A Comprehensive Photocatalysis Study of Promising Zirconia/Laser-Induced Graphene Nanocomposite for Wastewater Treatment-Based Methylene Blue Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterizations
3. Results and Discussion
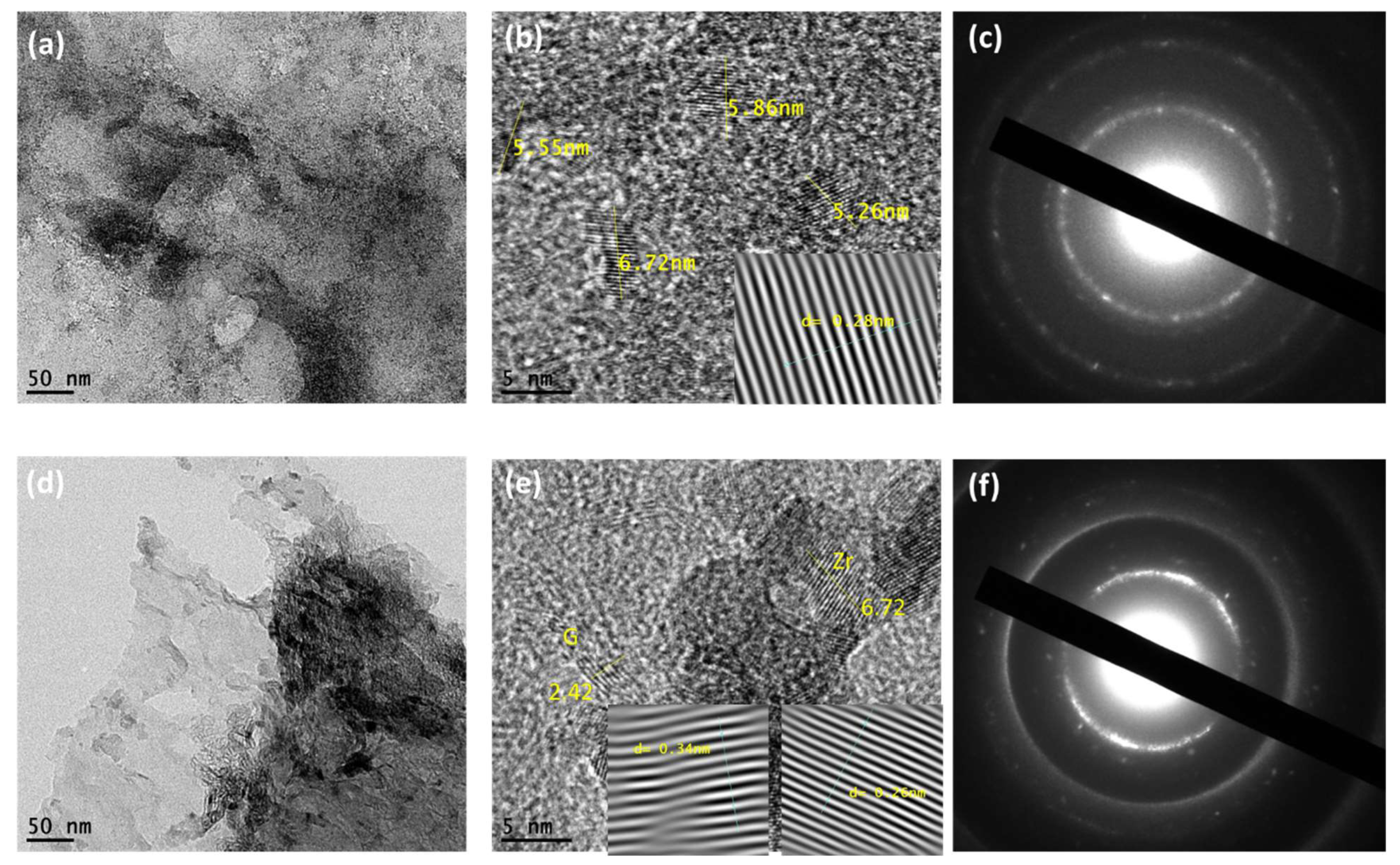
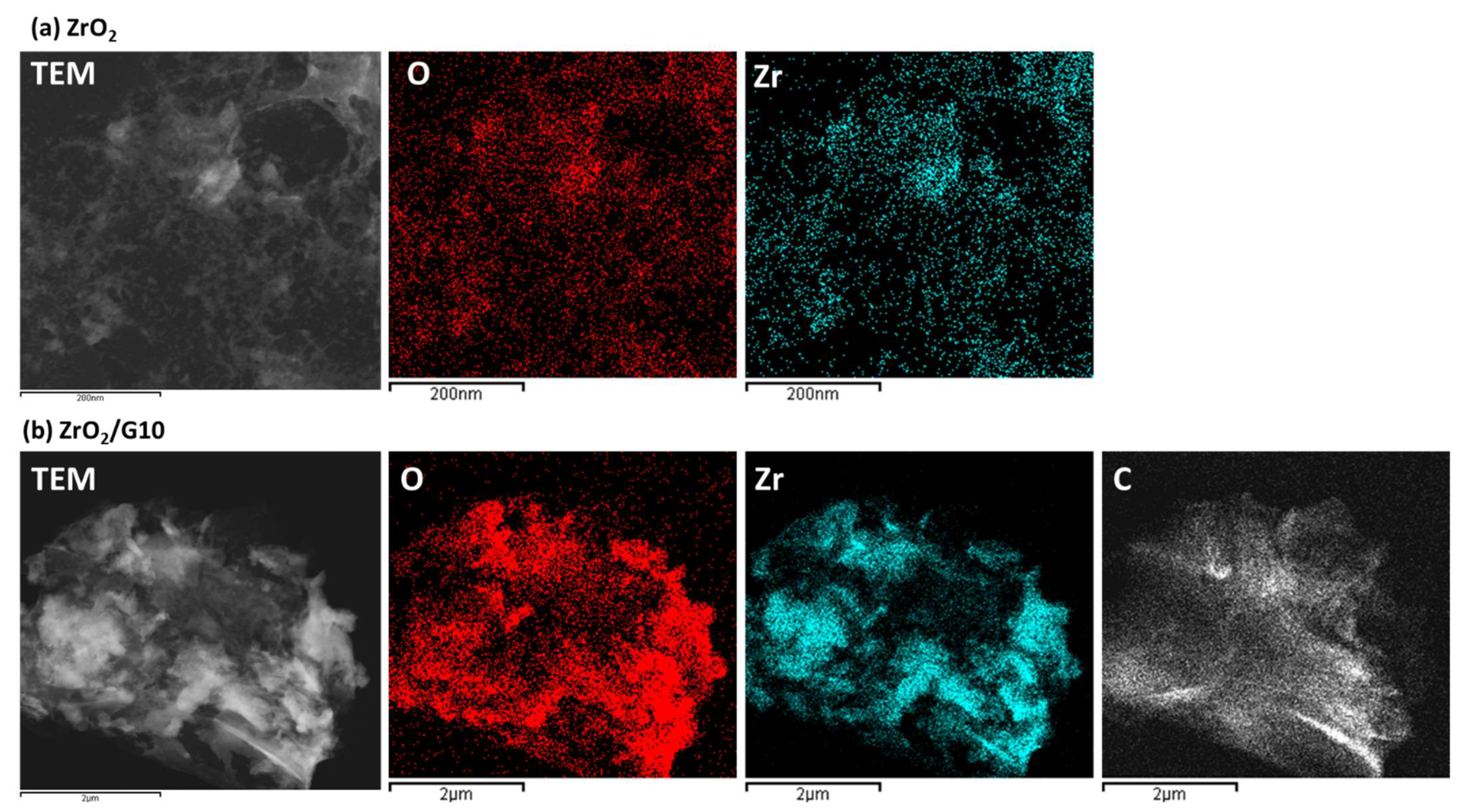
3.1. Structural Elemental Analysis
3.2. Raman Characterizations
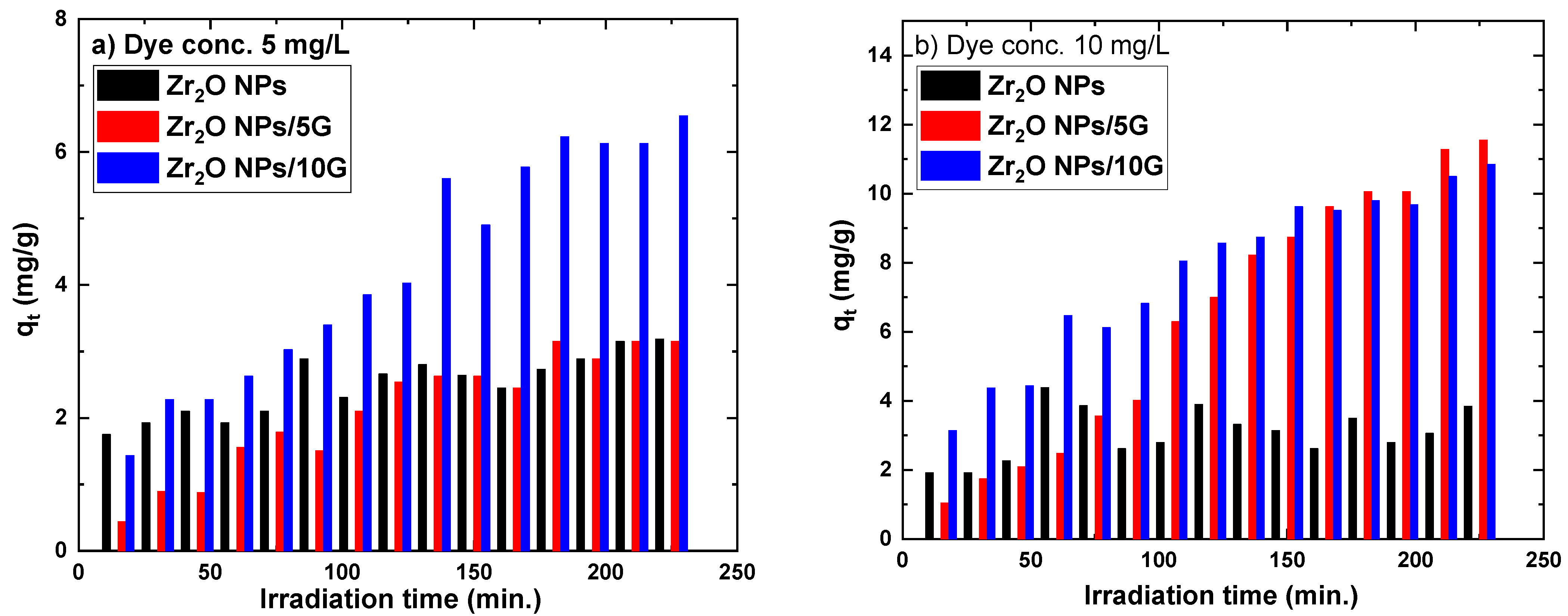
3.3. Photocatalytic Degradation Studies
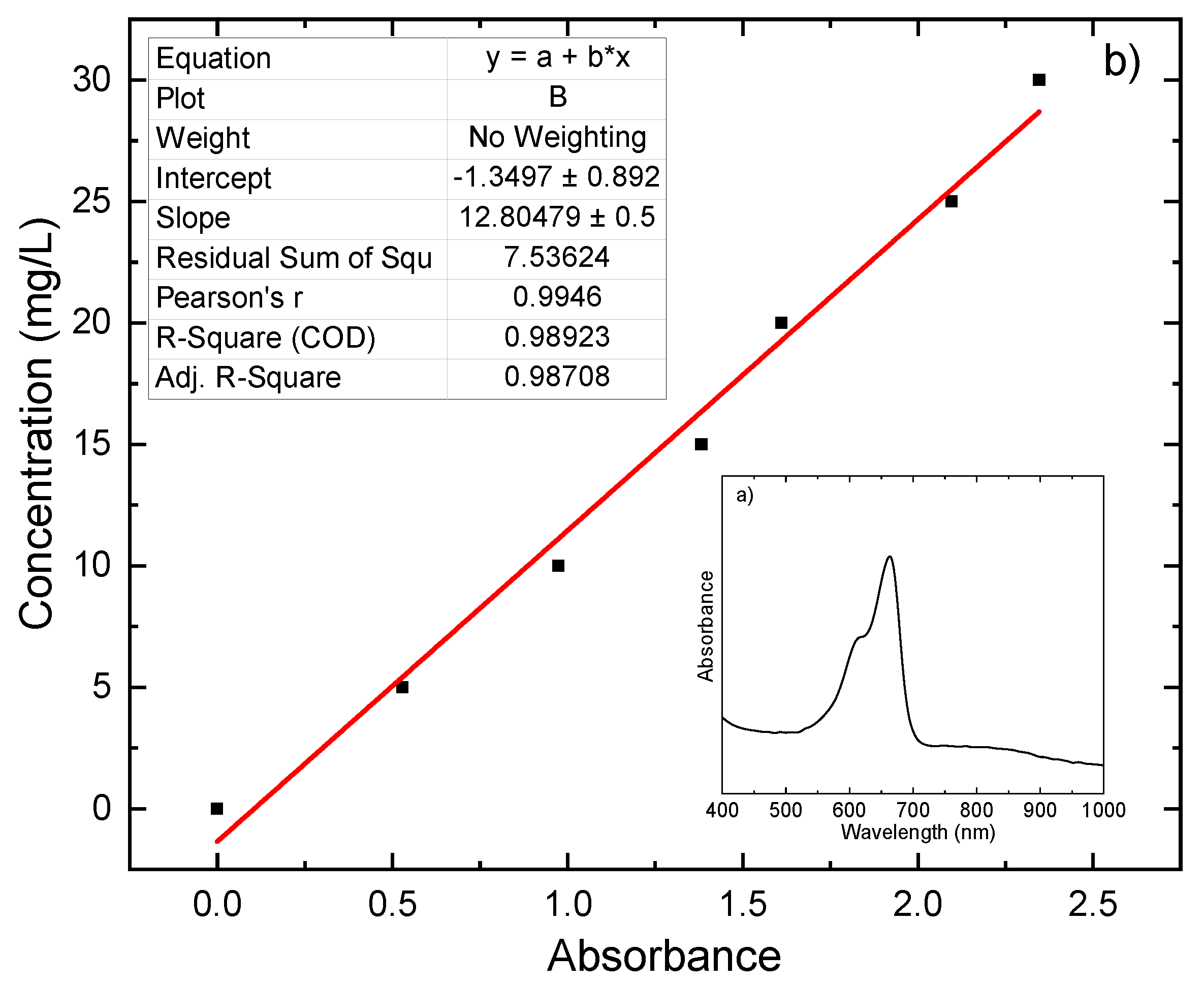
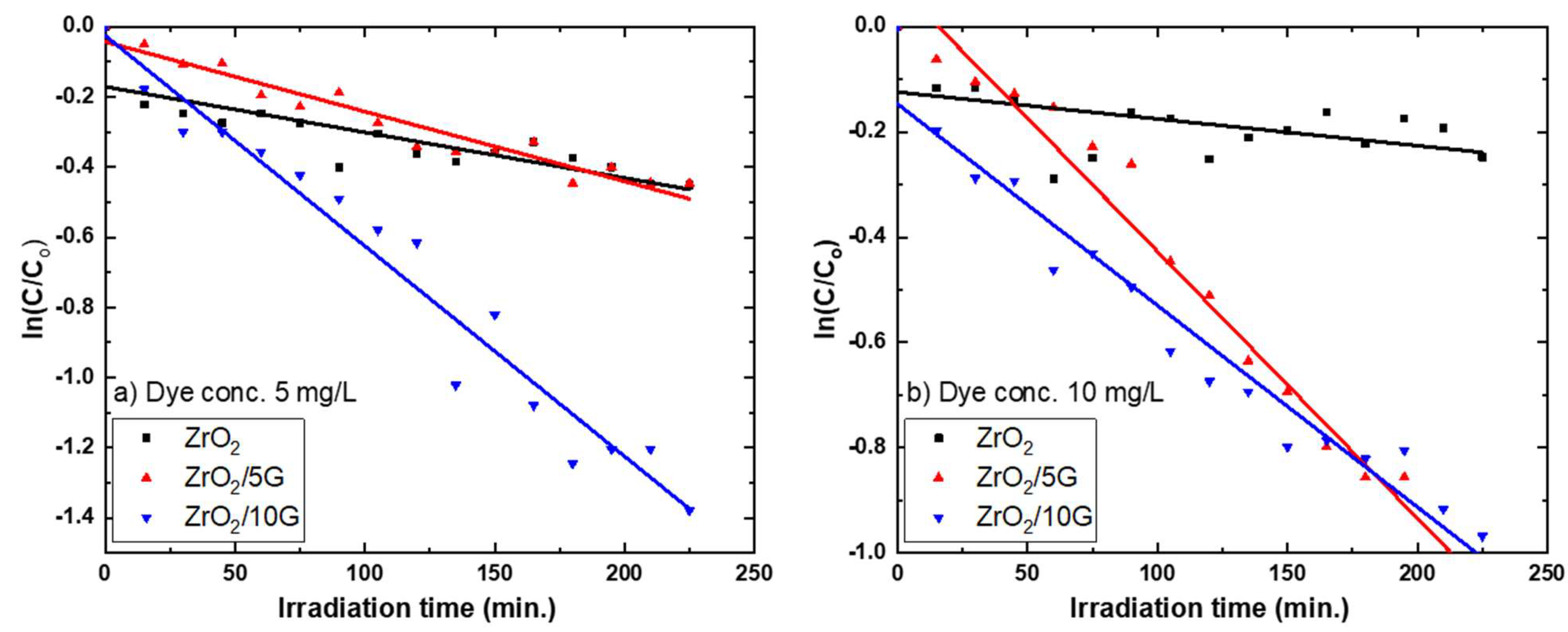
3.3.1. Photocatalytic Degradation Calculations
3.3.2. Photocatalytic Degradation of MB Pollution
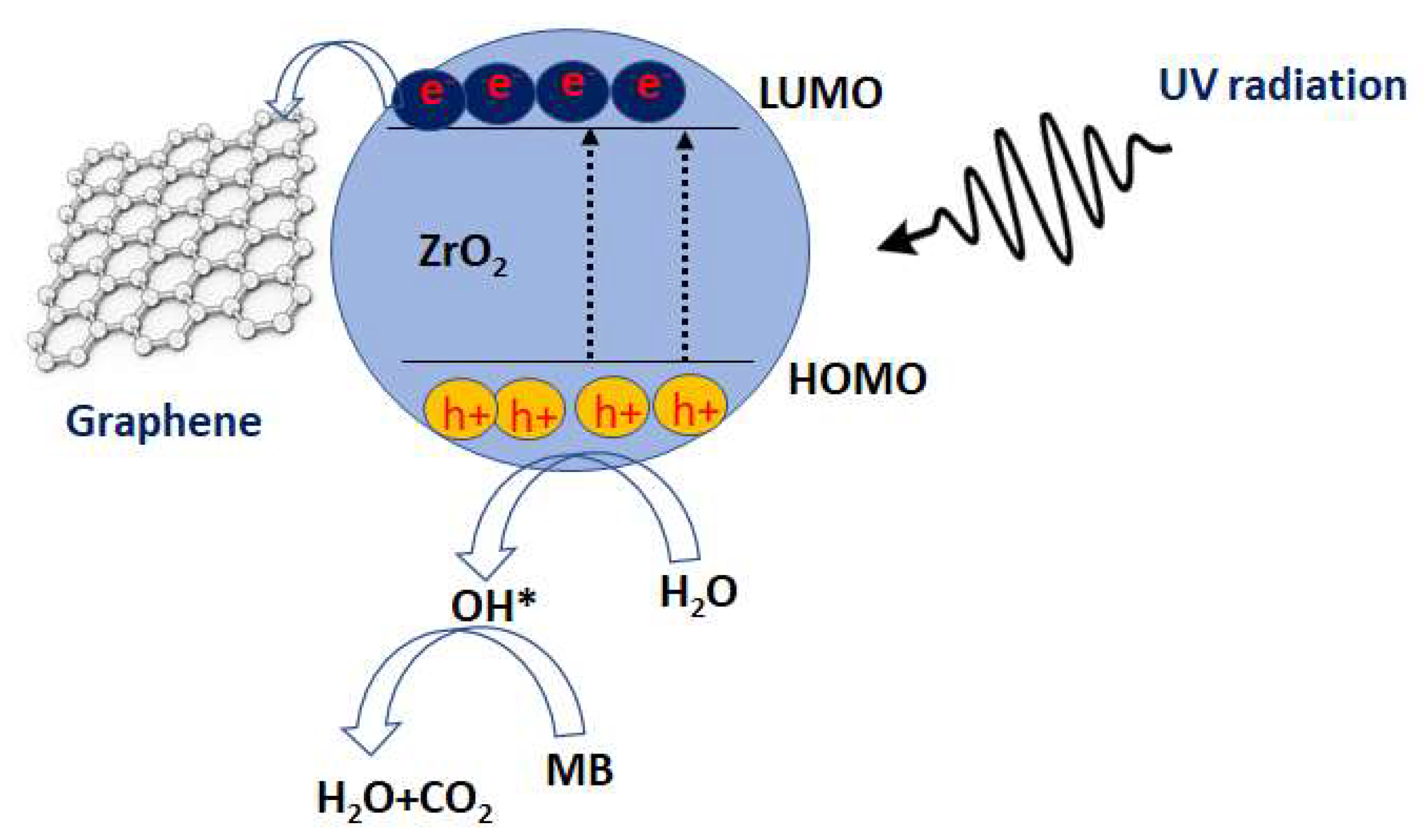
3.3.3. Mechanism of Catalysis
- (i)
- Electron/hole pair production: when a photon with energy (hν) greater than or equal to its bandgap energy falls on the ZrO2 surface, an electron from VB in ZrO2 will be photoexcited to CB, leaving positively charged holes in the VB, as follows:
- (ii)
- Hydroxyl radicals production: In this step, this electron (e−) and hole (h+) in the CB and VB of ZrO2 will move to the catalyst surface and react with oxygen molecules and hydroxyl group to yield superoxide radicals of O2− and hydroxyl radicals of OH* based on the following reaction:
