Single Cell Protein Production Using Different Fruit Waste: A Review
Abstract
:1. Introduction
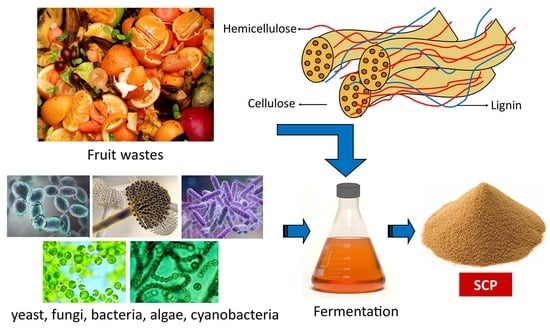
2. SCP
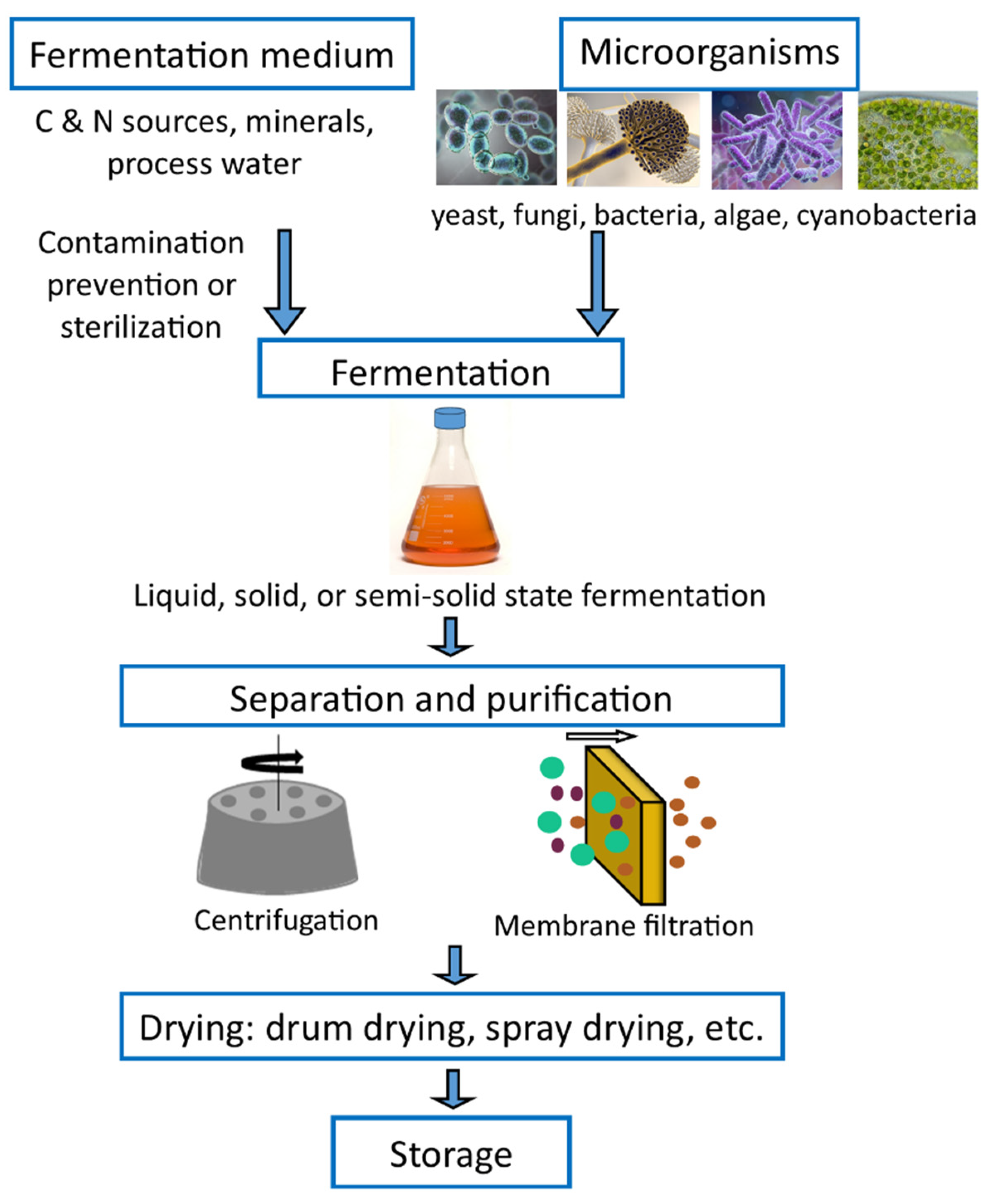
3. SCP Production Methods
4. Factors Affecting the SCP Production
5. Substrates for the Production of SCP
6. Fruit Production and Waste Generation
7. Physico-Chemical Properties of Fruit Waste
8. Fruit Waste as Substrate for SCP Production
9. Types of Fruit Waste
9.1. Fruit Wastes Rich in Simple Sugars
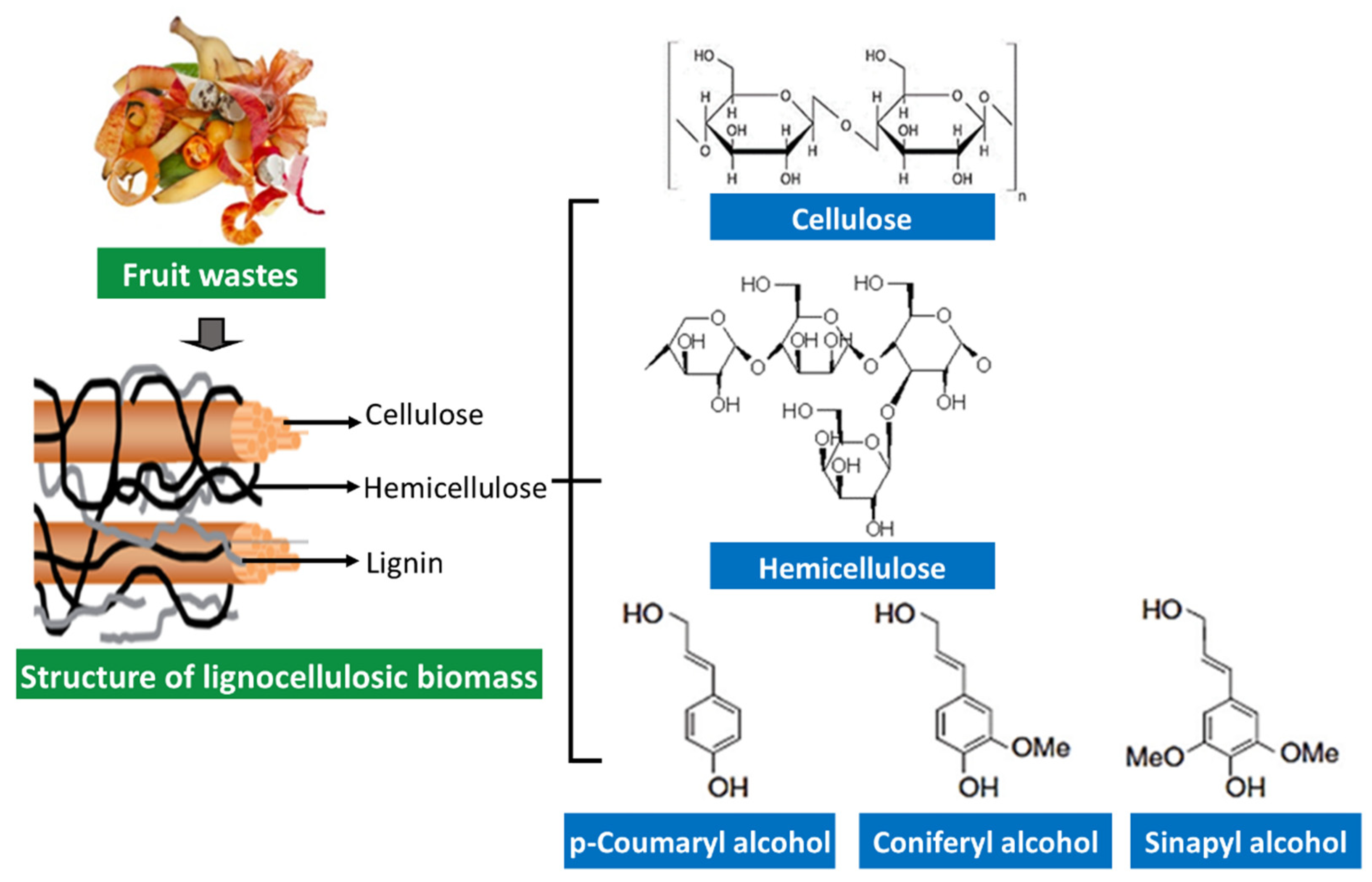
9.2. Fruit Waste Rich in Fibers
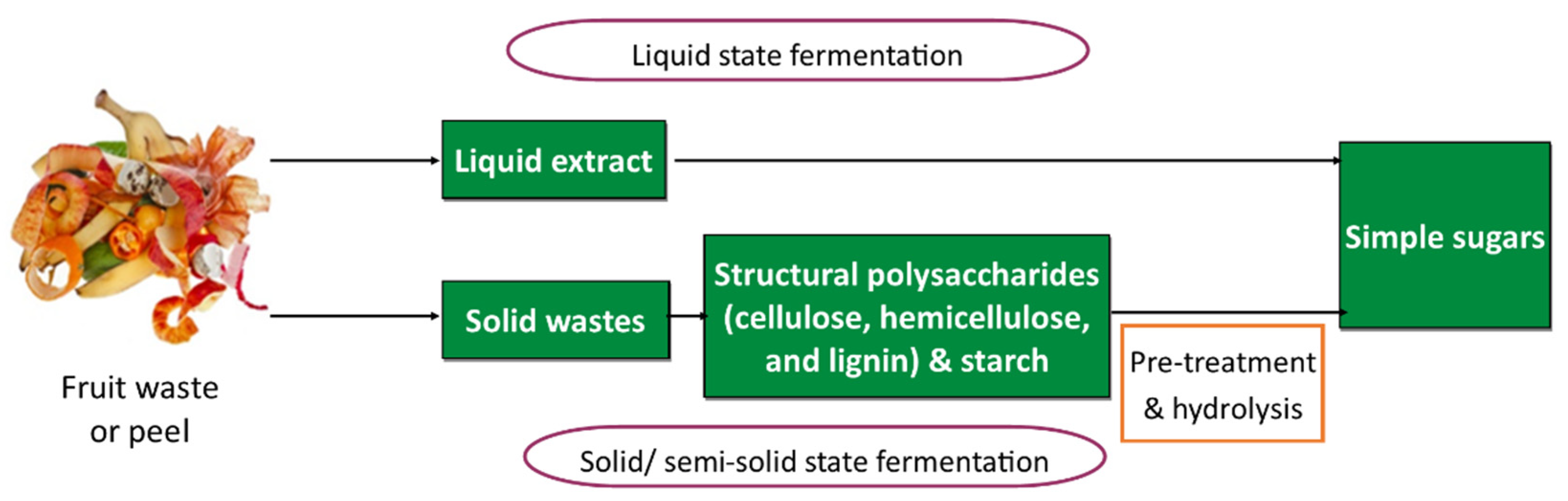
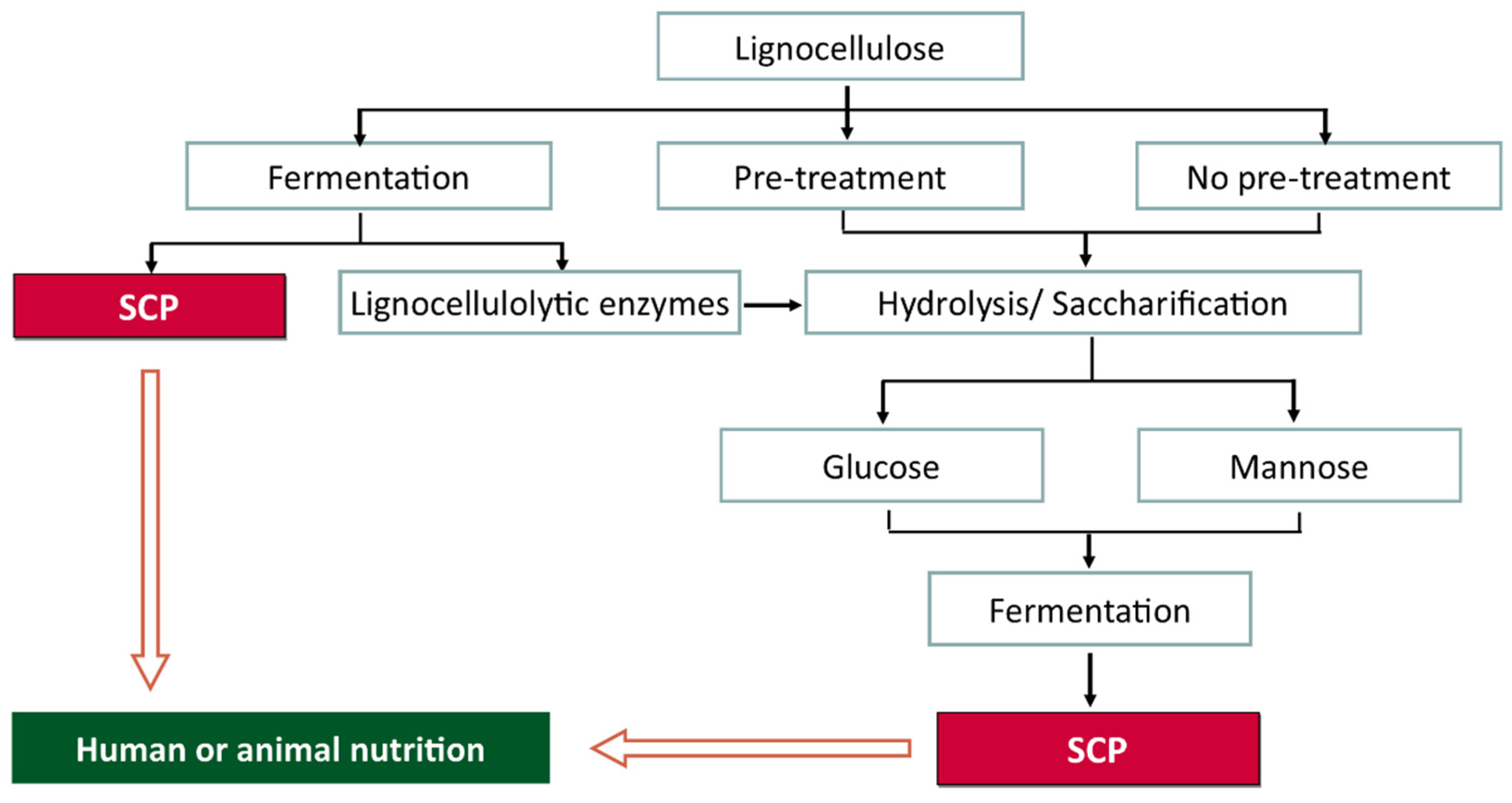
10. Bioconversion of Lignocellulosic Fruit Waste
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. SINGLE CELL PROTEIN | Yeasts and Bacteria. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 431–438. ISBN 978-0-12-384733-1. [Google Scholar]
- Nangul, A.; Bhatia, R. Microorganisms: A Marvelous Source of Single Cell Proteins. J. Microbiol. Biotechnol. Food Sci. 2013, 3, 15–18. [Google Scholar]
- Riesute, R.; Salomskiene, J.; Moreno, D.S.; Gustiene, S. Effect of Yeasts on Food Quality and Safety and Possibilities of Their Inhibition. Trends Food Sci. Technol. 2021, 108, 1–10. [Google Scholar] [CrossRef]
- Bajpai, P. (Ed.) Single Cell Protein Production from Lignocellulosic Biomass; Springer Briefs in Molecular Science; Springer: Singapore, 2017; ISBN 978-981-10-5873-8. [Google Scholar]
- El-Sayed, A.-F.M. Alternative Dietary Protein Sources for Farmed Tilapia, Oreochromis Spp. Aquaculture 1999, 179, 149–168. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative Proteins and EU Food Law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing Factors on Single-Cell Protein Production by Submerged Fermentation: A Review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Najafpour, G.D. CHAPTER 14-Single-Cell Protein. In Biochemical Engineering and Biotechnology; Najafpour, G.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 332–341. ISBN 978-0-444-52845-2. [Google Scholar]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. App. Sci 2015, 4, 251–262. [Google Scholar]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Ugalde, U.O.; Castrillo, J.I. Single Cell Proteins from Fungi and Yeasts. In Applied Mycology and Biotechnology; Khachatourians, G.G., Arora, D.K., Eds.; Agriculture and Food Production; Elsevier: Amsterdam, The Netherlands, 2002; Volume 2, pp. 123–149. [Google Scholar]
- Spalvins, K.; Zihare, L.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Comparison of Various Industrial by-Products. Energy Procedia 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Adoki, A. Factors Affecting Yeast Growth and Protein Yield Production from Orange, Plantain and Banana Wastes Processing Residues Using Candida Sp. Afr. J. Biotechnol. 2008, 7, 290–295. [Google Scholar] [CrossRef]
- Malav, A.; Dube, P. Single Cell Protein Production Using Various Microbial Mass: A Review. IJAR 2017, 5, 2190–2194. [Google Scholar] [CrossRef]
