Abstract
In an era where humanity is reinstating its lost hope and expectation on natural products, green tea occupies quite a position for what it has proven to be, in its endeavors for human welfare and health. Epigallocatechin-3-gallate (EGCG) is the key to the vast biological activities of green tea. Green tea is no longer in the backdrop; it has emerged as the most viral, trending bioactive molecule when it comes to health benefits for human beings. This review focuses on the use of various analytical techniques for the analysis of EGCG. That which has been achieved so far, in terms of in vitro, pure component analysis, as well as those spikes in biological fluids and those in vivo in animal and human samples, was surveyed and presented. The use of MS-based techniques for the analysis of EGCG is elaborately reviewed and the need for improvising the applications is explained. The review emphasizes that there is plenty of room to explore matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) applications in this subject area.
1. Introduction
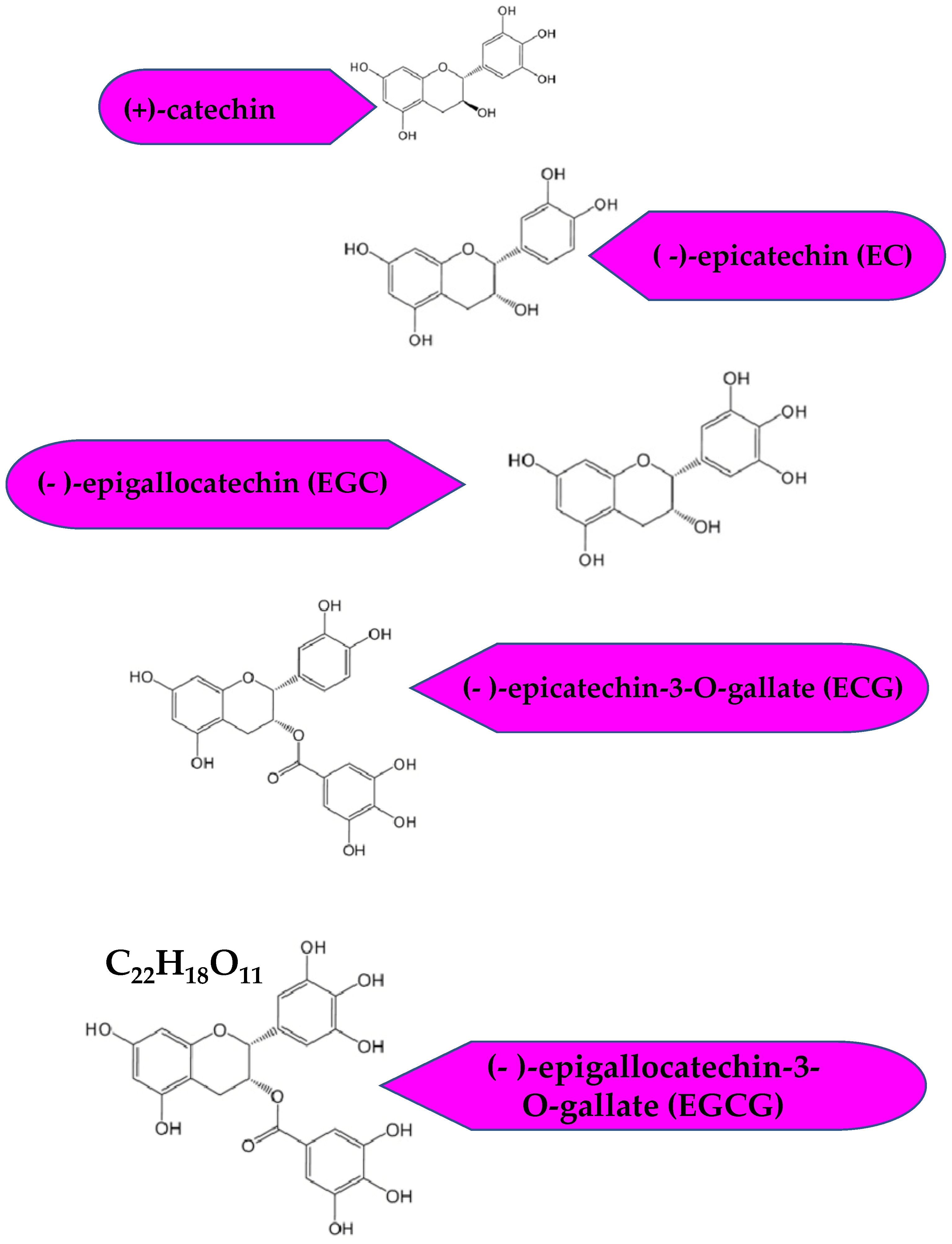
Tea is an age-old beverage steeped in the memoirs of history. Almost three billion kilograms of tea is produced each year. Camellia sinensis is predominantly consumed in China and Japan [1]. Green tea can be produced in various ways, which becomes the fundamental basis for the differentiation of various green tea types. Yet, the most fundamental method is via the steaming of fresh Camellia sinensis leaves for 1 min, followed by drying. The main components of green tea responsible for its health benefits are the active biocomponents, the catechins [2]. Green tea is the richest source of catechins, that account for nearly 30% of the leaf’s dry weight [1]. The other natural sources are red wine, broad beans, black grapes, apricots, and strawberries [3]. There are four predominant catechins in green tea, (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin-3-O-gallate (ECG) and (-)-epigallocatechin-3-O-gallate (EGCG). Amongst these, EGCG is the most abundant and most significant bioactive component [4,5]. Figure 1 shows the chemical structures of the predominant green tea catechins.
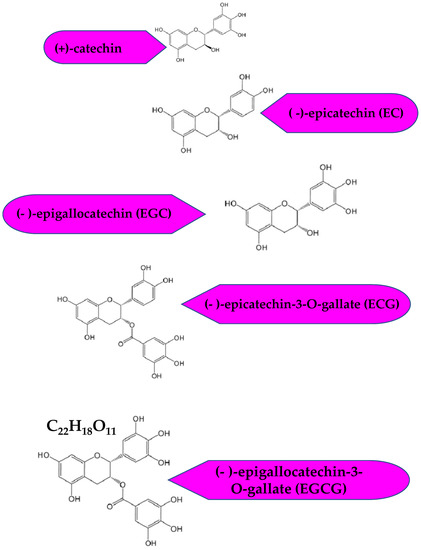
Figure 1.
Chemical structures of the green tea catechins.
Green and black teas are widely consumed worldwide and host a wide range of potential health benefits. Catechin flavonoids, which are around 60 mg/g of the dry leaf weight [6], possess antioxidant properties and other biological activities that are key players in the claimed therapeutic activity of green tea [7]. Green tea is a broad terminology which can be further divided and subdivided based on various factors: the processing style, the region grown, the time of harvest, the harvest season, the plant parts involved, and brewing method and the like. The three major popular Chinese green teas include: Biluochun, which is named based on its leaf shape which is curled like snails [8]; Chun Mee, which has a plum-like flavor [9]; Gunpowder tea, which is tumble-dried to look like a small pellet that resembles gunpowder [10]; Huangshan Maofeng, whose harvest includes two similar-sized leaves and buds together [10]; Longjing, which is pan-fired Chinese green tea [8]; Lu’an Melon Seed, which consists of a grassier flavor and harvested as two leaves separately from each branch, with no bud and stems [10]; Taiping Houkui, which is processed from unusually large leaves that are taken through a production process that flattens the tea leaves [10]; and Xinyang Maojian, which is a type of maojian tea grown in Xinyang, Henan province [11]. Maojian teas are harvested by plucking a bud and one leaf together [10]. Japanese green teas include: Bancha, which is a lower-grade tea with a bolder flavor, plucked after sencha production [12]; Genmaicha, which is a combination of sencha tea leaves with toasted puffs of rice; and Gyokuro, which is grown in shade for three weeks prior to plucking and is the most exclusive variety of Japanese tea [13,14]. Hōjicha, Kabusecha, Kukicha, Matcha [15], Sencha and Shincha are highly prized and expensive Japanese teas. Korean green teas are ideally classified based on the flush, or the time of the year when the leaves are plucked (also by leaf size). Korean green teas consist of Ujeon, Sejak, Jungjak [15], and Daejak [15] types. Table 1 gives the catechin composition of green tea.

Table 1.
Composition of Green tea.
