inflammatory Epoxyisoprostane Phospholipid in Mildly Oxidized Low Density Lipoprotein. J. Biol. Chem. 1999, 274, 24787–24798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berliner, J.A.; Gharavi, N.M. Endothelial Cell Regulation by Phospholipid Oxidation Products. Free Radic. Biol. Med. 2008, 45, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Springstead, J.R.; Gugiu, B.G.; Lee, S. Evidence for the Importance of OxPAPC Interaction with Cysteines in Regulating Endothelial Cell Function. J. Lipid Res. 2012, 53, 1304–1315. [Google Scholar] [CrossRef] [Green Version]
- Baier, J.; Maisch, T.; Maier, M. Direct Detection of Singlet Oxygen Generated by UVA Irradiation in Human Cells and Skin. J. Invest. Dermatol. 2007, 127, 1498–1506. [Google Scholar] [CrossRef]
- Miyamoto, S.; Martinez, G.R.; Rettori, D. Linoleic Acid Hydroperoxide Reacts with Hypochlorous Acid, Generating Peroxyl Radical Intermediates and Singlet Molecular Oxygen. Proc. Natl. Acad. Sci. USA 2006, 103, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Santrock, J.; Gorski, R.A.; O’Gara, J.F. Products and Mechanism of The Reaction of Ozone with Phospholipids in Unilamellar Phospholipid Vesicles. Chem. Res. Toxicol. 1992, 5, 134–141. [Google Scholar] [CrossRef]
- Belkner, J.; Wiesner, R.; Rathman, J. Oxygenation of Lipoproteins by Mammalian Lipoxygenases. Eur. J. Biochem. 1993, 213, 251–261. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J. Cytochrome C Acts as A Cardiolipin Oxygenase Required for Release of Proapoptotic Factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Kuhn, H.; Belkner, J.; Wiesner, R. Oxygenation of Biological Membranes by the Pure Reticulocyte Lipoxygenase. J. Biol. Chem. 1990, 265, 18351–18361. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New Tools and Applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenk, M.R. The Emerging Field of Lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Han, X. Tutorial on Lipidomics. Anal. Chim. Acta. 2019, 1061, 28–41. