Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Canola Meal
2.2. Preparation of Canola Meal Extracts (CMEs)
2.3. Total Phenolic Contents (TPC)
2.4. High-Performance Liquid Chromatography-Diode Array Detection with Online ABTS (2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic Acid) Diammonium Salt) Scavenging (HPLC-DAD-Online ABTS)
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS) for Identification of Canola Meal Extracts (CMEs)
2.6. ABTS Assay Radical Scavenging Activity
2.7. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. Antidiabetic Assay
2.9. Quantitative Analysis of Major Compounds from Canola Meal Extracts (CMEs)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Canola Extracts (CMEs) and Total Phenolic Content (TPC)
3.2. Characterisation of Phenolic Composition and Free Radical Scavenging Activity of Individual Compounds
3.3. Antioxidant Properties
3.4. Antidiabetic Activity
3.5. Recovery of Sinapine, Feruloyl Choline, Kaempherol-Sinapoyl-Trihexoside, Sinapic Acid, and Feruloyl Choline (4-O-8) Guaiacyl Disinapoyl
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obied, H.K.; Song, Y.; Foley, S.; Loughlin, M.; Rehman, A.U.; Mailer, R.; Masud, T.; Agboola, S. Biophenols and antioxidant properties of Australian canola meal. J. Agric. Food Chem. 2013, 61, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Taşpınar, H.; Elik, A.; Kaya, S.; Altunay, N. Optimization of green and rapid analytical procedure for the extraction of patulin in fruit juice and dried fruit samples by air-assisted natural deep eutectic solvent-based solidified homogeneous liquid phase microextraction using experimental design and computational chemistry approach. Food Chem. 2021, 358, 129817. [Google Scholar] [PubMed]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Centner, C.; Gollhofer, A.; König, D. Effects of Dietary Strategies on Exercise-Induced Oxidative Stress: A Narrative Review of Human Studies. Antioxidants 2021, 10, 542. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.A.; das Graças Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Its Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-interacting protein as a novel potential therapeutic target in diabetes mellitus and its underlying complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginter, E.; Simko, V. Global prevalence and future of diabetes mellitus. In Diabetes; Springer: Berlin, Germany, 2013; pp. 35–41. [Google Scholar]
- Weber, C.; Hung, J.; Hickling, S.; Li, I.; Murray, K.; Briffa, T. Changing age-specific trends in incidence, comorbidities and mortality of hospitalised heart failure in Western Australia between 2001 and 2016. Int. J. Cardiol. 2021, 343, 56–62. [Google Scholar] [CrossRef]
- Aynalem, S.B.; Zeleke, A.J. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: A cross sectional study. Int. J. Endocrinol. 2018, 2018, 9317987. [Google Scholar] [CrossRef] [PubMed]
- Clapham, J.C. Sixty years of drug discovery for type 2 diabetes: Where are we now? Type 2 Diabetes 2020, 2076, 1–30. [Google Scholar]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, K.J.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Jenkins, D.J.A.; Taylor, C.G.; Zahradka, P.; et al. Diets Enriched with Conventional or High-Oleic Acid Canola Oils Lower Atherogenic Lipids and Lipoproteins Compared to a Diet with a Western Fatty Acid Profile in Adults with Central Adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef]
- Kanikowska, D.; Kanikowska, A.; Rutkowski, R.; Wlochal, M.; Orzechowska, Z.; Juchacz, A.; Zawada, A.; Grzymislawski, M.; Roszak, M.; Sato, M.; et al. Amaranth (Amaranthus cruentus L.) and canola (Brassica napus L.) oil impact on the oxidative metabolism of neutrophils in the obese patients. Pharm. Biol. 2019, 57, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Rehman, A.U.; Luckett, D.J.; Blanchard, C.L.; Obied, H.K.; Strappe, P. Phenolic Compounds with Antioxidant Properties from Canola Meal Extracts Inhibit Adipogenesis. Int. J. Mol. Sci. 2019, 21, 1. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley: New York, NY, USA, 2018; Volume 11, pp. 77–106. [Google Scholar]
