GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oils from Two Viola Species Belonging to the V. calcarata Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Plant Material
2.2. Distillation of Essential Oils
2.3. SPME Sampling
2.4. GC-MS Analisys of EOs
2.5. DPPH Test
2.6. ABTS Test
2.7. Phytotoxicity Test
2.8. Statistical Analysis
3. Results
3.1. EO Chemical Composition
3.2. EO Antiradical Activity
3.3. EO Phytotoxic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butnariu, M.; Sarac, I. Essential oils from plants. Int. J. Biotechnol. Biomed. Sci. 2018, 1, 35–43. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Ecol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Sharif-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharif-Rad, M.; Valussi, M.; Tindis, R.; Sharif-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef]
- Azirak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. B Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Grul’ová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogal’ová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Variability of the essential oil of Viola etrusca. Ann. Bot. 2003, 91, 493–497. [Google Scholar] [CrossRef][Green Version]
- Hammami, I.; Kamoun, N.; Rebai, A. Biocontrol of Botrytis cinerea with essential oil and methanol extract of Viola odorata L. flowers. Arch. Appl. Sci. Res. 2011, 3, 44–51. [Google Scholar]
- Muhammad, N.; Saeed, M.; Awan, A.A.; Khan, H. Ethnomedicinal, phytochemical and pharmacological profile of genus Viola. Phytopharmacology 2012, 3, 214–226. [Google Scholar]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Pandey, H.K. Chemical composition of the essential oil of Viola serpens from Bageshwar (Shama), Uttarakhad, India. J. Med. Plant Res. 2017, 11, 513–517. [Google Scholar]
- Kirillov, V.; Stikhareva, T.; Atazhanova, G.; Vasiliv, A.M. Composition of essential oil of the aerial parts of Viola canina L. growing wild in Northern Kazakhistan. Nat. Prod. Res. 2019, 35, 2285–2288. [Google Scholar] [CrossRef]