- (iii)
- The degradation of the MB dye: In this step, the hydroxyl radicals and superoxide anion radicals formed degrade the MB dye molecules by breaking the N = N bond and other bonds. According to the results of a catalytic experiment, ZrO2 nanoparticles can lead to the degradation of MB dye.
3.4. Comparison of the Catalytic Degradation of MB Dye
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keiteb, A.S.; Saion, E.; Zakaria, A.; Soltani, N. Structural and optical properties of zirconia nanoparticles by thermal treatment synthesis. J. Nanomater. 2016, 2016, 1913609. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, E.C.; Maiti, H.S. Science and technology of zirconia. Adv. Ceram. 1981, 3, 1. [Google Scholar]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Masoud, M.S. Graphene oxide–metal oxide nanocomposites: Fabrication, characterization and removal of cationic rhodamine B dye. RSC Adv. 2018, 8, 13323–13332. [Google Scholar] [CrossRef] [Green Version]
- Emeline, A.; Kataeva, G.V.; Litke, A.S.; Rudakova, A.V.; Ryabchuk, V.K.; Serpone, N. Spectroscopic and photoluminescence studies of a wide band gap insulating material: Powdered and colloidal ZrO2 sols. Langmuir 1998, 14, 5011–5022. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Syed, F.A. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Tanugi, D.; Grossman, J.C. Nanoporous graphene as a reverse osmosis membrane: Recent insights from theory and simulation. Desalination 2015, 366, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Verma, V.; Kumar, R.; Sharma, S.; Kumar, R. Effect of structural and thermal disorder on the optical band gap energy of Cr2O3 nanoparticles. Mater. Res. Express 2019, 6, 85039. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation enhancement of methylene blue on ZnO nanocombs synthesized by thermal evaporation technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Taghdiri, M. Selective Adsorption and Photocatalytic Degradation of Dyes Using Polyoxometalate Hybrid Supported on Magnetic Activated Carbon Nanoparticles under Sunlight, Visible, and UV Irradiation. Int. J. Photoenergy 2017, 2017, 8575096. [Google Scholar] [CrossRef] [Green Version]
- Rashad, M.; L-Aoh, H.A.A. Promising adsorption studies of bromophenol blue using copper oxide nanoparticles. Desalin. Water Treat. 2019, 139, 360–368. [Google Scholar] [CrossRef]
