Targeted UHPLC-ESI-MS/MS Analysis of Selected Neurotransmitters, Tryptophan and Its Metabolite Kynurenine in Tau Transgenic Rat Brain Tissue: A Pivotal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Calibration Solutions
2.3. Animals and Sample Collection
2.4. Sample Preparation
2.5. Quality Control (QC) Samples Preparation
2.6. Instrumentation
2.7. Statistical Data Analysis
3. Results and Discussion
3.1. UHPLC-ESI-MS/MS Conditions
3.2. Method Validation
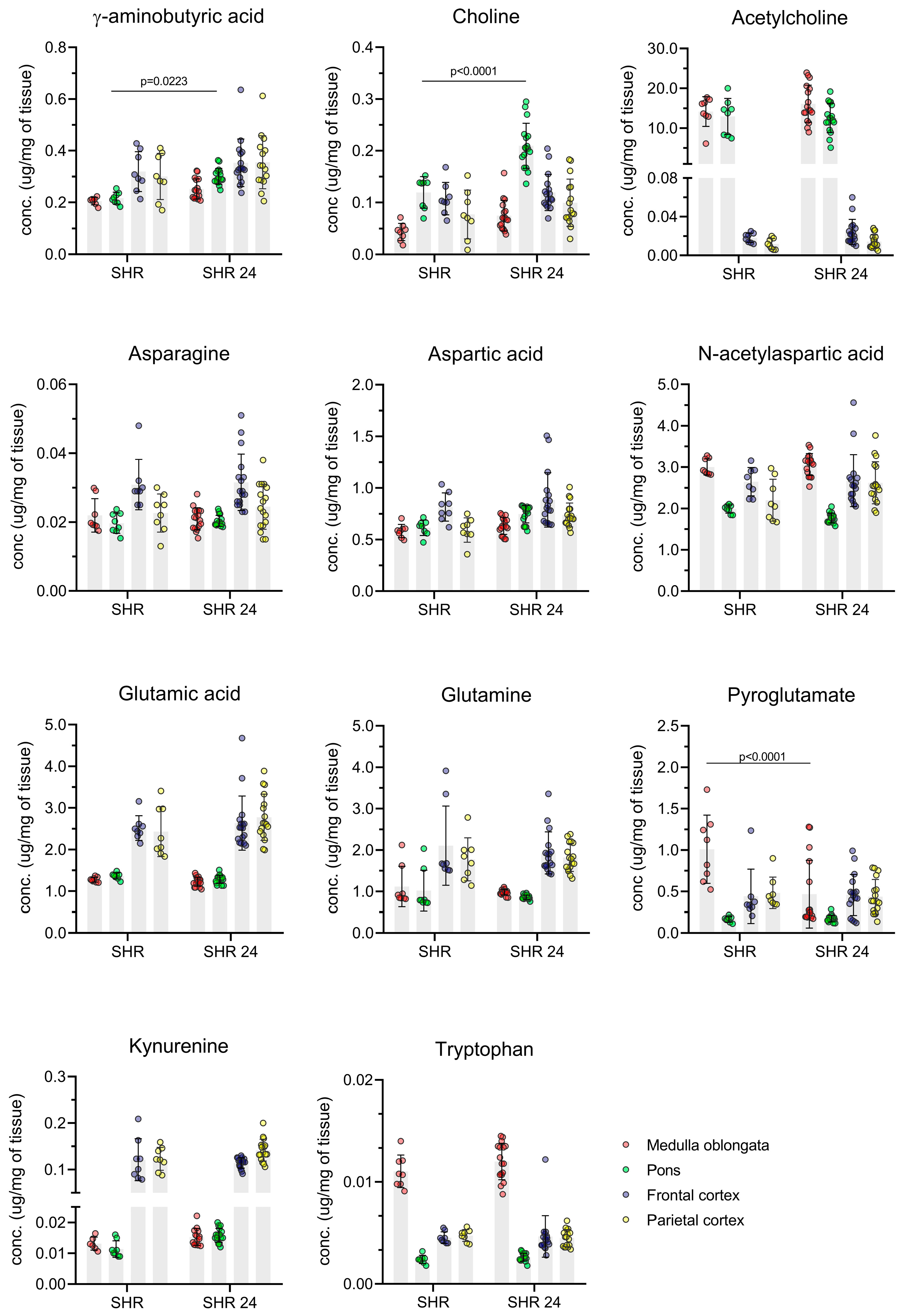
3.3. Method Application–Analysis of Brain Tissue Samples from Transgenic Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Orr, M.E.; Sullivan, A.C.; Frost, B. A brief overview of tauopathy: Causes, consequences, and therapeutic strategies. Trends Pharmacol. Sci. 2017, 38, 637–648. [Google Scholar] [CrossRef]
- Silva, M.C.; Haggarty, S.J. Tauopathies: Deciphering disease mechanisms to develop effective therapies. Int. J. Mol. Sci. 2020, 21, 8948. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Li, Q.-H.; Xu, Z.-D.; Dou, J.-J. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef] [Green Version]
- Frantzi, M.; Latosinska, A.; Kontostathi, G.; Mischak, H. Clinical proteomics: Closing the gap from discovery to implementation. Proteomics 2018, 18, 1700463. [Google Scholar] [CrossRef] [Green Version]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [Green Version]
- Dujardin, S.; Colin, M.; Buée, L. Animal models of tauopathies and their implications for research/translation into the clinic. Neuropathol. Appl. Neurobiol. 2015, 41, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Filipcik, P.; Zilka, N.; Bugos, O.; Kucerak, J.; Koson, P.; Novak, P.; Novak, M. First transgenic rat model developing progressive cortical neurofibrillary tangles. Neurobiol. Aging 2012, 33, 1448–1456. [Google Scholar] [CrossRef]
- Zilka, N.; Filipcik, P.; Koson, P.; Fialova, L.; Zilkova, M.; Skrabana, R.; Rolkova, G.; Kontsekova, E.; Novak, M. Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006, 580, 3582–3588. [Google Scholar] [CrossRef] [Green Version]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelborghs, S.; De Deyn, P.P. The neurochemistry of Alzheimer’s disease. Acta Neurol. Belg. 1997, 97, 67–84. [Google Scholar]
- Hebron, M.L.; Javidnia, M.; Moussa, C.E.-H. Tau clearance improves astrocytic function and brain glutamate-glutamine cycle. J. Neurol. Sci. 2018, 391, 90–99. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; Pletnikova, O.; O’Brien, R.; Yang, A.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Neurotransmitter imbalance in the brain and Alzheimer’s disease pathology. J. Alzheimers Dis. 2019, 72, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandimalla, R.; Reddy, P.H. Therapeutics of NT in Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1049–1069. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer’s disease and depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanari, A.; Amenta, F.; Silvestrelli, G.; Tomassoni, D.; Parnetti, L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech. Ageing Dev. 2006, 127, 158–165. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Torres-Cuevas, I.; Baquero, M.; Ferrer, I.; García, L.; Vento, M.; Cháfer-Pericás, C. Early neurotransmission impairment in non-invasive Alzheimer Disease detection. Sci. Rep. 2020, 10, 16396. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-Z. Cross-talk between oxidative stress and modifications of cholinergic and glutaminergic receptors in the pathogenesis of Alzheimer’s disease. Acta Pharmacol. Sin. 2008, 29, 773–780. [Google Scholar] [CrossRef]