- Yousuf, S.; Yousuf, T.; Bachheti, R.K.; Deshmukh, C. Reducing potential of essential oil extracted from Viola patrinii for some powerful oxidants like DPPH, FeCl3 and nitric oxide. Ann. Rom. Soc. Cell. Biol. 2021, 25, 20562–20571. [Google Scholar]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Preliminary observations on bioactive compounds: Antioxidant activity and phytochemical profile of two alpine subspecies. Agronomy 2021, 11, 2241. [Google Scholar] [CrossRef]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Khetwal, K.S.; Devrani, M.K.; Pandey, H.K. phytochemical and ethnomedicinal uses of family Violaceae. Curr. Res. Chem. 2015, 7, 44–52. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valntine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 3rd ed.; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Garzoli, S.; Laghezza Masci, V.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and Vapor Phase of Four Conifer-Derived Essential Oils: Comparison of Chemical Compositions and Antimicrobial and Antioxidant Properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitalini, S.; Apostolides, N.A.; El Beyrouthy, M. Chemical composition and antiradical capacity of essential oils from Lebanese medicinal plants. J. Ess. Oil Res. 2014, 26, 466–472. [Google Scholar] [CrossRef]
- Vitalini, S.; Orlando, F.; Iriti, M. Selective phytotoxic activity of eugenol towards monocot and dicot target species. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Al-Mudaris, M. Notes on various parameters recording the speed of seed germination. Der Trop. 1998, 99, 147–154. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973, 1, 630–633. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Analysis of the essential oil of the aerial parts of Viola etrusca from Monte Labbro (South Tuscany, Italy) and in vivo analysis of flower volatiles using SPME. Flavour Fragr. J. 2002, 17, 147–149. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, M.; Kohli, R.K.; Singh, S. Chemical characterization and phytotoxicity of volatile essential oil from leaves of Anisomeles indica (Lamiaceae). Biochem. Syst. Ecol. 2012, 41, 104–109. [Google Scholar] [CrossRef]
