Application of Chromatographic Technology to Determine Aromatic Substances in Tobacco during Natural Fermentation: A Review
Abstract
1. Introduction
2. Pretreatment Methods of Tobacco Aromatic Substances
3. Important Aromatic Components in the Fermentation Process and Analytical Methods
3.1. Maillard Reaction
3.2. Carotenoids
3.3. Cembranoids
3.4. Ladanum
3.5. Glycosides
4. Conclusions
5. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zappe, A.L.; de Oliveira, P.F.; Boettcher, R.; Rodriguez, A.L.; Machado, E.L. Human health risk and potential environmental damage of organic and conventional Nicotiana tobaccum production. Environ. Pollut. 2020, 266, 114820. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.F.; Darkis, F.R.; Wolf, F.A.; Hall, J.A.; Jones, E.P.; Gross, P.M. Natural Aging of Flue-Cured Cigarette Tobaccos. Ind. Eng. Chem. 1936, 28, 180–189. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Ou, M.; Wu, Y.; Fang, J. Research Progress in Tobacco Fermentation. J. Biosci. Med. 2018, 6, 105–114. [Google Scholar] [CrossRef]
- Frankenburg, W.G. Chemical changes in the harvested tobacco leaf.II. Chemical and enzymic conversions during fermentation and aging. Adv. Enzymol. Relat. Subj. Biochem. 1950, 10, 325–441. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Shi, S.; Zhou, J.; Zhao, Q.; Zi, W. Advance in Quality Changes of Redried Lamina of Flue-cured Tobacco during Natural Alcoholization. J. Henan Agric. Sci. 2015, 44, 7–12. (In Chinese) [Google Scholar] [CrossRef]
- Tang, C.; Xu, J.; Zhang, J.; Yao, H. Advances and assumptions in natural aging of tobacco leaves. Tob. Sci. China 1999, 20, 17–19. (In Chinese) [Google Scholar] [CrossRef]
- Huang, W.; Duan, Y.; Yang, J.; Zhe, W.; Gong, X. A Study on Major Aromatic Components in Flue-cured Tobacco and Correlation with Grades and Aging Stages of Tobacco. Acta Agric. Univ. Jiangxi Ensis 2010, 32, 0440–0445. (In Chinese) [Google Scholar] [CrossRef]
- Nongonierma, A.; Voilley, A.; Cayot, P.; Quéré, J.-L.; Springett, M. Mechanisms of Extraction of Aroma Compounds from Foods, Using Adsorbents.Effect of Various parameters. Food Rev. Int. 2006, 22, 51–94. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyeyinka, S.A.; Adebiyi, J.A.; Feng, X.; Wilkin, J.D.; Kewuyemi, Y.O.; Abrahams, A.M.; Tugizimana, F. Application of gas chromatography–mass spectrometry (GC-MS)-based metabolomics for the study of fermented cereal and legume foods: A review. Int. J. Food Sci. Technol. 2020, 56, 1514–1534. [Google Scholar] [CrossRef]
- Al-Rubaye, A.F.; Hameed, I.H.; Kadhim, M.J. A Review: Uses of Gas Chromatography-Mass Spectrometry (GC-MS) Technique for Analysis of Bioactive Natural Compounds of Some Plants. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 81–85. [Google Scholar] [CrossRef]
- Ibraimov, A.B.; Sergazina, M.M.; Alimzhanova, M.B.; Abilev, M.B. Analysis of tobacco products by chromatography methods: Review. Int. J. Biol. Chem. 2018, 11, 133–141. [Google Scholar] [CrossRef]
- Peters, V.C.T.; Dunkel, A.; Frank, O.; Rajmohan, N.; McCormack, B.; Dowd, E.; Didzbalis, J.; Gianfagna, T.J.; Dawid, C.; Hofmann, T. High-Throughput Flavor Analysis and Mapping of Flavor Alterations Induced by Different Genotypes of Mentha by Means of UHPLC-MS/MS. J. Agric. Food Chem. 2022, 70, 5668–5679. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qin, D.; Duan, J.; Li, H.; Sun, J.; Huang, M.; Sun, B. Characterization of benzenemethanethiol in sesame-flavour baijiu by high-performance liquid chromatography-mass spectrometry and sensory science. Food Chem. 2021, 364, 130345. [Google Scholar] [CrossRef] [PubMed]
