Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Solvents and Reagents
2.3. Plant Material
2.4. Sample Preparation and Derivatization
2.5. HPLC Analysis
2.6. GC–MS Analysis
2.7. Rancimat Test
2.8. Determination of Total Phenolic Content of Plant Extracts
2.9. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.10. Ferric-Reducing Antioxidant Power (FRAP)
2.11. Antimicrobial Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity
3.2. HPLC Analysis
3.3. GC–MS Analysis
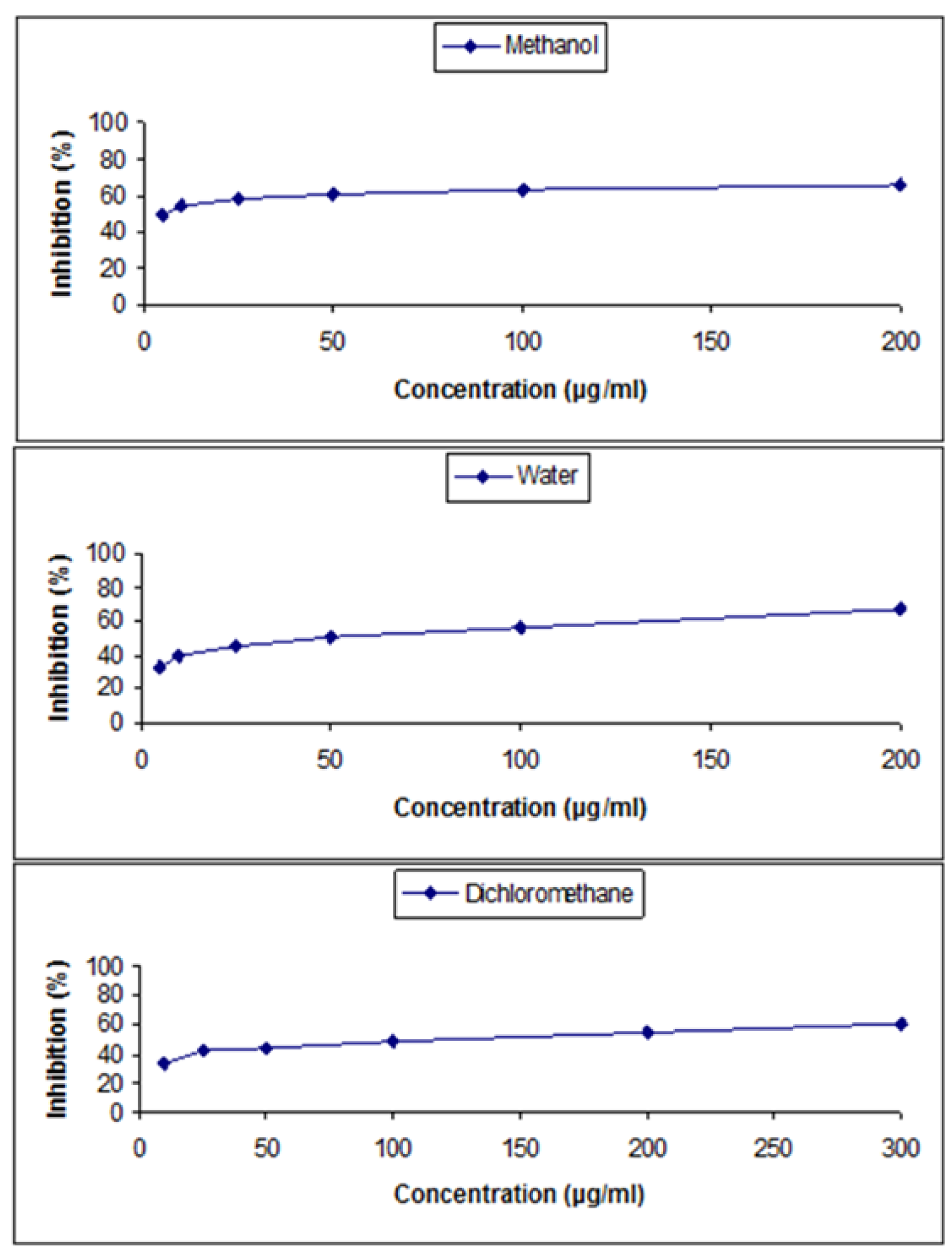
3.4. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Assay
3.5. Ferric-Reducing Antioxidant Power (FRAP)
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proestos, C. The Benefits of Plant Extracts for Human Health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catachins, caffeine and gallic acids in green, Oolong, black and pu-erch teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Chu, T.; Chang, C.; Liao, Y.; Chen, Y. Microwave-accelerated derivatization process for the determination of phenolic acids by gas chromatography—mass spectrometry. Talanta 2001, 54, 1163–1171. [Google Scholar] [CrossRef]
- Deng, Y.; Fan, X.; Delgado, A.; Nolan, C.; Furton, K.; Zuo, Y.; Jones, R.D. Separation and determination of aromatic acids in natural water with preconcentration by capillary zone electrophoresis. J. Chromatogr. A 1998, 817, 145–152. [Google Scholar] [CrossRef]
- Suarez, B.; Picinelli, A.; Mangas, J.J. Solid-phase extraction and high-performance liquid chromatographic determination of polyphenols in apple musts and ciders. J. Chromatogr. A 1996, 727, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods 2013, 2, 90–99. [Google Scholar] [CrossRef]
- Harborne, J.B. Phenolic Compounds. In Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1998; pp. 40–106. [Google Scholar]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.-J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Technol./Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm.-Wiss. Technol. Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 191, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: The Frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.F.; Szeto, Y.T. Total Antioxidant Capacity of Teas by the Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Mattila, P.; Astola, J.; Kumpulainen, J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agric. Food Chem. 2000, 48, 5834–5841. [Google Scholar] [CrossRef]