Mass spectrometry (MS) is a powerful, multi-faceted technique used in food safety, environmental, pharmaceutical, biological, and forensic investigations [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. This technique has brought about the detection of proteins, peptides, and lipids, and recently it has been extended to detect, quantitate, and localize metabolites and their counterparts. The unique ionization methods and the specificity and sensitivity of a mass analyzer make MS an attractive analytical option. A plethora of gas and liquid separation techniques are often used in conjunction with MS in order to increase sensitivity and ease interpretation; these include liquid chromatography (LC), gas chromatography (GC), capillary electrophoresis (CE), and gel electrophoresis (GE). The combination of methods is usually common in tandem with ICP-MS and DART-MS. Mass spectrometric imaging is an emerging powerful analytical methodology used for analyzing multiple molecules in complex samples without labeling, conferring a clear edge over preexisting methods for label-free and simultaneous drug and metabolite detection. There are a host of methods and ionization variants in MS. Gas phase methods include electron ionization (EI), chemical ionization (CI), direct analysis in real time (DART), and inductively coupled plasma (ICP); desorption methods consist of matrix-assisted laser desorption ionization (MALDI), fast atom bombardment (FAB), thermal ionization sources, plasma ionization sources, and liquid metal ion sources (LMIS); and spray methods include ultra-high electrospray ionization (ESI) and desorption electrospray ionization (DESI) [31,32,33,34,35,36,37]. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry profiling (MALDI-TOF MS profiling) is a promising approach for the rapid and high-throughput screening of biological samples, intact cells, and crude extracts [38]. This technique has been applied to clinically relevant medical applications, including cancer-disease-related biomarker identification and pathogenic microorganism diagnostics. It has now become a versatile tool for many applications. Clinical bacteria, mycobacteria, entomopathogenic soil fungi, yeasts, and viruses have been identified and clustered by MALDI-TOF MS profiling for quality control. MALDI-MS has also been successfully applied to quantitative studies of plant metabolites, plant alkaloids, anthocyanins, flavonoids, acetogenins, spirolides, curcuminoids, and rotenoids [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
The following review aims to catalogue the green tea catechins that are well known for their diverse social and health benefits, with special emphasis on EGCG, which is notorious for its bioactivity. The various analytical methods that are employed to assess and assay green tea catechins are briefly presented. The application of mass spectrometry for the analysis of EGCG has been elaborately discussed and the challenges in MS applications for this expertise and their future perspectives are presented.
2. A Snapshot of EGCG Applications
Green tea catechins are well known for their biological and pharmacological impacts: the anticarcinogenic [55] and antioxidant activities [56]; lowering of plasma lipid [57] and glucose levels [58]; reduction in obesity [59,60]; prevention of cardiovascular diseases; and antiarthritic, antibacterial, antioxidative, anti-inflammatory, antiangiogenic, antiviral, neuroprotective, and cholesterol-lowering actions [7,61]. EGCG not only holds the position of being the major polyphenolic component in green tea, but it also contributes predominantly towards green tea’s properties. In this review, we essentially focus on and highlight the biological functions of EGCG. It protects the system against free-radical-induced cellular damage, shields the cells from oxidative-stress-induced damage, and suppresses the pro-inflammatory activity of chemicals produced in the body. EGCG promotes heart health by reducing blood pressure, cholesterol, and plaque accumulation in blood vessels, which are triggers of cardiac diseases [62,63]. Catechins are also associated with the development of lung, gastric, and breast cancers [64,65,66]. A well-established connectivity between diabetes, improved immunity, and green tea is well-documented [67,68,69,70,71,72].
EGCG further inhibits inflammation and prevents chronic heart diseases, diabetes, and cancers. EGCG improves neurological function and prevents degenerative brain diseases and assists in the regeneration of neural cells in mice with spinal cord injuries [73,74]. In vitro studies validate the antibacterial property of EGCG, whereby they are able to suppress the growth of bacteria and lower the risk of infections [75,76,77,78]; there is a handful of evidence that green tea may reduce halitosis or bad breath [79,80]. Most importantly, EGCG has been associated with affecting age-related brain decline, as well as Alzheimer’s and Parkinson’s disease [81].
In the food industry, EGCG prevents lipid peroxidation of oily foods, scavenges free radicals, and inhibits the autooxidation of lipids as well as lipid degradation [82]. EGCG is almost 20 times more potent than vitamin E in preventing lipid peroxidation and four times higher than butylated hydroxyanisole (a food additive that preserves fat/oil) in this regard [83]. Furthermore, EGCG inhibits the formation of mutagens, which occur during the broiling or frying of meats and are known to increase the risk of cancers [84]. In the Asian food industry, EGCG has been used as functional food components, enhancing product shelf-life and adding health benefits for consumers [85]. Tea catechins have been used to fortify various food commodities and beverages [86]. In the pharmaceutical industry, applications such as mouthwashes, toothpastes, and breath fresheners to improve oral health are prevalent [86]. In addition, EGCG supplement tablets or drinks are commercialized to enhance consumer health. EGCG has been reported to be incorporated into air filters in ‘‘antiinfluenza’’ masks [86]. In the cosmetics industry, they have been added to shampoos, face masks, moisturizing creams, perfumes, and sunscreens, as they can relay soothing effects on the skin and protect it from free-radical damage [86].
The flip side of EGCG is also being carefully investigated; it is important to note that EGCG has been reported not to be 100% safe or risk-free. EGCG supplements have been associated with serious side effects such as [87]: liver and kidney failure, dizziness, low blood sugar, and anemia. Taking supplemental EGCG is not recommended for pregnant women, as it is confirmed to interfere with the metabolism of folate, which is a B vitamin needed for fetal growth and development, the deficiency of which results in birth defects such as spina bifida [88]. It is still ambiguous as to the safety of EGCG supplements for breastfeeding women [89]. EGCG interferes with the absorption of certain types of cholesterol-lowering and antipsychotic drugs [90]. The pro-oxidation action of EGCG is a crucial mechanism key to its protective functions, one of which includes the induction of adaptive responses and detoxification [91]. Intriguingly, it is also reported that EGCG at higher doses can induce hepatotoxicity in both animals and human beings. It is further postulated that EGCG may undergo auto-oxidation, generating reactive oxygen species (ROS), inducing toxicity [92]. These are a few of the health concerns that have been raised; there could be a host of other unknown aspects as well which, if disclosed, could guide us towards the better utilization of EGCG, knowing its pros and cons. Thus, it becomes essentially crucial to weigh their benefits against toxicity aspects to carefully work on discovering the safe physiological dosages of EGCG. Because of the high rates of tea consumption in the global population, even minor hazards could have seriously larger implications on public health. It is in this direction that the disposition of catechins within biological systems, involving their absorption, distribution, metabolism, and excretion, have been investigated in mice, rats, and humans [93]. The metabolism of catechins in animals and humans has been elaborately reviewed [94]. These pharmacokinetic profiles of catechins in rats and humans yield a clearer perspective on the movement and absorption of EGCG within a system; although, this is far from being fully elucidated.
3. Separation and Identification of Green Tea Catechins/EGCG
Green tea catechins consist of four major polyphenols, that include: epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epicatechin (EC) [2]. EGCG is the chieftain among the catechins; it constitutes about 50–80% of the total catechin content [95,96,97]. Catechins are not only soluble in water, but also in ethanol, methanol, and acetone. Pure catechins from tea are obtained following a separation protocol. Separation and isolation procedures for tea catechins involve extraction processes such as (i) treatment of the starting material, (ii) the extraction of tea constituents from tea leaves into infusions, (iii) the concentration of the extracted constituents, (iv) the separation and isolation of catechins from other impure components; and (v) drying of the catechins to obtain an extract powder for industrial use [98].
The specialized methods that have been applied for the analysis of tea catechins [99,100] in plasma samples include liquid chromatography (LC) connected to an ultraviolet detector (UVD), a chemiluminescence detector (CLD), a fluorescence detector (FLD), and an electrochemical detector (ECD) [101]. When it comes to the estimation of EGCG with marked specificity, spectrophotometry [102], HPLC [103,104], reverse-phase (RP) HPLC [100,101], ultra-performance liquid chromatography (UPLC) [103,104], ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) [105], electrospray ionization -mass spectrometry (ESI-MS), ultra-performance liquid chromatography-diode array detector-mass spectroscopy (UPLC-DAD-MS) [106,107], and ultra-performance liquid chromatography—time-of-flight—mass spectrometry (UPLC-TOF-MS) have been successfully applied [108].
3.1. MS-Coupled Chromatographic Techniques for Detection and Identification of Catechins/EGCG
The absorbance wavelengths of catechins lie in the range of 210 and 269–280 nm [109,110], and so, UV-spectrophotometry and diode array detectors have become easy and simple analytical methods for the determination of catechins. Various analytical methodologies have been used for identifying and quantifying tea catechins [111]. These methods determine the yield, concentration, and purity of catechins in the separated products. The identification and quantification of catechins are predominantly steered by chromatographic techniques, such as HPLC and CE associated with various UV, electrochemical, and MS detectors that enable the detection of individual catechins [112]. Near-infrared reflectance spectroscopy, high-speed countercurrent chromatography, TLC, and GC are the other alternate options [113], of which HPLC with ultraviolet (UV) detection is a rather simple, highly reproducible technique with low limits of detection (more than 500 ng/mL) [114]. HPLC with chemiluminescence detection overcame this detection limit, but its sample preparation and column preparation procedures are difficult [111]. Lee et al. reported the electrochemical detection (ECD) of catechins with HPLC [111]. This was a more sensitive approach, capable of detecting EGCG at concentrations as low as 0.5 ng/mL; the assay took almost 35 min. HPLC is the most common, as it leads to good separation and can be combined with many detectors [112]. A reverse-phase C18 column is mostly used for HPLC [112]. Ramakrishna et al. [113] used standard EGCG for EGCG optimization experiments using ultra-high-performance liquid chromatography (UHPLC). A preliminary trial run yielded good peak shapes and acceptable system suitability, leading to validation. The method was specific and had an acceptable recovery rate in the range of 99.1% to 100.4% with a relative standard deviation (less than 2%). This method was identified to be robust and rugged and was validated as a routine compliance test in the laboratory.