- Naghski, J.; Beinhart, E.G.; Couch, J.F. Fire Cured Tobaccos. Ind. Eng. Chem. 1944, 36, 556–559. [Google Scholar] [CrossRef]
- Rowland, L.R. Flue-cured Tobacco.Ⅱ.Neophytadiene. J. Am. Chem. Soc. 1957, 79, 5005–5010. [Google Scholar] [CrossRef]
- Stedman, L.R. Chemical composition of tobacco and tobacco smoke. Chem. Rev. 1968, 68, 153–207. [Google Scholar] [CrossRef]
- Wahlberg, I.; Karlsson, K.; Austin, D.J.; Junker, N.; Roeraade, J.; Enzell, C.R.; Johnson, W.H. Effects of flue-curing and ageing on the volatile, neutral and acidic constituents of Virginia tobacco. Phytochemisty 1977, 16, 1217–1232. [Google Scholar] [CrossRef]
- Wahlberg, I.; Karlsson, K.; Austin, D.J.; Junker, N.; Roeraade, J.; Enzell, C.R.; Johnson, W.H. Effects of flue-curing and ageing on the volatile basic constituents of Virginia tobacco. Phytochemisty 1977, 16, 1233–1235. [Google Scholar] [CrossRef]
- Chuman, T.; Noguchi, M. Acidic Aroma Constituents of Turkish Tobacco. Agric. Biol. Chem. 1977, 41, 1021–1030. [Google Scholar] [CrossRef]
- Sakakia, T.; Fukuharaa, K.; Niinoa, K.; Sakumaa, H.; Sugawara, S. Changes in the Composition of Headspace Volatiles of Flue-cured Tobacco by Aging. Agric. Biol. Chem. 1985, 49, 1785–1791. [Google Scholar] [CrossRef]
- Li, Y.; Xian, K. Analysis of the Neutral Flavor Constituents in Tobacco with Gas Co-Distillation Method. Acta Tab. Sin. 2002, 3, 1–9. (In Chinese) [Google Scholar]
- Xie, J.; Zhao, M.; Wu, M.; Zhao, X.; Wang, S.; Xie, F. Studies on Some Important Aroma Compositions in Burley Tobacco. Tob. Sci. Technol. 2002, 10, 3–16. (In Chinese) [Google Scholar]
- Jing, Y.; Gong, C.; Zhang, Y.; Chen, Z.; Li, Y. Research progress of aroma compositions of tobacco. Tob. Sci. China 2005, 26, 44–48. (In Chinese) [Google Scholar] [CrossRef]
- Guan, E.; Sun, F.; Wang, D.; Gao, K.; Wang, Y.; Li, H.; Yu, W. Changes of Sensory Quality of Flue-cured Tobacco During Natural and Artificial Aging. Chin. Tob. Sci. 2020, 41, 54–58. (In Chinese) [Google Scholar] [CrossRef]
- Kim, K.; Zlatkis, A.; Park, J.; Lee, U. Isolation of essential oils from tobacco by gas co-distillation solvent extraction. Chromatographia 1982, 15, 559–563. [Google Scholar] [CrossRef]
- Green, J.D.; Payne, B.P. Reproducibility of simultaneous distillation-extraction techniques used in the isolation of volatiles. Anal. Chim. Acta 1989, 226, 183–186. [Google Scholar] [CrossRef]
- Wu, L.; Liu, W.; Cao, J.; Li, Q.; Huang, Y.; Min, S. Analysis of the aroma components in tobacco using combined GC-MS and AMDIS. Anal. Methods 2013, 5, 1259–1263. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, H.; Du, W.; Wang, S.; Wang, C.; Chang, S.; Dong, S.; Xu, C.; Zhang, J. Optimization extraction process of aroma components in tobacco. J. Chromatogr. Sci. 2013, 51, 250–257. [Google Scholar] [CrossRef]
- Masao, M.; Kyôko, T.; Masae, K.; Shigeo, I.; Hajime, M. Comparison of roasted tobacco volatiles with tobacco essential oil and cigarette smoke condensate. Agric. Biol. Chem. 1985, 49, 3. [Google Scholar] [CrossRef]
- Takeshi, S.; Hirohiko, S.; Shiro, S. Studies on tobacco aroma. Part I. Analysis of the headspace volatiles of tobacco using an ether trap. Agric. Biol. Chem. 1984, 48, 2719–2724. [Google Scholar] [CrossRef]