- Saheed, O.K.; Jamal, P.; Karim, M.I.A.; Alam, M.Z.; Muyibi, S.A. Utilization of Fruit Peels as Carbon Source for White Rot Fungi Biomass Production under Submerged State Bioconversion. J. King Saud Univ.-Sci. 2016, 28, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Adedayo; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins: As Nutritional Enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Anupama; Ravindra, P. Value-Added Food: Single Cell Protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of Fruit Wastes in Producing Single Cell Protein. Int. J. Sci. Environ. 2012, 1, 430–438. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Azeem, A.M.; Sheir, D.H. Bioconversion of Lignocellulosic Residues into Single-Cell Protein (SCP) by Chaetomium. In Recent Developments on Genus Chaetomium; Abdel-Azeem, A.M., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 343–375. ISBN 978-3-030-31612-9. [Google Scholar]
- Hezarjaribi, M.; Ardestani, F.; Ghorbani, H.R. Single Cell Protein Production by Saccharomyces cerevisiae Using an Optimized Culture Medium Composition in a Batch Submerged Bioprocess. Appl. Biochem. Biotechnol. 2016, 179, 1336–1345. [Google Scholar] [CrossRef]
- Wikandari, R.; Manikharda; Baldermann, S.; Ningrum, A.; Taherzadeh, M.J. Application of Cell Culture Technology and Genetic Engineering for Production of Future Foods and Crop Improvement to Strengthen Food Security. Bioengineered 2021, 12, 11305–11330. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Sadler, M.J. Fungal Protein. In New and Developing Sources of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1994; pp. 343–362. ISBN 978-1-4615-2652-0. [Google Scholar]
- Upadhyaya, S.; Tiwari, S.; Arora, N.; Singh, D.P. Microbial Protein: A Valuable Component for Future Food Security. In Microbes and Environmental Management; Singh, J.S., Singh, D.P., Eds.; Studium Press: Houston, TX, USA, 2016; pp. 259–279. ISBN 978-93-80012-83-4. [Google Scholar]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial Protein: Future Sustainable Food Supply Route with Low Environmental Footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Oshoma, C.E.; Eguakun-Owie, S.O. Conversion of Food Waste to Single Cell Protein Using Aspergillus niger. J. Appl. Sci. Environ. Manag. 2018, 22, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single Cell Protein: Sources, Mechanism of Production, Nutritional Value and Its Uses in Aquaculture Nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Lu, Y.; Tait, S.; Batstone, D.J. Simultaneous Treatment and Single Cell Protein Production from Agri-Industrial Wastewaters Using Purple Phototrophic Bacteria or Microalgae–A Comparison. Bioresour. Technol. 2018, 254, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Kim, J.D.; Kim, J.E.; Kim, I.H. Amino Acid Digestibility of Single Cell Protein from Corynebacterium ammoniagenes in Growing Pigs. Anim. Feed. Sci. Technol. 2013, 180, 111–114. [Google Scholar] [CrossRef]
- Goldberg, I. Fermentation Processes for Microbial SCP Production. In Single Cell Protein; Goldberg, I., Ed.; Biotechnology Monographs; Springer: Berlin/Heidelberg, Germany, 1985; pp. 67–128. ISBN 978-3-642-46540-6. [Google Scholar]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of Food Grade Yeasts. Food Technol. Biotechnol. 2006, 44, 407–415. [Google Scholar]
- Bacha, U.; Nasir, M.; Khalique, A.; Anjum, A.; Jabbar, M. Comparative Assessment of Various Agro-Industrial Wastes for Saccharomyces cerevisiae Biomass Production and Its Quality Evaluation as Single Cell Protein. J. Anim. Plant Sci. 2011, 21, 844–849. [Google Scholar]