- Bi, H.H.; Zeng, R.S.; Su, L.M.; An, M.; Luo, S.M. Rice allelopathy induced by methyl jasmonate and methyl salicylate. J. Chem. Ecol. 2007, 33, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Van Poecke, R.M.P.; Posthumus, M.A.; Dicke, M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene expression analysis. J. Chem. Ecol. 2001, 27, 1911–1928. [Google Scholar] [CrossRef]
- Zhu, J.W.; Park, K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Shulaev, V.; Silverman, P.; Raskin, I. Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 1997, 385, 718–721. [Google Scholar] [CrossRef]
- Koo, Y.J.; Kim, M.A.; Kim, E.H.; Song, J.T.; Jung, C.; Moon, J.K.; Kim, J.H.; Seo, H.S.; Song, J.K.; Lee, J.S.; et al. Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant Mol. Biol. 2007, 64, 1–15. [Google Scholar] [CrossRef]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crops Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Oloyede, G.K. Toxicity, antimicrobial and antioxidant activities of methyl salicylate dominated essential oils of Laportea aestuans (Gaud). Arab. J. Chem. 2016, 9, 840–845. [Google Scholar] [CrossRef]
- Jayasekara, T.K.; Stevenson, P.C.; Hall, D.R.; Belmain, S.R. Effect of volatile constituents from Securidaca Longepedunculata on insect pests of stored grain. J. Chem. Ecol. 2005, 31, 303–313. [Google Scholar] [CrossRef]
- Yun, L.J.; Chen, W.L. SA and ROS are involved in methyl salicylate-induced programmed cell death in Arabidopsis thaliana. Plant Cell Rep. 2011, 30, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Brubacher, J.R.; Hoffman, R.S. Salicylism from topical salicylates review of the literature. J. Toxicol. Clin. Toxicol. 1996, 34, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.C. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis 1987, 39, 442–444. [Google Scholar] [PubMed]