- Wu, L.; He, Z.; Wu, Y.; Liu, J.; Li, C.; Cao, J.; Wang, B.; Min, S. Evaluation of Aroma Components in Chinese Southwest Tobacco by Headspace Gas Chromatography-Mass Spectrometry. Asian J. Chem. 2013, 25, 8853–8858. [Google Scholar] [CrossRef]
- Demole, E.; Berthet, D. A Chemical Study of Burley Tobacco Flavour (Nicotiana tabacum L.). I. Volatile to medium-volatile constituents. Helv. Chim. Acta 1972, 55, 1866–1882. [Google Scholar] [CrossRef]
- Demole, E.; Berthet, D. A Chemical Study of Burley Tobacco Flavour (Nicotiana tabacurn L.) Ⅱ.Medium-Volatile, free Acidic Constituents. Helv. Chim. Acta 1972, 55, 1898–1910. [Google Scholar] [CrossRef]
- Lloyd, R.A.; Mille, C.W.; Robert, D.L.; Giles, J.A.; Ayers, P.H. Flue-cured tobacco flavor. I. Essence and essential oil components. Tob. Sci. 1976, 20, 43–51. [Google Scholar]
- Schumacher, J.N. Flavor composition of Maryland tobacco. Beitr. Zur. Tab. Int. 1984, 12, 271–278. [Google Scholar] [CrossRef][Green Version]
- Fischer, M.; Jefferies, T.M. Optimization of Nicotine Extraction from Tobacco Using Supercritical Fluid Technology with Dynamic Extraction Modeling. J. Agric. Food Chem. 1996, 44, 1258–1264. [Google Scholar] [CrossRef]
- Smetena, S.S.Y.I. Determination of tobacco alkaloids using solid phase microextraction and GC-NPD. Chromatographia 1998, 47, 443–448. [Google Scholar]
- Zhu, X.; Gao, Y.; Chen, Z.; Su, Q. Development of a Chromatographic Fingerprint of Tobacco Flavor by Use of GC and GC-MS. Chromatographia 2009, 69, 735–742. [Google Scholar] [CrossRef]
- Xiang, Z.; Cai, K.; Liang, G.; Zhou, S.; Ge, Y.; Zhang, J.; Geng, Z. Analysis of volatile flavour components in flue-cured tobacco by headspace solid-phase microextraction combined with GC×GC-TOFMS. Anal. Methods 2014, 69, 735–742. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Liu, S.; Yu, H.; Dai, Y. Analytical method of free and conjugated neutral aroma components in tobacco by solvent extraction coupled with comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1280, 122–127. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Ji, H.; Wang, W.; Liu, J.; Wang, F.; Xie, F.; Yu, Y.; Qin, Y.; Wang, X. Metabolic profiling of tobacco leaves at different growth stages or different stalk positions by gas chromatography–mass spectrometry. Ind. Crops Prod. 2018, 116, 46–55. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Fekry, M.I.; Farag, M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Duan, C.; Shi, Y.; Zhu, B.; Javed, H.U.; Wang, J. Free and glycosidically bound volatile compounds in sun-dried raisins made from different fragrance intensities grape varieties using a validated HS-SPME with GC–MS method. Food Chem. 2017, 228, 125–135. [Google Scholar] [CrossRef]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Validation of SPME–GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta 2015, 134, 394–401. [Google Scholar] [CrossRef]
- Forrest, G.; Vilcins, G. Determination of tobacco carotenoids by resonance Raman spectroscopy. Agric. Food Chem. 1979, 27, 609–612. [Google Scholar] [CrossRef]
- Court, W.A.; Hendel, J.G. Determination of Nonvolatile Organic and Fatty Acids in Flue-Cured Tobacco by Gas-Liquid Chromatography. Chromatogr. Sci. 1978, 16, 314–317. [Google Scholar] [CrossRef]
- Waller, G.R.; Feather, M.S. The Maillard Reaction in Foods and Nutrition. Am. Chem. Soc. 1983, 215, 537–563. [Google Scholar]