- Labuza, T.P.; Santos, D.B.; Roop, R.N. Engineering Factors in Single-Cell Protein Production. I. Fluid Properties and Concentration of Yeast by Evaporation. Biotechnol. Bioeng. 1970, 12, 123–134. [Google Scholar] [CrossRef]
- Pandey, A. Recent Process Developments in Solid-State Fermentation. Process Biochem. 1992, 27, 109–117. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New Developments in Solid State Fermentation: I-Bioprocesses and Products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Ravichandran, S.; Vimala, R. Solid State and Submerged Fermentation for the Production of Bioactive Substances: A Comparative Study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Yamuna Rani, K.; Ramachandra Rao, V.S. Control of Fermenters—A review. Bioprocess Eng. 1999, 21, 77–88. [Google Scholar] [CrossRef]
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. Food Sec. 2019, 11, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Nalage, D.; Khedkar, G.; Kalyankar, A.; Sarkate, A.; Ghodke, S.; Bedre, V.B.; Khedkar, C.D. Single Cell Proteins. Encycl. Food Health 2016, 4, 790–794. [Google Scholar]
- Kamal, M.; Ali, M.; Shishir, M.R.I.; Saifullah, M.; Haque, M.; Mondal, S.C. Optimization of Process Parameters for Improved Production of Biomass Protein from Aspergillus Niger Using Banana Peel as a Substrate. Food Sci. Biotechnol. 2019, 28, 1693–1702. [Google Scholar] [CrossRef]
- Umesh, M.; Thazeem, B.; Preethi, K. Valorization of Pineapple Peels through Single Cell Protein Production Using Saccharomyces cerevisiae NCDC 364. Appl. Food Biotechnol. 2019, 6, 255–263. [Google Scholar] [CrossRef]
- Krishna, C. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23-Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. ISBN 978-0-444-63504-4. [Google Scholar]
- Mahato, N.; Sharma, K.; Sinha, M.; Dhyani, A.; Pathak, B.; Jang, H.; Park, S.; Pashikanti, S.; Cho, S. Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes 2021, 9, 220. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast Flavour Production by Solid State Fermentation of Orange Peel Waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Ahangangoda Arachchige, M.S.; Mizutani, O.; Toyama, H. Yeast Strains from Coconut Toddy in Sri Lanka Show High Tolerance to Inhibitors Derived from the Hydrolysis of Lignocellulosic Materials. Biotechnol. Biotechnol. Equip. 2019, 33, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Dissanayake, P.D.; Gao, B.; Liu, W.-J.; Lee, K.B.; Ok, Y.S. Review on Upgrading Organic Waste to Value-Added Carbon Materials for Energy and Environmental Applications. J. Environ. Manag. 2021, 296, 113128. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture–Statistical Yearbook 2021; FAO Statistical Yearbook–World Food and Agriculture; FAO: Rome, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, e3029295. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, U.K.; Kamarrudin, N.; Suzihaque, M.U.H.; Hashib, S.A. Local Fruit Wastes as a Potential Source of Natural Antioxidant: An Overview. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Miri, Malaysia, 1–3 December 2016; Volume 206, p. 012040. [Google Scholar] [CrossRef]
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Chapter 2-Fruit and Vegetable Processing Waste: Renewable Feed Stocks for Enzyme Production. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 23–59. ISBN 978-0-12-802392-1. [Google Scholar]