- Baron, J.A.; Raia, J.J.; Huang, Y.C. Evaluation of the effects of multiple-dose activated charcoal on the absorption of orally administered salicylate in a simulated toxic ingestion model. Ann. Emerg. Med. 1988, 17, 34–37. [Google Scholar] [CrossRef]
- Akhbari, M.; Batooli, H.; Kashi, F.J. Composition of essential oil and biological activity of extracts of Viola odorata L. from central Iran. Nat. Prod. Res. Form. Nat. Prod. Lett. 2012, 26, 802–809. [Google Scholar]
- Anca, T.; Philippe, V.; Ilioara, O.; Mircea, T. Composition of essential oils of Viola tricolor and V. arvensis from Romania. Chem. Nat. Comp. 2009, 45, 91–92. [Google Scholar] [CrossRef]
- Carranza, M.S.S.; Oyong, G.G.; Linis, V.C.; Ajero, M.D.M.; Tan, M.C.S. The antioxidant and antiproliferative agents from the bark of Philippine Alstonia scholaris (L.) R. Br. (Apocynaceae). Jordan J. Pharm. Sci. 2020, 13, 207–224. [Google Scholar]
- Moorthy, V.; Boominathan, M. The antimicrobial activities of crude extracts and fraction of Psidium guajava and Azadirachta indica against Staphylococcus aureus in chronic disease affected patients. Int. J. Univers. Pharm. Life Sci. 2011, 1, 2249–6793. [Google Scholar]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) swingle cultivated in Tunisia. Chem. Biodivers. 2014, 11, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.; Abd El-Gawad, A.M.; El-Amier, Y.A.; El Gendy, A.; Al-Rowaily, S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El Gendy, A.E.-N.G.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, 1900278. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.; El Gendy, A.; El-Amier, Y.; Gaara, A.; Omer, S.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef]
- Xuan, T.D.; Chung, I.M.; Khanh, T.D.; Tawata, S. Identification of phytotoxic substances from early growth of barnyard grass (Echinochloa crus-galli) root exudates. J. Chem. Ecol. 2006, 32, 895. [Google Scholar] [CrossRef]
- Mancini, E.; Arnold, N.A.; De Feo, V.; Formisano, C.; Rigano, D.; Piozzi, F.; Senatore, F. Phytotoxic effects of essential oils of Nepeta curviflora Boiss. and Nepeta nuda L. subsp. albiflora growing wild in Lebanon. J. Plant Interact. 2009, 4, 253–259. [Google Scholar] [CrossRef]