Saito et al. presented an HPLC analytical methodology development for the simultaneous determination and quantification of caffeine (CAF), catechin (C), epicatechin (EC), and epigallocatechin gallate (EGCG) in samples of Camellia sinensis (green tea) grown in Brazil and harvested in spring, summer, and autumn, in comparison to Brazilian black tea, to samples of Japanese and Chinese green tea, and to two standardized dry extracts of green tea [114]. Lambert et al. [115] detected intravenously administrated EGCG in the plasma of male CF-1 mice. The kinetics of EGCG in the liver, lung, intestine, and kidney of rats after intravenous administration of decaffeinated green tea was also reported [116]. The tissue levels of EGCG, EGC, and EC in rats, as well as the liver and lung levels in mice, after administration of green tea has been documented [116]. Wangkarn et al. [117] recently elaborately reviewed the HPLC-based detection of green tea catechins.
3.2. Direct MS Analysis of EGCG
MS-assisted methods for analysis of EGCG have also been reported; we present a brief overview of these reports in this section. EGCG gives a high intensity of the deprotonated molecular ion, [M-H]−, at m/z 457 in negative ESI analysis. Diverse methods to measure the catechin concentration in biological fluids have been reported for pharmacokinetic and metabolic studies [118,119,120,121,122]. This enables the tracking of catechin inside the biological system, assisting in a better understanding of the process of absorption, distribution, metabolism, and excretion via analyzing the plasma and urine levels. Numerous high-performance liquid chromatographic (HPLC) methods have been published on the analysis of tea catechins in blood plasma. Most of these methods involve the extraction of tea catechins from plasma or tissue homogenates using solvents such as ethyl acetate [123,124,125,126,127], acetonitrile [128], or methanol [129], followed by an HPLC separation coupled with electrochemical or MS detection [130]. Liquid chromatography coupled with mass spectrometry (LC/MS/MS) is another emerging analytical technique for the quantitative determination of metabolites in different biomatrices, due to its sensitivity and selectivity through MS/MS experiments and the fact that it enables structural identification [121,131,132,133].
Recently, HPLC with electrospray ionization (ESI) mass spectrometry (MS) was reported by Masukawa et al. [121]; a minimum detectable concentration of less than 1 ng/mL for EGCG in human plasma was achieved, but the assay time was over 50 min. However, in a more time-efficient assay, Lin et al. [134] detected EGCG in rat plasma and brain tissue using LC/MS/MS within 10 min. Despite the highly sensitive EGCG detection, quantification (LLOQ) is still a challenge. Ultraperformance liquid chromatography (UPLC) is a recently developed method in LC; this led to a marked separation time reduction and solvent consumption with high resolution [135].
LC-MS, for quite a while, had not been used for the detection of EGCG from actual plasma after ingestion; although, it has been used for analyzing EGCG in model plasma with a few spiked catechins. Columns packed with sub-2 m particles working at elevated pressure (UHPLC strategy) could improve the chromatographic performance. Identification required a liquid-liquid extraction procedure before UHPLC-UV analysis to decrease the complexity of the sample. UHPLC coupled with ESI-MS/MS could successfully enhance sensitivity and selectivity [136].
In another study [137], LC-ESI MS was used for the separation of eight catechins using an Inertsil ODS-2 column equipped with a gradient elution system. Detection using MS was performed under negative ESI. The key difference between positive and negative ionization in mass spectrometry is that positive ionization is the process that forms positively charged ions, whereas negative ionization is the process that forms negatively charged ions. In the negative-ion mode operation, peaks corresponding to deprotonated analyte molecules are observed. The negative mode allows better sensitivity for small-molecule detection compared to the positive mode. Eight catechins in human plasma after oral ingestion of a commercial green tea beverage were detected in human blood for the first time. Another rapid and valid method led to the simultaneous detection of catechin, epicatechin, and epicatechin gallate in rat plasmas. Three analytes were recovered; the lower limits of quantitation (LLOQ) in rat plasma for catechin, epicatechin, and epicatechin gallate were identified [124]. The results demonstrated that the present LC-MS/MS method was sensitive enough for pharmacokinetic study of catechins following oral administration of C. songaricum extract; since this method was successful in the detection of catechins that are less dominant than EGCG, this method can certainly be applied to EGCG as well.
The simultaneous detection and quantification of green tea catechins using UPLC/ESI-MS have also been reported. In 3.5 min analytical run time, EC, ECG, EGC, and EGCG were detected in rat plasma [138]. The assay was successfully applied to a pharmacokinetic tracking of catechins following intravenous and intragastric administrations of green tea extract in rats. Plasma concentrations of four catechins were detected up to 5–24 h after administration. LC-ESI-MS/MS was used to identify green tea catechin metabolites in plasma and urine, following oral intake of a green tea extract [139]. LC-ESI-MS/MS was applied for the identification and structural assignments of the polyphenolic extracts of green tea (Camellia sinensis) [140]. UPLC-MS/MS was used to obtain the concentration of catechins in blood after one-time ingestion of green tea extract (630 mg) with digoxin and consumption of 630 mg green tea for 15 days [114]. Green, oolong, and black tea samples were analyzed. Catechin standards were obtained from Cerilliant® (Round Rock, TX, USA). Liquid chromatography and mass spectrometry analysis was used for acquisition and data analysis [140].
Juang et al. [141] described the use of surface-assisted laser desorption/ionization mass spectrometry (SALDI-MS) for the detection of catechins. They incubated catechins with titanium dioxide (TiO2) nanoparticles (NPs) and graphene flakes (GF) and subjected the mixture to microwave irradiation for enriching the analytes. TiO2 nanoparticles enriched the catechins while GF enhanced the desorption/ionization efficiency. The dual nanomaterial matrix system yielded higher desorption/ionization efficiency, enhanced analyte enrichment, and reduced the run time to less than 10 min. Several tea samples were tested using the optimized method; good shot-to-shot, sample-to-sample, and quantitative linearity were obtained. (m/z 457 [M-H]−). Table 2 lists the reports involving MS-based identification/detection of EGCG.

Table 2.
MS-assisted identification of EGCG.
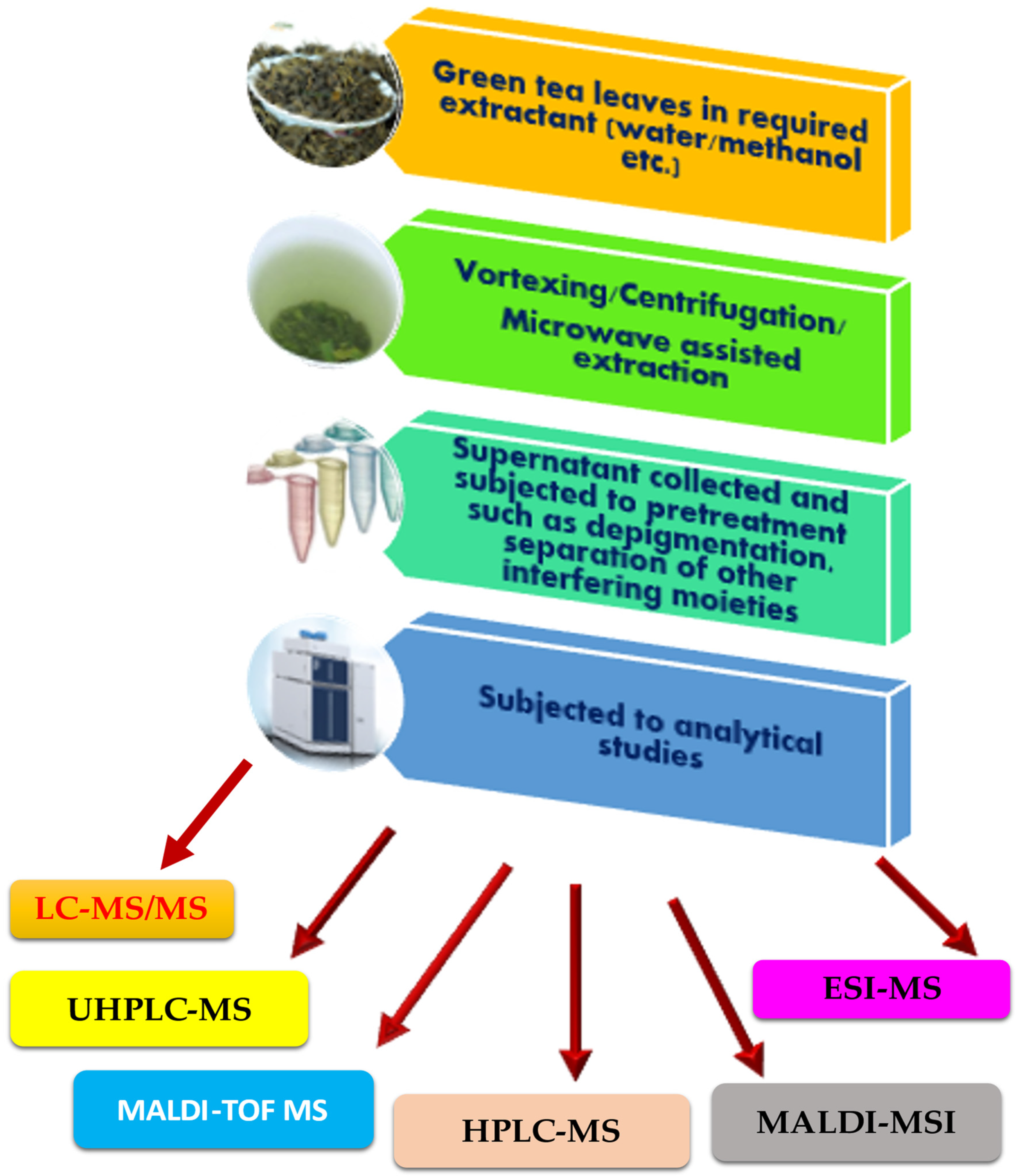
Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) is a trending offshoot of MALDI-TOF MS, which has been presently used for the physiological visualization of targets in organs. MALDI-MSI has been used as a visualization tool to view the intestinal absorption of polyphenols [170]. Nifedipine/phytic acid-aided MALDI-MSI was performed to visualize theaflavin-3′-O-gallate (TF3′G) and epicatechin-3-O-gallate (ECG) in the jejunum of rats. MALDI-MSI was also performed to determine the transport routes of the target metabolites. MALDI-MSI could provide critical spatial information on intestinal absorption of targets. Kim et al. [170] established a 1,5-diaminonaphthalene (1,5-DAN)-based MALDI-MSI technique for visualizing EGCG, within mammalian tissue microregions after oral dosing. Using a combination of label-free MALDI-MSI and a standard-independent metabolite identification method, the authors were able to achieve isotopic fine structure analysis using an ultra-high-resolution mass spectrometer. This led to the informative visualization of spatially resolved biotransformation based on the simultaneous mapping of EGCG and its phase II metabolites. The caliber of information derived hereby was unique. The only limitation of this technique was its detection sensitivity. An overview of the analytical analysis of EGCG, with the list of techniques, attempted so far, is shown in Figure 2.