- O′Dowd, Y.; Driss, F.; Dang, P.M.; Elbim, C.; Gougerot-Pocidalo, M.A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Miean, K.H.; Suhaila, M. Flavonoid (Myricetin, Quercetin, Kaempherol, Luteolin, and Apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry juice by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Diamandis, E.P.; Karumanchiri, A.; Goldberg, D.M. A multiresidue derivatization gas chromatographic assay for fifteen phenolic constituents with mass selective detection. Anal. Chem. 1997, 69, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolics in white free run juices and wines from penedés by high-performance liquid chromatography: Changes during vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]
- Deng, F.; Zito, S.W. Development and validation of a gas chromatographic-mass spectrometric method for simultaneous identification and quantification of marker compounds including bilobalide, ginkgolides and flavonoids in Ginkgo biloba L. extract and pharmaceutical preparations. J. Chromatogr. A 2003, 986, 121–127. [Google Scholar] [CrossRef]
- Cao, G.; Verdon, C.P.; Wu, A.H.B.; Wang, H.; Prior, R.L. Automated oxygen radical absorbance capacity assay using COBAS FARA II. Clin. Chem. 1995, 41, 1738–1744. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Vardar-Ünlü, G.; Candan, F.; Sökmen, A.; Daferera, D.; Polissiou, M.; Sökmen, M.; Dömnez, E.; Tepe, B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of thymus pectinatus fisch. et Mey. Var. pectinatus (Lamiaceae). J. Agric. Food Chem. 2003, 50, 63–67. [Google Scholar] [CrossRef]
- Thangadurai, D.; Anitha, S.; Pullaiah, T.; Reddy, P.N.; Ramachandraiah, O.S. Essential oil constituents and in vitro antimicrobial activity of decalepis hamiltonii roots against foodborne pathogens. J. Agric. Food Chem. 2002, 50, 3147–3149. [Google Scholar] [CrossRef] [PubMed]

| Species | Part Examined | Drying Method a | Total Phenolics b (mg Gallic Acid/ g ds) | PF c (Ground Sample) | PF (Methanol Extracts) | IC50 d (mg/L) |
|---|---|---|---|---|---|---|
| Filipendula ulmaria | Flower | Air | 77.4 ± 3.71 | 1.3 | 1.2 | 47.0 ± 2.1 |
| Salvia officinalis | Herb | Air | 234.7 ± 12.9 | 1.5 | 1.4 | 23.4 ± 1.4 |
| Rosmarinus officinalis | Herb | F/v | 190.2 ± 6.3 | 1 | 0.8 | 34.9 ± 2.2 |
| Sideritis scardica | Leaves | F/v | 75.3 ± 2.1 | 1.8 | 1.7 | 49.6 ± 0.4 |
| Geranium purpureum | Leaves | F/v | 120.5 ± 3.7 | 3.1 | 2.9 | 41.3 ± 2.1 |
| mg/ 100g ds a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant | GLA | GA | CA | pCA | VA | SA | FA | pHBA |
| Filipendula ulmaria | ND | ND | 6.5 ± 0.02 | 4.3 ± 0.02 | 4.8 ± 0.02 | ND | 13.2 ± 0.04 | ND |
| Salvia officinalis | 3.4 ± 0.01 | ND | ND | ND | ND | ND | 6.7 ± 0.02 | ND |
| Rosmarinus officinalis | 1.2 ± 0.02 | ND | 4.9 ± 0.01 | ND | ND | ND | 4.8 ± 0.01 | ND |
| Sideritis scardica | 3.7 ± 0.01 | ND | 5.3 ± 0.03 | ND | ND | ND | 4.2 ± 0.02 | ND |
| Geranium purpureum | 14 ± 0.02 | 3.2 ± 0.03 | 20 ± 0.03 | 4.1 ± 0.02 | 2 ± 0.02 | 1.1 ± 0.02 | ND | 5.4 ± 0.02 |