- Cruz-Estrada, A.; Ruiz-Sánchez, E.; Cristóbal-Alejo, J.; González-Coloma, A.; Fe Andrés, M.; Gamboa-Angulo, M. Medium-chain fatty acids from Eugenia winzerlingii leaves causing insect settling deterrent, nematicidal, and phytotoxic effects. Molecules 2019, 24, 1724. [Google Scholar] [CrossRef]
- Synowiec, A.; Kaemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Razavi, S.M. Chemical and allelopathic analyses of essential oils of Prangos pabularia Lindl. from Iran. Nat. Prod. Res. 2011, 26, 2148–2151. [Google Scholar] [PubMed]
- Abd-ELGawad, A.M.; Al-Rowaily, S.L.; Assaeed, A.M.; EI-Amier, Y.A.; El Gendy, A.E.-N.G.; Omer, E.; Al-Dosari, D.H.; Bonanomi, G.; Kassem, H.S.; Elshamy, A.I. Comparative chemical profiles and phytotoxic activity of essential oils of two ecospecies of Pulicaria undulata (L.) C.A.Mey. Plants 2021, 10, 2366. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Mancuso, R.; Lupini, A.; Giofrè, S.V.; Sunseri, F.; Gabriele, B.; Abenavoli, M.R. Phytotoxic potential and biological activity of three synthetic coumarin derivatives as new natural-like herbicides. Molecules 2015, 20, 17883–17902. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.R.; Dayan, F.E.; Duke, S.O. Phytotoxicity and volatile constituents from leaves of Callicarpa japonica Thunb. Phytochemistry 2002, 61, 37–40. [Google Scholar] [CrossRef]
- Çakmak, Y.S.; Aktumsek, A.; Duran, A. Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L. EXCLI J. 2012, 11, 178–187. [Google Scholar]
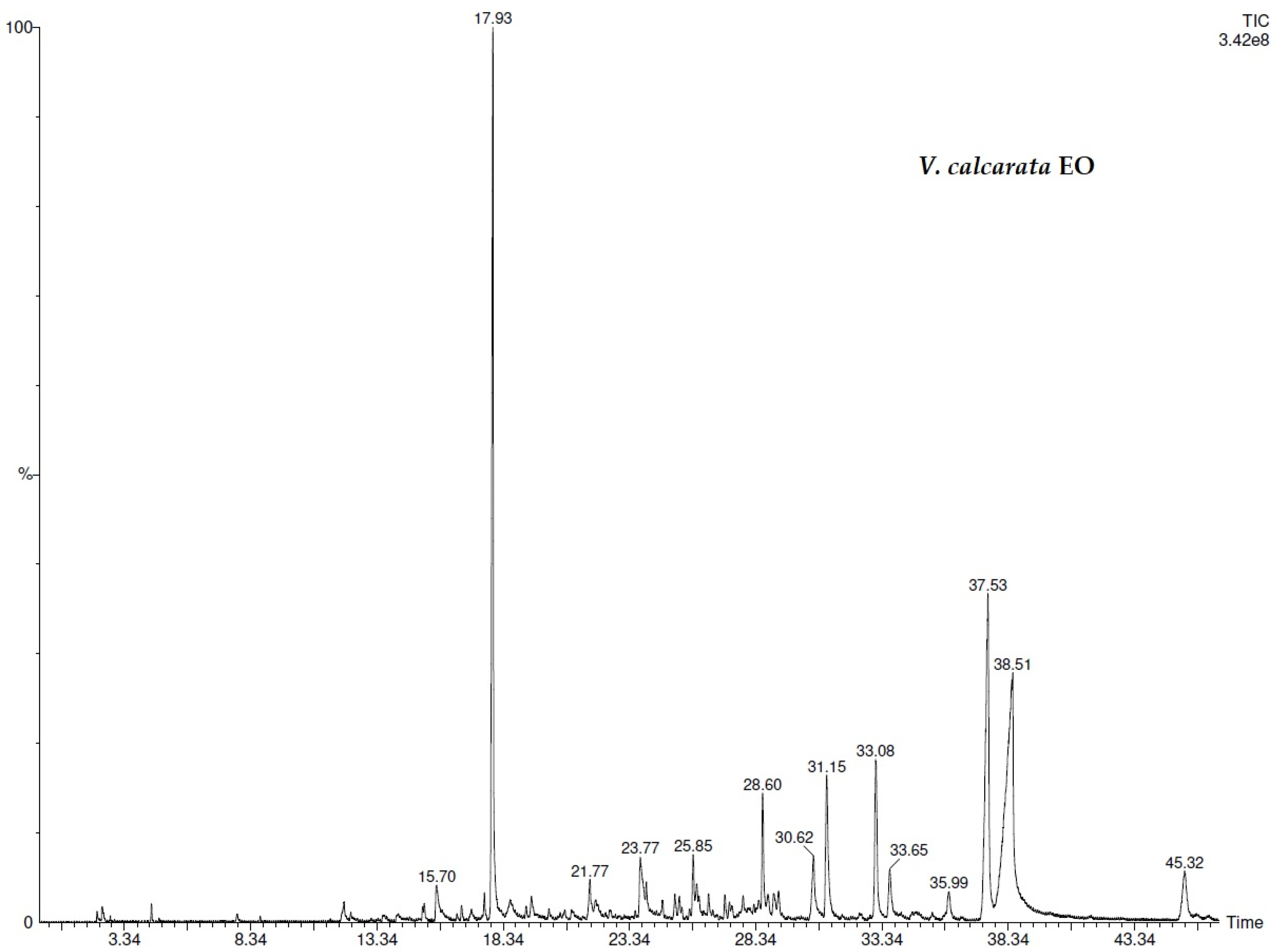
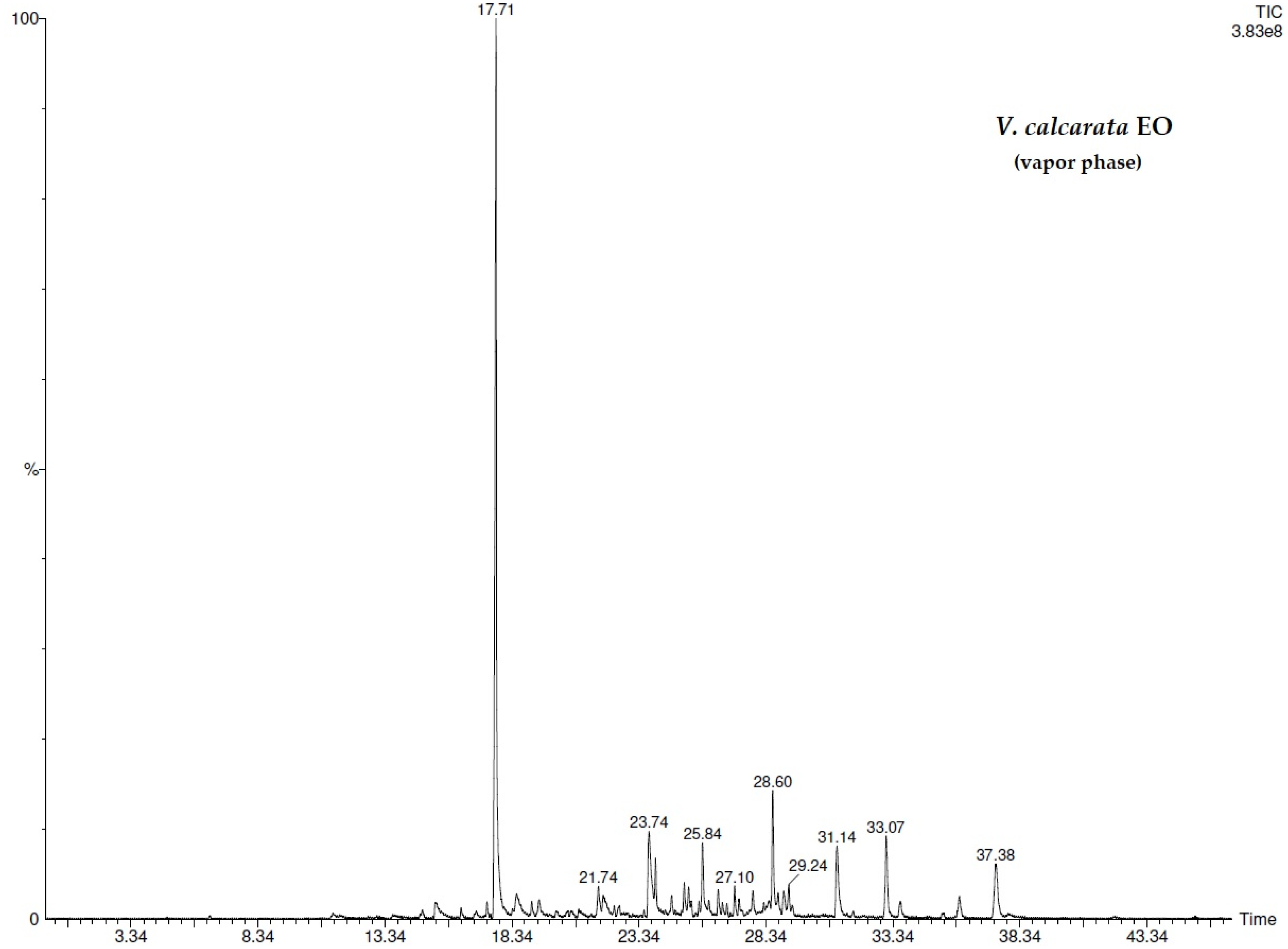
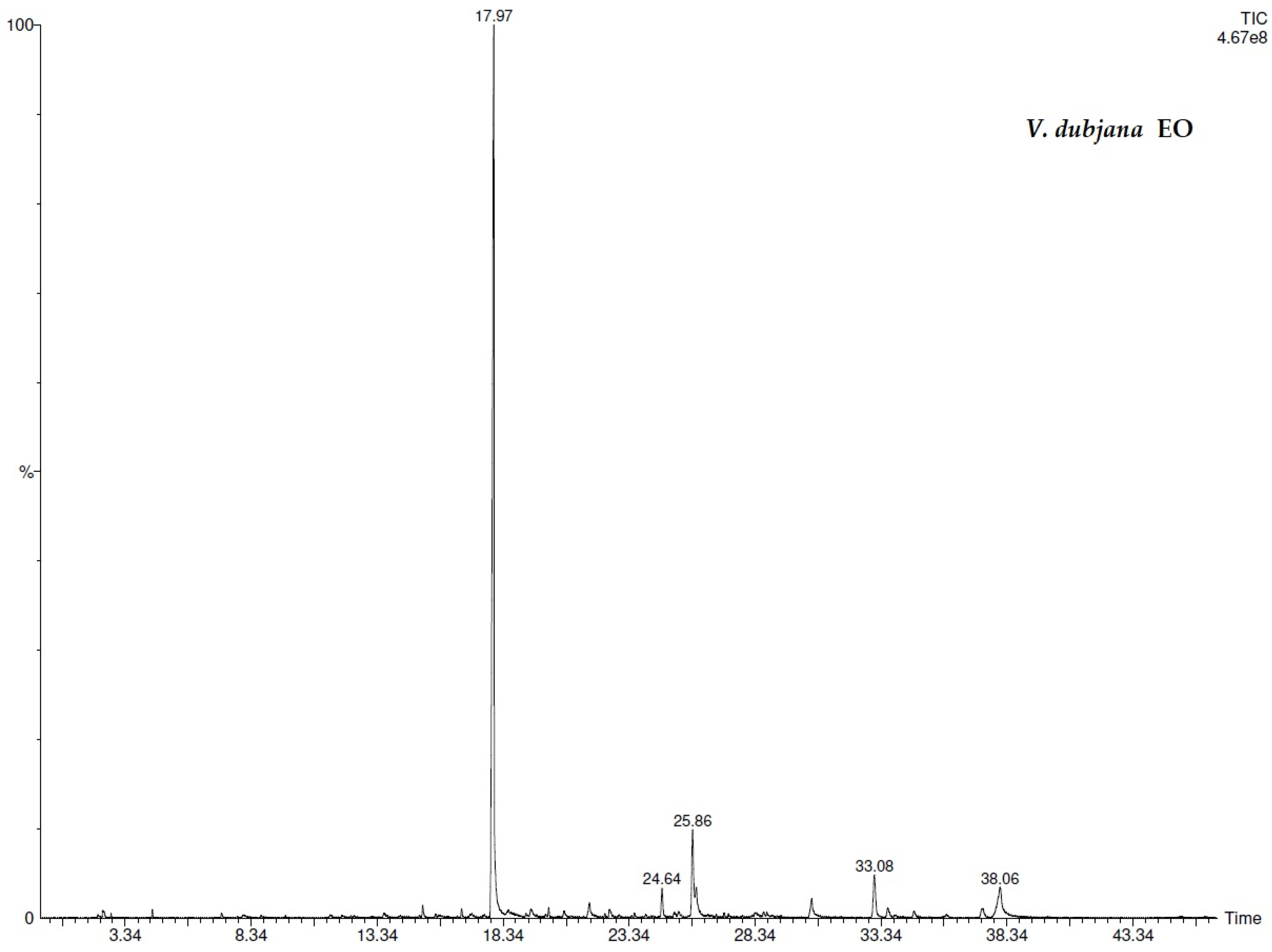
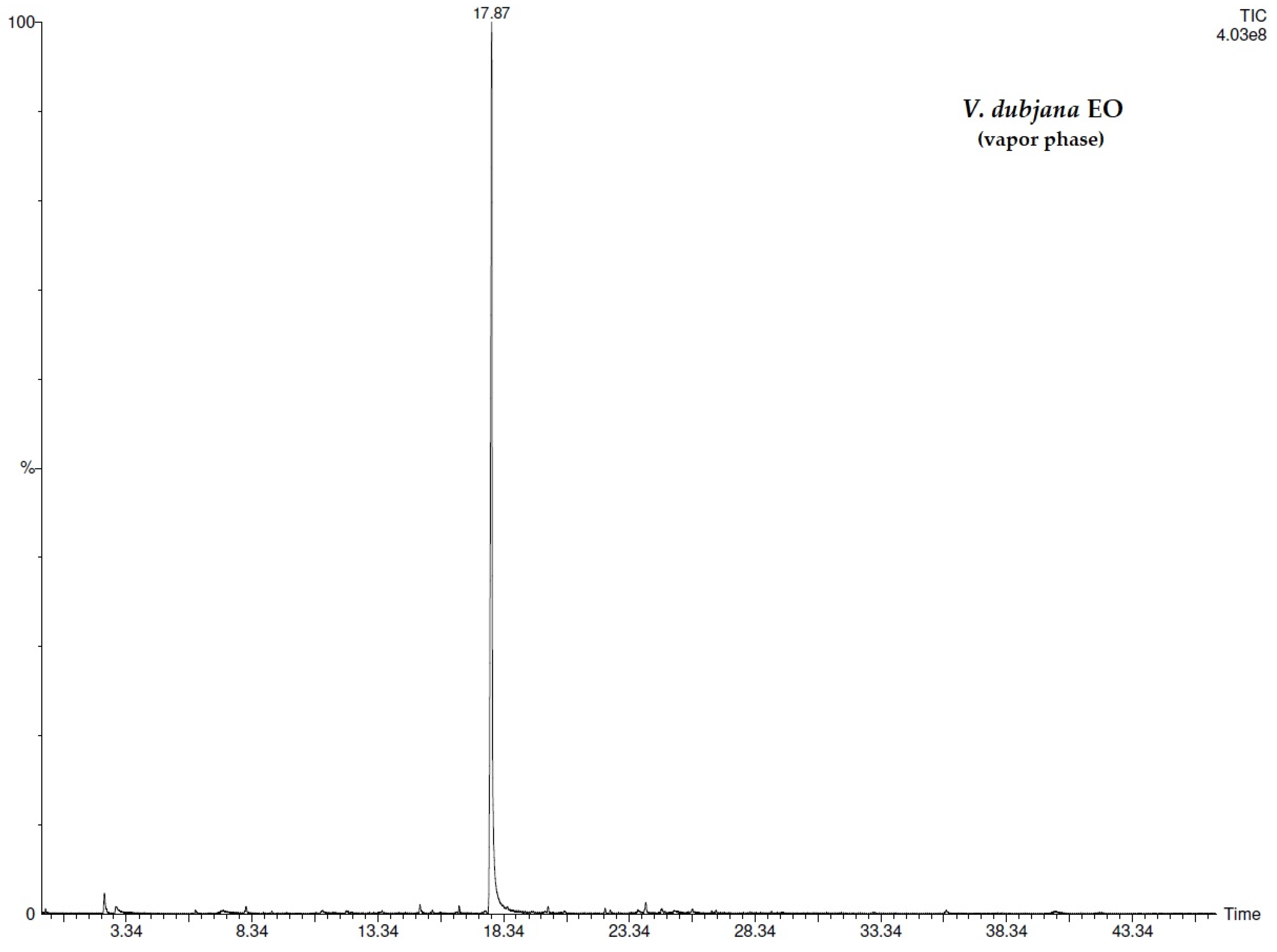
| N° | COMPONENT 1 | LRI 2 | V.d.3 (%) | V.d.4 (%) | V.c.5 (%) | V.c.6 (%) |
|---|---|---|---|---|---|---|
| 1 | hexanal | 770 | 0.3 ± 0.02 | - | - | - |
| 2 | furfural | 832 | 0.3 ± 0.02 | - | - | - |
| 3 | heptanal | 874 | 0.1 ± 0.01 | - | - | - |
| 4 | hexanoic acid | 976 | - | - | tr | 0.3 ± 0.02 |
| 5 | octanal | 1005 | 0.1 ± 0.00 | - | - | - |