- Merola, N.; Alonso, F.J.G.; Ros, G.; Castón, M.J.P. Antioxidant activity comparison between [6S]-5-methyltetrahydrofolic acid calcium salt and the related racemate form. Food Chem. 2013, 136, 984–988. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Dipeptidyl peptidase (DPP)-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of Different Extraction Solvents on Bioactive Compounds and Antioxidant Capacity from the Root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef] [Green Version]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy potential of different genotypes of walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [Green Version]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namiesnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography–diode array–electrospray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar] [CrossRef]
- Lelario, F.; Bianco, G.; Bufo, S.A.; Cataldi, T.R. Establishing the occurrence of major and minor glucosinolates in Brassicaceae by LC-ESI-hybrid linear ion-trap and Fourier-transform ion cyclotron resonance mass spectrometry. Phytochemistry 2012, 73, 74–83. [Google Scholar] [CrossRef]
- Lee, I.; Boyce, M.C. Extraction and Purification of Glucoraphanin by Preparative High-Performance Liquid Chromatography (HPLC). J. Chem. Educ. 2011, 88, 832–834. [Google Scholar] [CrossRef]
- Velasco, P.; Francisco, M.; Moreno, D.A.; Ferreres, F.; Garcia-Viguera, C.; Cartea, M.E. Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem. Anal. 2010, 22, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiyam-Hollander, U.; Aladedunye, F.; Logan, A.; Yang, H.; Diehl, B.W. Identification and quantification of canolol and related sinapate precursors in Indian mustard oils and Canadian mustard products. Eur. J. Lipid Sci. Technol. 2014, 116, 1664–1674. [Google Scholar] [CrossRef]
- Yang, S.-C.; Arasu, M.V.; Chun, J.-H.; Jang, Y.-S.; Lee, Y.-H.; Kim, I.H.; Lee, K.-T.; Hong, S.-T.; Kim, S.-J. Identification and Determination of Phenolic Compounds in Rapeseed Meals (Brassica napus L.). J. Agric. Chem. Environ. 2015, 4, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Reichelt, M.; Hidalgo, W.; Agnolet, S.; Schneider, B. Tissue-specific distribution of secondary metabolites in rapeseed (Brassica napus L.). PLoS ONE 2012, 7, e48006. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K. Biography of biophenols: Past, present and future. Funct. Foods Health Dis. 2013, 3, 230–241. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Dwiecki, K.; Kachlicki, P.; Nogala-Kalucka, M. Identification and antioxidant activity of sinapic acid derivatives in Brassica napus L. seed meal extracts. Eur. J. Lipid Sci. Technol. 2013, 115, 1130–1138. [Google Scholar] [CrossRef]
- Yates, K.; Pohl, F.; Busch, M.; Mozer, A.; Watters, L.; Shiryaev, A.; Kong Thoo Lin, P. Determination of sinapine in rapeseed pomace extract: Its antioxidant and acetylcholinesterase inhibition properties. Food Chem. 2019, 276, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Martinović, N.; Poklar Ulrih, N.; Abramovič, H. Sinapic acid and its derivatives increase oxidative stability in different model lipid systems. Eur. J. Lipid Sci. Technol. 2019, 121, 1800326. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ata-ur-rehman, D.L.; Blanchard, C.L. Can canola meal be used to treat cancer? New South Wales 2014, 89–209. Available online: https://www.researchgate.net/publication/282664401_Can_canola_meal_be_used_to_treat_cancer (accessed on 15 January 2022).