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Han, R.H. Novel Advances in Shotgun Lipidomics for Biology and Medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.; Domingues, P.; Domingues, M.R. Structural Motifs in Primary Oxidation Products of Palmitoyl-Arachidonoyl-Phosphatidylcholines by LC-MS/MS. J. Mass Spectrom. 2013, 48, 1207–1216. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, P.; Ferrer-Correia, A.J. Fragmentation Study of Short-Chain Products Derived from Oxidation of Diacylphosphatidylcholines by Electrospray Tandem Mass Spectrometry: Identification of Novel Short-Chain Products. Rapid Commun. Mass Spectrom. 2004, 18, 2849–2858. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, P.; Ferrer-Correia, A.J. Tandem Mass Spectrometry of Intact Oxidation Products of Diacylphosphatidylcholines: Evidence for The Occurrence of The Oxidation of the Phosphocholine Head and Differentiation of Isomers. J. Mass Spectrom. 2004, 39, 1513–1522. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, M.; Amado, F.M.; Ferrer-Correia, A.; Domingues, P. Separation of Peroxidation Products of Diacyl-Phosphatidylcholines by Reversed-Phase Liquid Chromatography–Mass Spectrometry. Biomed Chromatogr. 2005, 19, 129–137. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, P.; Ferrer-Correia, A.; Domingues, M. Identification of Free Radicals of Glycerophosphatidylcholines Containing ω-6 Fatty Acids Using Spin Trapping Coupled with Tandem Mass Spectrometry. Free Radic. Res. 2007, 41, 432–443. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, M.; Amado, F.; Ferrer-Correia, A.; Domingues, P. Radical Peroxidation of Palmitoyl-Lineloyl-Glycerophosphocholine Liposomes: Identification of Long-Chain Oxidised Products by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B. 2007, 855, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Bagdade, J.; Casaretto, A.; Albers, J. Effects of Chronic Uremia, Hemodialysis, and Renal Transplantation on Plasma Lipids and Lipoproteins in Man. J. Lab. Clin. Med. 1976, 87, 37–48. [Google Scholar]
- Norbeck, H.E.; Orö, L.; Carlson, L.A. Serum Lipid and Lipoprotein Concentrations in Chronic Uremia. Acta. Med. Scand. 1976, 200, 487–492. [Google Scholar] [CrossRef]