| 6 | linalol | 1078 | - | - | 0.5 ± 0.02 | 0.7 ± 0.02 |
| 7 | nonanal | 1091 | 0.6 ± 0.02 | 0.1 ± 0.02 | - | 0.2 ± 0.02 |
| 8 | camphor | 1157 | 0.4 ± 0.01 | 0.2 ± 0.01 | 0.5 ± 0.03 | 0.4 ± 0.01 |
| 9 | trans-sabinene hydrate | 1162 | 0.2 ± 0.01 | - | - | - |
| 10 | octanoic acid | 1172 | 0.2 ± 0.01 | 0.5 ± 0.02 | 0.3 ± 0.01 | 0.6 ± 0.01 |
| 11 | terpinen-4-ol | 1182 | - | - | 0.7 ± 0.02 | 1.1 ± 0.02 |
| 12 | β-cyclocitral | 1200 | 0.8 ± 0.02 | - | - | - |
| 13 | methyl salicylate | 1218 | 67.3 ± 0.02 | 68.0 ± 0.02 | 45.5 ± 0.02 | 46.3 ± 0.02 |
| 14 | coumaran | 1225 | - | - | 2.9 ± 0.02 | 0.2 ± 0.02 |
| 15 | nonanoic acid | 1263 | - | 0.9 ± 0.01 | 1.2 ± 0.01 | 1.4 ± 0.02 |
| 16 | p-vinylguaiacol | 1279 | 0.6 ± 0.02 | 0.5 ± 0.02 | tr | 0.4 ± 0.02 |
| 17 | bornyl acetate | 1280 | 0.4 ± 0.01 | 0.3 ± 0.02 | - | - |
| 18 | decanoic acid | 1349 | 1.4 ± 0.02 | 2.6 ± 0.02 | 1.6 ± 0.02 | 2.8 ± 0.01 |
| 19 | 2-(4-methoxyphenyl)-ethanol | 1360 | - | - | 1.6 ± 0.02 | 2.0 ± 0.02 |
| 20 | β-copaen-4α-ol | 1400 | 0.7 ± 0.02 | tr | - | - |
| 21 | coumarin | 1430 | - | 6.2 ± 0.02 | 5.8 ± 0.02 | |
| 22 | trans-geranyl acetone | 1437 | 0.2 ± 0.02 | 0.3 ± 0.02 | - | - |
| 23 | trans-β-ionone | 1463 | 1.8 ± 0.00 | 2.2 ± 0.02 | - | - |
| 24 | sesquicineole | 1500 | - | - | 0.4 ± 0.02 | 0.4 ± 0.02 |
| 25 | 2(4H)-benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 1530 | 6.6 ± 0.01 | 12.8 ± 0.01 | 4.2 ± 0.03 | 3.0 ± 0.02 |
| 26 | nerolidol | 1537 | - | - | 0.7 ± 0.01 | 0.8 ± 0.02 |
| 27 | dodecanoic acid | 1555 | 3.2 ± 0.02 | 1.4 ± 0.02 | - | - |
| 28 | spathulenol | 1572 | 0.2 ± 0.02 | 0.5 ± 0.02 | 1.1 ± 0.02 | 1.1 ± 0.02 |
| 29 | caryophyllene oxide | 1578 | - | - | 0.7 ± 0.01 | 0.6 ± 0.04 |
| 30 | humulene epoxide II | 1598 | - | - | 1.3 ± 0.02 | - |
| 31 | δ-cadinol | 1621 | 0.3 ± 0.02 | 1.0 ± 0.02 | - | - |
| 32 | bisabolol oxide B | 1660 | - | - | 6.0 ± 0.03 | 5.9 ± 0.02 |
| 33 | β-eudesmol | 1668 | - | - | 1.3 ± 0.02 | 1.3 ± 0.02 |
| 34 | α-cadinol | 1672 | 0.3 ± 0.02 | 0.4 ± 0.02 | - | - |
| 35 | α-bisabolol | 1681 | - | - | 1.2 ± 0.02 | 1.6 ± 0.02 |