- Abdullah; Mat, H.B. The Characteristic of Pineapple Waste from Canning Industry. Adv. Sci. Lett. 2017, 23, 5691–5693. [Google Scholar] [CrossRef]
- Murakonda, S.; Dwivedi, M. Powders from Fruit Waste. In Food Powders Properties and Characterization; Ermiş, E., Ed.; Food Engineering Series; Springer: Cham, Switzerland, 2021; pp. 155–168. ISBN 978-3-030-48908-3. [Google Scholar]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Chapter Five-Bioactive Potential of Fruit and Vegetable Wastes. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 157–225. [Google Scholar]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Monspart-Sényi, J. Fruit Processing Waste Management. In Handbook of Fruits and Fruit Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 315–331. ISBN 978-1-118-35253-3. [Google Scholar]
- Panda, S.K.; Ray, R.C.; Mishra, S.S.; Kayitesi, E. Microbial Processing of Fruit and Vegetable Wastes into Potential Biocommodities: A Review. Crit. Rev. Biotechnol. 2018, 38, 1–16. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, A.; Mandalari, G.; Arena, N.; Nucita, F.; Tripodo, M.M.; Lo Curto, R.B. SCP and Crude Pectinase Production by Slurry-State Fermentation of Lemon Pulps. Bioresour. Technol. 2002, 83, 89–94. [Google Scholar] [CrossRef]
- Sadhu, S.D.; Garg, M.; Kumar, A. 4-Major Environmental Issues and New Materials. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 77–97. ISBN 978-0-12-811033-1. [Google Scholar]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of Agro-Industrial Wastes in Solid-State Fermentation Processes; IntechOpen: London, UK, 2012; ISBN 978-953-51-0253-3. [Google Scholar]
- Saheed, O.K.; Jamal, P.; Kari, M.I.A.; Alam, Z.; Muyibi, S.A. Cellulolytic Fruits Wastes: A Potential Support for Enzyme Assisted Protein Production. J. Biol. Sci. 2013, 13, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Ani, P.N.; Abel, H.C. Nutrient, Phytochemical, and Antinutrient Composition of Citrus maxima Fruit Juice and Peel Extract. Food Sci. Nutr. 2018, 6, 653–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, P.G.I.; Sajiwanie, J.W.A.; Rathnayaka, R.M.U.S.K. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, S240–S251. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit Peel Waste: Characterization and Its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Orozco, R.S.; Hernández, P.B.; Morales, G.R.; Núñez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramírez, N.F.; Díaz, C.E.B.; Vázquez, P.C. Characterization of Lignocellulosic Fruit Waste as an Alternative Feedstock for Bioethanol Production. BioResources 2014, 9, 1873–1885. [Google Scholar]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, Hemicelluloses, Lignin and Ash Content of Some Organic Materials and Their Suitability for Use as Paper Pulp Supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kandari, V.; Gupta, S. Bioconversion of Vegetable and Fruit Peel Wastes in Viable Product. J. Microbiol. Biotechnol. Res. 2012, 2, 308–312. [Google Scholar]
- Akanni, G.; Ntuli, V.; Preez, D. Cactus Pear Biomass, a Potential Lignocellulose Raw Material for Single Cell Protein Production (SCP): A Review. Int. J. Curr. Microbiol. App. Sci 2014, 3, 171–197. [Google Scholar]
