Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Real Wastewater
2.2. Experimental Procedures
2.3. Analysis Methods
3. Results and Discussion
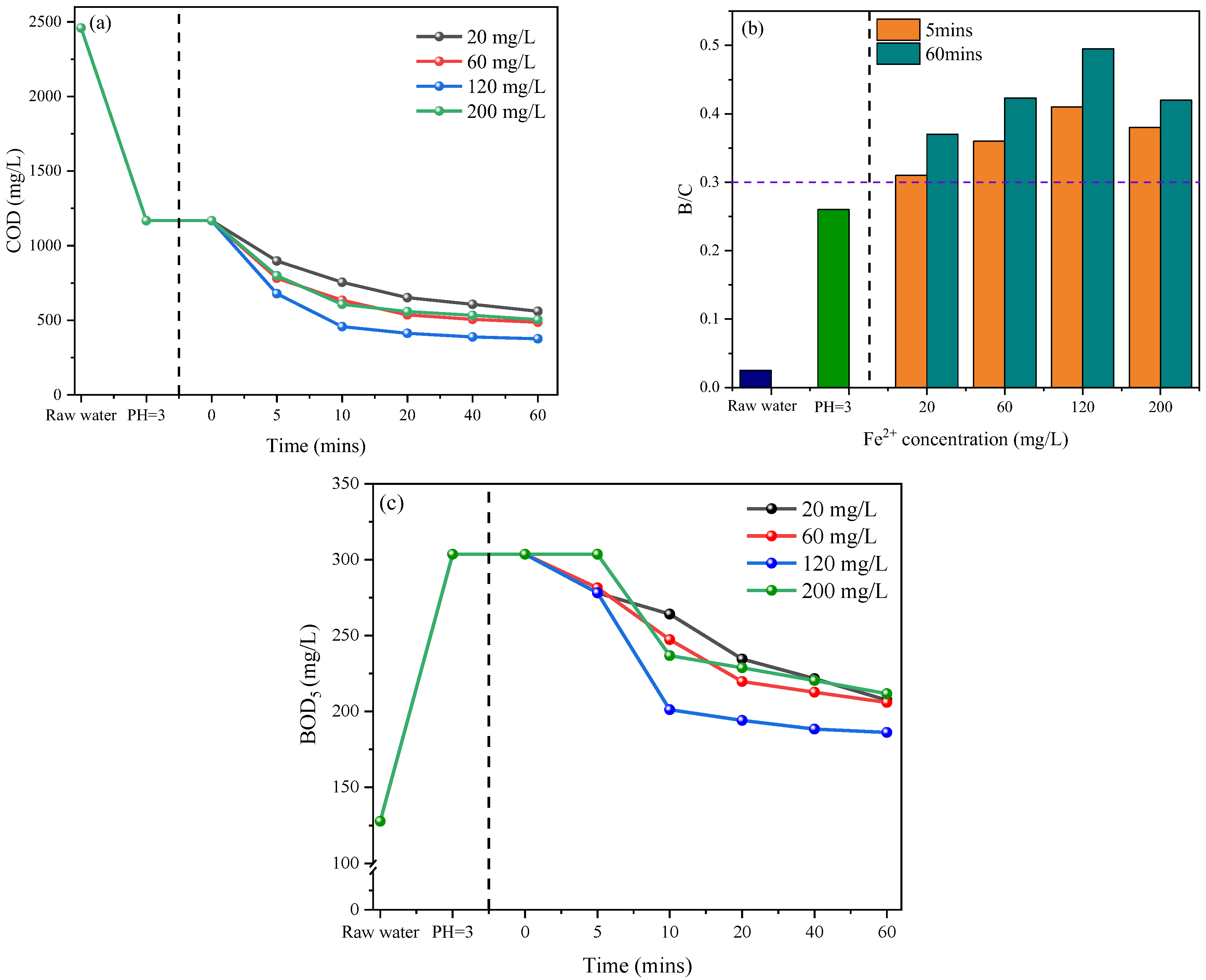
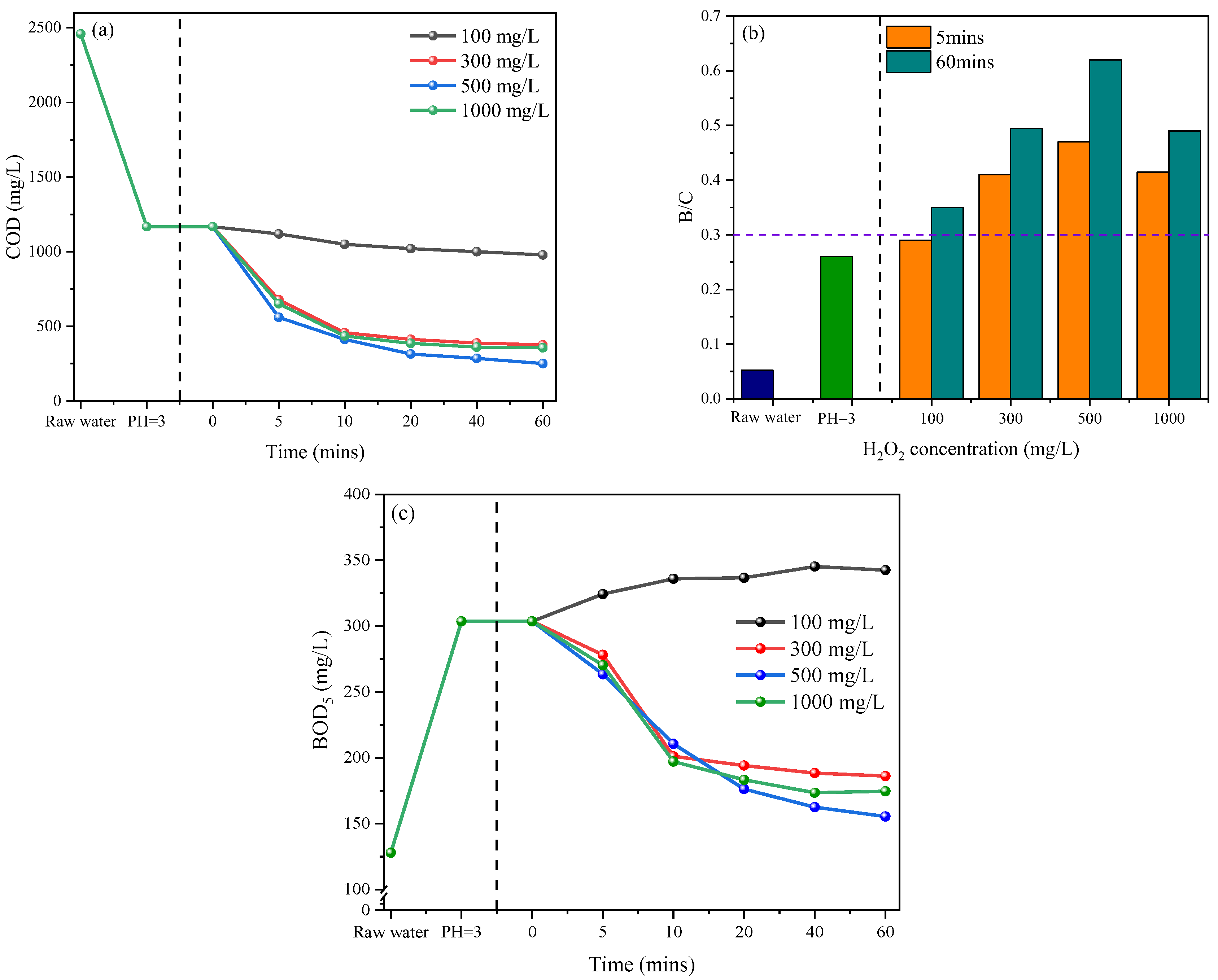
3.1. Pretreatment of Petrochemical Wastewater by Fenton Oxidation
3.1.1. Effect of Fe2+ Concentration
3.1.2. Effect of H2O2 Concentration
3.2. Pretreatment of Petrochemical Wastewater by Ozone Oxidation
3.3. Mechanisms of B/C Improvement
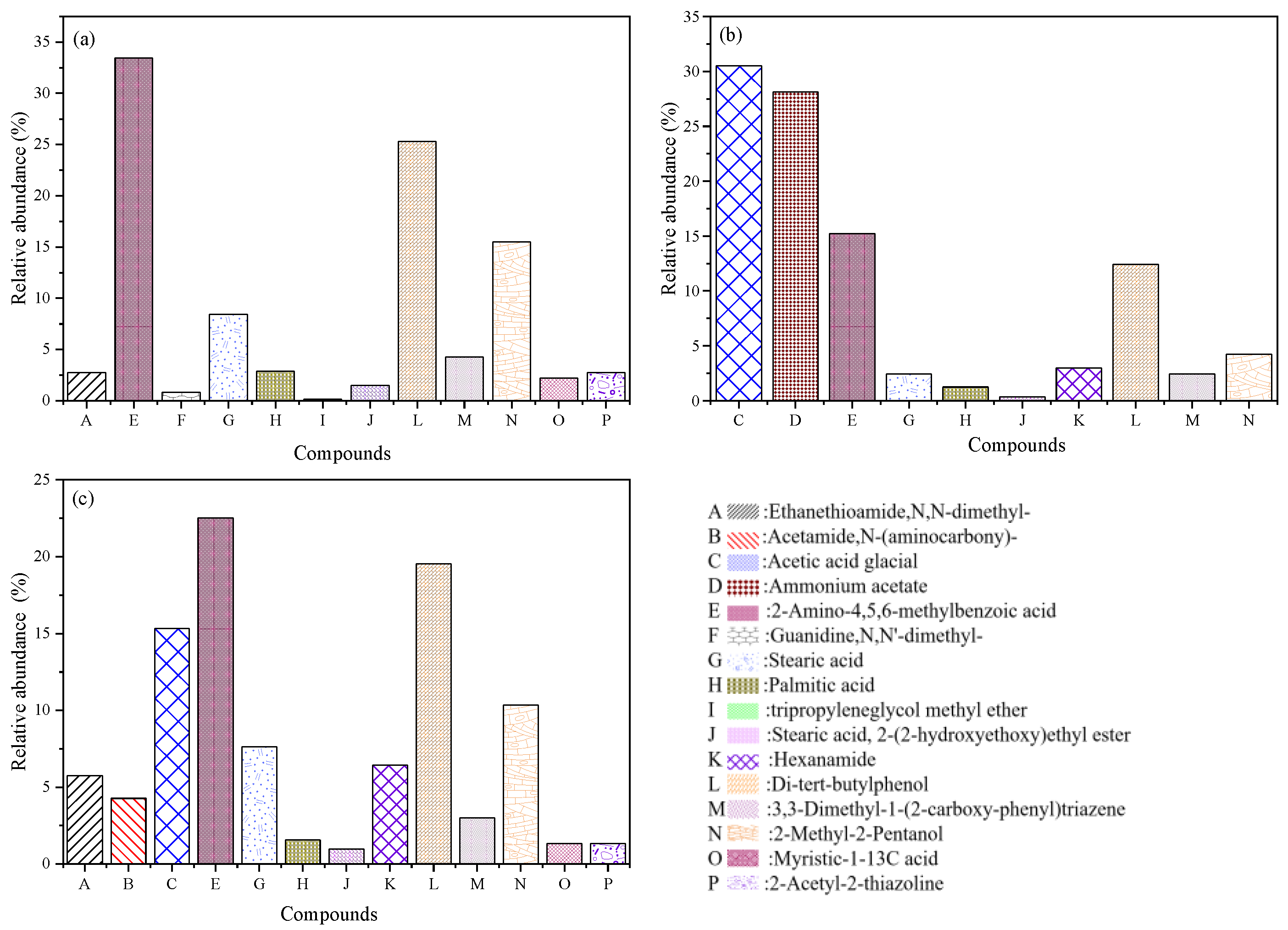
3.3.1. GC–MS Analysis of Wastewater
3.3.2. Biodegradability Improvement Analysis
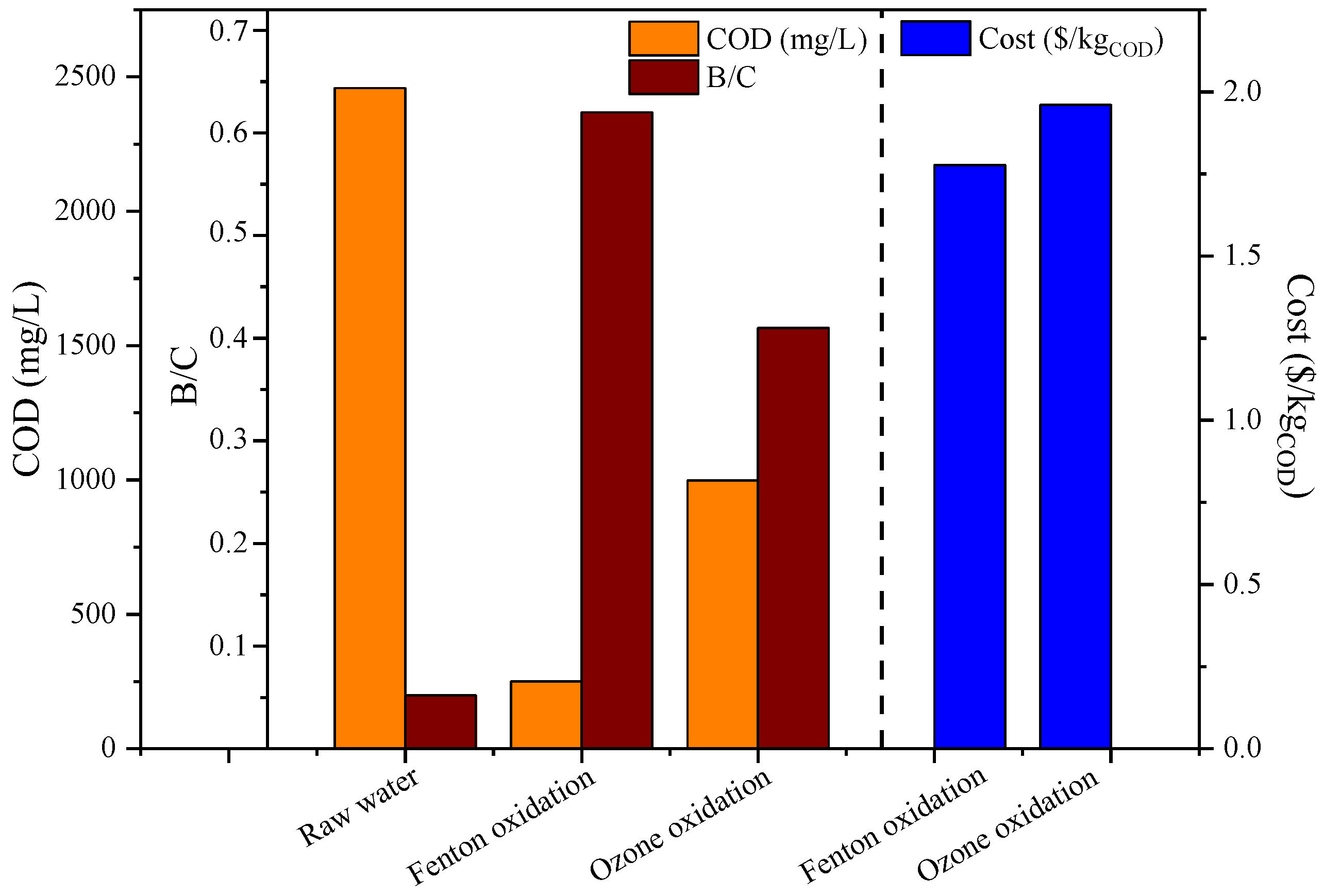
3.4. Treatment Costs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, T.; Banerjee, S.; Saha, A.; Bhattacharya, A.; Chanda, C.; Gantayet, L.M.; Bhadury, P.; Chaudhuri, S.R. Bacterial consortium based petrochemical wastewater treatment: From strain isolation to industrial effluent treatment. Environ. Adv. 2021, 7, 100132. [Google Scholar] [CrossRef]
- Hu, J.; Fu, W.; Ni, F.; Zhang, X.; Yang, C.; Sang, J. An integrated process for the advanced treatment of hypersaline petrochemical wastewater: A pilot study. Water Res. 2020, 182, 116019. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Wang, P.; Guo, S. Improving hydrolysis acidification by limited aeration in the pretreatment of petrochemical wastewater. Bioresour. Technol. 2015, 194, 256–262. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Lan, M.-Y.; Wang, F.; Yi, X.-H.; Wang, C.-C. ZIF-67-based catalysts in persulfate advanced oxidation processes (PS-AOPs) for water remediation. J. Environ. Chem. Eng. 2022, 10, 107997. [Google Scholar] [CrossRef]