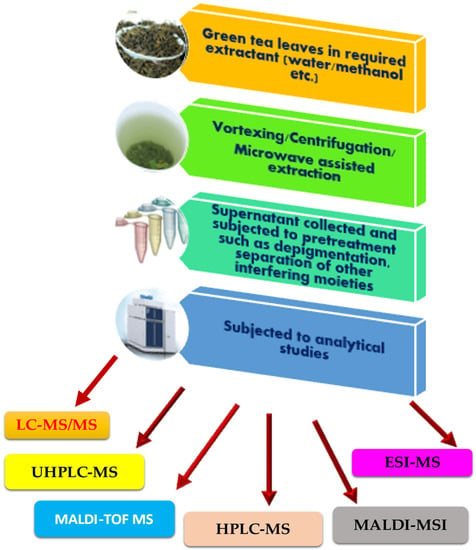
Figure 2.
Workflow of EGCG MS-based analysis.
4. Challenges and Future Perspective
The applications of EGCG have been briefly reviewed and the analytical instruments that have been used for the detection, identification, and analysis of green tea catechins and EGCG have been presented. Going with the theme of this review, more emphasis has been given to reviewing the MS-based analysis of EGCG. Through the course of the review, it has become exceedingly evident that green tea catechins and EGCG have huge potential and hold voluminous promises for human welfare. In spite of this reputation, the deployment of analytical techniques towards the analysis of EGCG in vitro and in vivo is represented by merely a handful of studies. In particular, MS-based applications for EGCG detection and analysis are less numerous. LC-MS is a somewhat more accomplished instrumentation when it comes to catechin detection, but this does not necessarily confirm the monopoly of this technique. The lack of availability of reports on other parallel techniques is the reason why such a comparison could not be raised.
MALDI-TOF MS is well known as a rapid and sophisticated technique which, except for a couple of studies in terms of EGCG detection, has not been well reported. MALDI-TOF MS is well established for its role in the detection of plant secondary metabolites. Rush et al. [171] reported the use of MALDI-TOF MS for the analysis of small molecules such as procyanidins, which constitute a unique class of polymeric plant secondary metabolites with a variety of biological properties including potent antioxidant activity. Its structural determination has been challenging, and the structures of many complex procyanidins remain uncertain. Negative-ion MALDI-TOF/TOF has expediated the characterization of procyanidins. The interpretation of the tandem mass spectra enabled the sequencing of A-type, B-type, mixed-type, linear, and branched procyanidins.
The identification of catechin oligomers from apple has been successfully demonstrated using MALDI-TOF MS and fast-atom-bombardment mass spectrometry. MALDI-TOF MS provided evidence for the pentadecamer (trans-3-indoleacrylic acid has been used as the matrix in the presence of silver ions). Given these facts, it is rather intriguing that the utility of MALDI-TOF MS has been proven for the identification, elucidation, and analysis of various bioactive small molecules, yet nearly nothing has been attempted to apply it in EGCG analysis [172]. This review stresses that there is much that MALDI-TOF MS can offer, especially in terms of reducing the elaborate sample preparation and instrumentation protocols that rise from LC-MS [147]. MALDI-TOF MS, as an option for EGCG analysis, should be strongly considered and explored; we, however, do not rule out the utility of standard chromatographic techniques that enable separation and the other MS-assisted chromatographic methods that enable detection. What we emphasize here is that MALDI-TOF MS is also able to contribute to EGCG detection, and more attention in the direction of checking what it has to offer is encouraged.
As evident from the previous section, LC-MS is unable to perform with respect to EGCG analysis as a standalone technique. LC/ESI MS and MS/MS; UPLC-MS/MS; LC/ESI-MS; and UPLC-DAD-MS are the various combinations of LC-MS and MS that have made progress in EGCG research. MALDI-TOF MS has the potential to function as a standalone technique, which will prove highly advantageous since this will simplify the preparative procedures as well as save time and resources and curtail involving multiple instrumentations [172].
Speculating why MALDI-TOF MS has not been used for the analysis of EGCG, it is understandable that chromatography methods enable the separation, leading to effective detection. Conventional MALDI-TOF MS did suffer from the interferences of the other compounds in an analyte, but with the introduction of the nanoparticle-based preconcentration techniques, as well as nanoparticle-based affinity-probe-assisted MALDI-TOF MS; other state-of-the-art techniques such as SALDI-MS, NALDI-MS, MALDI-TOF MSI; and other sophisticated LDI-MS platforms [173,174,175,176,177,178,179,180,181,182,183,184,185,186], MALDI-TOF MS has overcome its own limitations and is progressing rapidly in small-molecule analysis. This review urges the readers to utilize MALDI-TOF MS for the detection of EGCG and other catechins. MALDI-TOF MSI can be explored and applied, since it is a versatile and robust technique that can become very resourceful, especially when tracking the pharmacokinetics of EGCG in vivo. There can be more that MALDI-TOF MS and its variants have to offer, in terms of specificity, linearity, accuracy, system suitability, method precision, robustness, and ruggedness, which need to be investigated as soon as possible.
5. Conclusions
The benefits of green tea catechins, especially EGCG, were reviewed and the current status quo of the analytical techniques that have been used to analyze EGCG in vitro and in vivo were comprehensively presented and discussed. The use of MS-coupled chromatographic methods and direct MS methods for the analysis of EGCG were comprehensively presented. MS-based methods have proved useful when it comes to the analysis of EGCG; however, there are few MS techniques that are unattempted. In particular, MALDI-TOF MS has not been used much, in spite of its proven usefulness in small-molecule analysis. This is poised as a huge gap, since MALDI-TOF MS is a standalone technique and there are other, more evolved variants of MALDI-TOF MS that are in the field. Yet, as this review identified, this is an area which lacks research attention. This review prompts the need for exploring the available MALDI-TOF MS options for EGCG analysis.