- Zheng, W.; Xu, X. Research Progress on Maillard Reaction. Prog. Chem. 2005, 17, 122–129. (In Chinese) [Google Scholar]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. Engl. 2015, 45, 10316–10329. [Google Scholar] [CrossRef]
- Lee, K.W.; Simpson, G.; Ortwerth, B. A systematic approach to evaluate the modification of lens proteins by glycation-induced crosslinking. Biochim. Et. Biophys. Acta (BBA)-Mol. Basis Dis. 1999, 1453, 141–151. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Peng, X.; Cheng, K.W.; Kong, R.; Chu, I.K.; Chen, F.; Wang, M. Effects of melamine on the Maillard reaction between lactose and phenylalanine. Food Chem. 2010, 119, 1–6. [Google Scholar] [CrossRef]
- Wang, R.; Xie, Y.; Liang, Q.; Wang, Y.; Hu, P.; Luo, G. Simultaneous determination of five Amadori compounds in tobacco by high performance liquid chromatography-mass spectrometry. Chin. J. Chromatogr. 2013, 31, 1189–1193. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Zhang, J.; Fu, J.; Wang, y.; Lu, X.; Xu, G. Determination of twenty free amino acids in flue-cured tobacco leaves using ultra performance liquid chromatography-single quadruple mass spectrometry and pre-column derivatization. Chin. J. Chromatogr. 2013, 31, 1182–1188. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; David, F.; Tienpont, B.; Sandra, K.; Ochiai, N.; Tamura, H.; Sandra, P. Analysis of the reaction products from micro-vial pyrolysis of the mixture glucose/proline and of a tobacco leaf extract:Search for Amadori intermediates. J. Chromatogr. A 2015, 1422, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Xiu, L.; Mou, D.; Mao, D. Simultaneous Determination of Six Kind of Amadori Compounds in Tobaccos by LC-MS/MS. J. Chin. Mass Spectrom. Soc. 2015, 36, 45–51. [Google Scholar]
- Yuan, H.; Chen, M.; Wang, J. Simultaneous determination of 1-deoxy-1-L-proline-D-fructose and 2-deoxy-2-L-proline-D-glucose in Virginia type cigarette by HPLC-MS/MS. Tob. Sci. Technol. 2016, 49, 44–50. (In Chinese) [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Wang, S.; Liu, S.; Liu, H. Simultaneous quantification of ten Amadori compounds in tobacco using liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2017, 40, 849. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Yang, H.; Zhu, W.; Xiang, Z.; Geng, Z. Simultaneous determination of sixteen free amino acids and six Amadori products in tobacco by LC-MS/MS. Tob. Sci. Technol. 2017, 50, 58–65. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Liu, H.; Chen, Z.; Fan, Z.; Qin, Y.; Liu, H.; Pan, L.; Zhou, Y. Simultaneous determination of 22 Amadori compounds in tobacco leaves by HPLC-MS/MS. Tob. Sci. Technol. 2021, 54, 40–48. (In Chinese) [Google Scholar] [CrossRef]
- Britton, G.; Liaaen Jensen, S.; Pfander, H. Carotenoids: Isolation and analysis. Nutr. Health 1995, 5. [Google Scholar] [CrossRef]
- Bijttebier, S.; D′Hondt, E.; Noten, B.; Hermans, N.; Voorspoels, S.; Apers, S. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. J. Chromatogr. A 2014, 1332, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yue, Q.; LI, J.; Zhou, J.; Mao, J.; Liu, L. On Determination of Carotinoids in the Flue-cured Tobacco Leaves by High Performance Liquid Chromatography. J. Hunan Agric. Univ. 2006, 32, 6. (In Chinese) [Google Scholar] [CrossRef]
- Liu, S.; Chu, D.; Hong, S.; Tong, H.; Fang, L.; Zhou, G.; Chang, Y. Determination of carotenoids and isomers in tobacco leaves by RP-HPLC. Acta Tab. Sin. 2009, 15, 9. (In Chinese) [Google Scholar]