- Davarnejad, R.; Mohammadi, M.; Ismail, A.F. Petrochemical wastewater treatment by electro-Fenton process using aluminum and iron electrodes: Statistical comparison. J. Water Process Eng. 2014, 3, 18–25. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Van Hulle, S. Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Mohamadi, L.; Bazrafshan, E.; Rahdar, A.; Labuto, G.; Kamali, A.R. Nanostructured MgO-enhanced catalytic ozonation of petrochemical wastewater. Boletín Soc. Española Cerámica Vidr. 2020, 60, 391–400. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment-unexpected nitration side reactions-a serious environmental issue: A review. Chem. Eng. J. 2021, 430, 133002. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, X.; Li, R.; Mei, J.; Rao, T.; Ren, G.; Guo, H.; Wu, Z. In-situ slow production of Fe2+ to motivate electro-Fenton oxidation of bisphenol A in a flow through dual-anode reactor using current distribution strategy: Advantages, CFD and toxicity assessment. Electrochim. Acta 2022, 411, 140059. [Google Scholar] [CrossRef]
- Ma, X.; Rao, T.; Zhao, M.; Jia, Z.; Ren, G.; Liu, J.; Guo, H.; Wu, Z.; Xie, H. A novel induced zero-valent iron electrode for in-situ slow release of Fe2+ to effectively trigger electro-Fenton oxidation under neutral pH condition: Advantages and mechanisms. Sep. Purif. Technol. 2021, 283, 120160. [Google Scholar] [CrossRef]
- Dehboudeh, M.; Dehghan, P.; Azari, A.; Abbasi, M. Experimental investigation of petrochemical industrial wastewater treatment by a combination of integrated fixed-film activated sludge (IFAS) and electro-Fenton methods. J. Environ. Chem. Eng. 2020, 8, 104537. [Google Scholar] [CrossRef]