- Turdu, G.; Gao, H.; Jiang, Y.; Kabas, M. Plant dipeptidyl peptidase-IV inhibitors as antidiabetic agents: A brief review. Future Med. Chem. 2018, 10, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid. Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef] [Green Version]
- Zardo, I.; Rodrigues, N.P.; Sarkis, J.R.; Marczak, L.D. Extraction and identification by mass spectrometry of phenolic compounds from canola seed cake. J. Sci. Food Agric. 2020, 100, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Girish, V.; Keulen, D.; Wijngaard, H.; Lauteslager, X.; Ferreira, G.; Ottens, M. Recovery of sinapic acid from canola/rapeseed meal extracts by adsorption. Food Bioprod. Processing 2020, 120, 69–79. [Google Scholar] [CrossRef]
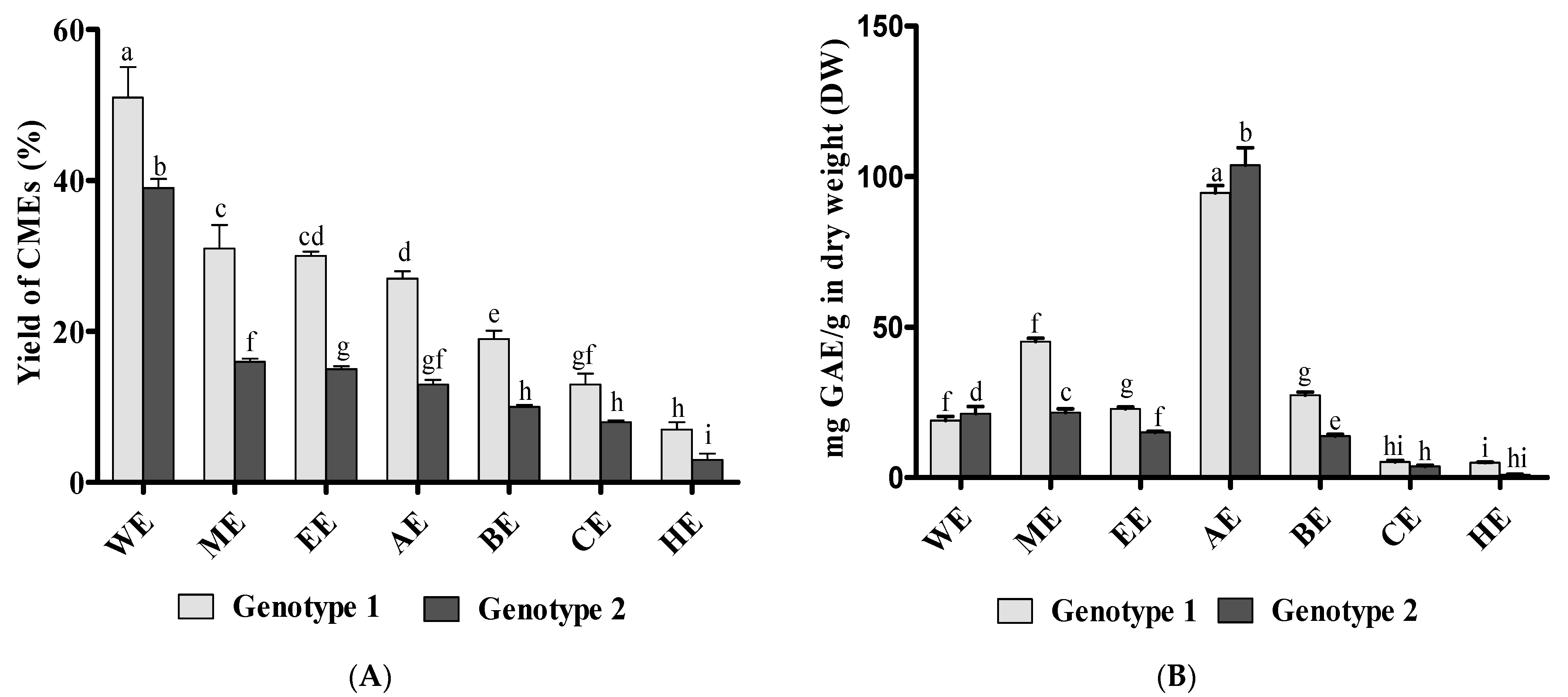
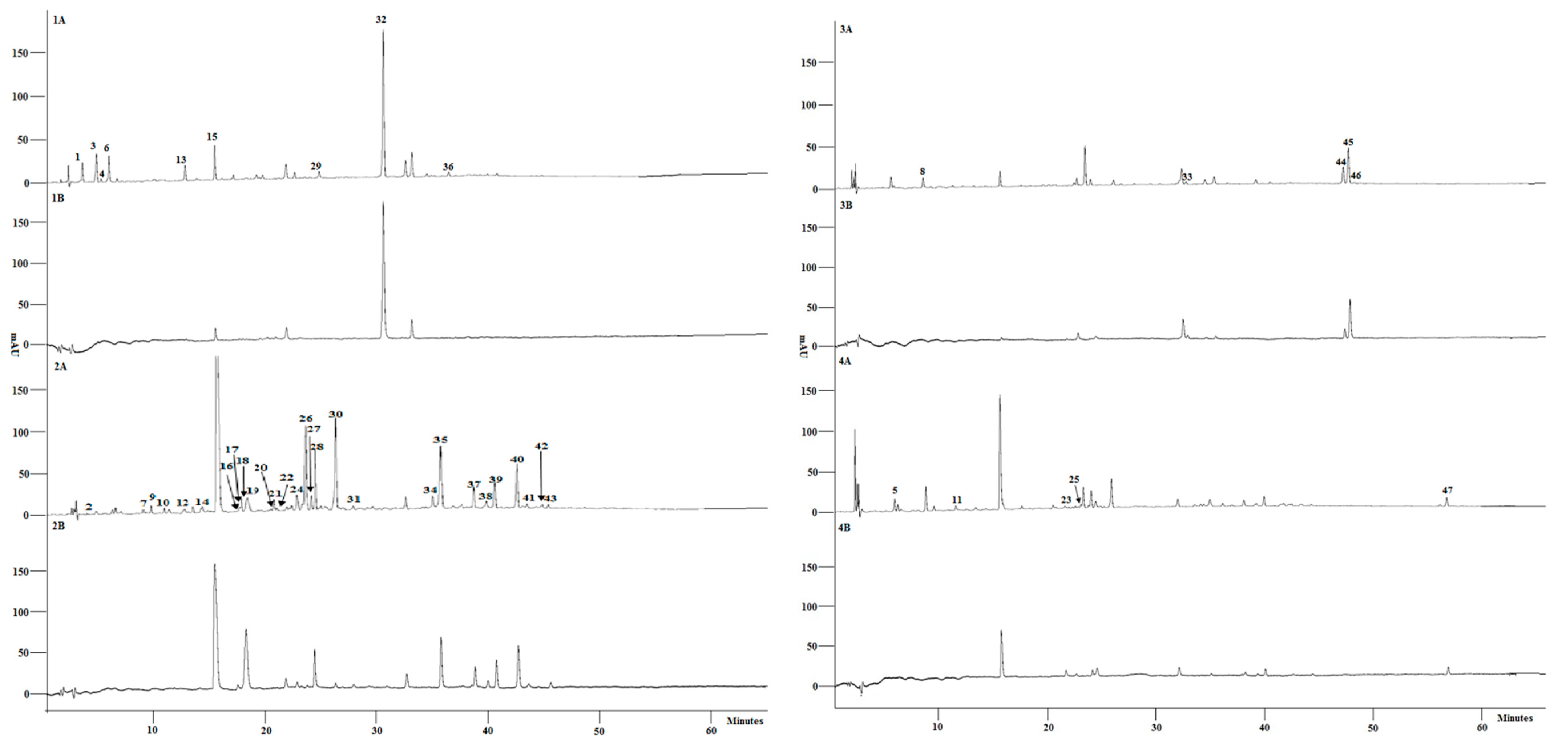
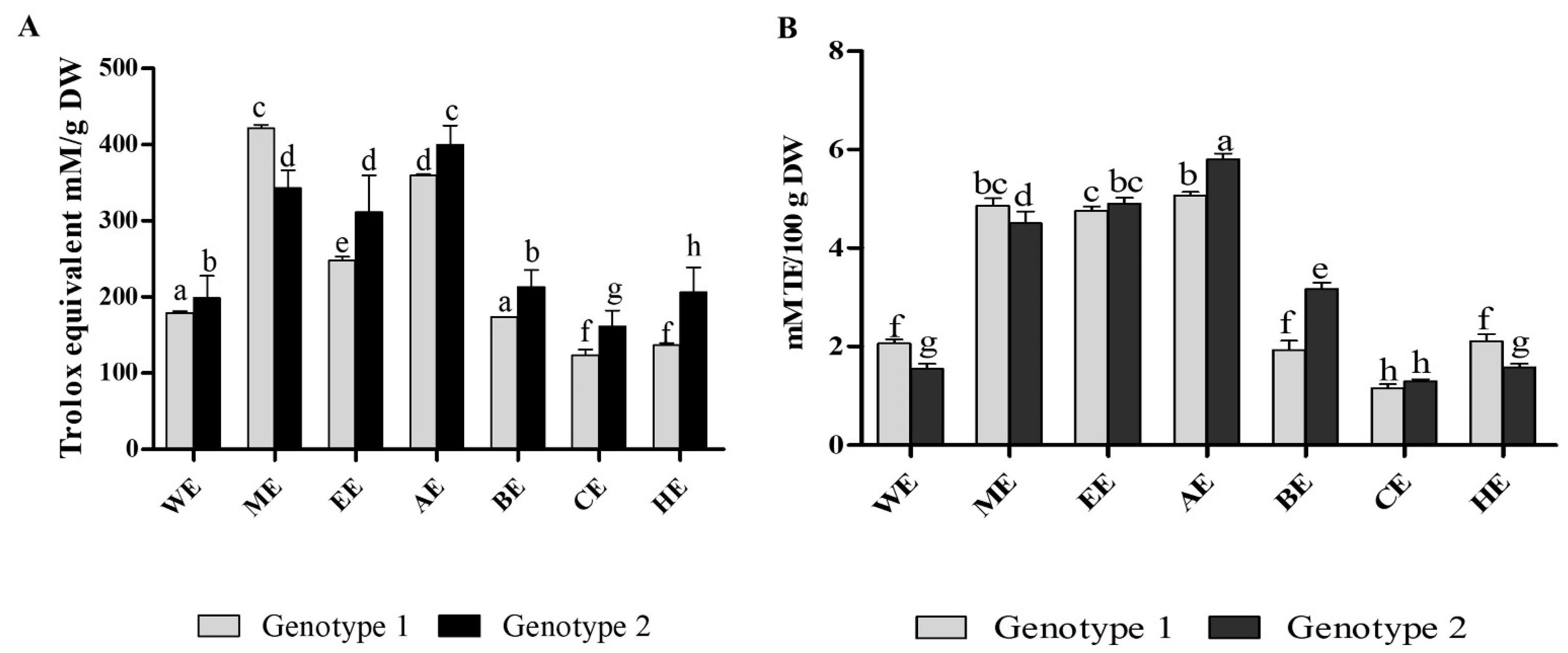
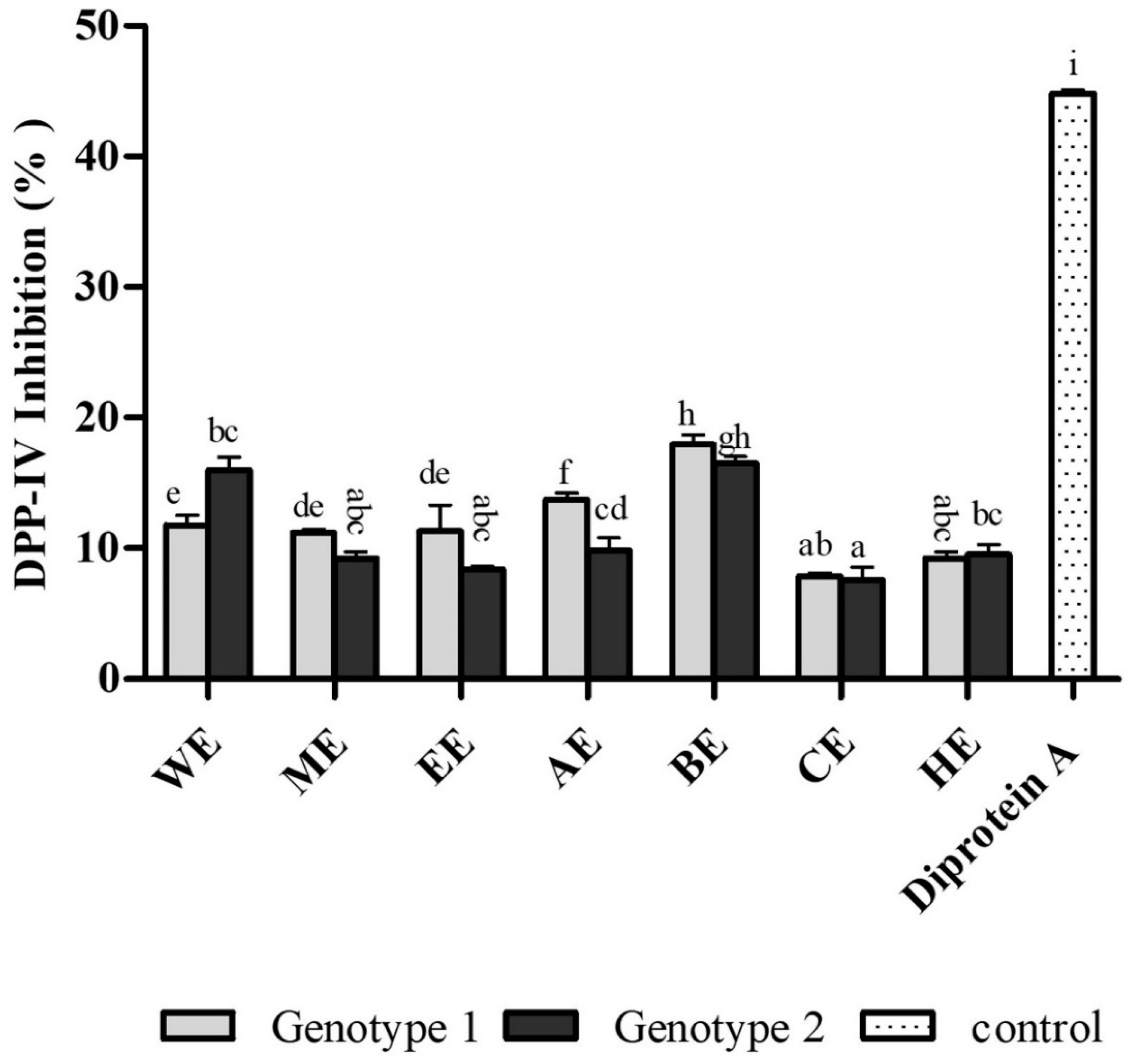
| P | Identity | GN | HE | CE | BE | AE | EE | ME | WE | RT | λmax | ABTS | ESI+ | ESI− | MW | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | GN2 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓✓✓ | 4.1 | 258 | − | 124 | 122 | 123 | − |
| 2 | Unknown | GN1 | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | 5.0 | 275 | − | 613 | 611 | 612 | − |
| 3 | Unknown | GN2 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓✓✓ | 5.5 | 249 | − | 137 | 135 | 136 | − |
| 4 | Unknown | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | 6.0 | 262 | − | 268 | 243 | 244 | − |
