Separation and Determination of Biophenols in Olive Oil Samples Based on the Official Method of the International Olive Council and Commission Regulation (EU) No. 432/2012
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Oil Samples
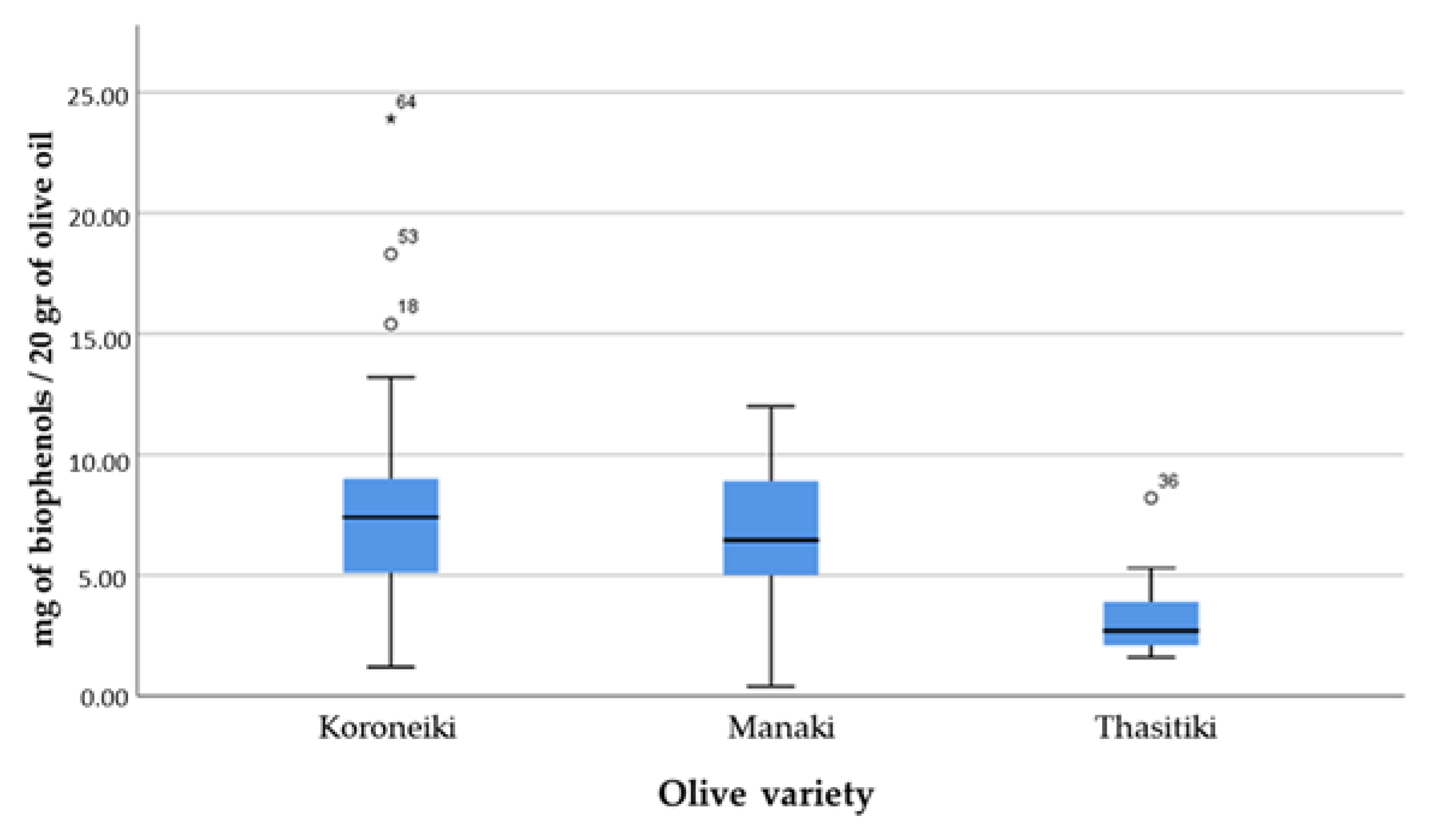
- Olive variety (Koroneiki, Manaki, and Thasitiki);