- Rashad, M.; Al-Ghamdi, S.A.; Alzahrani, A.O.M.; Al-Tabaa, K.; Al-Osemi, S.; Al-Atawi, O.; Al-Anzi, N.; Issa, S.A.M.; Abd-Elnaiem, A.M. Zinc oxide nanoparticles for adsorption of potassium permanganate from wastewater using shaking method. Desalin. Water Treat. 2021, 229, 227–234. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; al Sagheer, F.; Nahar, S. Controlled Synthesis of ZrO2 Nanoparticles with Tailored Size, Morphology and Crystal Phases via Organic/Inorganic Hybrid Films. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Colbea, C.; Avram, D.; Cojocaru, B.; Negrea, R.; Ghica, C.; Kessler, V.G.; Seisenbaeva, G.A.; Parvulescu, V.; Tiseanu, C. Full tetragonal phase stabilization in ZrO2 nanoparticles using wet impregnation: Interplay of host structure, dopant concentration and sensitivity of characterization technique. Nanomaterials 2018, 8, 988. [Google Scholar] [CrossRef] [Green Version]
- Azammi, A.M.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Asrofi, M.; Atiqah, A. Characterization studies of biopolymeric matrix and cellulose fibres based composites related to functionalized fibre-matrix interface. In Interfaces in Particle and Fibre Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–93. [Google Scholar]
- Inam, A.; Brydson, R.; Edmonds, D.V. Raman spectroscopy study of the crystallinity of graphite formed in an experimental free-machining steel. Mater. Charact. 2020, 163, 110264. [Google Scholar] [CrossRef]
- Bettinali, C.; Ferraresso, G.; Manconi, J.W. Thermoluminescence of ZrO2. J. Chem. Phys. 1969, 50, 3957–3961. [Google Scholar] [CrossRef]
- Puust, L.; Kiisk, V.; Utt, K.; Mändar, H.; Sildos, I. Afterglow and thermoluminescence of ZrO2 nanopowders. Cent. Eur. J. Phys. 2014, 12, 415–420. [Google Scholar] [CrossRef]
- Rani, S.; Aggarwal, M.; Kumar, M.; Sharma, S.; Kumar, D. Removal of methylene blue and rhodamine B from water by zirconium oxide/graphene. Water Sci. 2016, 30, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 21102. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Rashad, M. Performance efficiency and kinetic studies of water purification using ZnO and MgO nanoparticles for potassium permanganate. Opt. Quantum Electron. 2019, 51, 291. [Google Scholar] [CrossRef]
- Yan, H.; Hou, J.; Fu, Z.; Yang, B.; Yang, P.; Liu, K.; Wen, M.; Chen, Y.; Fu, S.; Li, F. Growth and photocatalytic properties of one-dimensional ZnO nanostructures prepared by thermal evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Aziz, A.; Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Ali, N.; Khan, H. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int. J. Biol. Macromol. 2020, 153, 502–512. [Google Scholar] [CrossRef]
- Kundu, A.; Mondal, A. Photodegradation of methylene blue under direct sunbeams by synthesized anatase titania nanoparticles. SN Appl. Sci. 2019, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Xiao, H.; Xu, H.; Ding, T.; Gu, Y. Photodegradation of Methylene Blue by TiO2-Fe3O4-Bentonite Magnetic Nanocomposite. Int. J. Photoenergy 2015, 2015, 591428. [Google Scholar] [CrossRef] [Green Version]
- Vig, A.S.; Gupta, A.; Pandey, O.P. Efficient photodegradation of methylene blue (MB) under solar radiation by ZrC nanoparticles. Adv. Powder Technol. 2018, 29, 2231–2242. [Google Scholar] [CrossRef]
- Yin, X.; Liu, L.; Ai, F. Enhanced Photocatalytic Degradation of Methylene Blue by WO3 Nanoparticles Under NIR Light Irradiation. Front. Chem. 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]




| Element | ZrO2 | ZrO2/G10 | ||
|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | |
| Zr | 69.39 | 28.45 | 72.16 | 29.08 |
| O | 30.61 | 71.55 | 18.56 | 42.55 |
| C | 0 | 0 | 9.28 | 28.37 |