- Li, X.; Xiao, L.; Yang, M. Rapid Determination of Carotenoids in Flue-cured Tobacco by Ultrasound Extraction and Ultra-high Performance Liquid Chromatography. Chem. Ind. Times 2019, 33, 10–13. [Google Scholar]
- Enzell, C.R.; Wahlberg, I.; Aasen, A.J. Isoprenoids and Alkaloids of Tobacco; Springer: Vienna, Austria, 1977. [Google Scholar]
- Donald, L.R.; Rowland, R.L. Macrocyclic Diterpenes, α- and β-4,8,13-Duvatriene-1,3-diols from Tobacco. J. Org. Chem. 1962, 27, 3989–3995. [Google Scholar] [CrossRef]
- Springer, J.P.; Clardy, J.; Cox, R.H.; Cutler, H.G.; Cole, R.J. The structure of a new type of plant growth inhibitor extracted from immature tobacco leaves. Tetrahedron Lett. 1975, 16, 2737–2740. [Google Scholar] [CrossRef]
- Asen, A.J.; Junker, N.; Enzell, C.R.; Berg, J.E.; Pilotti, A.M. Tobacco chemistry 36. Absolute configuration of tobacco thunberganoids. Tetrahedron Lett. 1975, 30, 2607–2610. [Google Scholar] [CrossRef]
- Chang, S.Y.; Grunwald, C. Duvatrienediols in cuticular wax of Burley tobacco leaves. J. Lipid Res. 1976, 17, 7–11. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Kemp, T.R.H.; Andersen, R.A.; Hildebrand, D.F. Headspace compounds from flowers of Nicotiana tabacum and related species. J. Agric. Food Chem. 1990, 38, 455–460. [Google Scholar] [CrossRef]
- Peng, F.; Sheng, L.; Liu, B.; Tong, H.; Liu, S. Comparison of different extraction methods:steam distillation, simultaneous distillation and extraction and headspace co-distillation, used for the analysis of the volatile components in aged flue-cured tobacco leaves. J. Chromatogr. A 2004, 1040, 1–17. [Google Scholar] [CrossRef]
- Shao, H.; Xu, Z.; Liu, L.; Lu, X.-P.; Xiao, B. Relationship between contents of total nitrogen as well as soluble protein and main volatile aroma components in flue-cured tobacco leaves. J. Northwest A F Univ. (Nat. Sci. Ed.) 2008, 36, 69–76. (In Chinese) [Google Scholar] [CrossRef]
- Sallaud, C.; Giacalone, C.; Tpfer, R.; Goepfert, S.; Tissier, A. Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 2012, 72, 1–17. [Google Scholar] [CrossRef]
- LI, G.; Zheng, Y.; Sun, Y.; Liu, M.; Wu, Z. Studies on the Aroma Volatilized Constituents of Ladaniferous. J. Instrum. Anal. 1991, 10, 12–18. [Google Scholar]
- Wang, L.; YU, R.; Shi, L.; Han, J.; Li, R. Analysis and Research of Volatile Aromatic Substances in Different Labdanum Concert with SED-GC-MS Method. J. Instrum. Anal. 2004, 23, 000083–000086. [Google Scholar]
- Cai, K.; Xiang, Z.; Pan, W.; Zhao, H.; Ren, Z.; Lei, B.; Geng, Z. Identification and quantitation of glycosidically bound aroma compounds in three tobacco types by gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1311, 149–156. [Google Scholar] [CrossRef]
- Li, W.; Dai, Y.; Wu, M.; Chen, X. Investigation of Five Glycosides in Tobacco by Liquid Chromatography-Mass Spectrometry(In Chinese). J. Instrum. Anal. 2009, 37, 82–88. [Google Scholar] [CrossRef]
- Cai, K.; Zhao, H.; Xiang, Z.; Cai, B.; Pan, W.; Lei, B. Enzymatic hydrolysis followed by gas chromatography-mass spectroscopy for determination of glycosides in tobacco and method optimization by response surface methodology. Anal. Methods 2014, 6, 7006–7014. [Google Scholar] [CrossRef]
- Pang, T.; Yuan, Z.; Dai, Y.; Wang, C.; Yang, J.; Peng, L.; Xu, G. Identification and determination of glycosides in tobacco leaves by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J. Sep. Sci. 2015, 30, 289–296. [Google Scholar] [CrossRef]