- Cultivation area (prefectures of Aitoloakarnania, Zakynthos, Argolida, Dodecanese, Kavala and Heraklion);
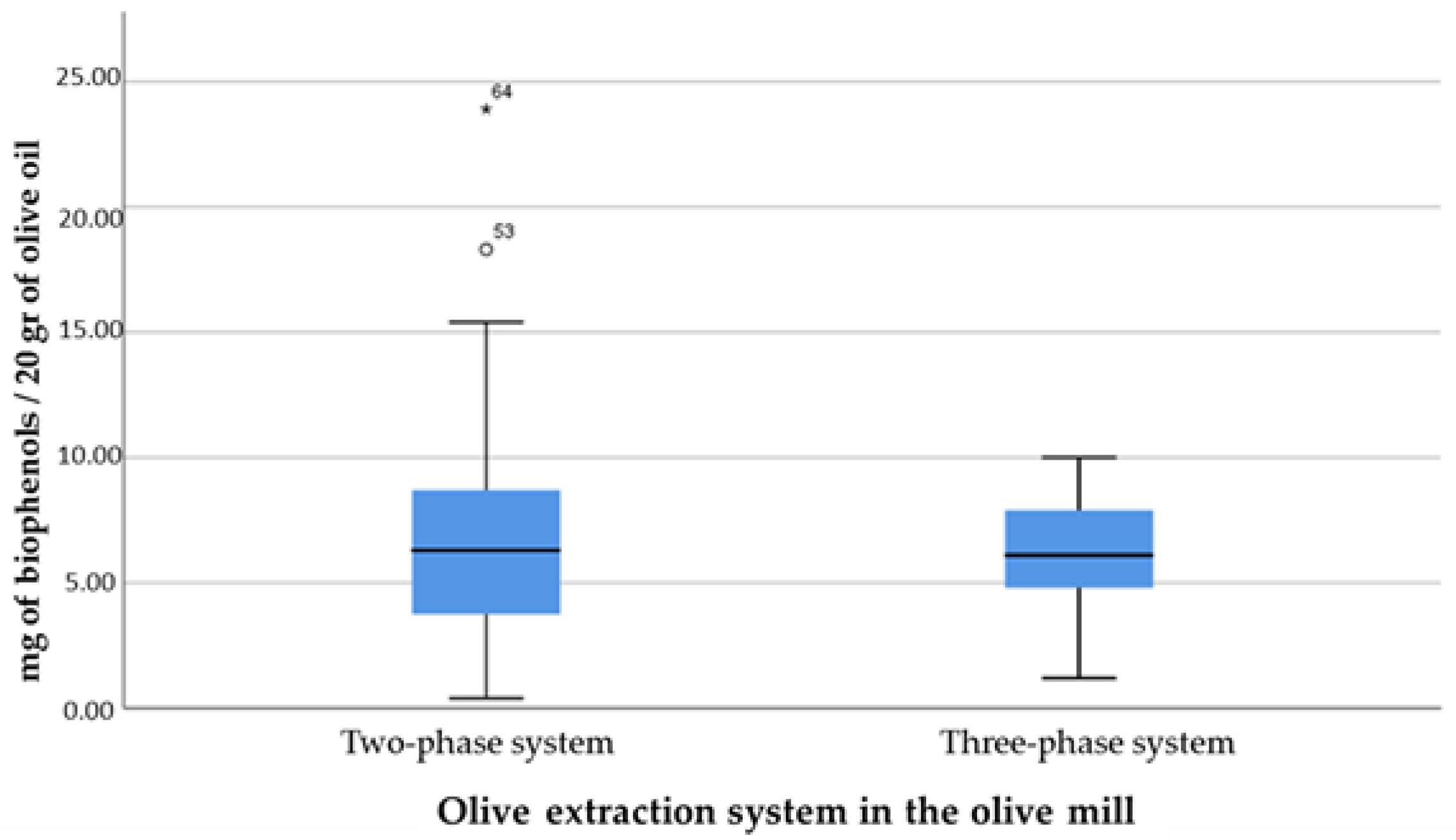
- Olive fruit extraction system in the mill (two-phase