Author Contributions
I.S., M.M. and J.G., preparation of original draft, revisions; S.S.C.P. and A.K., review and revisions; J.-W.O., participated in review and revisions and funding support. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food. Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Suga, K.; Nakachi, K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev. Med. 1997, 26, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea and Health: Studies in Humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Heiss, R.J.; Heiss, M.L. The Story of Tea: A Cultural History and Drinking Guide; Ten Speed Press: Berckley, CA, USA, 2007; p. 124. [Google Scholar]
- Chow, K.; Kramer, I. All the Tea in China; Dashwood, R.H., Ed.; China Books & Periodicals Inc.: San Francisco, CA, USA, 1990; p. 125. [Google Scholar]
- Battle, W. The World Tea Encyclopaedia: The World of Tea Explored and Explained from Bush to Brew; Troubador Publishing: Matador, UK, 2017; pp. 105–107, 162–164. [Google Scholar]
- Kim, Y.-M. (Ed.) Tradition—The Way of Tea: A Lifestyle Aesthetic for Learning the Depth and Enlightenment of Life; Pictorial Korea, Korean Overseas Culture and Information Service: Sejong-si, Korea, 2004; p. 26. [Google Scholar]
- Richardson, L.B. Modern Tea: A Fresh Look at an Ancient Beverage; Gilbut Publishing: Seoul, Korea, 2014; p. 51. [Google Scholar]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Heber, D. Catechin Content of 18 teas and a Green Tea extract supplement correlates with the antioxidant capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef]
- Gramza, A.; Khokhar, S.; Yoko, S.; Gliszczynska-Swiglo, A.; Hes, M.; Korczak, J. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur. J. Lipid Sci. Technol. 2006, 108, 351–362. [Google Scholar] [CrossRef]
- Enko, J.; Gliszczyńska-Świgło, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addit. Contam. Part A 2015, 32, 1234–1242. [Google Scholar] [CrossRef]
- Albrethsen, J. Reproducibility in Protein Profiling by MALDI-TOF Mass Spectrometry. Clin. Chem. 2007, 53, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, D.; Irizarry, R.; Califano, J.A.; Li, X.; Ren, H.; Benoit, N.; Mao, L. Serum protein MALDI profiling to distinguish upper aerodigestive tract cancer patients from control subjects. J. Natl. Cancer Inst. 2003, 95, 1711–1717. [Google Scholar] [CrossRef]
- Ilina, E.N.; Borovskaya, A.D.; Malakhova, M.M.; Vereshchagin, V.A.; Kubanova, A.A.; Kruglov, A.N.; Svistunova, T.S.; Gazarian, A.O.; Maier, T.; Kostrzewa, M.; et al. Direct bacterial profiling by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for identification of pathogenic Neisseria. J. Mol. Diagn. 2009, 11, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Eigner, U.; Holfelder, M.; Oberdorfer, K.; Betz-Wild, U.; Bertsch, D.; Fahr, A.M. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clin. Lab. 2009, 55, 289–296. [Google Scholar] [PubMed]
- Oswald-Richter, K.A.; Beachboard, D.C.; Seeley, E.H.; Abraham, S.; Shepherd, B.E.; Jenkins, C.A.; Culver, D.A.; Caprioli, R.M.; Drake, W.P. Dual Analysis for Mycobacteria and Propionibacteria in Sarcoidosis BAL. J. Clin. Immunol. 2012, 32, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zaidi-Ainouch, Z.; Gallien, S.; Domon, B. Mass spectrometry–based detection and quantification of plasma glycoproteins using selective reaction monitoring. Nat. Protoc. 2012, 7, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Ilag, L.; Termopoli, V.; Mendez, L. Advances in MS-Based Analytical Methods: Innovations and Future Trends. J. Anal. Methods Chem. 2018, 2018, 2084567. [Google Scholar] [CrossRef] [PubMed]
- Law, K.P.; Larkin, J.R. Recent advances in SALDI-MS techniques and their chemical and bioanalytical applications. Anal. Bioanal. Chem. 2011, 399, 2597–2622. [Google Scholar] [CrossRef]
- Wang, X.-N.; Tang, W.; Gordon, A.; Wang, H.-Y.; Xu, L.; Li, P.; Li, B. Porous TiO2 Film Immobilized with Gold Nanoparticles for Dual-Polarity SALDI MS Detection and Imaging. ACS Appl. Mater. Interface 2020, 12, 42567–42575. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Oradu, S.; Ifa, D.R.; Cooks, R.G.; Kräutler, B. Direct Plant Tissue Analysis and Imprint Imaging by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2011, 83, 5754–5761. [Google Scholar] [CrossRef]
- Hemalatha, R.G.; Pradeep, T. Understanding the Molecular Signatures in Leaves and Flowers by Desorption Electrospray Ionization Mass Spectrometry (DESI MS) Imaging. J. Agric. Food Chem. 2013, 61, 7477–7487. [Google Scholar] [CrossRef]
- Granborg, J.R.; Handler, A.H.; Janfelt, C. Mass spectrometry imaging in drug distribution and drug metabolism studies—Principles, applications and perspectives. TrAC Trends Anal. Chem. 2022, 146, 116482. [Google Scholar] [CrossRef]
- Dai, W.; Hu, Z.; Xie, D.; Tan, J.; Lin, Z. A novel spatial-resolution targeted metabolomics method in a single leaf of the tea plant (Camellia sinensis). Food Chem. 2020, 311, 126007. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Liu, Y.; He, H.; Han, M.; Li, Y.; Zeng, M.; Wang, X. Recent advances in matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) for in situ analysis of endogenous molecules in plants. Phytochem. Anal. 2018, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Cooks, R.G.; Ouyang, Z.; Takats, Z.; Wiseman, J.M. Ambient Mass Spectrometry. Science 2006, 311, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.M.; Cooks, R.G. Ambient mass spectrometry using desorption electrospray ionization (DESI): Instrumentation, mechanisms and applications in forensics, chemistry, and biology. J. Mass Spectrom. 2005, 40, 1261–1275. [Google Scholar]
- Shiea, J.; Huang, M.Z.; Hsu, H.J.; Lee, C.Y.; Yuan, C.H.; Beech, I.; Sunner, J. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun. Mass Spectrom. 2005, 19, 3701–3704. [Google Scholar] [CrossRef]
- Cody, R.B.; Laramee, J.A.; Durst, H.D. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- McEwen, C.N.; McKay, R.G.; Larsen, B.S. Analysis of Solids, Liquids, and Biological Tissues Using Solids Probe Introduction at Atmospheric Pressure on Commercial LC/MS Instruments. Anal. Chem. 2005, 77, 7826–7831. [Google Scholar] [CrossRef] [PubMed]
- Takats, Z.; Cotte-Rodriguez, I.; Talaty, N.; Chen, H.W.; Cooks, R.G. Direct, trace level detection of explosives on ambient surfaces by desorption electrospray ionizationmass spectrometry. Chem. Commun. 2005, 15, 1950–1952. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.B.; Faria, M.; Souza, D.A.; Bloch, C., Jr.; Silva, L.P.; Humber, R.A. MALDI-TOF mass spectrometry ap-plied to identifying species of insect-pathogenic fungi from Metarhizium anisopliae complex. Mycologia 2014, 106, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Augustini, B.; Silva, L.P.; Bloch, C. Evaluation of MALDI-TOF mass spectrometry for identification of environmental yeasts and development of supplementary database. Appl. Microbiol. Biotechnol. 2014, 98, 5645–5654. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Arcangeletti, M.C.; Rodighiero, I.; Buttrini, M.; Gorrini, C.; Motta, F.; Germini, D.; Medici, M.C.; Chezzi, C.; De Conto, F. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry applied to virus identification. Sci. Rep. 2014, 4, 6803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kliks, M.M.; Qu, W.; Jun, S.; Shi, G.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI-TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef] [PubMed]
- Bonatto, C.C.; Silva, L.P. Cocoa content influences chocolate molecular profile investigated by MALDI-TOF mass spectrometry. J. Sci. Food Agric. 2015, 95, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Enfissi, E.M.A.; Goodfellow, M.; Eguchi, T.; Bramley, P.M. Metabolite profiling of plant carotenoids using the matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Plant. J. 2007, 49, 552–564. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, Y.; Wang, H.; Chen, G. Qualitative and quantitative analysis of quaternary ammonium alkaloids from Rhizoma Corydalis by matrix-assisted laser desorption/ionization Fourier transform mass spectrometry coupled with a selective precipitation reaction using Reinecke salt. Anal. Chim. Acta 2006, 555, 269–277. [Google Scholar] [CrossRef]
- Abell, D.C.; Sporns, P. Rapid quantitation of potato glycoalkaloids by matrix-assisted laser desorption/ionization time-of-fight mass spectrometry. J. Agric. Food Chem. 1996, 44, 2292–2296. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, D.K. Quantitative determination of nicotinic acid in micro liter volume of urine sample by drop-to-drop solvent microextraction coupled to matrix assisted laser desorption/ionization mass spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 253–257. [Google Scholar] [CrossRef]
- Wang, J.; Kalt, W.; Sporns, P. Comparison between HPLC and MALDI-TOF MS analysis of anthocyanins in highbush Blueberries. J. Agric. Food Chem. 2000, 48, 3330–3335. [Google Scholar] [CrossRef]