- Khan, M.; Khan, S.; Zafar, A.; Tanveer, A. Production of Single Cell Protein from Saccharomyces cerevisiae by Utilizing Fruit Wastes. Nanobiotechnica Univers. 2010, 1, 127–132. [Google Scholar]
- Thiviya, P.; Kapilan, R.; Madhujith, T. Bioconversion of Fruit Wastes of Papaya, Watermelon, and Banana into Single Cell Protein Production. Trop. Agric. Res. 2021, 32, 503–514. [Google Scholar] [CrossRef]
- Rages, A.A.; Haider, M.M. Alkaline Hydrolysis of Olive Fruits Wastes for the Production of Single Cell Protein by Candida lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999. [Google Scholar] [CrossRef]
- Nigam, J.N. Single Cell Protein from Pineapple Cannery Effluent. World J. Microbiol. Biotechnol. 1998, 14, 693–696. [Google Scholar] [CrossRef]
- Rosma, A.; Ooi, K.I. Production of Candida utilis Biomass and Intracellular Protein Content: Effect of Agitation Speed and Aeration Rate. MJM 2006, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Munawar, R.; Irfan, M.; Nadeem, M.; Syed, Q.; Siddique, Z. Biosynthesis of Single Cell Biomass of Candida Utuilis by Submerged Fermentation. Pak. J. Sci. 2010, 62, 1–5. [Google Scholar]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A.S. Production of Single Cell Protein (SCP) and Essentials Amino Acids from Candida utilis FMJ12 by Solid State Fermentation Using Mango Waste Supplemented with Nitrogen Sources. AJB 2018, 17, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Jiru, T.M.; Melku, B. Single Cell Protein Production from Torula Yeast (Cyberlindnera Sp.) Using Banana Peel Hydrolysate. J. Adv. Microbiol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Ziino, M.; Lo Curto, R.B.; Salvo, F.; Signorino, D.; Chiofalo, B.; Giuffrida, D. Lipid Composition of Geotrichum candidum Single Cell Protein Grown in Continuous Submerged Culture. Bioresour. Technol. 1999, 67, 7–11. [Google Scholar] [CrossRef]
- Stabnikova, O.; Wang, J.-Y.; Bo Ding, H. Joo-HwaTay Biotransformation of Vegetable and Fruit Processing Wastes into Yeast Biomass Enriched with Selenium. Bioresour. Technol. 2005, 96, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Abarshi, M.M.; Mada, S.B.; Amin, M.I.; Salihu, A.; Garba, A.; Mohammad, H.A. Effect of Nutrient Supplementation on Single Cell Protein Production from Watermelon and Pineapple Peels. Niger. J. Basic Appl. Sci. 2017, 25, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein Enrichment of Yam Peels by Fermentation with Saccharomyces cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37. [Google Scholar] [CrossRef]
- Mujdalipah, S.; Putri, M.L. Utilization of Pineapple Peel and Rice Washing Water to Produce Single Cell Proteins Using Saccharomyces cerevisiae. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 9–10 October 2019; Volume 472, p. 012029. [Google Scholar] [CrossRef]
- Nurmalasari, A.; Maharani, S. Addition of Carbon Sources to Pineapple Waste Media in the Production of Single Cell Protein Biomass Saccharomyces Cerevisiae. J. Ris. Biol. Dan Apl. 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Umesh, M.; Priyanka, K.; Thazeem, B.; Preethi, K. Production of Single Cell Protein and Polyhydroxyalkanoate from Carica papaya Waste. Arab. J. Sci. Eng. 2017, 42, 2361–2369. [Google Scholar] [CrossRef]