system; three-phase system);
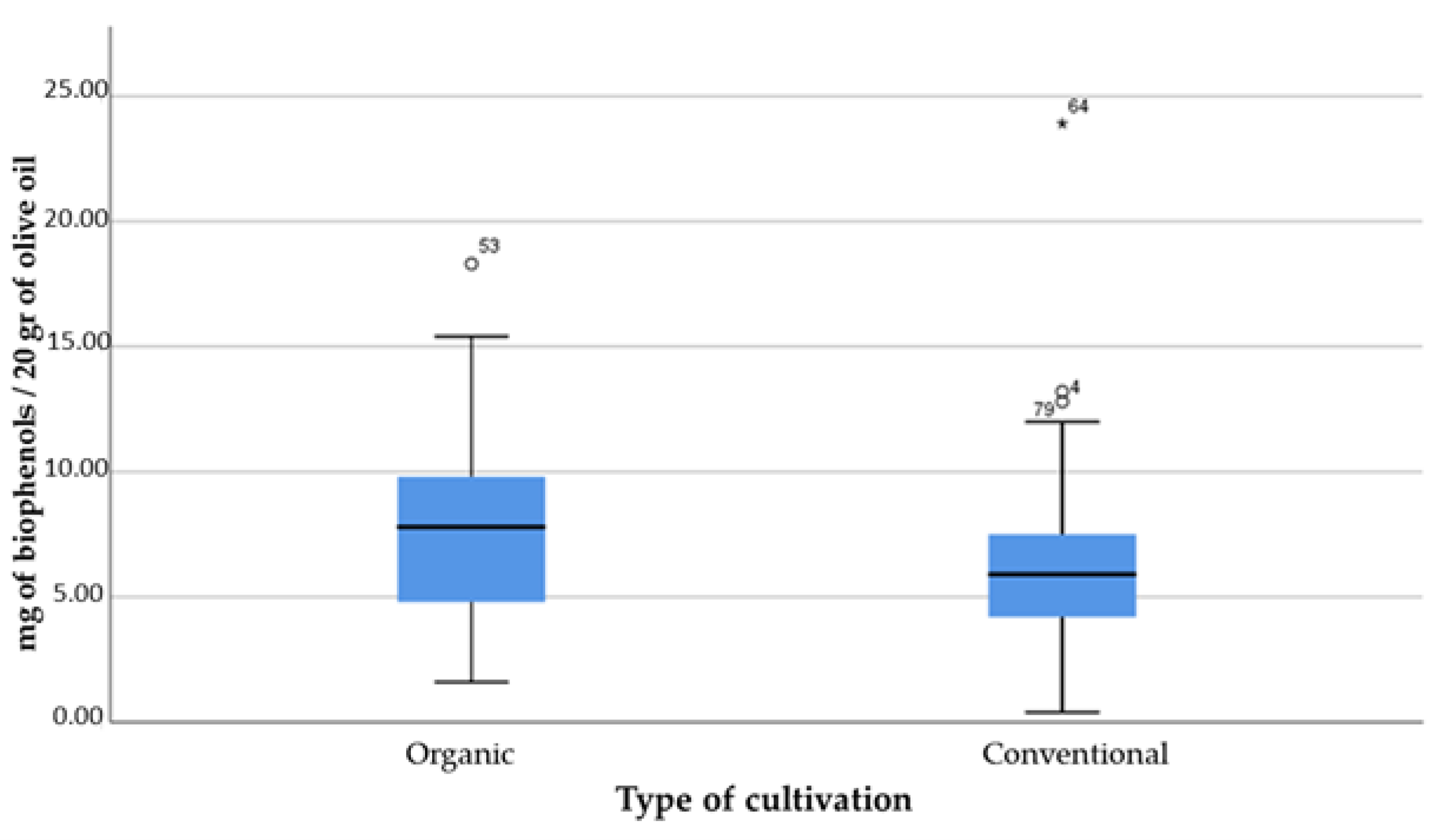
- Type of cultivation (organic; conventional);
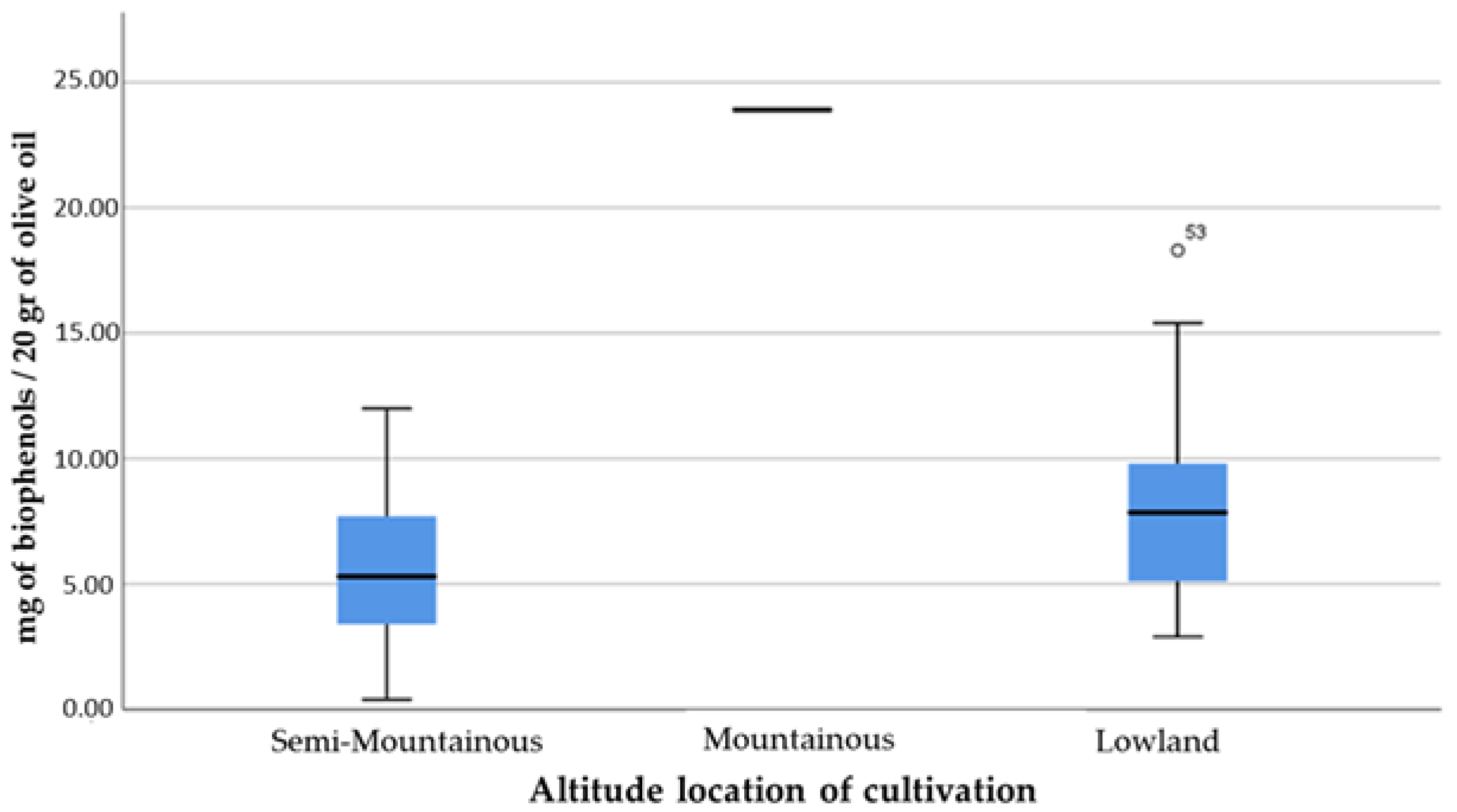