- Wang, J.; Sporns, P. Analysis of anthocyanins in red wine and fruit juice using MALDI-MS. J. Agric. Food Chem. 1999, 47, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Marczak, Ł.; Kachlicki, P.; Koźniewski, P.; Skirycz, A.; Krajewski, P.; Stobiecki, M. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry monitoring of anthocyanins in extracts from Arabidopsis thaliana leaves. Rapid Commun. Mass Spectrom. 2008, 22, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Frison-Norrie, S.; Sporns, P. Identification and Quantification of Flavonol Glycosides in Almond Seedcoats Using MALDI-TOF MS. J. Agric. Food Chem. 2002, 50, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Melot, A.; Guérineau, V.; Gleye, C.; Höglinger, G.U.; Ruberg, M.; Lannuzel, A.; Laprévote, O.; Laurens, A.; Hocquemiller, R. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in Guadeloupe. Mov. Disord. 2005, 20, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Sleno, L.; Volmer, D.A. Toxin screening in phytoplankton: Detection and quantitation using MALDI triple quadrupole mass spectrometry. Anal. Chem. 2005, 77, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- May, L.A.; Tourkina, E.; Hoffman, S.R.; Dix, T.A. Detection and quantitation of curcumin in mouse lung cell cultures by matrix-assisted laser desorption ionization time of flight mass spectrometry. Anal. Biochem. 2005, 337, 62–69. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. Simultaneous quantitation of naturally occurring insecticides, acaricides, and piscicides in rapeseed oil by UV-MALDI mass spectrometry. J. Food Meas. Charact. 2014, 8, 15–28. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef]
- Okuda, T.; Kimura, Y.; Yoshida, T.; Hatano, T.; Okuda, H.; Arichi, S. Studies on the activities of tannins and related compounds from medicinal plants and drugs. I. Inhibitory effects on lipid peroxidation in mitochondria and microsomes of liver. Chem. Pharm. Bull. 1983, 31, 1625–1631. [Google Scholar] [CrossRef]
- Muramatsu, K.; Fukuyo, M.; Hara, Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 1986, 32, 613–622. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ishigaki, F.; Ishigaki, A.; Iwashima, H.; Hara, Y. Reduction of blood glucose levels by tea catechin. Biosci. Biotechnol. Biochem. 1993, 57, 525–527. [Google Scholar] [CrossRef]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-induced Obesity by Up-Regulation of mRNA Levels of the Enzymes Involved in beta-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef]
- Mendilaharsu, M.; de Stefani, E.; Deneo-Pellegrini, H.; Carzoglio, J.C.; Ronco, A. Consumption of tea and coffee and the risk of lung cancer in cigarette-smoking men: A case–control study in Uruguay. Lung Cancer 1998, 19, 101–107. [Google Scholar] [CrossRef]
- Wang, M.; Guo, C.; Li, M. A case-control study on the dietary risk factors of upper digestive tract cancer. Zhonghua Yixeuehui Zazhishe 1999, 20, 95–97. [Google Scholar]
- Wu, A.H.; Tseng, C.C.; Van Den Berg, D.; Yu, M.C. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003, 63, 7526–7529. [Google Scholar] [PubMed]
- Yang, Y.C.; Lu, F.H.; Wu, J.S.; Wu, C.H.; Chang, C.J. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med. 2004, 164, 1534–1540. [Google Scholar] [CrossRef]
- Davies, M.J.; Judd, J.T.; Baer, D.J.; Clevidence, B.A.; Paul, D.R.; Edwards, A.J.; Wiseman, S.A.; Muesing, R.A.; Chen, S.C. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J. Nutr. 2003, 133, 3298S–3302S. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T. Anti-cariogenic properties of tea (Camellia sinensis). J. Med. Microbiol. 2001, 50, 299–302. [Google Scholar] [CrossRef]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Sabitha, K.E.; Srinivasan, P.; Shyamaladevi, C.S. Green tea attenuates diabetes induced Maillard-type fluorescence and collagen cross-linking in the heart of streptozotocin diabetic rats. Pharmacol. Res. 2007, 55, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Sharangi, A.B. Medicinal and Therapeutic Potentialities of Tea (Camellia sinensis L.)—A Review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Ruzicka, J.; Karova, K.; Kloudova, A.; Svobodova, B.; Amin, A.; Dubisova, J.; Schmidt, M.; Kubinova, S.; Jhanwar-Uniyal, M.; et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 2017, 126, 213–223. [Google Scholar] [CrossRef]
- Renno, W.M.; Al-Khaledi, G.; Mousa, A.; Karam, S.M.; Abul, H.; Asfar, S. (−)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and, chronically injured spinal cord of adult rats. Neuropharmacology 2014, 77, 100–119. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Juneja, L.R.; Taniguchi, M. Antimicrobial effects of green tea polyphenols on thermophilic spore-forming bacteria. J. Biosci. Bioeng. 2000, 90, 81–85. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, Y.N.; Youn, H.N.; Lee, D.H.; Kwak, J.H.; Seong, B.L.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult. Sci. 2012, 91, 66–73. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Lodhia, P.; Yaegaki, K.; Khakbaznejad, A.; Imai, T.; Sato, T.; Tanaka, T.; Murata, T.; Kamoda, T. Effect of green tea on volatile sulfur compounds in mouth air. J. Nutr. Sci. Vitaminol. 2008, 54, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.P.; Bedran, T.B.; Fournier-Larente, J.; Haas, B.; Azelmat, J.; Grenier, D. Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei. BMC Complement. Altern. Med. 2015, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J. Natural antioxidants for food use. Trends Food Sci. Technol. 1991, 2, 223–227. [Google Scholar] [CrossRef]
- Hara, Y. Green Tea: Health Benefits and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Weisburger, J.H.; Veliath, E.; Larios, E.; Pittman, B.; Zang, E.; Hara, Y. Tea polyphenols inhibit the formation of mutagens during the cooking of meat. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2002, 516, 19–22. [Google Scholar] [CrossRef]
- Bozkurt, H. Utilization of natural antioxidants: Green tea extract and thymbra spicata oil in Turkish dry-fermented sausage. Meat Sci. 2006, 73, 442–450. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Technol. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Yazdy, M.M.; Tinker, S.C.; Mitchell, A.A.; Demmer, L.A.; Werler, M.M. Maternal tea consumption during early pregnancy and the risk of spina bifida. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 756–761. [Google Scholar] [CrossRef]
- Merhav, H.; Amitai, Y.; Palti, H.; Godfrey, S. Tea drinking and microcytic anemia in infants. Am. J. Clin. Nutr. 1985, 41, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.A.; Markowitz, J.S. An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis). Planta Med. 2017, 83, 496–508. [Google Scholar] [CrossRef]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Nair, S.; Lin, W.; Yang, C.S.; Chan, J.Y.; Kong, A.N. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm. Res. 2005, 22, 1805–1820. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di, S.A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Sagesaka, Y.M.; Kakuda, T. Analysis of tea catechins in human plasma by high-performance liquid chromatography with solid-phase extraction. J. Agric. Food Chem. 2005, 53, 9885–9889. [Google Scholar] [CrossRef]
- Oh, C.J.; Yang, E.S.; Shin, S.W.; Choi, S.H.; Park, C.I.; Yang, C.H.; Park, J.-W. Epigallocatechin gallate, a constituent of green tea, regulates high glucose-induced apoptosis. Arch. Pharm. Res. 2008, 31, 34–40. [Google Scholar] [CrossRef]
- Kim, S.J.; Li, M.; Jeong, C.W.; Bae, H.B.; Kwak, S.H.; Lee, S.H.; Lee, H.J.; Heo, B.H.; Yook, K.B.; Yoo, K.Y. Epigallocatechin-3-gallate, a green tea catechin, protects the heart against regional ischemia–reperfusion injuries through activation of risk survival pathways in rats. Arch. Pharm. Res. 2014, 37, 1079–1085. [Google Scholar] [CrossRef]
- Secretan, P.-H.; Thirion, O.; Sadou Yayé, H.; Damy, T.; Astier, A.; Paul, M.; Do, B. Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations. Pharmaceuticals 2021, 14, 1242. [Google Scholar] [CrossRef]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Amirthalingam, M.; Reddy, M.S.; Udupa, N.; Mutalik, S. Development and Validation of RP-HPLC Method for Estimation of Epigallocatechin −3-gallate (EGCG) in Lipid based Nanoformulations. Res. J. Pharm. Technol. 2016, 9, 725–730. [Google Scholar] [CrossRef]
- He, Q.; Yao, K.; Jia, D.; Fan, H.; Liao, X.; Shi, B. Determination of total catechins in tea extracts by HPLC and spectrophotometry. Nat. Prod. Res. 2009, 23, 93–100. [Google Scholar] [CrossRef]
- Hirun, S.; Roach, P.D. An improved solvent extraction method for the analysis of catechins and caffeine in green tea. J. Food Nutr. Res. 2011, 50, 160–166. [Google Scholar]
- Fernando, C.D.; Soysa, P. Simple isocratic method for simultaneous determination of caffeine and catechins in tea products by HPLC. Springerplus 2016, 5, 970–974. [Google Scholar] [CrossRef]
- Pan, H.B.; Zhang, D.; Li, B.; Wu, Y.Y.; Tu, Y.Y. A Rapid UPLC Method for Simul-taneous Analysis of Caffeine and 13 Index Polyphenols in Black Tea. J. Chromatogr. Sci. 2017, 55, 495–496. [Google Scholar] [CrossRef]
- El-Kayal, M.O.; Sayed, M.N.; Mortada, N.D.; Elkheshen, S. Development and vali-dation of a simple and rapid UPLC method for the in-vitro estimation of (-)-epigallocatechin-3-gallate in lipid-based formulations. Eur. J. Med. Chem. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, T.E.; Shin, K.H. Quantitative Analysis of Four Catechins from Green Tea Extract in Human Plasma Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Pharmacokinetic Studies. Molecules 2018, 23, 984. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef]