- Pourehie, O.; Saien, J. Homogeneous solar Fenton and alternative processes in a pilot-scale rotatable reactor for the treatment of petroleum refinery wastewater. Process Saf. Environ. Prot. 2020, 135, 236–243. [Google Scholar] [CrossRef]
- Ahmadi, M.; Kakavandi, B.; Jaafarzadeh, N.; Babaei, A.A. Catalytic ozonation of high saline petrochemical wastewater using PAC@ FeIIFe2IIIO4: Optimization, mechanisms and biodegradability studies. Sep. Purif. Technol. 2017, 177, 293–303. [Google Scholar] [CrossRef]
- Bavasso, I.; Montanaro, D.; Petrucci, E. Ozone-based electrochemical advanced oxidation processes. Curr. Opin. Electrochem. 2022, 34, 101017. [Google Scholar] [CrossRef]
- Lin, C.-K.; Tsai, T.-Y.; Liu, J.-C.; Chen, M.-C. Enhanced biodegradation of petrochemical wastewater using ozonation and bac advanced treatment system. Water Res. 2000, 35, 699–704. [Google Scholar] [CrossRef]
- Ye, H.; Yang, B.; Wang, Q.; How, Z.T.; Nie, C.; Chelme-Ayala, P.; Guo, S.; Chen, C.; El-Din, M.G. Influences of integrated coagulation-ozonation pretreatment on the characteristics of dissolved organic pollutants (DOPs) of heavy oil electric desalting wastewaters. J. Environ. Manag. 2021, 300, 113756. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, M.; Xu, Z.; Zhang, D.; Li, L. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst. Sep. Purif. Technol. 2018, 211, 269–278. [Google Scholar] [CrossRef]
- Gong, C.; Ren, X.; Han, J.; Wu, Y.; Gou, Y.; Zhang, Z.; He, P. Toxicity reduction of reverse osmosis concentrates from petrochemical wastewater by electrocoagulation and Fered-Fenton treatments. Chemosphere 2021, 286, 131582. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, S.; Zhang, Y.; Ren, G.; Pan, Y.; Zhang, Q.; Zhou, M. Simultaneous removal of tetracycline and disinfection by a flow-through electro-peroxone process for reclamation from municipal secondary effluent. J. Hazard. Mater. 2019, 368, 771–777. [Google Scholar] [CrossRef]
- Rao, T.; Ma, X.; Yang, Q.; Cheng, S.; Ren, G.; Wu, Z.; Sirés, I. Upgrading the peroxi-coagulation treatment of complex water matrices using a magnetically assembled mZVI/DSA anode: Insights into the importance of ClO radical. Chemosphere 2022, 303, 134948. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Zhang, Q.; Xu, X.; Li, Y.; Su, P.; Paidar, M.; Bouzek, K. Cost-efficient improvement of coking wastewater biodegradability by multi-stages flow through peroxi-coagulation under low current load. Water Res. 2019, 154, 336–348. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Su, P.; Liang, L.; Yang, W.; Mousset, E. Highly energy-efficient removal of acrylonitrile by peroxi-coagulation with modified graphite felt cathode: Influence factors, possible mechanism. Chem. Eng. J. 2018, 343, 467–476. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Liu, M.; Ma, L.; Yang, H. A novel vertical-flow electro-Fenton reactor for organic wastewater treatment. Chem. Eng. J. 2016, 298, 55–67. [Google Scholar] [CrossRef]
- Pacheco-Álvarez, M.; Benítez, R.P.; Rodríguez-Narváez, O.M.; Brillas, E.; Peralta-Hernández, J.M. A critical review on paracetamol removal from different aqueous matrices by Fenton and Fenton-based processes, and their combined methods. Chemosphere 2022, 303, 134883. [Google Scholar] [CrossRef]