- Altitude location of cultivation (ountainous; semi-mountainous; lowland);
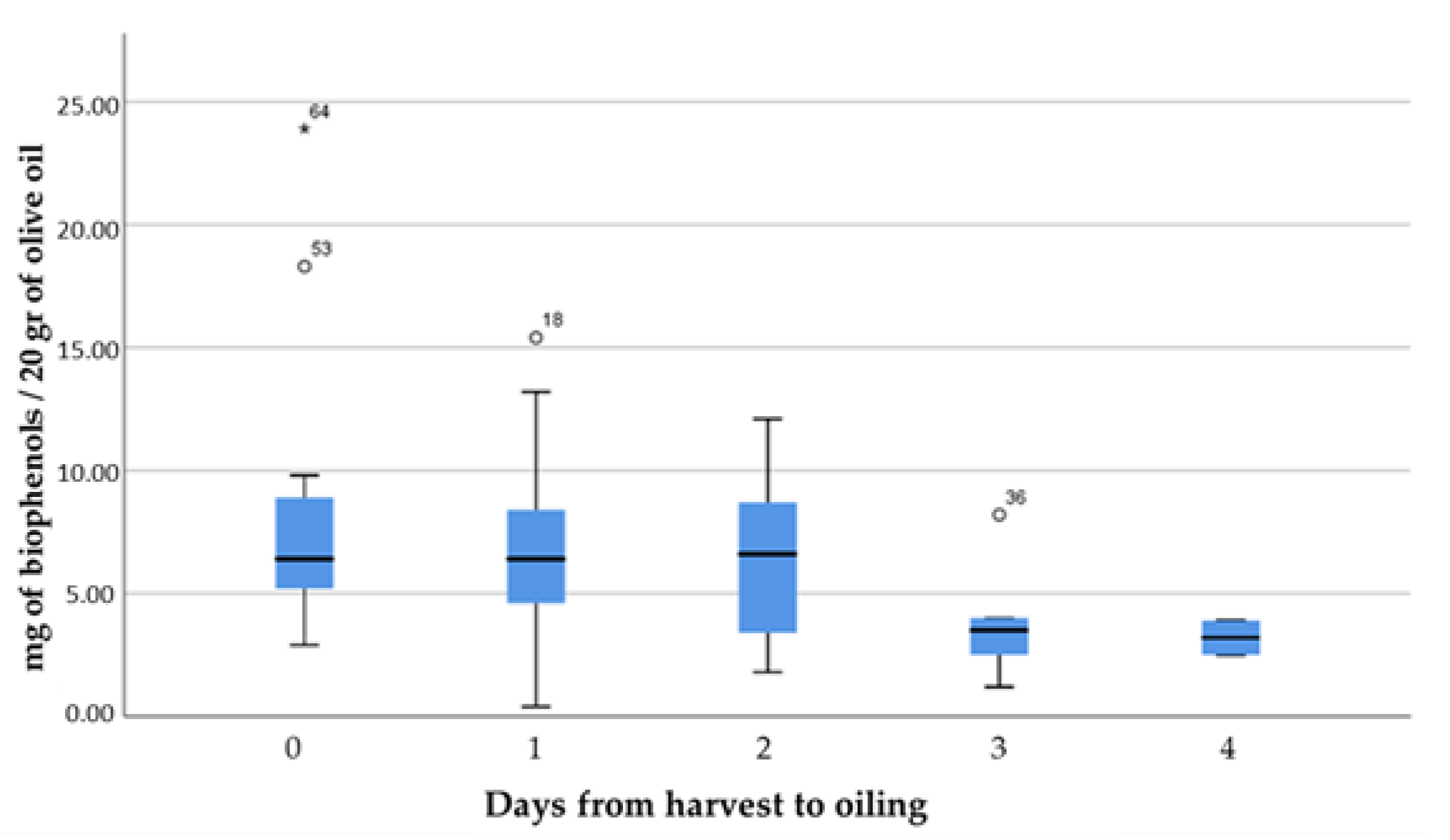
- The time interval between harvest and oiling in days (0, 1, 2, 3, and 4).