- Pongsuwan, W.; Bamba, T.; Harada, K.; Yonetani, T.; Kobayashi, A.; Fukusaki, E. High-Throughput Technique for Comprehensive Analysis of Japanese Green Tea Quality Assessment Using Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC/TOF MS). J. Agric. Food Chem. 2008, 56, 10705–10708. [Google Scholar] [CrossRef]
- Ninomiya, M.; Unten, L.; Kim, M.; Yamamoto, T.; Juneja, L.R.; Chu, D.C.; Kim, M. (Eds.) Chemistry and Applications of Green Tea; CRC Press: Boca Raton, FL, USA, 1997; pp. 23–36. [Google Scholar]
- Sharma, V.; Gulati, A.; Ravindranath, S.D. Extractability of tea catechins as a function of manufacture procedure and temperature of infusion. Food Chem. 2005, 93, 141–148. [Google Scholar] [CrossRef]
- Wong, C.C.; Cheng, K.; Chao, J.; Peng, X.; Zheng, Z.; Wu, J.; Chen, F.; Wang, M.; Ho, C.-T.; Lin, J.-K.; et al. (Eds.) Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2009; pp. 77–110. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gacía-Viguera, C.; Tomás-Barberán, F.A.; Santos-Buelga, C.; Williamson, G. (Eds.) Methods in Polyphenol Analysis; RSC: Cambridge, UK, 2003; pp. 92–127. [Google Scholar]
- Ramakrishna, U.V.; Sunder, R.S.; Kumar, K.R.; Sinha, S.N. Method development and validation for rapid identification of epigallocatechin gallate using ultra-high performance liquid chromatography. PLoS ONE 2020, 15, e0227569. [Google Scholar]
- Saito, S.; Welzel, A.; Suyenaga, E.; Bueno, F. A method for fast determination of epigallocatechin gallate (EGCG), epicatechin (EC), catechin (C) and caffeine (CAF) in green tea using HPLC. Food Sci. Technol. 2006, 26, 394–400. [Google Scholar] [CrossRef]
- Lambert, J.; Lee, M.-J.; Lu, H.; Meng, X.; Ju, J.; Seril, D.; Sturgill, M.; Yang, C. Epigallocatechin-3-Gallate Is Absorbed but Extensively Glucuronidated Following Oral Administration to Mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.J.; Hong, J.; Li, C.; Smith, T.J.; Yang, G.Y.; Seril, D.N.; Yang, C.S. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer 2000, 37, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Wang, X.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of Plasma and Urinary tea Polyphenols in Human Subjects. Cancer Epidemiol. Biomark. Prev. 1995, 4, 393–399. [Google Scholar] [PubMed]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence-high-performance liquid chromatographic determination of tea catechin, ()-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Pharmacokinetics and system linearity of tea catechins in rat. Xenobiotica 2001, 31, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, Y.; Matsui, Y.; Shimizu, N.; Kondou, N.; Endou, H.; Kuzukawa, M.; Hase, T. Determination of green tea catechins in human plasma using liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B 2006, 834, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mata-Bilbao Mde, L.; Andres-Lacueva, C.; Roura, E.; Jauregui, O.; Escribano, E.; Torre, C.; Lamuela-Raventos, R.M. Absorption and pharmacokinetics of green tea catechins in beagles. Br. J. Nutr. 2008, 100, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-H.; Wang, W.-B.; Li, J.; Chang, Y.-X.; Wang, Y.-F.; Zhang, J.; Zhang, B.-L.; Gao, X.-M. Simultaneous determination of catechin, epicatechin and epicatechin gallate in rat plasma by LC–ESI-MS/MS for pharmacokinetic studies after oral admin-istration of Cynomorium songaricum extract. J. Chromatogr. B 2012, 880, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Van Amelsvoort, J.M.; van het Hof, K.H.; Mathot, J.N.J.J.; Mulder, T.P.J.; Wiersma, A.; Tijnerg, L.B.M. Plasma concen-trations of individual tea catechins after a single oral dose in humans. Xenobiotica 2001, 31, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Serafini, M.; Salucci, M.; Azzini, E.; Ferreo-Luzzi, A. Application of a new high-performance liquid chromato-graphic method for measuring selected polyphenols in human plasma. J. Chromatogr. B 1997, 692, 311–317. [Google Scholar] [CrossRef]
- Pietta, P.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazoni, P.; Bombardelli, E. Cate-chin metabolites after intake of green tea infusions. Biochem. Mol. Biol. Int. 1998, 46, 895–903. [Google Scholar] [PubMed]
- Lee, M.J.; Prabhu, S.; Meng, X.; Li, C.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Anal. Biochem. 2000, 279, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kotani, A.; Miyashita, N.; Kusu, F. Determination of catechins in human plasma after commercial canned green tea ingestion by high-performance liquid chromatography with electrochemical detection using a microbore column. J. Chromatogr. B 2003, 788, 269–275. [Google Scholar] [CrossRef]
- Umegaki, K.; Sugisawa, A.; Yamada, K.; Higuchi, M. Analytical method of measuring tea catechins in human plasma by solidphase extraction and HPLC with electrochemical de-tection. J. Nutr. Sci. Vitaminol. 2001, 47, 402–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roura, E.; Andres-Lacueva, C.; Jauregui, O.; Badia, E.; Estruch, R.; Izquierdo-Pulido, M.; Lamuela-Raventos, R.M. Rapid liquid chromatography tandem mass spectrometry assay to quantify plasma (-)-epicatechin metabolites after ingestion of a standard portion of co-coa beverage in humans. J. Agric. Food Chem. 2005, 53, 6190–6194. [Google Scholar] [CrossRef]
- Murphy, A.T.; Bonate, P.L.; Kasper, S.C.; Gillespie, T.A.; Delong, A.F. Determination of xanomeline in human plasma by ionspray tandem mass-spectrometry. Mass Spectrom. 1994, 23, 621–625. [Google Scholar] [CrossRef]
- Mata, M.L.; Lacueva, C.A.; Roura, E.; Jáuregui, O.; Escribano, E.; Torre, C.; Lamuela-Raventós, R.M. Absorption and phar-macokinetics of grapefruit flavanones in beagles. Br. J. Nutr. 2007, 98, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Urpí-Sardà, M.; Jáuregui, O.; Lamuela-Raventós, R.M.; Jaeger, W.; Miksits, M.; Covas, M.I.; Andrés-Lacueva, C. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal. Chem. 2005, 77, 3149–3155. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food. Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Plumb, R.; Castro-Perez, J.; Granger, J.; Beattie, I.; Joncour, K.; Wright, A. Ultra-performance liquid chromatography coupled to quadrupole-orthogonal time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Guillarme, D.; Casetta, C.; Bicchi, C.; Veuthey, J.-L. High throughput qualitative analysis of polyphenols in tea samples by ultra-high pressure liquid chromatography coupled to UV and mass spectrometry detectors. J. Chromatogr. A 2010, 1217, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Kimura, J.; Watanabe, H.; Yamada, S. Development of rapid and simultaneous quantitative method for green tea catechins on the bioanalytical study using UPLC/ESI-MS. Biomed. Chromatogr. 2013, 27, 1–6. [Google Scholar] [CrossRef]
- De Lourdes Mata-Bilbao, M.; Andrés-Lacueva, C.; Roura, E.; Jáuregui, O.; Torre, C.; Lamuela-Raventós, R.M. A new LC/MS/MS rapid and sensitive method for the determination of green tea catechins and their metabolites in biological samples. J. Agric. Food Chem. 2007, 55, 8857–8863. [Google Scholar] [CrossRef]
- Miketova, P.; Schram, K.H.; Whitney, J.; Li, M.; Huang, R.; Kerns, E.; Valcic, S.; Timmermann, B.N.; Rourick, R.; Klohr, S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000, 35, 860–869. [Google Scholar] [CrossRef]
- Liquid Chromatography/Mass Spectrometry, Application Note? LC/MS Applications Team PerkinElmer, Inc.: Waltham, MA, USA. Available online: https://www.perkinelmer.com/category/liquid-chromatography-mass-spectrometry-lc-ms (accessed on 2 July 2022).
- Juang, Y.M.; Chien, H.J.; Chen, C.J.; Lai, C.C. Graphene flakes enhance the detection of TiO2-enriched catechins by SALDI-MS after microwave-assisted enrichment. Talanta 2016, 153, 347–352. [Google Scholar] [CrossRef]
- Rajapaksha, S.; Shimizu, N. Pilot-scale extraction of polyphenols from spent black tea by semi-continuous subcritical solvent extraction. Food Chem. X 2022, 13, 100200. [Google Scholar] [CrossRef] [PubMed]
- Wasai, M.; Fujimura, Y.; Nonaka, H.; Kitamura, R.; Murata, M.; Tachibana, H. Postprandial glycaemia-lowering effect of a green tea cultivar Sunrouge and cultivar-specific metabolic profiling for determining bioactivity-related ingredients. Sci. Rep. 2018, 8, 16041. [Google Scholar] [CrossRef]
- Fujimura, Y.; Watanabe, M.; Mori-kawa-Ichinose, T.; Fujino, K.; Yamamo-to, M.; Nishioka, S.; Inoue, C.; Ogawa, F.; Yonekura, M.; Nakasone, A.; et al. Metabolic Profiling for Evaluating the Dipeptidyl Peptidase-IV Inhibitory Po-tency of Diverse Green Tea Cultivars and Determining Bioactivity-Related Ingredients and Combinations. J. Agric. Food Chem. 2022, 70, 6455–6466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, N.; Li, Y.; Chen, S.-W. Efficient enzymatic modification of epi-gallocatechin gallate in ionic liquids. Green Chem. Lett. Rev. 2021, 14, 415–424. [Google Scholar] [CrossRef]
- Song, Y.; Sun, H.J.; Xiao, J.; Wang, F.; Ding, Y.; Zhao, J.Y.; Wen, A.D. Development of a liquid chromatography-tandem mass spectrometric (LC-MS/MS) method for simultaneous determination of epigallocate-chin-3-gallate, silibinin, and curcumin in plasma and different tissues after oral dosing of Protandim in rats and its ap-plication in pharmacokinetic and tissue distribution studies. J. Pharmaceut. Biomed. 2019, 170, 54–62. [Google Scholar]