- Sun, M.; Zou, L.; Wang, P.; Fan, X.; Pan, Z.; Liu, Y.; Song, C. Nano valent zero iron (NZVI) immobilized CNTs hollow fiber membrane for flow-through heterogeneous Fenton process. J. Environ. Chem. Eng. 2022, 10, 107806. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G. Challenges and pitfalls in the investigation of the catalytic ozonation mechanism: A critical review. J. Hazard. Mater. 2022, 436, 129157. [Google Scholar] [CrossRef]
- Yousefi, N.; Pourfadakari, S.; Esmaeili, S.; Babaei, A.A. Mineralization of high saline petrochemical wastewater using Sonoelectro-activated persulfate: Degradation mechanisms and reaction kinetics. Microchem. J. 2019, 147, 1075–1082. [Google Scholar] [CrossRef]
- Kakavandi, B.; Ahmadi, M. Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem. 2019, 56, 25–36. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Y.; Li, F. Heterogeneous Fenton system with dual working mechanisms for aqueous pollutants degradation. J. Environ. Chem. Eng. 2022, 10, 107686. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Zheng, H.; Zheng, Y.; Jing, T.; Ma, J.; Nan, J.; Leong, Y.K.; Chang, J.-S. Advanced oxidation process based on hydroxyl and sulfate radicals to degrade refractory organic pollutants in landfill leachate. Chemosphere 2022, 297, 134214. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; Amu-Darko, J.N.-O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants―A review. Chemosphere 2021, 289, 133208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, J.; Wang, X.; Wang, J.; Mo, S.; Fu, M.; Chen, L.; Ye, D. Ozone-enhanced deep catalytic oxidation of toluene over a platinum-ceria-supported BEA zeolite catalyst. Mol. Catal. 2018, 460, 7–15. [Google Scholar] [CrossRef]