2.2. Chemical Analysis of Olive Oil
3. Results
3.1. Comparison of Concentrations of Biophenolic Components of the Samples with the Various Parameters
- Heraklion 33.3%;
- Aitoloakarnania 77.8%;
- Dodecanese 80.0%;
- Zakynthos 89.5%.
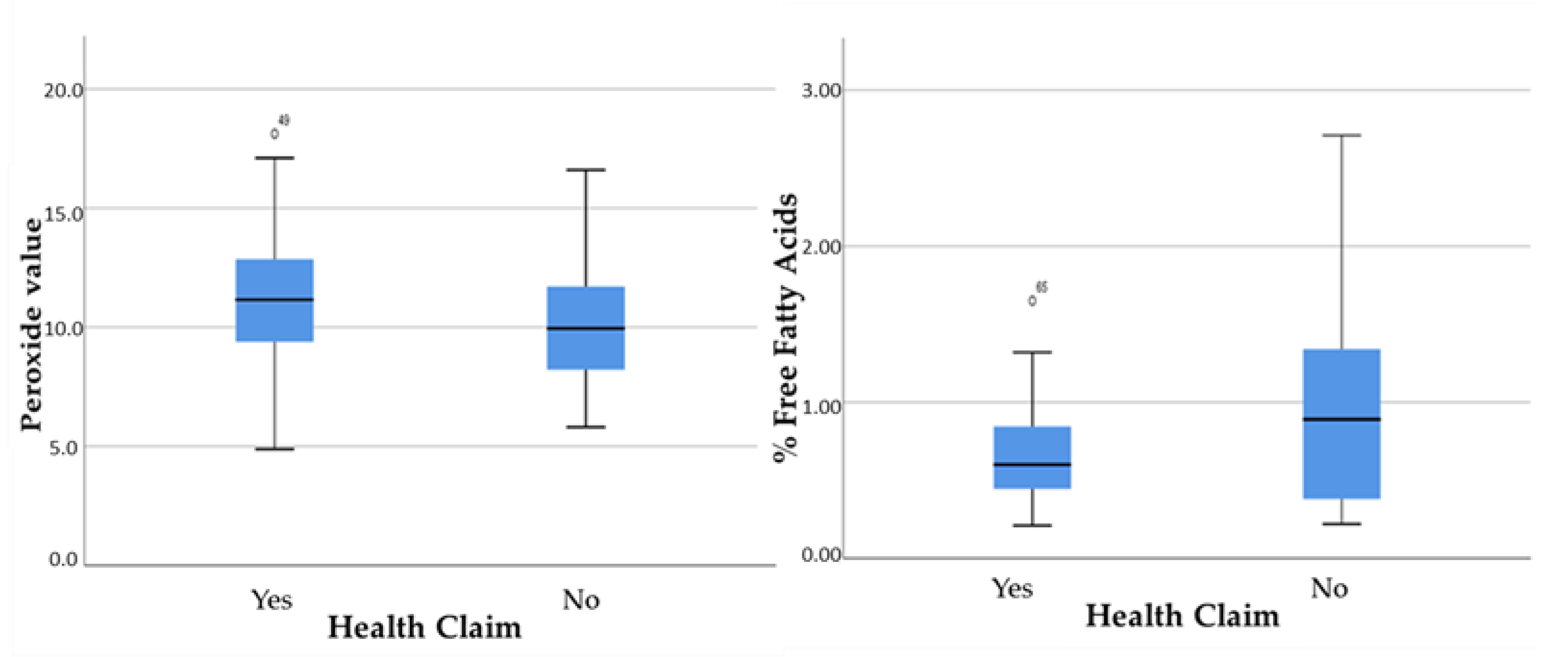
3.2. Comparison of Some Quality Characteristics of Olive Oil with the Results for the Health Claim
4. Discussion
4.1. Summary of Results
- In terms of variety, Koroneiki showed the lowest levels of free fatty acids and the highest concentrations of biophenolic components, presenting a plethora of extra virgin olive oils with the health claim. This was followed by the Manaki variety with quite good results, while the Thasitiki had high levels of free fatty acids and 88.9% failure in the health claim; however, it had the lowest peroxide value;
- In Argolida, we only had samples from Manaki and in Kavala from Thasitiki. Thus, from the other areas with the Koroneiki variety, Zakynthos showed the best results and the highest percentage in the health claim, followed by Aitoloakarnania;
- None of the quality characteristics were significantly affected by the way the olive oil was produced in the mill and the extraction system. However, the two-phase system had slightly higher concentrations of phenolic components and three very high values. It should be noted that in a two-phase system, all the samples of the Thasitiki variety significantly lowered the average value;
- Based on the cultivation technique, the olives from organic olive cultivations had lower values of % free acidity, higher concentrations of phenolic compounds and a higher percentage of olive oils with health claims compared to conventional cultivations. The same applies to the lowland region in relation to the semi-mountainous location of the cultivations;
- A very important factor for the quality of olive oils, as the results showed, is the interval between the harvest of the olive fruit and its oiling. The shorter this period, the better the quality of the oil. After the intervals of 0 and 1 day, the lowest values of % free acidity and the highest concentrations of bioactive phenolic components appear, while at intervals of 3 and 4 days these characteristics change dramatically in the opposite;
- Finally, a general observation is that the number of peroxides did not appear to be affected by the changing factors, except for the variety. In contrast, the content of bioactive phenolic compounds and the values of % free acidity are equally affected by all parameters.