- Susanti, E.; Ratnawati, R.; Rudijanto, A. Qualitative analysis of catechins from green tea GMB-4 clone using HPLC and LC-MS/MS. Asian Pac. J. Trop. Biomed. 2015, 5, 1046–1050. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Z.; Wen, M.; Ho, C.T.; Jiang, Z.; Wang, Y.; Xu, N.; Xie, Z.; Zhang, J.; Zhang, L.; et al. Screening of α-glucosidase inhibitors in large-leaf yellow tea by offline bioassay coupled with liquid chromatography tandem mass spectrometry. Food Sci. Hum. Wellness 2022, 11, 627–634. [Google Scholar] [CrossRef]
- Farag, M.A.; Shakour, Z.T.A.; Elmassry, M.M.; Donia, M.S. Metabolites profiling reveals gut microbiome-mediated bio-transformation of green tea polyphenols in the presence of N-nitrosamine as pro-oxidant. Food Chem. 2022, 371, 131147. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temper-ature. LWT 2022, 161, 113394. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Yi, L.; Hoegger, P.; Arroo, R.; Bajpai, V.K.; Prieto, M.-A.; Simal-Gandara, J.; Wang, S.; Cao, H. Stability and antioxidant capacity of epigallocatechin gallate in Dulbecco’s modified eagle medium. Food Chem. 2022, 366, 130521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, J.; Xu, Y.; Wang, H.; Lu, L.; Song, R.; Zou, J. Epigallocate-chin-3-gallate inhibits replication of white spot syndrome virus in the freshwater crayfish Procambarus clarkii. J. Fish Dis. 2022, 45, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Cui, Y.; Granato, D.; Wen, M.; Han, Z.; Zhang, L. Free, soluble conjugated and insoluble bonded phenolic acids in Keemun black tea: From UPLC-QQQ-MS/MS method development to chemical shifts monitoring during processing. Food Res. Int. 2022, 155, 111041. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhang, Q.; Li, Q.; Geng, B.; Bi, K. Development of a UFLC-MS/MS method for the simultaneous determination of seven tea catechins in rat plasma and its application to a pharmacokinetic study after administration of green tea extract. J. Pharm. Biomed. Anal. 2016, 125, 229–235. [Google Scholar] [CrossRef]
- Jing, J.; Shi, Y.Z.; Zhang, Q.F.; Wang, J.; Ruan, J.Y. Prediction of Chinese green tea ranking by metabolite profiling using ul-tra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS). Food Chem. 2017, 221, 311–316. [Google Scholar] [CrossRef]
- Tu, L.J.; Sun, H.J.; He, S.D.; Zhu, Y.S.; Yu, M.; Sun, X.B.; Zhang, Z.Y. Isolation of Epigallocatechin Gallate from Green Tea and its Effects on Probiotics and Pathogenic Bacteria. Curr. Top. Nutraceutical Res. 2019, 17, 69–77. [Google Scholar]
- Del Rio, D.; Calani, L.; Cordero, C.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and catabolism of green tea fla-van-3-ols in humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef]
- Tao, W.; Zhou, Z.; Zhao, B.; Wei, T. Simultaneous determination of eight catechins and four theaflavins in green, black and oolong tea using new HPLC–MS–MS method. J. Pharm. Biomed. Anal. 2016, 131, 140–145. [Google Scholar] [CrossRef]
- Guo, P.-C.; Shen, H.-D.; Fang, J.-J.; Ding, T.-M.; Ding, X.-P.; Liu, J.-F. On-line high-performance liquid chromatography coupled with biochemical detection method for screening of α-glucosidase inhibitors in green tea. Biomed. Chromatogr. 2018, 32, e4281. [Google Scholar] [CrossRef]
- Ong, C.; Annuar, M.S.M. Polyphenolic composition and in vitro antioxidant activities of native- and tannase-treated green tea extracts. Int. J. Food Sci. Technol. 2017, 52, 748–756. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between Epigallocatechin-3-gallate (EGCG) and Human Gut Microbiota In vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Fu, Y.-Q.; Chen, J.-X.; Wang, F.; Feng, Z.-H.; Yin, J.-F.; Zeng, L.; Xu, Y.-Q. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Lane, G.A.; Otter, D.E.; Hemar, Y.; Quek, S.-Y.; Harrison, S.J.; Rasmussen, S. Analysis of metabolic markers of tea origin by UHPLC and high-resolution mass spectrometry. Food Res. Int. 2013, 53, 827–835. [Google Scholar] [CrossRef]
- Ge, J.; Tan, B.-X.; Chen, Y.; Yang, L.; Peng, X.-C.; Li, H.-Z.; Lin, H.-J.; Zhao, Y.; Wei, M.; Cheng, K.; et al. Interaction of green tea polyphenol epigallocatechin-3-gallate with sunitinib: Potential risk of diminished sunitinib bioavailability. J. Mol. Med.-Jmm. 2011, 89, 595–602. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, Y.; Zhang, H.; Zhong, L.; Qian, X. Systematic characterization of the covalent interactions between (−)-epigallocatechin gallate and peptides under physiological conditions by mass spectrometry. Rapid. Commun. Mass Spectrom. 2009, 23, 1147–1157. [Google Scholar] [CrossRef]
- Bae, M.J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (−)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mori, T.; Tanaka, T.; Mizuno, D.; Yamaji, R.; Kumazawa, S.; Nakayama, T.; Akagawa, M. Covalent modification of pro-teins by green tea polyphenol epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008, 45, 1384–1394. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Tanaka, M.; Li, B.; Ueno, T.; Matsuda, H.; Matsui, T. Novel in situ visualisation of rat intestinal absorption of polyphenols via matrix-assisted laser desorption/ionisation mass spectrometry imaging. Sci. Rep. 2019, 9, 3166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Fujimura, Y.; Hagihara, T.; Sasaki, M.; Yukihira, D.; Nagao, T.; Miura, D.; Yamaguchi, S.; Saito, K.; Tanaka, H.; et al. In situ label-free imaging for visualizing the biotransformation of a bioactive polyphenol. Sci. Rep. 2013, 3, 2805. [Google Scholar] [CrossRef] [PubMed]
- Rush, M.D.; Rue, E.A.; Wong, A.; Kowalski, P.; Glinski, J.A.; van Breemen, R.B. Rapid Determination of Procyanidins Using MALDI-ToF/ToF Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 11355–11361. [Google Scholar] [CrossRef]
- Ohnishi-Kameyama, M.; Yanagida, A.; Kanda, T.; Nagata, T. Identification of catechin oligomers from apple (Malus pumila cv. Fuji) in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and fast-atom bombardment mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 31–36. [Google Scholar] [CrossRef]
- Go, E.P.; Apon, J.V.; Luo, G.; Saghatelian, A.; Daniels, R.H.; Sahi, V.; Dubrow, R.; Cravatt, B.F.; Vertes, A.; Siuzdak, G. Desorption/ionization on silicon nanowires. Anal. Chem. 2005, 77, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-J.; Shin, J.-H.; Song, J.-Y.; Han, S.-Y. Effects of ZnO nanowire length on surface-assisted laser desorption/ionization of small molecules. J. Am. Soc. Mass Spectrom. 2010, 21, 989. [Google Scholar] [CrossRef]
- Walker, B.N.; Razunguzwa, T.; Powell, M.; Knochenmuss, R.; Vertes, A. Nanophotonic ion production from silicon microcolumn arrays. Angew. Chem. Int. Ed. Engl. 2009, 48, 1669–1672. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Li, J.; Chi, L.; Guo, X.; Lu, N. Biomimetic antireflective siliconnanocones array for small molecules analysis. J. Am. Soc. Mass Spectrom. 2013, 24, 66. [Google Scholar] [CrossRef]
- Gulbakan, B.; Park, D.; Kang, M.; Kececi, K.; Martin, C.R.; Powell, D.H.; Tan, W. Laser desorption ionization mass spectrometry on silicon nanowell arrays. Anal. Chem. 2010, 82, 7566–7575. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Fernandes, A.M.A.P.; Santos, V.G.; Alberici, R.M.; Araldi, D.; Parada, C.A.; Braguini, W.; Veronez, L.; Bisson, G.S.; Reis, F.H.Z.; et al. Nanoassisted laser desorption-ionization-MS imaging oftumors. Anal. Chem. 2012, 84, 6341–6345. [Google Scholar] [CrossRef]
- Wyatt, M.F.; Ding, S.; Stein, B.K.; Brenton, A.G.; Daniels, R.H. Analysis of variousorganic and organometallic compounds using nanostructure-assisted laserdesorption/ionization time-of-flight mass spectrometry (NALDI-TOFMS). J. Am. Soc. Mass Spectrom. 2010, 21, 1256. [Google Scholar] [CrossRef][Green Version]
- Shen, Z.; Thomas, J.; Averbuj, C.; Broo, K.; Engelhard, M.; Crowell, J.; Finn, M.; Siuzdak, G. Porous silicon as a versatile platform for laser desorption/ionization mass spectrometry. Anal. Chem. 2001, 73, 612–619. [Google Scholar] [CrossRef]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption-ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [CrossRef]
- Northen, T.R.; Yanes, O.; Northen, M.T.; Marrinucci, D.; Uritboonthai, W.; Apon, J.; Golledge, S.L.; Nordström, A.; Siuzdak, G. Clathrate nanostructures for mass spectrometry. Nature 2007, 449, 1033–1036. [Google Scholar] [CrossRef]
- Stolee, J.A.; Walker, B.N.; Chen, Y.; Vertes, A. Nanophotonic Ion Sources. AIP Conf. Proc. 2010, 1278, 98–110. [Google Scholar]
- Her, T.H.; Finlay, R.J.; Wu, C.; Mazur, E. Femtosecond laser-induced formation of spikes on silicon. Appl. Phys. A Mater. Sci. Process. 2000, 70, 383–385. [Google Scholar] [CrossRef]
- Walker, B.N.; Stolee, J.A.; Vertes, A. Nanophotonic ionization for ultratrace and single-cell analysis by mass spectrometry. Anal. Chem. 2012, 84, 7756–7762. [Google Scholar] [CrossRef] [PubMed]
- Stopka, S.A.; Rong, C.; Korte, A.R.; Yadavilli, S.; Nazarian, J.; Razunguzwa, T.T.; Morris, N.J.; Vertes, A. Molecular imaging of Biological Samples on Nanophotonic LaserDesorption Ionization Platforms. Angew. Chem. Int. Ed. Engl. 2016, 55, 4369–4612. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).