| 5 | Unknown | Both | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓ | 6.2 | 256 | − | 233 | 231 | 232 | − |
| 6 | Progoitrin | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | 6.7 | 220 | − | NI | 388 | 389 | [30] |
| 7 | Sinigrin | Both | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | 7.8 | 285 | − | NI | 358 | 359 | [30] |
| 8 | Unknown | Both | ✓✓ | ✓✓✓ | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | 8.8 | 252, 270s | − | 277 | NI | 276 | − |
| 9 | Glucoalyssin | Both | ✗ | ✗ | ✗ | ✓✓ | ✓✓ | ✓✓✓ | ✗ | 9.5 | 225 | − | 452 | 450 | 451 | [31] |
| 10 | Glucoraphanin | Both | ✗ | ✗ | ✗ | ✓✓ | ✓ | ✓ | ✓ | 10.5 | 230 | − | NI | 435 | 436 | [32] |
| 11 | Gluconapoleiferin | Both | ✗ | ✗ | ✗ | ✓✓ | ✓✓ | ✓✓ | ✓ | 11.1 | 275 | − | NI | 402 | 403 | [33] |
| 12 | Gluconapin | Both | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | 11.3 | 220 | − | NI | 372 | 373 | [1] |
| 13 | Unknown | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | 13.7 | 278 | − | 451 | 449 | 450 | − |
| 14 | Unknown | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓✓ | 13.7 | 288 | − | NI | 315 | 316 | − |
| 15 | Sinapine | Both | ✓ | ✓ | ✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓ | 15.7 | 237, 328 | ++ | 310 | 294, 663 | 310 | [34] |
| 16 | Caffeoyl dihexoside | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 17.2 | 297s, 327 | ++ | NI | 503 | 504 | [1] |
| 17 | 4-hydroxyglucobrassicin | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 17.6 | 292 | − | NI | 463 | 464 | [31] |