- Yi, L.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic neurotransmission in Alzheimer’s disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar]
- Chen, K.H.; Reese, E.A.; Kim, H.W.; Rapoport, S.I.; Rao, J.S. Disturbed neurotransmitter transporter expression in Alzheimer’s disease brain. J. Alzheimer’s Dis. 2011, 26, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Huey, E.D.; Putnam, K.T.; Grafman, J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006, 66, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Khamis, M.M.; Adamko, D.J.; El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 2017, 36, 115–134. [Google Scholar] [CrossRef]
- de Souza, L.P.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Forgacsova, A.; Galba, J.; Garruto, R.M.; Majerova, P.; Katina, S.; Kovac, A. A novel liquid chromatography/mass spectrometry method for determination of neurotransmitters in brain tissue: Application to human tauopathies. J. Chromatogr. B 2018, 1073, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Fuertig, R.; Ceci, A.; Camus, S.M.; Bezard, E.; Luippold, A.H.; Hengerer, B. LC-MS/MS-based quantification of kynurenine metabolites, tryptophan, monoamines and neopterin in plasma, cerebrospinal fluid and brain. Bioanalysis 2016, 8, 1903–1917. [Google Scholar] [CrossRef] [Green Version]
- Tareke, E.; Bowyer, J.F.; Doerge, D.R. Quantification of rat brain neurotransmitters and metabolites using liquid chromatography/electrospray tandem mass spectrometry and comparison with liquid chromatography/electrochemical detection. Rapid Commun. Mass Spectrom. 2007, 21, 3898–3904. [Google Scholar] [CrossRef]
- Wang, L.-S.; Zhang, M.-D.; Tao, X.; Zhou, Y.-F.; Liu, X.-M.; Pan, R.-L.; Liao, Y.-H.; Chang, Q. LC-MS/MS –based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J. Chromatogr. B 2019, 1112, 24–32. [Google Scholar] [CrossRef] [PubMed]
- González, R.R.; Fernández, R.F.; Vidal, J.L.M.; Frenich, A.G.; Pérez, M.L.G. Development and validation of an ultra-high performance liquid chromatography–tandem mass-spectrometry (UHPLC–MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. J. Neurosci. Methods 2011, 198, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Q.; Bi, K. Rapid HPLC-ESI-MS/MS analysis of neurotransmitters in the brain tissue of Alzheimer’s disease rats before and after oral administration of Xanthoceras sorbifolia bunge. Molecules 2018, 23, 3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, Z.; Zhu, L.; Sui, Z.; Bi, W.; Liu, R.; Bi, K.; Li, Q. Targeted neurotransmitters profiling identifies metabolic signatures in rat brain by LC-MS/MS: Application in insomnia, depression and Alzheimer’s disease. Molecules 2018, 23, 2375. [Google Scholar] [CrossRef] [Green Version]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.W.; Rodger, J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef]