- Yue, Y.; Rong, Z.; Li, D.; Cheng, L.; Li, G. Simultaneous quantitative assessment of nine glycosides in tobacco by liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2018, 41, 1009–1016. [Google Scholar] [CrossRef]


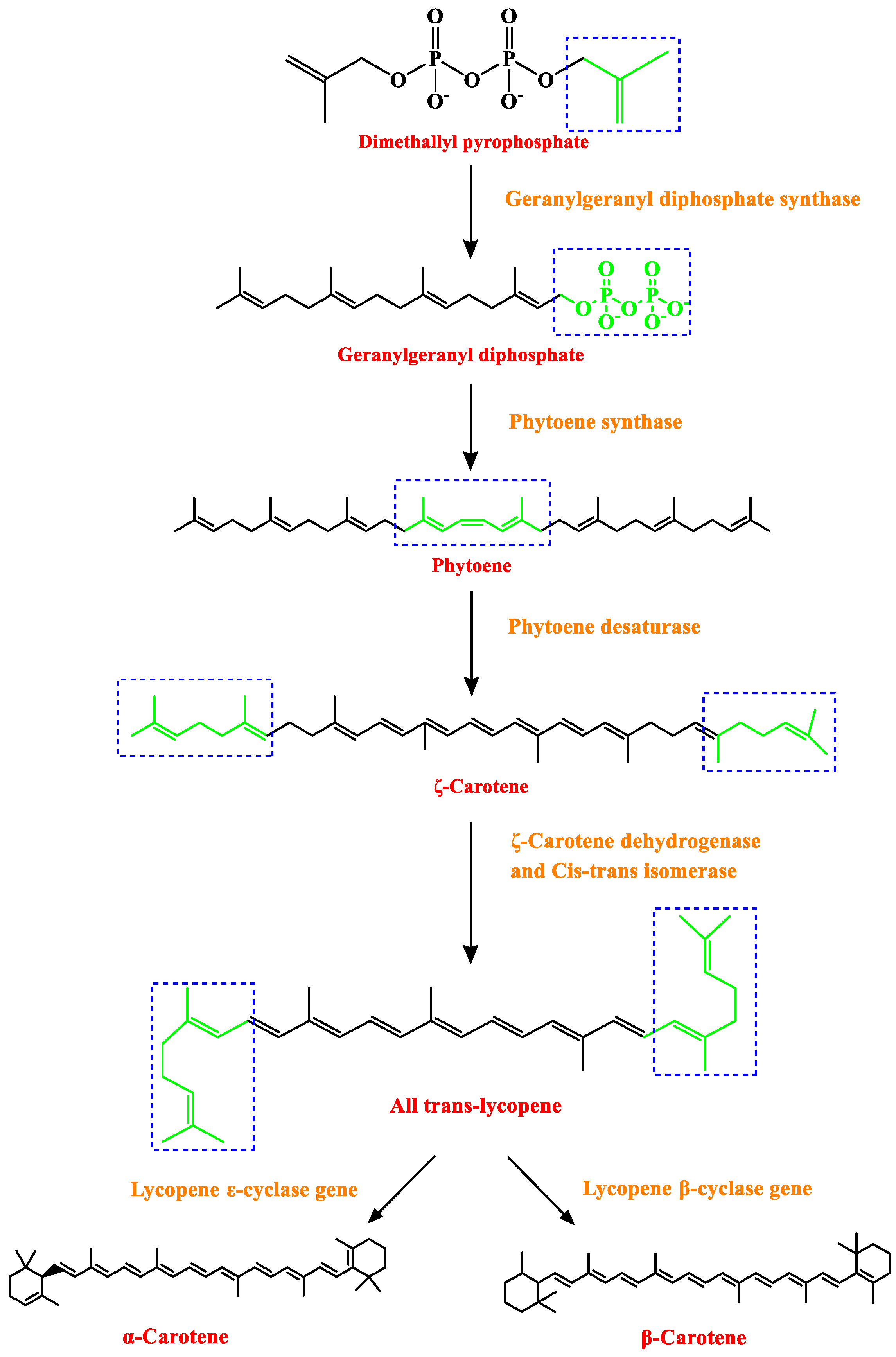
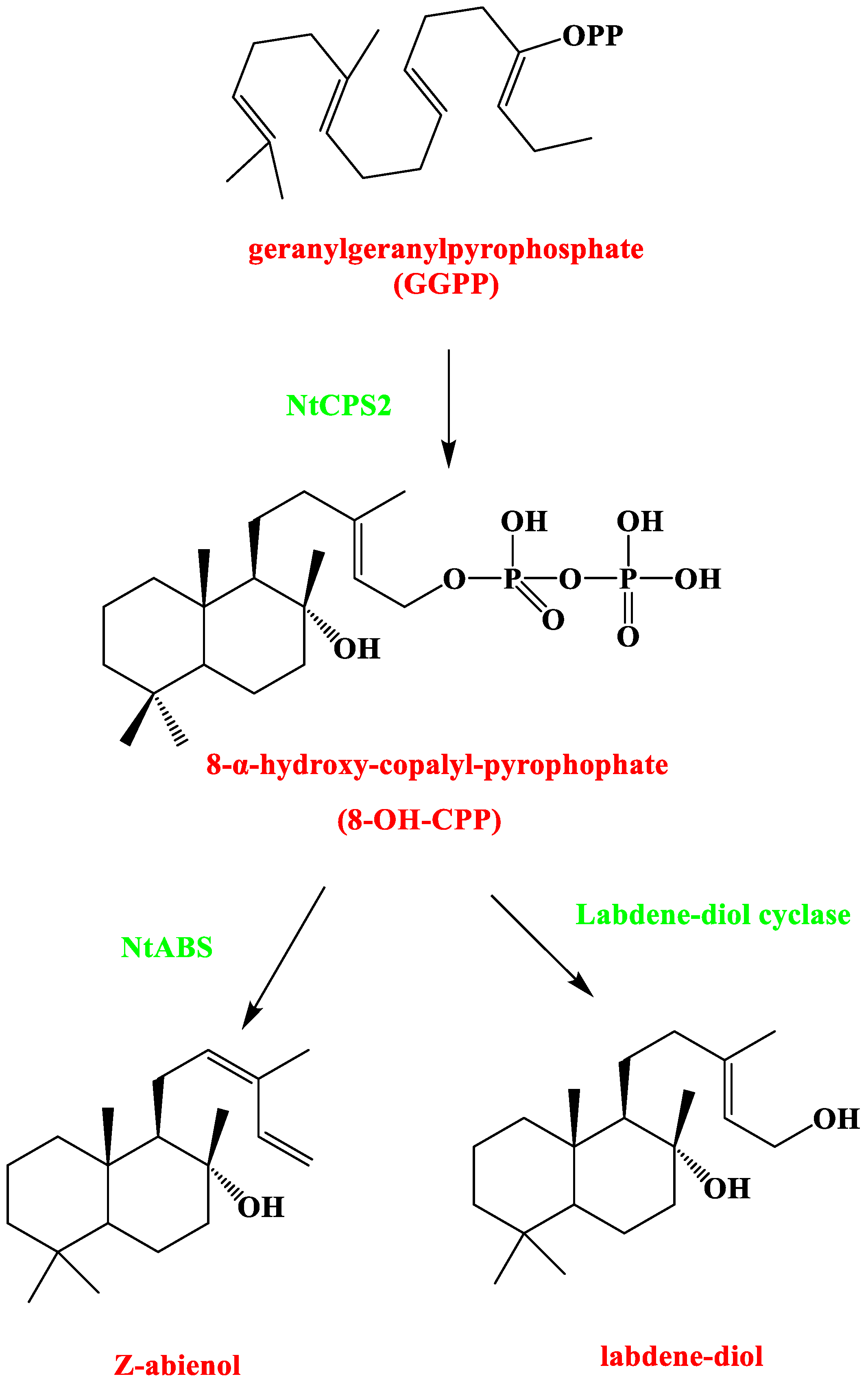
| Method | Detectable Component | Sample Preparation | Methodological Conditions | Reference |
|---|---|---|---|---|
| HPLC-QQQ-MS | Fru-Ala, Fru-Val, Fru-Pro, Fru-Phe, Fru-Trp | ① Weigh 25 mg dried tobacco. ② Soak in 95 mL water. ③ Ultrasonic extraction in bath. ④ Add appropriate amount of methanol. ⑤ Centrifuge. | Column: X BridgeTM Amide (250 mm × 4.6 mm, 3.5 μm); Phase A: methanol; Phase B: water | [52] |
| GC-MS LC-MS | Volatile compounds, Semi-volatile compounds, Non-volatile compounds | ① Weigh 1 g dried tobacco. ② Add 50 mL distilled water. ③ Supersonic simulation. | LC-MS: Column: Develosil ODS-SR-3; Mobile Phase A: 5 mM ammonium acetate; Mobile Phase B: acetonitrile GC-MS: HP-5 MS column (30 m × 250 m I.D., 0.25 μm df) | [54] |
| LC-MS/MS | Fru-Ala, Fru-Pro, Fru-Thr, Fru-Glu, Fru-Gly, Fru-Amb | ① Weigh 2 g tobacco powder. ② Add 70% methanol aqueous solution. ③ Ultrasonic extraction. ④ Filtration. ⑤ Concentrated to dry by rotary evaporation at 30 °C under reduced pressure. | Column: Thermo Hypersil Gold (100 mm × 2.1 mm × 1.9 μm); Mobile Phase: methanol–water solution | [55] |
| HPLC-MS/MS | Pro-Fru, Pro-Glu | ① Remove tobacco from flue-cured tobacco cigarette samples. ② Freeze-dried at −50 °C for 24 h. ③ Weigh 0.2 g samples. ④ Place in 200 mL volumetric flask. ⑤ Add 150 mL water. ⑥Ultrasonic mixing 20 min. ⑦ Add water volume to scale. | Column: Agilent ZORBAX C18 (4.6 mm × 250 mm, 5 μm) temperature: 30 °C; Mobile Phase A: acetonitrile; Mobile Phase B: water | [56] |
| LC-MS/MS | Glucosamine, Fru-Ala, Fru-Asn, Fru-Glu, Fru-Pro, Fru-Val, Fru-Lle, Fru-Leu, Fru-Phe, Fru-Trp | ① 100 mg tobacco powder was weighed. ② 3 mL of a 30: 70 v/v methanol/water solution was added. ③ The solution was extracted by ultrasonic wave for 25 min. ④ Centrifuged at 10000 rpm for 10 min. | Column: Atlantis T3 (2.1 × 250 mm, 5 μm); Phase A: aqueous formic acid; Phase B: acetonitrile; Gradients: 0.00 min, 89.5% A; 0.10 min, 78% A; 10.00 min, 78% A; V = 600 mL/min | [57] |
| LC-MS/MS | Fru-Ala, Fru-Pro, Fru-Val, Fru-Leu, Fru-Phe, Fru-Trp | ① Weigh 0.1 g tobacco samples in centrifuge tube. ② Add internal standard reserve liquid. ③ Ultrapure water; ultrasonic extraction 10 min, extraction 2 mL. ④Centrifuged for 15 min, 0.22 μm microporous membrane. | Column: Acclaim Explosive E2 column (250 mm × 4.6 mm, 5 μm); Mobile Phase A: methanol; Mobile Phase B: 0.1% formic acid aqueous solution | [58] |
| HPLC-Q-TOF-MS | Fru-Ala, Fru-GABA, Fru-Arg, Fru-Asn Fru-Asp, Fru-Cys, Fru-Gln, Fru-Glu, Fru-Gly, Fru-His, Fru-Leu, Fru-Lle, Fru-Lys, Fru-Met, Fru-Phe, Fru-Pro, Fru-Ser, Fru-Thr, Fru-Trp, Fru-Tyr, Fru-Val | ① 1 g tobacco powder. ② Added 30 mL 30% methanol-water solution. ③ Ultrasonic extraction 25 min. ④ The supernatant was filtered through an organic filter membrane. ⑤ 50 μL was taken in a 10 mL volumetric flask, diluted with methanol; 1 mL was taken for HPLC-MS/MS analysis. | Column: Atlantis T3 liquid column; Mobile Phase: 0.2% formic acid aqueous solution (phase A) and acetonitrile (phase B); Gradient Elution Program: 0–0.1 min 89.5% A ~78.0% A, 0.1–10.0 min 78.0% A. The initial flow phase was used to balance two samples for 8 min. | [59] |