| 18 | Feruloyl choline (4-0-8’) guiacyl | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 17.7 | 270b, 325b | +++ | 476 | NI | 476 | [35] |
| 19 | Feruloyl choline guiacyl isomer | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | 18.3 | 270b, 325b | ++ | 476 | NI | 476 | [35] |
| 20 | 4’-glucosylsinapic acid | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓✓ | 20.6 | 317b | − | NI | 385 | 386 | [1] |
| 21 | Sinapoyl dihexoside | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓✓ | 19.0 | 328.54 | + | NI | 547 | 548 | [1] |
| 22 | Cyclic spermidin derivative | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | 20.8 | 316 | − | 496 | 494 | 495 | [36] |
| 23 | Cyclic spermidin derivative | Both | ✓ | ✓ | ✓✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | 21.7 | 320 | − | 496 | 494 | 495 | [36] |
| 24 | Sinapoyl hexoside | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 22.7 | 330 | ++ | NI | 385 | 386 | [37] |
| 25 | Sinapoyl hexoside isomer | GN1 | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 23.1 | 330 | ++ | NI | 385 | 386 | [37] |
| 26 | Unknown | GN1 | ✓ | ✓✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | 23.5 | 305b | − | 533 | 531 | 532 | − |
| 27 | Unknown | Both | ✗ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 23.6 | 310b | − | 449 | 447 | 448 | − |
| 28 | Feruloyl choline (5-8’) guaiacyl | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 24.4 | 328, 280s | ++ | 457 | 458 | 459 | [35] |
| 29 | Unknown | Both | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓✓✓ | 24.6 | 260s, 280s, 290s | ++ | 429 | 427 | 428 | − |