- Han, X.-M.; Qin, Y.-J.; Zhu, Y.; Zhang, X.-L.; Wang, N.-X.; Rang, Y.; Zhai, X.-J.; Lu, Y.-N. Development of an underivatized LC-MS/MS method for quantitation of 14 neurotransmitters in rat hippocampus, plasma and urine: Application to CUMS induced depression rats. J. Pharm. Biomed. Anal. 2019, 174, 683–695. [Google Scholar] [CrossRef]
- Zhao, X.E.; Ruo, Y.R. Simultaneous determination of monoamine and amino acid neurotransmitters in rat endbrain tissues by pre-column derivatization with high-performance liquid chromatographic fluorescence detection and mass spectrometric identification. Talanta 2008, 76, 690–697. [Google Scholar] [CrossRef]
- De Freitas Silva, D.M.; Ferrez, V.P.; Ribeiro, A.M. Improved high-performance liquid chromatographic method for GABA and glutamate determination in regions of the rodent brain. J. Neurosci. Methods 2009, 177, 289–293. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM). Guidance for Industry: Bioanalytical Method Validation; FDA: Rockville, MD, USA, 2018.
- Nowak, P.M.; Kościelniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
- Fayed, N.; Modrego, P.J.; Rojas-Salinas, G.; Aguilar, K. Brain glutamate levels are decreased in Alzheimer’s disease: A magnetic resonance spectroscopy study. Am. J. Alzheimers Dis. Other Dement. 2011, 26, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Lin, H.; Zhang, Q.; Zheng, L.; Zheng, Y.; Yin, X.; Li, Z.; Liang, S.; Huang, S. Meta-analysis of neurochemical changes estimated via magnetic resonance spectroscopy in mild cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 738971. [Google Scholar] [CrossRef]
- Koson, P.; Zilka, N.; Kovac, A.; Kovacech, B.; Korenova, M.; Filipcik, P.; Novak, M. Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur. J. Neurosci. 2008, 28, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zilka, N.; Stozicka, Z.; Kovac, A.; Pilipcinec, E.; Bugos, O.; Novak, M. Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy. J. Neuroimmunol. 2009, 209, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Liu, H.; Leak, R.K.; Hu, X. Neurotransmitter receptors on microglia. Stroke Vacs. Neurol. 2016, 1, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, S.A.; van Landeghem, K.H.; Zacharias, R.; Färber, K.; Rappert, A.; Pavlovic, S.; Hoffmann, A.; Nolte, C.; Kettenmann, H. Microgila express GABAB receptors to modulate interleukin release. Mol. Cell. Neurosci. 2004, 25, 312–322. [Google Scholar] [CrossRef]
- Mead, E.L.; Mosley, M.; Eaton, S.; Dobson, L.; Heales, S.J.; Pocock, J.M. Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial-neuronal interactions. J. Neurochem. 2012, 121, 287–301. [Google Scholar] [CrossRef]
- Axford, J.S.; Howe, F.A.; Heron, C.; Griffiths, J.R. Sensitivity of quantitative (1)H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Ann. Rheum. Dis. 2001, 60, 106–111. [Google Scholar] [CrossRef]
- Emmer, B.J.; van der Bijl, A.E.; Huizinga, T.W.J.; Breedveld, F.C.; Steens, S.C.A.; Bosma, G.P.T.; van Buchem, M.A.; van der Grond, J. Brain involvement in rheumatoid arthritis: A magnetic resonance spectroscopy study. Arthritis Rheum. 2009, 60, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Ichesco, E.; Ratai, E.-M.; Gonzalez, R.G.; Burdo, T.; Loggia, M.L.; Harris, R.E.; Napadow, V. