Abstract
Laurus azorica (Seub.) Franco is an endemic species from the Azores, traditionally used in all the islands as a seasoning in cooking. The studies carried out with this species refer mainly to its essential oils. The study that was developed here allowed, for the first time, to determine the chemical composition and biological activities of the ethanol extract, fractions, and pure compounds from L. azorica. The hexane fraction was analyzed by GC–MS and revealed the presence of 48 compounds, comprising mainly fatty acids, fatty alcohols and terpenes, the family of fatty alcohols identified here for the first time in the genus Laurus. Three sesquiterpene lactones—costunolide, 11,13-dehydrosantonin and reynosin—were isolated for the first time in L. azorica from the same fraction, and structurally characterized using spectroscopic techniques. The compounds identified belong to families known to have relevant medicinal and nutritional properties. Regarding antioxidant activities, the results obtained showed a moderate radical scavenging effect of extracts and fractions, while in the β-carotene bleaching assay, costunolide was shown to be the most active (IC50 = 4.08 ± 0.76 μg/mL), about 3.6 times more active than the standard, gallic acid, which presented IC50 = 14.56 ± 0.13 μg/mL. Although the inhibition of extracellular matrix-degrading enzymes was not detected, the ethanol extract showed good inhibitory activity of tyrosinase, with an IC50 of 12.04 ± 0.23 μg/mL, only 6.6-fold lower than the control kojic acid. The results presented deepen the knowledge about a little studied species, opening new perspectives for the development of value-added applications in the food and cosmeceutical fields.
1. Introduction
Plant extracts are proving to be a considerable source of phytochemicals with beneficial effects [1]. They are excellent in the development of, for example, food additives [2,3], new medicines and cosmetic products [4,5]. These phytochemicals have been explored due to their diversified medicinal effects and nutritional value, showing the potential to replace synthetic ones [6,7]. Despite this, much remains to be investigated regarding the isolation and identification of secondary metabolites from poorly studied natural sources, as well as the investigation of new applications for plants, extracts and secondary metabolites.
Laurus azorica (Seub.) Franco, known in the Azores as “louro-das-ilhas” and “louro-bravo”, is an important member of the native highland subtropical forest species, Laurisilva, although it is not always the dominant tree species [8]. It belongs to the genus Laurus and the family Lauraceae [9], and it is one of those plants little studied from the chemical point of view and whose biological activities are important to value. On the other hand, it belongs to the same genus as Laurus nobilis L., native to Mediterranean countries, well known as a condiment to traditional dishes and whose chemical composition and health/nutritional effects are already studied [10,11,12]. The great diversity of documented beneficial effects of L. nobilis suggests similar properties for Laurus target species.
Laurus azorica has traditionally been used in all the Azores archipelago as a seasoning in cooking [13]. This species has also been used as a disinfectant [13] and the oil from the ripe berries has been used to treat wounds, as well as being used for illumination [14].
The few published studies involving Azorean L. azorica are focused on its chemical composition of essential oils (mainly composed of monoterpenes and sesquiterpenes [15]), and its bioactivities (hepatoprotective [16], insecticidal [17], fumigant [18], and molluscicide [19] effects). The antithrombin activity of dichloromethane and methanol extracts of L. azorica [20] and the antioxidant activities of ethanol, aqueous and hydroalcoholic extracts from the leaves [21] are the only published studies not involving the essential oils of this species.
Separation and hybrid methods are essential to isolate, purify, identify, and even quantify the secondary metabolites from natural sources [22,23], while sensitive bioassays allow for the assessment of potential applications. Therefore, this study was designed to (using gas chromatography coupled to mass spectrometry (GC–MS), thin layer chromatography (TLC) and nuclear magnetic resonance (NMR)) isolate, identify and quantify secondary metabolites, and then evaluate the antioxidant and enzymatic inhibition potential of lipophilic constituents of L. azorica. This will contribute to increasing the knowledge that we have of this species, both from the point of view of both its chemical profile and potential biological effects.
2. Materials and Methods
2.1. Chemicals
All the solvents used in preparative chromatography (TLC and CC) were of analytical grade. Solvents used for GC–MS analysis were purchased from Panreac and Acros Organics and were of analytical grade. Chemicals such as N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (99%) and trimethylsilyl chloride (TMSCl) (99%), purchased from Sigma-Aldrich, were used as derivatization agents. Octadecane (99%), myristic acid (99%), ethyl heptadecanoate (>99%), (9Z) octadecenoic acid (99%), (9Z,12Z) octadecadienoic acid (99%), β-sitosterol (99%), phytol (97%), squalene (98%) and 1-monolaurin (99%) were purchased from Sigma-Aldrich and used as standards for qualitative and quantitative GC–MS analysis.
2.2. Plant Material
Aerial parts of Laurus azorica (Seub.) Franco were collected in a small area around GPS coordinates (37°78′53.57″ N, −25°46′46.18″ W) in Estrada das Lombadas, Ribeira Grande, São Miguel Island, Azores, in February 2020. The species was taxonomically identified by Prof Luís Silva, responsible for the Ruy Telles Palhinha Herbarium (AZB), where a voucher specimen number AZB 3669 was deposited. The leaves were separated from the stems and air-dried in the absence of light.
2.3. Extract Preparation and Liquid/Liquid Partition
About 200 g of dried and ground L. azorica leaves were extracted with EtOH (10% w/v) by maceration at room temperature, under mechanical agitation and in the absence of light, by 3 cycles of 72 h each, with solvent renewal at the end of each cycle. The extraction yield was 13.7%.
About 10 g of dry EtOH extract was suspended in water (200 mL) and fractionated by liquid–liquid partition using the solvent n-hexane (3 × 200 mL). The solvent of these combined hexane fractions was evaporated to dryness in a rotary evaporator, under vacuum, at a temperature of 40 °C, obtaining the lipophilic fraction of L. azorica (fraction A).
2.4. Isolation of Secondary Metabolites
Fraction A (2.56 g) was fractionated by column chromatography (CC) using silica gel 230–400 Mesh as the stationary phase, and mixtures of hexane: ethyl acetate (Hx:AcOEt) of increasing polarity (9:1; 8:2; 7:3; 1:1; 3:7; 0:10) as the mobile phase. Sixty subfractions were collected (FA.1–FA.60).
Subfraction FA.7 was purified by preparative TLC (20 × 20 cm) on silica gel 60 with a fluorescent indicator (GF254), using CHCl3 as eluent, yielding 4 fractions (FA.7.1–FA.7.4), while FA.8 was rechromatographed on similar conditions (TLC on silica gel 60 GF254, eluted with CHCl3 (twice), giving 4 fractions (FA.8.1–FA.8.4). The fractions FA.7.1 (20.3 mg) and FA.8.1 (8.0 mg) are pure, consisting of compound 1 (28.3 mg).
Subfractions FA.32 and FA.33 were purified by preparative TLC, using as eluents Hx:AcOEt (1:1) and Hx:AcOEt (4:6), respectively, giving rise to two fractions each, FA.32.1–FA.32.2 and FA.33.1–FA.33.2. The fractions FA.32.2 (1 mg) and FA.33.2 (0.4 mg) are pure fractions, which led to obtaining compound 2 (1.4 mg).
Subfraction FA.34 was rechromatographed on a similar preparative TLC, eluted with Hx:AcOEt (1:1) and gave two fractions (FA.34.1–FA.34.2). Compound 3 was identified on the pure fraction FA.34.2 (1.2 mg).
2.5. Identification of Isolated Compounds by Spectroscopic Techniques
Compounds 1, 2 and 3 were identified by analyzing 1D (1H, 13C, DEPT 90 and 135) and 2D (COSY, HSQC, HMBC (71 ms) and H2BC) NMR spectra, obtained at room temperature using a Bruker Avance NMR apparatus operating at 300.13 MHz and 500.13 MHz for 1H. Chemical shifts (δ) are expressed in ppm, with respect to the internal tetramethylsilane reference, while coupling constants (J) are expressed in Hz.
The mass spectra of the pure compounds were obtained using an LXQ Linear Ion Trap mass spectrometer (ThermoFinnigan) and positive-mode electrospray (ESI(+)). The ESI conditions were as follows: 5 kV, capillary temperature at 275 °C and gas flow at 5 U. Data collection and analysis were performed using the Xcalibur Data System version 2.0.
Compound 1: Costunolide, whitish solid; 1H, 13C NMR data in Table 1. MS ESI(+) m/z 233 [M + H]+, m/z 255 [M + Na]+, (calcd for C15H20O2 232).

Table 1.
1H, 13C, COSY, HMBC and H2BC NMR data of compound 1 (in CDCl3; 300.13 MHz; δ = ppm).
Compound 2: 11,13-Dehydrosantonin, yellow solid; 1H, 13C NMR data in Table 2. MS ESI(+) m/z 245 [M + H]+ and m/z 267 [M + Na]+, (calcd for C15H16O3 244).

Table 2.
1H, 13C, COSY, HMBC and H2BC NMR data of compound 2 (in CDCl3; 500.13 MHz; δ = ppm).
Compound 3: Reynosin, white solid; 1H, 13C NMR data in Table 3. MS ESI(+) m/z 249 [M + H]+ and m/z 271 [M + Na]+, (calcd for C15H20O3 248).

Table 3.
1H, 13C, COSY, HMBC and H2BC NMR data of compound 3 (in CDCl3; 500.13 MHz; δ = ppm).
2.6. GC–MS Analysis
Four aliquots (ca. 20 mg each) of a dried hexane fraction of L. azorica (fraction A) were dissolved in four screw-cap glass tubes with 1 mL of dichloromethane. To each tube was added the internal standard (300 μL of octadecane standard solution at 17.9 mg/10.0 mL), 250 μL of pyridine, 250 μL of BSTFA and 50 μL of TMSCl; all of them were silylated at 70 °C for 30 min [24,25]. Each silylated tube was analyzed, in duplicate, by GC–MS using the QP2010 Ultra Shimadzu instrument. Separation of compounds was performed on a DB-5 J&W capillary column (30 m × 0.25 mm internal diameter, 0.25 µm film thickness) using helium as the carrier gas (1.16 mL/min). The chromatographic conditions were as follows: initial temperature 100 °C for 2 min; temperature rise at 10 °C/min to 150 °C; at 3 °C/min to 235 °C; at 10 °C/min to 260 °C; at 2.3 °C/min to 300 °C, which was held for 5 min. The inlet temperature was 320 °C; the transfer line temperature was 300 °C, using splitless 1:50.
The mass spectrometer detector was operated in electron impact (EI) mode with an energy level of 70 eV, and data were collected at a rate of 1 scan/s in a range of m/z 50–1000. The ion source was maintained at 200 °C. The chromatography performance lasted 60.22 min and the fraction components were identified by comparison with the MS of authentic compounds obtained in the same experimental conditions, or with the MS library supplied with the equipment (NIST 14 Mass Spectral and Wiley Registry of Mass Spectral Data), as well as by detailed analysis of the mass spectrum obtained, seeking to identify typical fragmentation patterns of natural compound families described in the literature.
A calibration curve for each identified compound of fraction A was achieved using standard solutions of different concentrations (4 to 5 different concentrations), consisting of at least one standard compound from each family of organic compounds identified (namely, myristic acid, heptadecanoic acid ethyl ester, phytol, monolaurin, oleic acid, squalene, linoleic acid and sitosterol) and the internal standard (octadecane). The standard solutions, before being injected into the GC–MS, were subjected to the derivatization process described above. The quantitative analysis was performed by interpolation on the calibration curves, with the correlation coefficient r2 ≥ 0.99.