| 36 | bisabolone oxide A | 1700 | - | - | 1.2 ± 0.01 | 1.3 ± 0.02 |
| 37 | bisabolol oxide A | 1710 | - | - | 5.8 ± 0.02 | 5.7 ± 0.03 |
| 38 | tetradecanoic acid | 1755 | 1.5 ± 0.01 | 1.1 ± 0.01 | - | - |
| 39 | hexahydrofarnesyl acetone | 1844 | 4.2 ± 0.02 | 2.9 ± 0.02 | 5.4 ± 0.04 | 4.7 ± 0.02 |
| 40 | diisobutyl phthalate | 1868 | 0.9 ± 0.02 | 0.7 ± 0.02 | 1.1 ± 0.03 | 2.2 ± 0.04 |
| 41 | dibutyl phthalate | 1911 | 1.3 ± 0.02 | 0.2 ± 0.02 | 2.5 ± 0.01 | 3.3 ± 0.02 |
| 42 | hexadecanoic acid | 1975 | 6.1 ± 0.01 | 3.0 ± 0.01 | 1.6 ± 0.03 | 1.9 ± 0.04 |
| 43 | epimanool | 2061 | - | - | 2.2 ± 0.02 | 4.0 ± 0.02 |
| SUM | 100.0 | 99.6 | 97.7 | 100.0 | ||
| Terpenoids | 8.2 | 13.3 | 5.9 | 5.2 | ||
| Sesquiterpenoids | 5.7 | 4.8 | 10.8 | 10.5 | ||
| Fatty acids | 12.4 | 9.5 | 4.7 | 7.0 | ||
| Other | 73.7 | 72.0 | 76.3 | 77.3 |
| EO | ABTS (μM Trolox eq/mL) | DPPH (μM Trolox eq/mL) |
|---|---|---|
| Viola calcarata | 1.03 ± 0.01 | 0.29 ± 0.01 |
| Viola dubyana | 1.32 ± 0.03 | 0.37 ± 0.01 |
| Viola calcarata | |||||||
|---|---|---|---|---|---|---|---|
| Target Species | EO Doses (μL) | G (%) | CVG | MGT | SVI | Root (mm) | Shoot (mm) |
| Lolium multiflorum | 2 | 92.0 ± 9.0 | 101.0 ± 8.0 | 4.8 ± 0.3 | 10,332 ± 954 | 62.3 ± 8.9 | 50.0 ± 5.3 |
| 20 | 90.0 ± 7.0 | 99.0 ± 5.0 | 4.8 ± 0.2 | 10,368 ± 686 | 64.1 ± 22.0 | 51.1 ± 7.23 | |
| 50 | 80.0 ± 8.0 | 79.0 ± 4.0 | 5.0 ± 0.2 | 6240 ± 555 | 34.7 ± 7.4 | 43.3 ± 12.4 | |
| 100 | 73.0 ± 5.0 | 42.0 ± 6.0 | 5.5 ± 0.3 | 1022 ± 286 | 5.1 ± 1.4 | 8.9 ± 1.9 | |
| CTRL | 97.0 ± 5.0 | 105.0 ± 9.0 | 4.8 ± 0.1 | 10,796 ± 731 | 61.4 ± 7.9 | 49.9 ± 4.0 | |
| Sinapis alba | 2 | 83.0 ± 5.0 | 115.0 ± 6.0 | 4.2 ± 0.2 | 4001 ± 301 | 26.1 ± 7.7 | 22.1 ± 5.5 |
| 20 | 80.0 ± 8.0 | 108.0 ± 7.6 | 4.2 ± 0.3 | 3200 ± 143 | 21.7 ± 9.2 | 18.3 ± 4.0 | |
| 50 | 53.0 ± 5.0 | 60.0 ± 7.3 | 4.3 ± 0.3 | 912 ± 69 | 7.3 ± 3.9 | 9.9 ± 2.2 | |
| 100 | 47.0 ± 3.0 | 48.0 ± 3.3 | 4.5 ± 0.0 | 785 ± 98 | 7.3 ± 2.5 | 9.4 ± 1.4 | |
| CTRL | 87.0 ± 5.0 | 119.0 ± 9.8 | 4.1 ± 0.1 | 4359 ± 353 | 27.8 ± 3.4 | 22.3 ± 6.8 | |