- Muniz, C.E.S.; Santiago, Â.M.; Gusmão, T.A.S.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Solid-State Fermentation for Single-Cell Protein Enrichment of Guava and Cashew by-Products and Inclusion on Cereal Bars. Biocatal. Agric. Biotechnol. 2020, 25, 101576. [Google Scholar] [CrossRef]
- Azam, S.; Khan, Z.; Bashir, A.; Khan, I.; Ali, J. Production of Single Cell Protein from Orange Peels Using Aspergillus niger and Saccharomyces cerevisiae. Glob. J. Biotechnol. Biochem. 2014, 9, 14–18. [Google Scholar] [CrossRef]
- Hamdy, H.S. Production of Mini-Food by Aspergillus niger, Rhizopus oryzae and Saccharomyces cerevisiae Using Orange Peels. Rom. Biotechnol. Lett. 2013, 18, 7929–7946. [Google Scholar]
- Rashad, M.M.; Moharib, S.A.; Jwanny, E.W. Yeast Conversion of Mango Waste or Methanol to Single Cell Protein and Other Metabolites. Biol. Wastes 1990, 32, 277–284. [Google Scholar] [CrossRef]
- Yabaya, A.; Ado, S.A. Mycelial Protein Production by Aspergillus niger Using Banana Peels. Sci. World J. 2008, 3, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Bind, A.; Kumar, M.; Singh, D. Optimization of SCP Production of Aspergillus niger Using Different Fruit Peels-Indian Journals. Int. J. Bioinform. Biol. Sci. 2013, 1, 1–8. [Google Scholar]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of Agro Industrial Wastes as Immobilization Carrier for Solid-State Fermentation. Ind. Crops Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Jaganmohan, P.; Daas, B.P.; Prasad, S.V. Production of Single Cell Protein (SCP) with Aspergillus terreus Using Solid State Fermentation. Eur. J. Biol. Sci. 2013, 5, 38–43. [Google Scholar] [CrossRef]
- Scerra, V.; Caridi, A.; Foti, F.; Sinatra, M.C. Influence of Dairy Penicillium Spp. on Nutrient Content of Citrus Fruit Peel1Contribution from the Ministry of Scientific Research and Technology–Research Fund 60%: M. C. Sinatra.1. Anim. Feed. Sci. Technol. 1999, 78, 169–176. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.S.; Ahmed, Z.; Tanveer, A. Production of Fungal Single Cell Protein Using Rhizopus Oligosporus Grown on Fruit Wastes. Biol. Forum 2009, 1, 26–28. [Google Scholar]
- Ahmadi, F.; Zamiri, M.J.; Khorvash, M.; Banihashemi, Z.; Bayat, A.R. Chemical Composition and Protein Enrichment of Orange Peels and Sugar Beet Pulp after Fermentation by Two Trichoderma Species. Iran. J. Vet. Res. 2015, 16, 25–30. [Google Scholar]
- Mahan, K.M.; Le, R.K.; Wells, T., Jr.; Anderson, S.; Yuan, J.S.; Stoklosa, R.J.; Bhalla, A.; Hodge, D.B.; Ragauskas, A.J. Production of Single Cell Protein from Agro-Waste Using Rhodococcus Opacus. J. Ind. Microbiol. Biotechnol. 2018, 45, 795–801. [Google Scholar] [CrossRef]
- Patel, N.; Patel, A.; Patel, H.; Patel, M.; Patel, U. Production of Single Cell Protein from Mix Fruits Waste Using Lactobacillus. Int. J. Pharm. Biol. Sci. 2019, 9, 164–168. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.; Bondar, M.; Palolo, I.; Queirós, O.; de Almeida, C.D.; Cesário, M.T. Xylose Metabolism in Bacteria—Opportunities and Challenges towards Efficient Lignocellulosic Biomass-Based Biorefineries. Appl. Sci. 2021, 11, 8112. [Google Scholar] [CrossRef]
- Sandle, T. 22-Microbiological Challenges to the Pharmaceuticals and Healthcare. In Pharmaceutical Microbiology; Sandle, T., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 281–294. ISBN 978-0-08-100022-9. [Google Scholar]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-Industrial Lignocellulosic Biomass a Key to Unlock the Future Bio-Energy: A Brief Review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Kalaichelvan, P.T.; Arulpandi, I. Bioprocess Technology; MJP Publishers: Chennai, India, 2019; ISBN 978-81-8094-032-3. [Google Scholar]