4.2. Future Suggestions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Code of Food and Beverage and Common Objects: Edible Fats and Oils, 18th ed.; General Chemical State Laboratory of Greece: Athens, Greece, 2015; Article 71; p. 1.
- Pérez-Jiménez, F.; Ruano, J.; Perez-Martinez, P.; Lopez-Segura, F.; Lopez-Miranda, J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Wiesman, Z. Desert Olive Oil Cultivation: Advanced Bio Technologies, 1st ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 3–17. [Google Scholar]
- Kiritsakis, A.; Shahidi, F. Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Beauchamp, G.; Keast, R.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.; Watson, R. General Aspects of Olives and Olive Oil. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V., Watson, R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; Section 1.1, Chapter 1; pp. 5–15. [Google Scholar]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Brenes, M.; Hidalgo, F.J.; García, A.; Rios, J.J.; García, P.; Zamora, R.; Garrido, A. Pinoresinol and 1-acetoxypinoresinol, two new phenolic compounds identified in olive oil. J. Am. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- Okogeri, O.; Tasioula-Margari, M. Changes occurring in phenolic compounds and α-tocopherol of virgin olive oil during storage. J. Agric. Food Chem. 2002, 50, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Ranalli, A.; Modesti, G.; Patumi, M.; Fontanazza, G. The compositional quality and sensory properties of virgin olive oil from a new olive cultivar—I-77. Food Chem. 2000, 69, 37–46. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of malaxation temperature and time on the quality of virgin olive oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; De La Torre, R.; Francés, F.; Cabezas, C.; del Carmen López-Sabater, M.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. Integrase inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 872–878. [Google Scholar] [CrossRef]
- Leenen, R.; Roodenburg, A.J.; Vissers, M.N.; Schuurbiers, J.A.; van Putte, K.P.; Wiseman, S.A.; van de Put, F.H. Supplementation of plasma with olive oil phenols and extracts: Influence on LDL oxidation. J. Agric. Food Chem. 2002, 50, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EU) No. 432/2012. Off. J. Eur. Union 2012, 136, 22.