- Janicki, K.; Solski, J.; Janicka, L.; Kimak, E.; Bednarek-Skublewska, A.; Stettner, S.; Molas, G. Lipid and Apolipoproteins (ApoAI, ApoB, Apo CIII, ApoE) Disturbance in Hemodialysis (HD) and Renal Transplant (Tx) Patients. Ann. Univ. Mariae. Curie. Sklodowska Med. 2004, 59, 459–466. [Google Scholar]
- Kes, P.; Reiner, Z.; Brunetta, B. Lipoprotein Disorders in Chronic Kidney Failure, Nephrotic Syndrome and Dialysis. Lijec. Vjesn. 2002, 124, 372–377. [Google Scholar] [PubMed]
- Rao, A.M.; Bitla, A.; Reddy, E.; Sivakumar, V.; Rao, P.S. Lipid Abnormalities, Lipoprotein (a) and Apoprotein Pattern in Non-dialyzed Patients with Chronic Kidney Disease. Indian. J. Clin. Biochem. 2010, 25, 47–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecino, A.; Teruel, J.L.; Navarro, J.L.; Cesar, J.M. Plasma Phospholipids and Platelet Function in Uremic Patients. Am. J. Nephrol. 1996, 16, 409–411. [Google Scholar] [CrossRef]
- Stübiger, G.; Aldover-Macasaet, E.; Bicker, W.; Sobal, G.; Willfort-Ehringer, A.; Pock, K.; Bochkov, V.; Widhalm, K.; Belgacem, O. Targeted Profiling of Atherogenic Phospholipids in Human Plasma and Lipoproteins of Hyperlipidemic Patients Using MALDI-QIT-TOF-MS/MS. Atherosclerosis 2012, 224, 177–186. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Oxidized LDL to Autoantibodies Against oxLDL Ratio–The new Biomarker Associated with Carotid Atherosclerosis and Cardiovascular Complications in Dialyzed patients. Atherosclerosis 2012, 224, 252–257. [Google Scholar] [CrossRef]


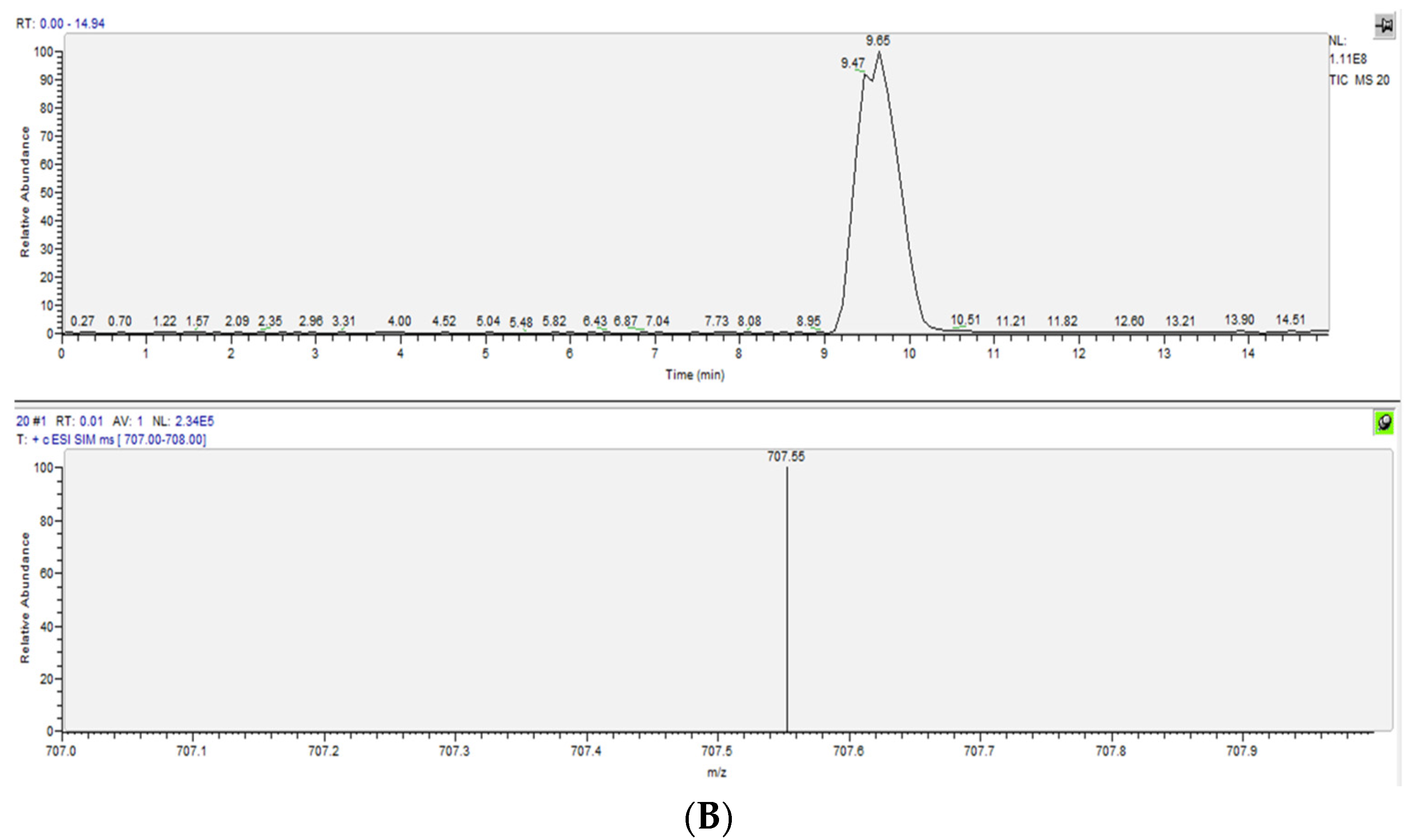
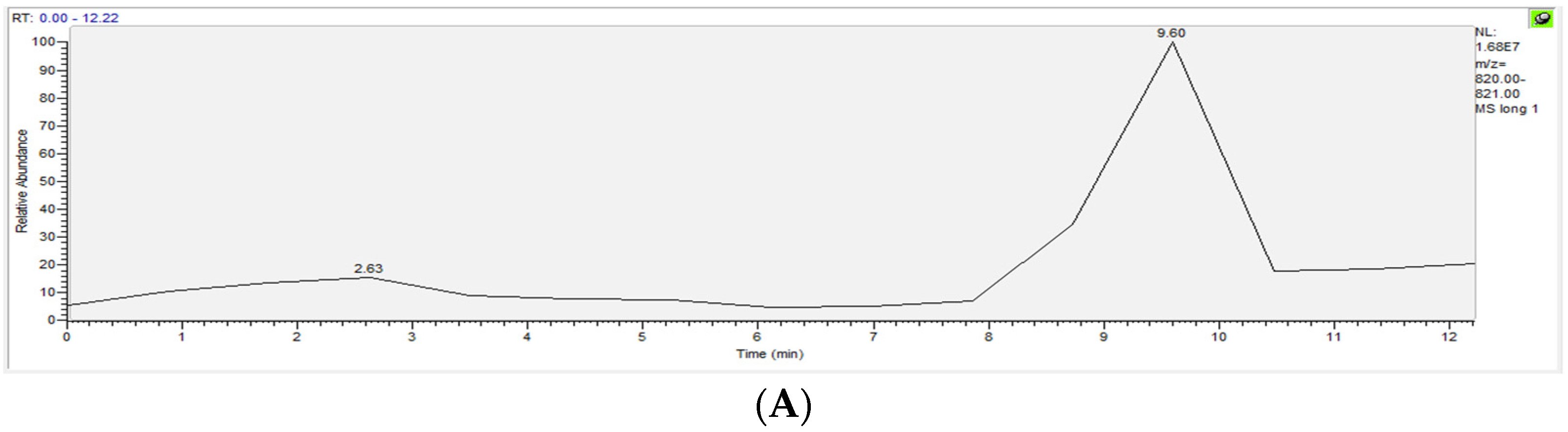

| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 820.5 | 11.71 ± 2.00 | 16.36 ± 3.13 *** |
| m/z 828.5 | 10.14 ± 1.52 | 12.88 ± 3.24 * |
| m/z 836.5 | 10.25 ± 1.63 | 13.15 ± 3.34 * |
| m/z 846.5 | 10.19 ± 1.41 | 12.00 ± 1.86 * |
| m/z 850.5 | 10.59 ± 1.80 | 13.78 ± 3.55 * |
| m/z 852.5 | 11.10 ± 2.56 | 13.41 ± 3.59 |
| m/z 868.5 | 10.08 ± 1.24 | 12.28 ± 1.89 ** |
| m/z 884.5 | 9.82 ± 1.17 | 11.83 ± 1.73 ** |