- Jin, X.; Wu, C.; Fu, L.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. J. Environ. Sci. 2022, 124, 330–349. [Google Scholar] [CrossRef]
- Pérez, J.S.; Arzate, S.; Soriano-Molina, P.; Sánchez, J.G.; López, J.C.; Plaza-Bolaños, P. Neutral or acidic pH for the removal of contaminants of emerging concern in wastewater by solar photo-Fenton? A techno-economic assessment of continuous raceway pond reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]

| Wastewater | pH | COD (mg/L) | BOD5 (mg/L) | B/C |
|---|---|---|---|---|
| Petrochemical wastewater | 6.26 | 2458.75 | 127.86 | 0.052 |
| No. | Compound | Molecular Structure | Elemental Composition | tR (min) | [M + H] + m/z | Wastewater | Fenton Oxidation | Ozone Oxidation |
|---|---|---|---|---|---|---|---|---|
| A | Ethanethioamide,N,N-dimethyl- |  | C4H9NS | 4.173 | 103.19 | √ | √ | |
| B | Acetamide,N-(aminocarbony)- |  | C3H6N2O2 | 4.485 | 102.09 | √ | ||
| C | Acetic acid glacial | 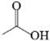 | C2H4O2 | 4.666 | 60.05 | √ | √ | |
| D | Ammonium acetate | 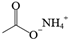 | C2H7NO2 | 4.804 | 77.08 | √ | ||
| E | 2-Amino-4,5,6-methylbenzoic acid | 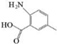 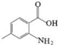  | C8H9NO2 | 5.378 | 151.16 | √ | √ | √ |
| F | Guanidine,N,N’-dimethyl- | 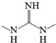 | C3H9N3 | 6.532 | 87.12 | √ | ||
| G | Stearic acid | 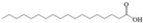 | C18H36O2 | 9.213 | 284.48 | √ | √ | √ |
| H | Palmitic acid |  | C16H32O2 | 14.13 | 256.42 | √ | √ | √ |
| I | tripropyleneglycol methyl ether |  | C10H22O4 | 17.70 | 206.28 | √ | ||