To statistically compare the mean in independent aliquots, a one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test, was applied using GraphPad Software (GraphPad Prism version 7 for Windows) (p < 0.001 was considered statistically significant in all analyses).
2.7. Biological Activities
2.7.1. DPPH Radical Scavenging Activity
Antioxidant activity was determined using the DPPH spectrophotometric method modified from Blois [26]. In a 96-well plate, serial dilutions were performed in methanol at different concentrations, between 250 μg/mL and 0.244 μg/mL for the extract, fraction and compounds. DPPH was added to the microwells so that the final concentration was 45 µg/mL. After 30 min in the dark, absorbance (Abs) was measured at 515 nm. The percentage of antioxidant activity (% AA) was calculated with the following formula:
where Abscontrol is the absorbance of the control and Abssample is the absorbance of the sample. Results were expressed as IC50 (sample concentration required for 50% inhibition/scavenging of the DPPH radical), calculated by interpolation from the % AA vs. concentration curve. Trolox was used as a positive control of antioxidant activity.
% AA = [(Abscontrol − Abssample)]/(Abscontrol)] × 100
2.7.2. ABTS Radical Scavenging Activity
The assay of antioxidant activity by the ABTS method was performed using the method described by Re et al. [27] and adapted by Zárate et al. [28]. An ABTS solution was prepared by mixing 7 mM ABTS and 2.4 mM potassium persulfate in equal amounts and placing in the dark for 12–16 h. 1 mL of the solution was diluted with the necessary amount of methanol to obtain an Abs of 0.7 at 734 nm. In a 96-well plate, serial dilutions were performed in methanol at different concentrations, between 250 μg/mL and 0.244 μg/mL for the extract, fractions, and compounds. The ABTS solution was added to the microwells and, after 8 min of incubation, Abs was measured at 405 nm. The % AA was calculated with the following formula:
where Abscontrol is the absorbance of the control and Abssample is the absorbance of the sample. Results were expressed as IC50 calculated by interpolation from the % AA vs. concentration curve. Trolox was used as a positive control of the antioxidant activity.
% AA = [(Abscontrol − Abssample)]/(Abscontrol)] × 100
2.7.3. Inhibition of β-Carotene Bleaching Assay
Antioxidant activity was determined according to the methods described in Barreira et al. [29] and Lu et al. [30] and adapted by Zárate et al. [28]. A solution of β-carotene (2 mg of β-carotene in 10 mL of chloroform) was prepared. In a 100 mL flask, 2 mL of the solution, 40 mg of linoleic acid and 400 mg of Tween 80 were placed. The resulting mixture was evaporated under vacuum at 40 °C in order to remove the chloroform, and 100 mL of distilled water was added. In a 96-well plate, serial dilutions were performed in methanol at different concentrations, between 75 μg/mL and 0.146 μg/mL for the extract, and between 250 μg/mL and 0.244 μg/mL for the fractions and compounds. 160 µL of β-carotene solution was added and the Abs was measured at 490 nm. The plate was incubated at 50 °C for 120 min and the Abs was measured again. The inhibition of lipid peroxidation was calculated using the following formula:
where “a” is the initial absorbance (0 min), “b” is the absorbance at 120 min and “t” is the total assay time (120 min). The % AA was calculated with the following formula:
Degradation rate (DR) = ln(a/b) × 1/t
% AA = (DRcontrol − DRsample) DRcontrol × 100
Results were expressed as IC50, calculated by interpolation from the % AA vs. concentration curve. Gallic acid was used as a control of inhibition of the β-carotene bleaching activity.
2.7.4. Tyrosinase Inhibition Assay
The assay was performed according to the method described by Shimizu et al. [31], modified by Manosroi et al. [32] and adapted by Zárate et al. [28]. In a 96-well plate, serial dilutions were performed at different concentrations, between 250 μg/mL and 0.488 μg/mL for the extract, fraction and compounds, in 100 mM phosphate buffer, pH 6.8. To the same 96-well plate, 25 µL of buffer and 25 µL of tyrosinase enzyme solution were added and incubated for 20 min at 37 °C. Then, 50 µL of the 1.66 mM tyrosine solution was added and the Abs was measured at 490 nm every 10 min for 30 min. The % enzyme inhibition was calculated using the following formula:
where Abscontrol is the absorbance of buffer + tyrosinase + solvent and Abssample is the Abs of buffer + tyrosinase + sample. The results were expressed as IC50 (concentration that inhibits 50% of the enzyme activity), calculated by interpolation from the inhibition of tyrosinase vs. a sample concentration curve. Kojic acid was used as the standard inhibitor.
% of tyrosinase inhibition = [(Abscontrol − Abssample)/Abscontrol] × 100
2.7.5. Statistical Analysis
The assays were carried out in triplicate. The results were expressed as mean values ± standard deviations. The data were analyzed using a one-way ANOVA with Tukey’s test; p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Structural Characterization of Isolated Compounds
The analysis of a lipophilic fraction from L. azorica leaves (fraction A) by preparative chromatographic techniques led to the isolation of three compounds. The chemical structures of the isolated compounds, shown in Figure 1, were determined by analyzing the NMR and ESIMS spectra.
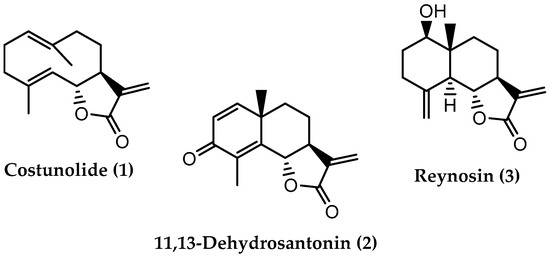
Figure 1.
Chemical structures of compounds isolated from a lipophilic fraction of the ethanolic extract of L. azorica leaves.
The analysis of the NMR data (Table 1) obtained in compound 1, and comparing them with the 1H and 13C data described in the literature [33,34,35,36], allowed us to elucidate the chemical structure of compound 1, which corresponds with costunolide (C15H20O2). The MS spectrum of compound 1 exhibits signals at m/z 233 [M + H]+ and at m/z 255 [M + Na]+, data consistent with the chemical formula of costunolide.
Costunolide (1) is a sesquiterpene lactone from the germacranolide subclass, isolated for the first time from the root of the species Saussurea costus (Falc.) Lipsch (syn Saussurea lappa Clarke) [37,38]. The natural occurrence of costunolide (1) is also reported in Laurus novocanariensis [36,39] and in L. nobilis [34,35,40,41,42], while in L. azorica, costunolide (1) was identified here for the first time. The spectroscopic data indicated in Table 1 are in accordance with the previously reported data, which also confirm the chiral centers configuration indicated in compound 1 (Figure 1).
Costunolide has been described as having several therapeutic effects [38], confirmed by in vivo studies, such as an anticancer effect, by several different pathways against gastric adenocarcinoma and osteosarcoma in xenografted mice models [37], and an anti-inflammatory effect [38].
As for compound 2, the MS data showed peaks at m/z 245 [M + H]+ and m/z 267 [M + Na]+, which means that the compound has a molecular mass of 244, suggesting the molecular formula C15H16O3. The chemical structure of compound 2 was elucidated based on NMR data (Table 2), which are in agreement with those indicated in the literature [43,44], confirming that compound 2 corresponds to 11,13-dehydrosantonin, another sesquiterpene lactone from the eudesmanolide subclass, biosynthesized from α-santonin [10,45]. In L. azorica, it was identified for the first time in this study, having already been isolated from the dichloromethane extract of the leaves of L. nobilis harvested in Turkey [41,46]. Studies carried out with 11,13-dehydrosantonin have mainly evaluated its cytotoxic activity [43,45,46].
Regarding compound 3, the analysis of the spectroscopic data (Table 3) and the comparison with those reported in the literature [35,47] allowed the structural elucidation of compound 3 (Figure 1), identifying it as reynosin. The MS spectrum shows two peaks at m/z 249 [M + H]+ and m/z 271 [M + Na]+, which indicates that the compound has a molecular mass of 248, consistent with the molecular formula C15H20O3 of reynosin.
Like 11,13-dehydrosantonin (2), reynosin (3) is also a sesquiterpene lactone from the eudesmanolide subgroup [10], having been isolated first from the species Ambrosia confertiflora [48]. In this study, it was identified in L. azorica for the first time, having already been isolated and identified in the species L. nobilis [35,41,42,46] and L. novocanariensis [39]. Reynosin is described as a cytotoxic agent against A2780 human ovarian cancer cell lines [41], and able to inhibit nitric oxide production in lipopolysaccharide (LPS)-activated murine macrophages [49].
3.2. GC–MS Analysis of Fraction A
The hexane fraction of the ethanolic extract from L. azorica leaves, called fraction A, was also analyzed by GC–MS to further assess the lipophilic profile of this species, both with regard to volatile compounds and fatty acids whose reference method of analysis is GC–MS. This analysis allowed the identification and quantification of 48 compounds (Table 4).

Table 4.
Results of qualitative and quantitative analysis of a lipophilic fraction of L. azorica leaves by GC–MS.
Considering that fraction A, before being injected into the GC–MS, was derivatized, compounds that have hydroxyl groups in their chemical structure were detected as silylated derivatives. However, the compounds existing in the fraction and identified in Table 4 are the corresponding non-silylated ones.
Table 4 shows fraction A as a complex mixture of metabolites belonging to the families of fatty acids, long-chain alcohols, terpenes, sterols, esterified fatty acids and a small quantity of other kinds of compounds.
The results of quantitative analysis, indicated in Table 4, show that the total mass of compounds corresponds to 74.6% (SD = 4.47) of the mass of fraction A analyzed. Thus, these results are representative of the chemical composition of fraction A.
The five most abundant compounds in fraction A are deacetyllaurenobiolide (Rt 29.0 min, 137 mg/100 g fresh leaves), reynosin (Rt 28.2 min, 134 mg/100 g fresh leaves), palmitic acid (Rt 24.0 min, 66.3 mg/100 g fresh leaves), 1-hexacosanol (Rt 44.0 min, 69.0 mg/100 g fresh leaves) and phytol (Rt 27.6 min, 65.8 mg/100 g fresh leaves), belonging to four different classes of natural compounds: two sesquiterpene lactones, one of them isolated by preparative chromatography and characterized by spectroscopic techniques (Section 3.1, compound 3), a saturated fatty acid, a long-chain alcohol and a diterpene.
Table 4 presents for the first time the fatty acids profile of L. azorica leaves. Fatty acids represent 26.0% of all compounds identified (Figure S1 in Supplementary Material).
The most abundant fatty acids are palmitic acid (C16:0) and α-linolenic acid (C18:3. Ω-3). The preponderance of these two acids agrees with what was observed in wild L. nobilis leaves [50]. α-Linolenic acid is an essential polyunsaturated acid in the human diet, being described as having anti-inflammatory, antioxidant, antitumor, neuroprotective and anti-obesity effects [51,52]. Palmitic acid is a saturated fatty acid, also present in the human diet, that has been described as having harmful effects on health [53], such as increasing the risk of cardiovascular diseases. However, this negative effect has been questioned [54].