| 30 | Kaempherol-sinapoyl-trihexoside | Both | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 26.3 | 268, 333 | + | 979 | 978 | 977 | [38] |
| 31 | Gluconasturtiin | Both | ✗ | ✗ | ✗ | ✓ | ✓ | ✓✓ | ✗ | 29.4 | 230 | − | NI | 422 | 423 | [31] |
| 32 | Trans-Sinapic acid | Both | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | 32.6 | 324 | ++ | NI | 223 | 224 | [1] |
| 33 | Kaempherol 3-dihexoside-7-sinapoyl-hexoside | Both | ✓ | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 33.0 | 266, 323 | ++ | 979 | 978 | 977 | [37] |
| 34 | Cis-sinapic acid | Both | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓✓ | 35.3 | 266, 323 | ++ | NI | 223 | 224 | [37] |
| 35 | Feruloyl choline (4-O-8’) guaiacyl-di-sinapoyl | GN1 | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 36.1 | 323 | ++ | 682 | NI | 682 | [35] |
| 36 | Unknown (methyl sinapate dihexoside) | Both | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓✓ | 36.4 | 329 | ++ | NI | 561 | 562 | − |
| 37 | Disinapoyl dihexoside | Both | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 38.6 | 230, 330 | ++ | NI | 753 | 754 | [1] |
| 38 | Trisinapoyl dihexoside 1 | Both | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 39.7 | 327 | ++ | NI | 959 | 960 | [1] |
| 39 | Disinapoyl hexoside | Both | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓ | 40.7 | 330 | ++ | NI | 591 | 592 | [1] |
| 40 | Tetrasinapoyl dihexoside | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓✓ | ✓ | 41.9 | 326 | ++ | NI | 1183 | 1184 | [37] | |