| Sample | ID/G | IG’/G |
|---|---|---|
| ZrO2 | - | - |
| ZrO2/5G | 0.87 | 0.45 |
| ZrO2/10G | 0.84 | 0.19 |
| MB 5 mg/L | |||
|---|---|---|---|
| qe(exp) | k | K | |
| ZrO2 | 3.18 | 0.039 | 0.034 |
| ZrO2/5G | 3.15 | 0.228 | 0.009 |
| ZrO2/10G | 6.55 | 1.135 | 0.005 |
| MB 10 mg/L | |||
|---|---|---|---|
| qe(exp) | k | K | |
| ZrO2 | 3.325 | 0.02 | 0.025 |
| ZrO2/5G | 11.03 | 0.32 | 0.016 |
| ZrO2/10G | 10.68 | 0.13 | 0.029 |
| Samples | MB Concentration (mg/L) | Degradation (%) | Irradiation | Time (min) | Ref. |
|---|---|---|---|---|---|
| Ti-S-500 NPs | 2 × 10−5 M | 44 | direct sunbeams | 90 min | [29] |
| Ti-D-500 NPs | 37 | ||||
| Ti-500 NPs | 27 | ||||
| ZrC NPs | 80 | UV | 5 h | [31] | |
| WO3 NPs | 10 mg/L | 55 | NIR light | 50 min | [32] |
| 43 | UV | ||||
| ZnO at 0.15 Torr | 1 × 10−6 M | 81 | sun simulator | 90 min | [10] |
| ZnO at 0.30 Torr | 80 | ||||
| ZnO at 0.70 Torr | 65 | ||||
| ZnO at 1.00 Torr | 56 | ||||
| TiO2-Fe3O4- bentonite NPs | 30 mg/L | 90 | UV | 90 min | [30] |
| ZrO2 NPs | 5 mg/L | 30 | UV | 240 min | Current work |
| ZrO2/G5 NPs | 30 | ||||
| ZrO2/G10 NPs | 80 | ||||
| ZrO2 NPs | 10 mg/L | 20 | |||
| ZrO2/G5 NPs | 60 | ||||