The results obtained also show that saturated fatty acids correspond to 68.2% of the total fatty acids, a higher percentage than that of unsaturated acids, which is in opposition to the results obtained by Dias et al. [50] for wild L. nobilis leaves, but in agreement with those obtained by the same research group in cultivated plants. This means that the saturated:unsaturated acid proportion is variable and dependent, not only on the species, but also on other factors, such as being cultivated or wild.
Regarding terpenes, this is the most abundant family, corresponding to 42.8% of the mass of analyzed fraction A. Here were identified and quantified two monoterpenes, corresponding to 0.845% of the total mass of terpenoids; two open-chain diterpenes, corresponding to 15.3%; and seven identified sesquiterpene lactones that constitute 72.5% of the total terpenes in fraction A (Figure S2 in Supplementary Material).
It should be noted that, in Table 4, seven compounds included in the unidentified category exhibit mass spectra with a fragmentation pattern very similar to that observed in the spectra of the identified hydroxylated sesquiterpene lactones, suggesting that they belong to the same class. If we take these into account, then sesquiterpene lactones constitute 82.3% of the total mass of terpenes in the lipophilic fraction of L. azorica.
The most abundant are two sesquiterpene lactones, deacetyllaurenobiolide and reynosin, previously identified in L. nobilis [10].
Terpenes in general and sesquiterpene lactones, in particular, are structurally very diverse and exhibit a wide variety of biological activities. Some of the sesquiterpene lactones identified, as mentioned in the previous section, exhibit antitumor, anti-inflammatory, antimalarial, antimicrobial, antioxidant and antidiabetic properties [37,55].
Concerning the family of fatty alcohols, it is the third most abundant family in the lipophilic fraction of L. azorica (14.7%). Interestingly, this is the first time that compounds belonging to this family have been identified in the genus Laurus. The most abundant is 1-hexacosanol, described as being able to be used to prevent diabetes-induced nephropathy [56].
3.3. Biological Activities
Oxidative stress occurs due to an imbalance between the production of free radicals and the body’s ability to neutralize their harmful effects [57]. One way to help reduce the harmful effects of oxidative stress is to provide the body with antioxidants. Additionally, the enzyme tyrosinase is involved in melanogenesis, so the inhibition of this enzyme, overactivated in the aging process, helps to counteract the appearance of blemishes on the skin.
The ethanolic extract from L. azorica leaves (EtOH extract), its hexane fraction (fraction A) and the pure costunolide (compound 1) isolated from fraction A were evaluated for their antioxidant potential by different methods (DPPH and ABTS and the inhibition of β-carotene bleaching), and inhibition of the enzyme tyrosinase. Regarding compounds 2 and 3, it was not possible to assess their potential since the amounts were not sufficient to carry out the tests. The obtained results are presented in Table 5.

Table 5.
IC50 values (μg/mL) of antioxidant activity by the DPPH and ABTS methods and the inhibition of β-carotene bleaching.
The ferric chelating and inhibition activities of collagenase, elastase and hyaluronidase enzymes were also tested, using the methods described in Zárate et al. [28], but none of the samples showed activity at the maximum concentration of 250 µg/mL.
As for the antioxidant activity by the DPPH method (Table 5), it was not possible to calculate the IC50 values for fraction A and compound 1 because the % AA was <50% at 250 μg/mL (13.10% and 7.70%, respectively), while the EtOH extract at the same concentration scavenged the DPPH radical by more than 50%, yielding an IC50 = 59.19 µg/mL, about 2.93 times lower than Trolox.
Regarding the antioxidant activity by the ABTS method (Table 5), only compound 1 was inactive at the maximum tested concentration (250 µg/mL). The EtOH extract was the most active sample, with IC50 = 6.78 µg/mL, whereas fraction A showed relatively low activity compared to the standard compound (IC50 = 170.63 µg/mL).
The results related to the bleaching inhibition activity of β-carotene showed that all samples tested showed activity. IC50 values were calculated and, according to Table 5, the EtOH extract and compound 1 were more active than the reference compound, with compound 1 being the most active (4.08 μg/mL). Fraction A showed lower activity, with IC50 = 14.74 µg/mL, comparable to the value obtained for the reference compound gallic acid. The β-carotene bleaching assay can be a measure of the ability of compounds to protect membrane fatty acids against oxidation and is particularly appropriate for lipophilic matrices [58]. These results corroborate the results of Cheong et al. [59], who detected a protective effect of costunolide in assays using live cells against H2O2-induced cell death by reducing intracellular ROS. The effect of dehydrocostus lactone against osteoblast damage induced by AMA was also partly attributed to its antioxidant effect by Seo and Choi [60]. It should be pointed out that dehydrocostus lactone and other sesquiterpene lactones (which represent the most abundant compounds in the extract) could be responsible for this bioactivity, since sesquiterpene lactones cause changes in cell redox balance reducing oxidative stress. There are, however, other antioxidant compounds present in the crude ethanolic extract which explain its antioxidant activity in all the antioxidant assays carried out in the present work. The results obtained here confirm the antioxidant potential of L. azorica suggested by other authors [21]. However, it is not possible to quantitatively compare the results obtained here with others reported in the literature. In fact, this work is the first to present adequately quantified antioxidant results regarding the concentration of extracts and compounds, in parallel with standards of antioxidant activity, and express the activity in units that can be compared with other studies, thus allowing a reliable quantitative assessment of this activity.
Concerning the anti-tyrosinase activity, the results are presented in Table 6.

Table 6.
IC50 values (μg/mL) of tyrosinase inhibition activity.
The tyrosinase inhibition activity by the EtOH extract was approximately 6.6-fold lower than the activity of kojic acid, the standard inhibitor compound. Neither fraction A nor compound 1 were active against this enzyme; therefore, it is likely that the compounds responsible for this activity were present in more polar fractions.
According to the literature, there is no data on the potential of L. azorica and costunolide (1) as tyrosinase inhibitors, or on the other compounds identified in the present work, so this study is the first to assay extracts from this species and compound 1 for their ability to inhibit this enzyme. Okguchi et al. [61] detected an inhibition of melanin synthesis in mouse B16 melanoma cells, but this was apparently due to the suppression of tyrosinase expression and not to the inhibition of the enzyme.
Although compound 1, costunolide, presented the highest IC50 value for antioxidant activity in the β-carotene assay, the EtOH extract is quite interesting, since it is active on all the antioxidant assays and also as a tyrosinase inhibitor. Therefore, it is relevant to find out if these activities are caused by cumulative and/or synergistic effects of several compounds, or if there is one compound that is responsible for each activity. Compound 1 seems to be the main compound causing the β-carotene bleaching, since its activity is more than 2-fold the activity of the original EtOH extract, although a more thorough phytochemical characterization and the bioactivity determinations of isolated compounds are needed to make reliable conclusions on that aspect.
4. Conclusions
In this study, the ethanolic extract from leaves of L. azorica and its lipophilic fraction (fraction A) were prepared and analyzed by different chromatographic methods.
For the first time in this species, three compounds belonging to the terpene family, the subfamily of sesquiterpene lactones, namely costunolide, 11,13-dehydrosantonin and reynosin, were isolated and identified by spectroscopic techniques.
The analysis of fraction A by GC–MS led to the identification of 48 compounds belonging to the families of fatty acids, long-chain alcohols, terpenes, sterols, esterified fatty acids and a small quantity of other kinds of compounds. The terpenes are the most abundant class of compounds (435 mg/100 g of fresh leaves), followed by the classes of fatty acids (265 mg/100 g of fresh leaves) and fatty alcohols (150 mg/100 g of fresh leaves). Deacetyllaurenobiolide, reynosin, palmitic acid, 1-hexacosanol and phytol are the five most abundant compounds in fraction A. The compounds belonging to the fatty alcohol family, which is the third most abundant class of compounds (150 mg/100 g of fresh leaves), were identified here for the first time in the genus Laurus.
Regarding biological activities, the ethanol extract showed excellent activity in the ABTS assay, and the isolated compound 1, identified as costunolide, proved to be the most active in the β-carotene assay, more active than the standard compound. For the first time, in this study, L. azorica extracts as well as their constituent costunolide (1) were tested for their ability to inhibit the tyrosinase enzyme, where the ethanol extract was shown to be active, proving its interest in the protection against melanogenesis deregulation. Other activities were tested, such as ferric chelating and inhibition activities of collagenase, elastase and hyaluronidase enzymes, but none of the samples showed activity at the maximum concentration of 250 µg/mL.
The results obtained show that the investigation in this species must continue, seeking to isolate and chemically and biologically characterize the secondary metabolites present in more polar fractions of the ethanol extract.
This work opens new horizons for the valorization of Laurus azorica as a source of metabolites of high commercial value (such as sesquiterpene lactones), which are structurally diverse and biologically active.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9080211/s1, Figure S1: Graphic representation of chemical composition of L. azorica lipophilic fraction (%) by natural compounds families; Figure S2. Graphic representation of the terpenoid composition of L. azorica lipophilic fraction.
Author Contributions
Conceptualization, M.C.B. and A.M.L.S.; Funding acquisition, M.C.B.; Investigation, M.M.V.; Supervision, M.C.B. and A.M.L.S.; Writing—original draft, M.M.V.; Writing—review and editing, M.M.V., M.C.B. and A.M.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project MACBIOPEST (MAC2/1.1a/289), Interreg MAC 2014–2020, co-financed by DRCT (Azores Regional Government), as well as by FCT—Fundação para a Ciência e Tecnologia, the European Union, QREN, FEDER and COMPETE, through funding the cE3c center-CHANGE (UIDB/00329/2020) and the LAQV-REQUIMTE (UIDB/50006/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks are due to the University of Azores, Roberto Resendes and the University of Aveiro.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seca, A.M.L.; Moujir, L. Natural Compounds: A Dynamic Field of Applications. Appl. Sci. 2020, 10, 4025. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. How Are Medicinal Plants Useful When Added to Foods? Medicines 2020, 7, 58. [Google Scholar] [CrossRef]
- Ding, A.; Zheng, S.; Huang, X.; Xing, T.; Wu, G.; Sun, H.; Qi, S.; Luo, H. Current Perspective in the Discovery of Anti-aging Agents from Natural Products. Nat. Prod. Bioprospect. 2017, 7, 335–404. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 779. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural Zonal Vegetation of the Azores Islands: Characterization and Potential Distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- WFO (2021): World Flora Online. Published on the Internet. Available online: http://www.worldfloraonline.org (accessed on 2 May 2022).
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and Biological Activities of Laurel Tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef]
- Rodilla, J.M.; Tinoco, M.T.; Morais, J.C.; Gimenez, C.; Cabrera, R.; Martín-Benito, D.; Castillo, L.; Gonzalez-Coloma, A. Laurus novocanariensis essential oil: Seasonal variation and valorization. Biochem. Syst. Ecol. 2008, 36, 167–176. [Google Scholar] [CrossRef]
- Mansour, O.; Darwish, M.; Ismail, G.; Douba, Z.A.; Ismaeel, A.; Eldair, K.S. Review Study on the Physiological Properties and Chemical Composition of the Laurus nobilis. Pharma. Chem. J. 2018, 5, 225–231. [Google Scholar]
- Braga, T. Plantas Usadas Na Medicina Popular, 2nd ed.; Amigos dos Açores: Ponta Delgada, Portugal, 2006; p. 47. [Google Scholar]
- Pontes, G.; Braga, T. Plantas Nativas Dos Açores; Amigos dos Açores: Ponta Delgada, Portugal, 2004; p. 32. [Google Scholar]
- Pedro, L.G.; Santos, P.A.G.; Silva, J.A.; Figueiredo, A.C.; Barroso, J.G.; Deans, S.G.; Looman, A.; Scheffer, J.J.C. Essential oils from Azorean Laurus azorica. Phytochemistry 2001, 57, 245–250. [Google Scholar] [CrossRef]
- Candeias, F.; Tinoco, M.T.; Morais, J.C. Actividade Hepatoprotectora Do Óleo Essencial De Laurus Azorica (Seub.) J. Franco. Livro de Resumos I Congresso Das Plantas Aromáticas E Medicinais Dos Paı́ses De Lı́ngua Oficial Portuguesa. Available online: https://cat.biblioteca.ipbeja.pt/cgi-bin/koha/opac-detail.pl?biblionumber=25923 (accessed on 15 May 2022).