| 41 | Methyl sinapate | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓✓ | ✓ | 42.5 | 325 | ++ | NI | 237 | 238 | [1] |
| 42 | Unknown | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓ | 43.1 | 325 | + | NI | 545 | 546 | [36] | |
| 43 | Disinapoyl hexoside isomer | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | 45 | 326 | + | NI | 591 | 592 | [1] |
| 44 | Unknown | Both | ✗ | ✓✓✓ | ✗ | ✗ | ✗ | ✗ | ✗ | 47.2 | 320 | ++ | 275 | NI | 274 | − |
| 45 | Unknown | GN1 | ✓ | ✓✓✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 47.6 | 325 | ++ | 454 | NI | 453 | − |
| 46 | Unknown | Both | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | 48.7 | 325 | + | 245 | NI | 244 | − |
| 47 | Unknown | Both | ✓✓ | ✓ | ✓✓✓ | ✗ | ✗ | ✗ | ✗ | 58.2 | 327 | + | 303 | 279 | 280 | − |
| Recovery mg SAE/g DW | |||||
|---|---|---|---|---|---|
| Extract (Sample) | Sinapine | Feruloyl Choline | Kaempherol-Sinapoyl-Trihexoside | Sinapic Acid * | Feruloyl Choline (4-O-8) Guaiacyl di Sinapoyl |
| WE (GN-1) | 2.2 ± 0.0 a | traces | 0.6 ± 0.0 a | 1.5 ± 0.0 a | 0.6 ± 0.0 a |
| WE (GN-2) | 2.2 ± 0.1 a | traces | 0.6 ± 0.0 a | 1.7 ± 0.0 a,d | 0.7 ± 0.0 a |
| ME (GN-1) | 7.0 ± 0.1 b | 0.8 ± 0.0 a | 1.4 ± 0.0 b | 0.2 ± 0.0 b | 1.0 ± 0.0 a |
| ME (GN-2) | 12.1 ± 0.2 c | 1.1 ± 0.0 a | 2.2 ± 0.0 c | 0.9 ± 0.0 c | 1.4 ± 0.0 b |
| EE (GN-1) | 7.7 ± 0.0 b | 0.8 ± 0.0 a | 1.5 ± 0.0 b | 0.2 ± 0.0 b | 1.3 ± 0.0 b |
| EE (GN-2) | 10.1 ± 0.0 d | 0.7 ± 0.0 a | 1.7 ± 0.1 b | 1.0 ± 0.0 c | 0.9 ± 0.0 a |