| Content, mg/100g Dry Sample a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant | Quercetin | Apigenin | Luteolin | Naringenin | Eriodictyol | Rutin | (+)-Catechin Hydrated | (−)-Epicatechin | Hydroxytyrosol |
| Filipendula ulmaria | 1.5 ± 0.01 | ND | ND | ND | ND | 1.3 ± 0.01 | ND | ND | ND |
| Salvia officinalis | 3.6 ± 0.01 | 4 ± 0.01 | ND | 1.7 ± 0.02 | ND | 2.6 ± 0.01 | ND | ND | ND |
| Rosmarinus officinalis | ND | ND | 3.1 ± 0.02 | ND | 0.3 ± 0.02 | ND | ND | ND | ND |
| Sideritis scardica | 3.6 ± 0.02 | ND | ND | ND | ND | 1.9 ± 0.01 | ND | 4.5 ± 0.04 | ND |
| Geranium purpureum | 11.2 ± 0.02 | ND | ND | ND | ND | 4.5 ± 0.01 | 1.5 ± 0.01 | 5.6 ± 0.02 | 11.2 ± 0.04 |
| Compound * | MW (Silylated Compounds) | Identified Ions (m/z) |
|---|---|---|
| p-HBA | 282 | 267 (100%), 193, 223, 282 |
| VA | 312 | 149 (100%), 312, 223, 165 |
| GA | 370 | 355 (100%), 281, 147, 223, 267, 370 |
| GLA | 458 | 281 (100%), 458, 179, 147 |
| pCA | 308 | 219 (100%), 293, 308, 249 |
| FA | 338 | 338 (100%), 308, 323, 249, 293, 219, 279 |
| CA | 396 | 219 (100%), 396, 381, 191 |
| Quercetin | 647 | 575 (100%), 647, 487 |
| Catechin | 650 | 368 (100%), 355, 650, 267, 383, 179, 297 |
| Hydroxytyrosol | 370 | 267 (100%), 193, 179, 370 |
| SA | 342 | 327 (100%), 312, 297, 342 |
| Epicatechin | 650 | 368 (100%), 355, 267, 147, 649 |
| p-Hydroxyphenylacetic acid | 296 | 179 (100%), 164, 149, 296 |
| Protocatechuic acid | 370 | 193 (100%), 223, 370, 267 |
| Cinnamic acid | 220 | 131 (100%), 205, 103 161, 220 |
| Plant Extracts | Escherichia coli 0157:H7 NCTC12900 | Salmonella enteritidis PT4 | Staphylococcus aureus ATCC 6538 | Listeria monocytogenes ScottA | Bacillus cereus FSS134 | Pseudomonas putida AMF178 |
|---|---|---|---|---|---|---|
| Filipendula ulmaria | - a | - | ~ b | ++ d | ~ | + c |
| Salvia officinalis | ~ | - | - | ++ | ~ | ~ |
| Rosmarinus officinalis | - | - | - | + | - | + |
| Sideritis scardica | + | - | - | ++ | - | + |
| Geranium purpureum | ++ | - | + | ++ | + | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papastavropoulou, K.; Oz, E.; Oz, F.; Proestos, C. Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece. Separations 2022, 9, 186. https://doi.org/10.3390/separations9080186
Papastavropoulou K, Oz E, Oz F, Proestos C. Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece. Separations. 2022; 9(8):186. https://doi.org/10.3390/separations9080186
Chicago/Turabian StylePapastavropoulou, Konstantina, Emel Oz, Fatih Oz, and Charalampos Proestos. 2022. "Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece" Separations 9, no. 8: 186. https://doi.org/10.3390/separations9080186
APA StylePapastavropoulou, K., Oz, E., Oz, F., & Proestos, C. (2022). Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece. Separations, 9(8), 186. https://doi.org/10.3390/separations9080186