- Rosa, J.S.; Mascarenhas, C.; Oliveira, L.; Teixeira, T.; Barreto, M.C.; Medeiros, J. Biological activity of essential oils from seven Azorean plants against Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Appl. Entomol. 2010, 134, 346–354. [Google Scholar] [CrossRef]
- Furtado, R.; Baptista, J.; Lima, E.; Paiva, L.; Barroso, J.G.; Rosa, J.S.; Oliveira, L. Chemical composition and biological activities of Laurus essential oils from different Macaronesian Islands. Biochem. Syst. Ecol. 2014, 55, 333–341. [Google Scholar] [CrossRef]
- Teixeira, T.; Rosa, J.S.; Rainha, N.; Baptista, J.; Rodrigues, A. Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra (Müller, 1774). Chemosphere 2012, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.M.R.; Macedo, M.; Contancia, J.P.; Nguyen, C.; Cunningham, G.; Miles, D.H. Antithrombin activity of medicinal plants of the Azores. J. Ethnopharmacol. 2000, 72, 157–165. [Google Scholar] [CrossRef]
- Vinha, A.F.; Guido, L.F.; Costa, A.M.L.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Monomeric and oligomeric flavan-3-ols and antioxidant activity of leaves from different Laurus sp. Food Funct. 2015, 6, 1944–1949. [Google Scholar] [CrossRef]
- Ahmad Dar, A.; Sangwan, P.L.; Kumar, A. Chromatography: An important tool for drug discovery. J. Sep. Sci. 2020, 43, 105–119. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Sayed, A.M.; Elmaidomy, A.H. Natural Products’ Extraction and Isolation-Between Conventional and Modern Techniques. Front. Nat. Produc. 2022, 1, 873808. [Google Scholar] [CrossRef]
- Halket, J.M.; Zaikin, V.G. Derivatization in mass spectrometry-1. Silylation. Eur. J. Mass Spectro. 2003, 9. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Lesenfants, M.L.; Rosa, G.P.; Barreto, M.C.; Silva, A.M.S.; Seca, A.M.L. GC- and UHPLC-MS Profiles as a Tool to Valorize the Red Alga Asparagopsis armata. Appl. Sci. 2022, 12, 892. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zárate, R.; Portillo, E.; Teixidó, S.; Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.B.P.; Pereira, J.A. Antioxidant activities of the extracts from Chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Lu, Y.; Khoo, T.J.; Wiart, C. Antioxidant activity determination of citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol. Pharm. 2014, 5, 395. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.H.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 1998, 64, 408–412. [Google Scholar] [CrossRef]
- Manosroi, A.; Jantrawut, P.; Akihisa, T.; Manosroi, W.; Manosroi, J. In Vitro anti-aging activities of Terminalia chebula gall extract. Pharm. Biol. 2010, 48, 469–481. [Google Scholar] [CrossRef]
- El-Feraly, F.S.; Benigni, D.A. Sesquiterpene Lactones of Laurus nobilis Leaves. J. Nat. Prod. 1980, 43, 527–531. [Google Scholar] [CrossRef]
- Hibasami, H.; Yamada, Y.; Moteki, H.; Katsuzaki, H.; Imai, K.; Yoshioka, K.; Komiya, T. Sesquiterpenes (Costunolide and Zaluzanin D) Isolated from Laurel (Laurus nobilis L.) Induce Cell Death and Morphological Change Indicative of Apoptotic Chromatin Condensation in Leukemia HL-60 Cells. Int. J. Mol. Med. 2003, 12, 147–151. [Google Scholar] [CrossRef]
- Fang, F.; Sang, S.; Chen, K.Y.; Gosslau, A.; Ho, C.; Rosen, R.T. Isolation and Identification of Cytotoxic Compounds from Bay Leaf (Laurus nobilis). Food Chem. 2005, 93, 497–501. [Google Scholar] [CrossRef]
- Ferrari, B.; Castilho, P.; Tomi, F.; Rodrigues, A.I.; Costa, M.C.; Casanova, J. Direct Identification and Quantitative Determination of Costunolide and Dehydrocostuslactone in the Fixed Oil of Laurus novocanariensis by 13C-NMR Spectroscopy. Phytochem. Anal. 2005, 16, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide–A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef]
- Fraga, B.M.; Terrero, D.; Cabrera, I.; Reina, M. Studies on the Sesquiterpene Lactones from Laurus novocanariensis Lead to the Clarification of the Structures of 1-epi-Tatridin B and its Epimer Tatridin, B. Phytochemistry 2018, 153, 48–52. [Google Scholar] [CrossRef]
- Matsuda, H.; Kagerura, T.; Toguchida, I.; Ueda, H.; Morikawa, T.; Yoshikawa, M. Inhibitory Effects of Sesquiterpenes from Bay Leaf on Nitric Oxide Production in Lipopolysaccharide-Activated Macrophages: Structure Requirement and Role of Heat Shock Protein Induction. Life Sci. 2000, 66, 2151–2157. [Google Scholar] [CrossRef]