- Chen, H. Chemical Composition and Structure of Natural Lignocellulose. In Biotechnology of Lignocellulose: Theory and Practice; Chen, H., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. ISBN 978-94-007-6898-7. [Google Scholar]
- Tanaka, M.; Matsuno, R. Conversion of Lignocellulosic Materials to Single-Cell Protein (SCP): Recent Developments and Problems. Enzym. Microb. Technol. 1985, 7, 197–206. [Google Scholar] [CrossRef]
- Parekh, V.J.; Rathod, V.K.; Pandit, A.B. 2.10-Substrate Hydrolysis: Methods, Mechanism, and Industrial Applications of Substrate Hydrolysis. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, Canada, 2011; pp. 103–118. ISBN 978-0-08-088504-9. [Google Scholar]
- Jahid, M.; Gupta, A.; Sharma, D.K. Production of Bioethanol from Fruit Wastes (Banana, Papaya, Pineapple and Mango Peels) Under Milder Conditions. J. Bioprocess. Biotech. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Chapter 2-Pretreatment of Lignocellulosic Biomass for Efficient Enzymatic Saccharification of Cellulose. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 17–65. ISBN 978-0-12-815936-1. [Google Scholar]
- Kutshik, J.R.; Usman, A.M.; Ali-Dunkrah, U. Comparative Study of Protein Enrichment of Lignocellulose Wastes Using Baker’s Yeast (Saccharomyces Cerevisiae) for Animal Feeds. IOSR J. Biotechnol. Biochem. 2016, 2, 73–77. [Google Scholar]
- Refaat, A.A. 5.13-Biofuels from Waste Materials. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; pp. 217–261. ISBN 978-0-08-087873-7. [Google Scholar]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic Hydrolysis of Lignocellulosic Biomass: Converting Food Waste in Valuable Products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Pocan, P.; Bahcegul, E.; Oztop, M.H.; Hamamci, H. Enzymatic Hydrolysis of Fruit Peels and Other Lignocellulosic Biomass as a Source of Sugar. Waste Biomass Valor. 2018, 9, 929–937. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial Cellulases and Their Industrial Applications. Enzyme Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [Green Version]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Chapter 7-Biological Pretreatment of Lignocellulosic Biomass—Current Trends and Future Perspectives. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. ISBN 978-0-12-815162-4. [Google Scholar]
- Rodrigues, A.C.; Haven, M.Ø.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic® CTec2: Saccharification/Fermentation of Wheat Straw, Solid–Liquid Partition and Potential of Enzyme Recycling by Alkaline Washing. Enzym. Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taherzadeh, M.J.; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Howard, R.L.; Abotsi, E.; Van Rensburg, E.J.; Howard, S. Lignocellulose Biotechnology: Issues of Bioconversion and Enzyme Production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
| Microorganisms | Protein | Fat | Ash | Nucleic Acid |
|---|---|---|---|---|
| Fungi | 30–45 | 2–8 | 9–14 | 7–10 |
| Algae | 40–60 | 7–20 | 8–10 | 3–8 |
| Yeast | 45–55 | 2–6 | 5–10 | 6–12 |
| Bacteria | 50–65 | 1–3 | 3–7 | 8–12 |