| J | Stearic acid, 2-(2-hydroxyethoxy)ethyl ester |  | C22H44O4 | 18.30 | 372.58 | √ | √ | √ |
| K | Hexanamide | 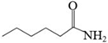 | C6H13NO | 19.92 | 115.17 | √ | √ | |
| L | Di-tert-butylphenol | 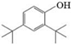 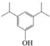 | C14H22O | 20.63 | 206.32 | √ | √ | √ |
| M | 3,3-Dimethyl-1-(2-carboxyphenyl)triazene | 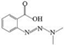 | C9H11N3O2 | 21.12 | 193.2025 | √ | √ | √ |
| N | 2-Methyl-2-Pentanol | 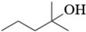 | C6H14O | 21.91 | 102.17 | √ | √ | √ |
| O | Myristic-1-13C Acid |  | C13(13C)H28O2 | 25.85 | 229.36 | √ | √ | |
| P | 2-Acetyl-2-thiazoline |  | C5H7NOS | 25.85 | 129.18 | √ | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Ran, X.; Ren, G.; Wei, Z.; Wang, Z.; Rao, T.; Li, R.; Ma, X. Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism. Separations 2022, 9, 179. https://doi.org/10.3390/separations9070179
Cheng S, Ran X, Ren G, Wei Z, Wang Z, Rao T, Li R, Ma X. Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism. Separations. 2022; 9(7):179. https://doi.org/10.3390/separations9070179
Chicago/Turabian StyleCheng, Siyu, Xiaomeng Ran, Gengbo Ren, Zizhang Wei, Zhimin Wang, Tiantong Rao, Ruixuan Li, and Xiaodong Ma. 2022. "Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism" Separations 9, no. 7: 179. https://doi.org/10.3390/separations9070179
APA StyleCheng, S., Ran, X., Ren, G., Wei, Z., Wang, Z., Rao, T., Li, R., & Ma, X. (2022). Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism. Separations, 9(7), 179. https://doi.org/10.3390/separations9070179