| m/z 900.5 | 9.78 ± 1.15 | 12.01 ± 2.01 * |
| m/z 782.5 | 11.65 ± 3.45 | 12.23 ± 2.91 |
| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 478 | 16.05 ± 4.25 | 35.56 ± 36.70 |
| m/z 496 | 9.56 ± 0.53 | 10.89 ± 2.21 ** |
| m/z 594 | 10.67 ± 1.39 | 12.48 ± 1.58 ** |
| m/z 610 | 10.85 ± 1.48 | 12.71 ± 1.80 * |
| m/z 616 | 11.80 ± 2.10 | 14.91 ± 2.80 ** |
| m/z 620 | 11.27 ± 1.93 | 14.50 ± 3.02 ** |
| m/z 622 | 11.50 ± 2.38 | 18.58 ± 9.08 * |
| m/z 632 | 12.23 ± 2.23 | 15.55 ± 3.08 ** |
| m/z 636 | 11.82 ± 2.16 | 14.94 ± 2.34 ** |
| m/z 650 | 10.39 ± 1.22 | 12.08 ± 1.38 ** |
| m/z 666 | 10.42 ± 1.17 | 12.47 ± 1.34 *** |
| m/z 690 | 10.29 ± 1.12 | 12.39 ± 1.81 * |
| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 820.5 | 4.92 ± 0.89 | 4.49 ± 0.21 |
| m/z 828.5 | 4.53 ± 0.16 | 4.48 ± 0.32 |
| m/z 836.5 | 4.66 ± 0.28 | 4.53 ± 0.38 |
| m/z 846.5 | 4.58 ± 0.34 | 4.40 ± 0.09 |
| m/z 850.5 | 4.64 ± 0.38 | 4.44 ± 0.10 |
| m/z 852.5 | 4.62 ± 0.23 | 4.66 ± 0.70 |
| m/z 868.5 | 4.57 ± 0.22 | 4.50 ± 0.32 |
| m/z 884.5 | 4.49 ± 0.11 | 4.43 ± 0.22 |
| m/z 900.5 | 4.56 ± 0.34 | 4.44 ± 0.27 |
| m/z 782.5 | 4.45 ± 0.14 | 4.42 ± 0.04 |
| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 478 | 6.22 ± 0.61 | 5.06 ± 0.39 *** |
| m/z 496 | 4.41 ± 0.04 | 4.38 ± 0.06 |
| m/z 594 | 4.69 ± 0.43 | 4.57 ± 0.39 |
| m/z 610 | 4.62 ± 0.19 | 4.50 ± 0.23 |
| m/z 616 | 4.91 ± 0.73 | 4.66 ± 0.54 |
| m/z 620 | 4.75 ± 0.58 | 4.54 ± 0.36 |
| m/z 622 | 4.66 ± 0.43 | 4.50 ± 0.28 |
| m/z 632 | 5.41 ± 1.32 | 4.97 ± 0.85 |
| m/z 636 | 5.52 ± 1.77 | 4.87 ± 0.66 |
| m/z 650 | 4.60 ± 0.34 | 4.52 ± 0.34 |
| m/z 666 | 4.58 ± 0.31 | 4.47 ± 0.31 |
| m/z 690 | 4.60 ± 0.41 | 4.50 ± 0.33 |
| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 820.5 | 2.67 ± 0.07 | 2.56 ± 0.03 *** |
| m/z 828.5 | 2.58 ± 0.04 | 2.54 ± 0.02 ** |
| m/z 836.5 | 2.64 ± 0.06 | 2.55 ± 0.03 ** |
| m/z 846.5 | 2.59 ± 0.04 | 2.54 ± 0.02 ** |
| m/z 850.5 | 2.61 ± 0.06 | 2.54 ± 0.02 ** |
| m/z 852.5 | 2.61 ± 0.05 | 2.54 ± 0.02 ** |
| m/z 868.5 | 2.63 ± 0.07 | 2.56 ± 0.03 ** |
| m/z 884.5 | 2.58 ± 0.06 | 2.54 ± 0.02 * |