| Microorganism | Substrate (Fruit Waste) | Type of Fermentation Medium | Reference |
|---|---|---|---|
| Yeast | |||
| Yarrowia lipolytica (formerly Candida lipolytica, or Saccharomyces lipolytica) | Olive fruits wastes | SF/LSF | [81] |
| Candida utilis | Pineapple cannery effluent | SF/LSF | [82] |
| Pineapple waste | SF/LSF | [83] | |
| Mixture of the banana and orange waste | SF/LSF | [84] | |
| Orange peel | SF/LSF | [85] | |
| Mango wastes | SSF | [86] | |
| Cyberlindnera spp. | Banana peel hydrolysate | SF/LSF | [87] |
| Geotrichum candidum | Orange peel | SF/LSF | [88] |
| Saccharomyces cerevisiae | Watermelon, mixture of fruit wastes | SF/LSF | [89] |
| Watermelon, pineapple | SF/LSF | [90] | |
| Yam peel | SF/LSF | [91] | |
| Apple, orange peel | SF/LSF | [36] | |
| Cucumber peel, orange peel | SF/LSF | [20] | |
| Pineapple waste | SF/LSF | [11,46,92,93] | |
| Papaya waste | SF/LSF | [94] | |
| Apple, papaya, banana | SF/LSF | [77] | |
| Guava peels and cashew bagasse | SSF | [95] | |
| Rind of pomegranate, mango, banana, apple, sweet orange peel | SSF | [79] | |
| Orange peels | SSF | [96,97] | |
| Pichia pinus | Mango waste | SF/LSF | [98] |
| Fungi | |||
| Aspergillus niger | Banana peel, orange peel, cucumber peel, pineapple peel, watermelon peel | SF/LSF | [29] |
| Banana peel | SF/LSF | [99] | |
| Banana peel | SF/LSF | [45] | |
| Banana, papaya, orange | SF/LSF | [100] | |
| Lemon peel, orange peel, apple pomace | SSF | [101] | |
| Aspergillus niger Rhizopus oryzae | Orange peels | SSF | [97] |
| Aspergillus niger Saccharomyces cerevisiae | Orange peel | SSF | [96] |
| Aspergillus terreus | Banana peel | SSF | [102] |
| Penicillium roqueforti, Penicillium camemberti | Bergamot fruit (citrus fruit) peel | SSF | [103] |
| Phanerochaete chrysosporium, Panus tigrinus | Banana peel, pineapple peel, papaya peel | SF/LSF | [16] |
| Phanerochaete chrysosporium | Banana peels, pineapple peels, and papaya peels | SF/LSF | [16] |
| Rhizopus oligosporus | Papaya waste, cucumber peelings, pomegranate fruit rind, pineapple fruit skin, and watermelon skin. | SSF | [104] |
| Trichoderma viride, Trichoderma reesei | Orange peel | SSF | [105] |
| Bacteria | |||
| Rhodococcus opacus | Orange wastes, lemon wastes | SF/LSF | [106] |
| Other natural sources/mixed cultures | |||
| Natural microorganisms in Palmyrah toddy | Papaya, watermelon, and banana peel | SF/LSF | [80] |
| Lactobacillus culture isolated from curd | Mix fruit wastes such as pineapple peel residue, pomegranate waste, apple waste, and pear waste | SF/LSF | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. https://doi.org/10.3390/separations9070178
Thiviya P, Gamage A, Kapilan R, Merah O, Madhujith T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations. 2022; 9(7):178. https://doi.org/10.3390/separations9070178
Chicago/Turabian StyleThiviya, Punniamoorthy, Ashoka Gamage, Ranganathan Kapilan, Othmane Merah, and Terrence Madhujith. 2022. "Single Cell Protein Production Using Different Fruit Waste: A Review" Separations 9, no. 7: 178. https://doi.org/10.3390/separations9070178
APA StyleThiviya, P., Gamage, A., Kapilan, R., Merah, O., & Madhujith, T. (2022). Single Cell Protein Production Using Different Fruit Waste: A Review. Separations, 9(7), 178. https://doi.org/10.3390/separations9070178