| Viola dubyana | |||||||
|---|---|---|---|---|---|---|---|
| Target Species | EO Doses (μL) | G (%) | CVG | MGT | SVI | Root (mm) | Shoot (mm) |
| Lolium multiflorum | 2 | 90.0 ± 14.0 | 104.5 ± 9.3 | 4.9 ± 0.2 | 11,601 ± 877 | 74.9 ± 11.2 | 54.0 ± 9.4 |
| 20 | 83.0 ± 9.0 | 101.0 ± 15.0 | 4.9 ± 0.1 | 10,234 ± 941 | 69.9 ± 12.8 | 53.4 ± 8.3 | |
| 50 | 80.0 ± 5.0 | 74.0 ± 2.8 | 5.2 ± 0.1 | 6320 ± 458 | 38.0 ± 6.6 | 41.0 ± 9.8 | |
| 100 | 80.0 ± 0.0 | 47.0 ± 4.5 | 5.9 ± 0.0 | 1192 ± 237 | 7.8 ± 2.6 | 7.1 ± 1.7 | |
| CTRL | 100.0 ± 5.0 | 108.0 ± 4.0 | 4.8 ± 0.1 | 13,100 ± 687 | 76.3 ± 10.6 | 54.7 ± 11.2 | |
| Sinapis alba | 2 | 83.0 ± 8.0 | 102.0 ± 9.0 | 4.2 ± 0.1 | 4116 ± 301 | 29.4 ± 6.7 | 20.2 ± 4.5 |
| 20 | 57.0 ± 5.0 | 63.0 ± 2.0 | 4.2 ± 0.0 | 1533 ± 143 | 12.8 ± 4.5 | 14.1 ± 2.6 | |
| 50 | 20.0 ± 5.0 | 20.0 ± 6.0 | 4.1 ± 0.1 | 258 ± 69 | 5.1 ± 1.1 | 7.8 ± 1.5 | |
| 100 | 0.0 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CTRL | 87.0 ± 5.0 | 112.0 ± 11.0 | 4.2 ± 0.1 | 4829 ± 353 | 34.3 ± 8.5 | 21.2 ± 5.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitalini, S.; Iriti, M.; Garzoli, S. GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oils from Two Viola Species Belonging to the V. calcarata Complex. Separations 2022, 9, 39. https://doi.org/10.3390/separations9020039
Vitalini S, Iriti M, Garzoli S. GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oils from Two Viola Species Belonging to the V. calcarata Complex. Separations. 2022; 9(2):39. https://doi.org/10.3390/separations9020039
Chicago/Turabian StyleVitalini, Sara, Marcello Iriti, and Stefania Garzoli. 2022. "GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oils from Two Viola Species Belonging to the V. calcarata Complex" Separations 9, no. 2: 39. https://doi.org/10.3390/separations9020039
APA StyleVitalini, S., Iriti, M., & Garzoli, S. (2022). GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oils from Two Viola Species Belonging to the V. calcarata Complex. Separations, 9(2), 39. https://doi.org/10.3390/separations9020039