| m/z 900.5 | 2.57 ± 0.05 | 2.53 ± 0.01 * |
| m/z 782.5 | 2.55 ± 0.02 | 2.60 ± 0.04 ** |
| ox-PAPC | Healthy Controls (n = 10) µg/mL Average ± SD | Uremic Patients (n = 10) µg/mL Average ± SD |
|---|---|---|
| m/z 478 | 3.98 ± 0.96 | 2.88 ± 0.21 ** |
| m/z 496 | 2.56 ± 0.02 | 2.54 ± 0.05 |
| m/z 594 | 2.62 ± 0.05 | 2.55 ± 0.03 *** |
| m/z 610 | 2.68 ± 0.09 | 2.57 ± 0.05 ** |
| m/z 616 | 2.66 ± 0.06 | 2.58 ± 0.03 ** |
| m/z 620 | 2.59 ± 0.03 | 2.55 ± 0.02 ** |
| m/z 622 | 2.57 ± 0.02 | 2.54 ± 0.03 ** |
| m/z 632 | 3.43 ± 0.26 | 2.88 ± 0.26 *** |
| m/z 636 | 3.40 ± 0.25 | 2.80 ± 0.24 *** |
| m/z 650 | 2.72 ± 0.12 | 2.56 ± 0.03 ** |
| m/z 666 | 2.57 ± 0.04 | 2.54 ± 0.02 * |
| m/z 690 | 2.60 ± 0.05 | 2.56 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-T.; Chang, I.-T.; Hsia, M.-H.; Wang, C.-C.; Chen, C.-J.; Zou, H.-B.; Chen, Y.-Q.; Chiu, W.-C.; Lin, Z.-R.; Liu, M.-Y. Analysis of Oxidized 1-Palmitoyl-2-Arachidonoyl-Sn-Glycero-3 Phosphocholine Products in Uremic Patients by LC-ESI/MS. Separations 2022, 9, 192. https://doi.org/10.3390/separations9080192
Chang C-T, Chang I-T, Hsia M-H, Wang C-C, Chen C-J, Zou H-B, Chen Y-Q, Chiu W-C, Lin Z-R, Liu M-Y. Analysis of Oxidized 1-Palmitoyl-2-Arachidonoyl-Sn-Glycero-3 Phosphocholine Products in Uremic Patients by LC-ESI/MS. Separations. 2022; 9(8):192. https://doi.org/10.3390/separations9080192
Chicago/Turabian StyleChang, Chiz-Tzung, I-Ting Chang, Min-Hui Hsia, Chun-Cheng Wang, Chao-Jung Chen, Hsin-Bai Zou, Yu-Qing Chen, Wen-Chien Chiu, Zhi-Ru Lin, and Mine-Yine Liu. 2022. "Analysis of Oxidized 1-Palmitoyl-2-Arachidonoyl-Sn-Glycero-3 Phosphocholine Products in Uremic Patients by LC-ESI/MS" Separations 9, no. 8: 192. https://doi.org/10.3390/separations9080192
APA StyleChang, C.-T., Chang, I.-T., Hsia, M.-H., Wang, C.-C., Chen, C.-J., Zou, H.-B., Chen, Y.-Q., Chiu, W.-C., Lin, Z.-R., & Liu, M.-Y. (2022). Analysis of Oxidized 1-Palmitoyl-2-Arachidonoyl-Sn-Glycero-3 Phosphocholine Products in Uremic Patients by LC-ESI/MS. Separations, 9(8), 192. https://doi.org/10.3390/separations9080192