| ZrO2/G10 NPs | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.M.; Rashad, M.; Saber, O.; Alshoaibi, A.; Awada, C. A Comprehensive Photocatalysis Study of Promising Zirconia/Laser-Induced Graphene Nanocomposite for Wastewater Treatment-Based Methylene Blue Pollution. Separations 2022, 9, 185. https://doi.org/10.3390/separations9080185
Shaalan NM, Rashad M, Saber O, Alshoaibi A, Awada C. A Comprehensive Photocatalysis Study of Promising Zirconia/Laser-Induced Graphene Nanocomposite for Wastewater Treatment-Based Methylene Blue Pollution. Separations. 2022; 9(8):185. https://doi.org/10.3390/separations9080185
Chicago/Turabian StyleShaalan, Nagih M., Mohamed Rashad, Osama Saber, Adil Alshoaibi, and Chawki Awada. 2022. "A Comprehensive Photocatalysis Study of Promising Zirconia/Laser-Induced Graphene Nanocomposite for Wastewater Treatment-Based Methylene Blue Pollution" Separations 9, no. 8: 185. https://doi.org/10.3390/separations9080185
APA StyleShaalan, N. M., Rashad, M., Saber, O., Alshoaibi, A., & Awada, C. (2022). A Comprehensive Photocatalysis Study of Promising Zirconia/Laser-Induced Graphene Nanocomposite for Wastewater Treatment-Based Methylene Blue Pollution. Separations, 9(8), 185. https://doi.org/10.3390/separations9080185