- Barla, A.; Topçu, G.; Öksüz, S.; Tümen, G.; Kingston, D.G.I. Identification of Cytotoxic Sesquiterpenes from Laurus nobilis L. Food Chem. 2007, 104, 1478–1484. [Google Scholar] [CrossRef]
- Turk, A.; Ahn, J.H.; Jo, Y.H.; Song, J.Y.; Khalife, H.K.; Gali-Muhtasib, H.; Kim, Y.; Hwang, B.Y.; Lee, M.K. NF-κB Inhibitory Sesquiterpene Lactones from Lebanese Laurus nobilis. Phytochem. Lett. 2019, 30, 120–123. [Google Scholar] [CrossRef]
- Arantes, F.F.P.; Barbosa, L.C.A.; Alvarenga, E.S.; Demuner, A.J.; Bezerra, D.P.; Ferreira, J.R.O.; Costa-Lotufo, L.V.; Pessoa, C.; Moraes, M.O. Synthesis and Cytotoxic Activity of α-Santonin Derivatives. Eur. J. Med. Chem. 2009, 44, 3739–3745. [Google Scholar] [CrossRef]
- Komiya, T.; Yamada, Y.; Moteki, H.; Katsuzaki, H.; Imai, K.; Hibasami, H. Hot Water Soluble Sesquiterpenes [Anhydroperoxy-Costunolide and 3-Oxoeudesma-l,4 (15),11 (13)triene-12,6α-olide] Iso-lated from Laurel (Laurus nobilis L.) Induce Cell Death and Morphological Change Indicative of Apoptotic Chromatin Con-densation in Leucemia Cells. Oncol. Rep. 2003, 11, 85–88. [Google Scholar]
- Rosselli, S.; Bruno, M.; Raimondo, F.M.; Spadaro, V.; Varol, M.; Koparal, A.T.; Mggio, A. Cytotoxic Effect of Eudesmanolides Isolated from Flowers of Tanacetum vulgare ssp. Siculum. Molecules 2012, 17, 8186–8195. [Google Scholar] [CrossRef] [PubMed]
- Julianti, E.; Jang, K.H.; Lee, S.; Lee, D.; Mar, W.; Oh, K.; Shin, J. Sesquiterpenes from the leaves of Laurus nobilis L. Phytochemistry 2012, 80, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Aceves, E.W.; Velázquez, C.; Robles-Zepeda, R.E.; Jiménez-Estrada, M.; Hernández-Martínez, J.; Gálvez-Ruiz, J.C.; Garibay-Escobar, A. Reynosin and Santamarine: Two Sesquiterpene Lactones from Ambrosia confertiflora with Bactericidal Activity Against Clinical Strains of Mycobacterium tuberculosis. Pharm. Biol. 2016, 54, 2623–2628. [Google Scholar] [CrossRef]
- Yoshioka, H.; Renold, W.; Fischer, N.H.; Higo, A.; Mabry, T.J. Sesquiterpene lactones from Ambrosia confertiflora (Compositae). Phytochemistry 1970, 9, 823–832. [Google Scholar] [CrossRef]
- Marino, S.; Borbone, N.; Zollo, F.; Ianaro, A.; Meglio, P.D.; Iorizzi, M. New Sesquiterpene Lactones from Laurus nobilis Leaves as Inhibitors of Nitric Oxide Production. Planta Med. 2005, 71, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueñas, M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Nutritional and Antioxidant Contributions of Laurus nobilis L. Leaves: Would Be More Suitable a Wild or a Cultivated Sample? Food Chem. 2014, 156, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Terao, R.; Kaneko, H. Protective Effects and Molecular Signalling of n-3 Fatty Acids on Oxidative Stress and Inflammation in Retinal Diseases. Antioxidants 2020, 9, 920. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The Review of Alfa-Linolenic Acid: Sources, Metabolism, and Pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic Acid and Health: Introduction. Crit. Rev. Food Sci. Nutr. 2016, 56, 1941–1942. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Santos, C.N. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kinoshita, Y.; Satoh, I.; Shinbori, C.; Kono, T.; Hanada, T.; Uemasu, J.; Suzuki, H.; Yamada, M.; Satoh, K. n-Hexacosanol Ameliorates Streptozotocin-Induced Diabetic Rat Nephropathy. Eur. J. Pharmacol. 2006, 544, 132–137. [Google Scholar] [CrossRef]
- Tavares, W.R.; Seca, A.M.L. Inula, L. Secondary Metabolites against Oxidative Stress-Related Human Diseases. Antioxidants 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Murado, M.A.; Rodríguez-Amado, I.; Vázquez, J.A. β-Carotene Assay Revisited. Application to Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.-U.; Yeh, C.-S.; Hsieh, Y.-W.; Lee, Y.-R.; Lin, M.-Y.; Chen, C.-Y.; Lee, C.-H. Protective Effects of Costunolide Against Hydrogen Peroxide-Induced Injury in PC12 cells. Molecules 2016, 21, 898. [Google Scholar] [CrossRef]
- Seo, M.S.; Choi, E.M. The effects of dehydrocostus lactone on osteoblastic MC3T3-E1 cells in redox changes and PI3K/Akt/CREB. Immunopharmacol. Immunotoxicol. 2012, 34, 810–814. [Google Scholar] [CrossRef]
- Ohguchi, K.; Ito, M.; Yokoyama, K.; Iinuma, M.; Itoh, T.; Nozawa, Y.; Akao, Y. Effects of Sesquiterpene Lactones on Melanogenesis in Mouse B16 Melanoma Cells. Biol. Pharm. Bull. 2009, 32, 308–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).