| Method | Detectable Component | Sample Preparation | Methodological Conditions | Reference |
|---|---|---|---|---|
| HPLC | β-carotene, lutein | ① Twelve portions of 1.0 g tobacco leaf samples were weighed. ② Divided into two groups without shading and with black cloth shading. ③ Each group of samples was added with butylated hydroxytoluene (BHT) at 0, 0.01%, 0.05%, 0.10%, 0.15%, and 0.20%. ④ The samples were subjected to constant temperature oscillation and constant volume. | Column: Diamonsil-ODS-C18 column; Mobile Phase A: ethyl acetate ester; Mobile Phase B: 90% acetonitrile; Flow Rate: 0.8 mL/min | [62] |
| RP-HPLC-DAD | Neoxanthin, cis-neoxanthin, violaxanthin, luteoxanthin, cis-luteoxanthin, cis-violaxanthin, lutein, zeaxanthin, cis-lutein a, cis-lutein b, β-carotene | ① 0.5 g smoke sample was accurately weighed and placed in a 100 mL conical flask with a grinding mouth, 25 mL acetone was added, and 50 μg internal standard was added. ② The sample was wrapped and sealed with aluminum foil for 2 h. ③ The oscillation extraction the filter residue was filtered quickly, washed with acetone, and the filtrate was combined. | Column: Zorbax SB C18 column (4.6 × 150 mm, 5 μm); Flow Mobile Phase: gradient elution of acetonitrile, water, and ethyl acetate; Column Temperature: 25 °C; Flow Rate: 1.0 mL/min | [63] |
| UHPLC | Lutein, β-carotene | ① 0.10 g sample was weighed and added with 2 mL internal standard solution. ② Filled with N2 for exhaust. ③ Ultrasonic extraction. ④ Centrifugation. | Column: ACQUITY UPLC BEH C18 reversed-phase chromatography (100 mm × 2.1 mm, 1.7 μm); Mobile Phase A: acetonitrile–water; Mobile Phase B: ethyl acetate | [64] |
| Method | Detectable Component | Sample Preparation | Methodological Conditions | Reference |
|---|---|---|---|---|
| Solvent extraction | α-4,8,13-Duvatriene-1,3-diols, β-4,8,13-Duvatriene-1,3-diols | ① The hexane was removed in vacuo and the residue was chromatographed on silicic acid. ② The silicic acid columns were eluted with hexane, with 10%, 25%, and 50% ether–hexane mixtures. ③ The duvatrienediols were in fractions eluted with 50% ether-hexane. | NMR and IR | [66] |
| GC-MS | 2-hexanol, (E)β-ocimene, hexanol, 4-methylhexanol, 6-methylheptanol, linalool, caryophyllene, humulene, benzyl alcohol, neophytadiene, caryophyllene epoxide, eugenol | ① Flowers are put in a 5 L flask, and part of the flask is immersed in a 30 °C water bath. ② Connect the high-purity compressed air to the flask through the headspace device at a speed of 500 mL/min through a Teflon tube. | Column: Supelcowax10, capillary, (60 m × 0.32); GC Conditions: inlet temperature, 220 °C | [70] |
| 1. Steam distillation/ solvent extraction 2. Simultaneous distillation and extraction 3. Headspace co-distillation | Aliphatic alcohols, aromatics, ionol derivatives, furfurals, ketols, terpenoids, furanones, pyranone, damascone, ionone derivatives, volatile fatty acids, semi-volatile fatty acids, anhydrides, esters aliphatics | ① The dichloromethane solutions of volatile components obtained by three methods were extracted twice with 20 mL of a 5 wt % aqueous solution of sodium hydroxide and re-extracted twice with 10 mL dichloromethane to obtain the acidic fraction. ② The combined dichloromethane solution was extracted twice with 20 mL of a 5 wt % aqueous solution of hydrochloric acid and extracted twice with 10 mL dichloromethane to obtain the basic fraction. | Chromatographic separations were performed on a HP-5 MS column (30 m × 0.25 mm, 0.25 μm) | [71] |