- Esti, M.; Cinquanta, L.; La Notte, E. Phenolic compounds in different olive varieties. J. Agric. Food Chem. 1998, 46, 32–35. [Google Scholar] [CrossRef]
- Ouni, Y.; Taamalli, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Zarrouk, M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC–ESI–TOF-MS method. Food Chem. 2011, 127, 1263–1267. [Google Scholar] [CrossRef]
- Kiralan, M.; Ozkan, G.; Koyluoglu, F.; Ugurlu, H.A.; Bayrak, A.; Kiritsakis, A. Effect of cultivation area and climatic conditions on volatiles of virgin olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 552–557. [Google Scholar] [CrossRef]
- Romero, M.P.; Tovar, M.J.; Girona, J.; Motilva, M.J. Changes in the HPLC phenolic profile of virgin olive oil from young trees (Olea europaea L. Cv. Arbequina) grown under different deficit irrigation strategies. J. Agric. Food Chem. 2002, 50, 5349–5354. [Google Scholar] [CrossRef]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea europaea L.: Hojiblanca and Arbequina varieties. J. Agric. Food Chem. 2010, 58, 12924–12933. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and Hydrolyzable Phenolic Compounds in Virgin Olive Oil. 1. Their Extraction, Separation, and Quantitative and Semiquantitative Evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/ (accessed on 10 March 2022).
- Reboredo-Rodríguez, P.; Valli, E.; Bendini, A.; Di Lecce, G.; Simal-Gándara, J.; Gallina Toschi, T. A widely used spectrophotometric assay to quantify olive oil biophenols according to the health claim (EU Reg. 432/2012). Eur. J. Lipid Sci. Technol. 2016, 118, 1593–1599. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [Green Version]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]

| N | Sample | n | Country | Method | Purpose | Compound | Statistical Analysis | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | EVOO | 12 | Italy Spain | FC HPLC | Comparison of the effectiveness of analysis methods for the determination of olive oil biophenols based on EU Regulation 432/2012. | Biophenols | t-test | [29] |
| 2 | EVOO | 108 | Italy | HPLC-DAD | Comparison of hydroxytyrosol and tyrosol levels based on EFSA health claim, from variations in acid hydrolysis. | Biophenols | ANOVA F-test | [30] |
| 3 | EVOO OO | 25 | Italy | (HPLC-DAD-ESI/MS) HPLC-DAD FC | Development of a method for the determination of polyphenols and comparison of its results with those of the IOC and FC method. | Biophenols | ANOVA | [31] |
| 4 | EVOO | 284 | Italy | HPLC-DAD FC | Comparison of biophenolic contents in PDO and non-PDO olive oils based on the regulation on the EU health claim 432/2012. | Biophenols | t-test | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papastavropoulou, K.; Pasias, I.N.; Dotsika, E.; Oz, E.; Oz, F.; Proestos, C. Separation and Determination of Biophenols in Olive Oil Samples Based on the Official Method of the International Olive Council and Commission Regulation (EU) No. 432/2012. Separations 2022, 9, 101. https://doi.org/10.3390/separations9040101
Papastavropoulou K, Pasias IN, Dotsika E, Oz E, Oz F, Proestos C. Separation and Determination of Biophenols in Olive Oil Samples Based on the Official Method of the International Olive Council and Commission Regulation (EU) No. 432/2012. Separations. 2022; 9(4):101. https://doi.org/10.3390/separations9040101
Chicago/Turabian StylePapastavropoulou, Konstantina, Ioannis N. Pasias, Elissavet Dotsika, Emel Oz, Fatih Oz, and Charalampos Proestos. 2022. "Separation and Determination of Biophenols in Olive Oil Samples Based on the Official Method of the International Olive Council and Commission Regulation (EU) No. 432/2012" Separations 9, no. 4: 101. https://doi.org/10.3390/separations9040101
APA StylePapastavropoulou, K., Pasias, I. N., Dotsika, E., Oz, E., Oz, F., & Proestos, C. (2022). Separation and Determination of Biophenols in Olive Oil Samples Based on the Official Method of the International Olive Council and Commission Regulation (EU) No. 432/2012. Separations, 9(4), 101. https://doi.org/10.3390/separations9040101