| AE (GN-1) | 13.0 ± 0.3 c | 1.6 ± 0.0 b | 2.4 ± 0.0 c | 0.3 ± 0.0 b | 0.6 ± 0.1 a |
| AE (GN-2) | 12.6 ± 0.3 c | 1.2 ± 0.0 a | 2.2 ± 0.0 c | 1.0 ± 0.0 c | 1.4 ± 0.0 b |
| BE(GN-1) | 5.7 ± 0.0 e | 0.7 ± 0.0 a | 1.3 ± 0.1 b | 0.4 ± 0.0 b | 0.4 ± 0.0 c |
| BE (GN-2) | 5.9 ± 0.1 e | 0.7 ± 0.0 a | 1.6 ± 0.0 b | 0.8 ± 0.0 c | 0.6 ± 0.0 a |
| CE (GN-1) | 0.3 ± 0.0 f | traces | 0.2 ± 0.0 a | 0.7 ± 0.0 c | 0.2 ± 0.0 c |
| CE (GN-2) | 2.0 ± 0.0 a | traces | 0.6 ± 0.0 a | 1.8 ± 0.0 a | 0.6 ± 0.0 a |
| HE (GN-1) | 0.2 ± 0.0 f | traces | 0.2 ± 0.1 b | 0.7 ± 0.0 c | 0.3 ± 0.0 c |
| HE (GN-2) | 0.5 ± 0.0 f | traces | 0.4 ± 0.0 a | 2.0 ± 0.0 d | 0.6 ± 0.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Rehman, A.U.; Obied, H.K.; Luckett, D.J.; Blanchard, C.L. Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal. Separations 2022, 9, 38. https://doi.org/10.3390/separations9020038
Hussain S, Rehman AU, Obied HK, Luckett DJ, Blanchard CL. Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal. Separations. 2022; 9(2):38. https://doi.org/10.3390/separations9020038
Chicago/Turabian StyleHussain, Saira, Ata Ur Rehman, Hassan K. Obied, David J. Luckett, and Christopher L. Blanchard. 2022. "Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal" Separations 9, no. 2: 38. https://doi.org/10.3390/separations9020038
APA StyleHussain, S., Rehman, A. U., Obied, H. K., Luckett, D. J., & Blanchard, C. L. (2022). Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal. Separations, 9(2), 38. https://doi.org/10.3390/separations9020038