| GC-MS | Degradation products of aromatic amino acids, degradation products of cembrane-like compounds, neophytadiene, carotenoids | ① The aroma components in tobacco leaves were extracted by steam synchronous distillation device, and the extract was extracted with dichloromethane. | Column: DB-5 (30 m × 0.25 mm × 0.25 μm) | [72] |
| Method | Detectable Component | Sample Preparation | Methodological Conditions | Reference |
|---|---|---|---|---|
| LC-MS | α-Ionol-β-D-glucopyranoside, 3-Oxo-α-ionol-β-D-glucopyranoside, 4-OH-α-ionol-β-D-glucopyranoside, 3-OH-β-damasenone-β-D-glucopyranoside, Lolidide-β-D-glucopyranoside | ① Tobacco leaves were dried in an oven at 40 °C for 4 h and ground for later use. | Column: C18 (250 mm × 4.6 mm) Mobile Phase A: methanol; Mobile Phase B: water | [75] |
| GC-MS | Aliphatic alcohols, fatty acids, aromatic compounds, C13 norisoprenoids, terpenoids, polyphenols | ① Glycosides were concentrated to dryness and re-dissolved in 15 mL of 0.2 M disodium hydrogen phosphate–citric acid buffer (pH = 5.6). ② Before adding the enzyme, the remaining volatiles were extracted with 20 mL of pentane–diethyl ether (1:1, v/v). | Column: HP-5 ms capillary (60 m × 0.25 mm i.d. × 0.25 mm); Toven = 60 °C (τheld = 1 min)–230 °C (τheld = 4 min), V = 2 °C/min | [76] |
| HPLC-APCI-MS | Scopolin, rutin, quercetin-3-glycoside | ① The tobacco powder (0.5 g) was placed in a 10 mL capped flask. ② 5 mL of methanol was added. ③ The extraction was carried out for 10 min in an ultrasonic washer. ④ The extract was centrifuged for 2 min at 3000 rpm at room temperature. ⑤ The supernatant was transferred into a beaker. | Column: Hypersil C18 (5 μm; 4.6 × 250 mm2); Mobile Phase A: 1% mic acid water; Mobile Phase B: CAN | [77] |
| LC-MS/MS | Phenolic, glycosides, benzenoid, glycosides, sesquiterpene, glycosides | ① The dried powders of flue-cured tobacco leaves (26.5 mg) were accurately weighed. ② Ultrasonic extraction with 5 mL of 72.4% methanol with 8.0 µg/mL of IS in a 25 °C water bath for 51.4 min. | Column: BEH-C18, (100 mm × 2.1 mm I.D., 1.7 µm); Mobile Phase A: water containing 0.1% formic acid | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Bin, J.; Yan, X.; Ding, M.; Yang, M. Application of Chromatographic Technology to Determine Aromatic Substances in Tobacco during Natural Fermentation: A Review. Separations 2022, 9, 187. https://doi.org/10.3390/separations9080187
Li X, Bin J, Yan X, Ding M, Yang M. Application of Chromatographic Technology to Determine Aromatic Substances in Tobacco during Natural Fermentation: A Review. Separations. 2022; 9(8):187. https://doi.org/10.3390/separations9080187
Chicago/Turabian StyleLi, Xuefeng, Jun Bin, Xiufang Yan, Mengjiao Ding, and Min Yang. 2022. "Application of Chromatographic Technology to Determine Aromatic Substances in Tobacco during Natural Fermentation: A Review" Separations 9, no. 8: 187. https://doi.org/10.3390/separations9080187
APA StyleLi, X., Bin, J., Yan, X., Ding, M., & Yang, M. (2022). Application of Chromatographic Technology to Determine Aromatic Substances in Tobacco during Natural Fermentation: A Review. Separations, 9(8), 187. https://doi.org/10.3390/separations9080187






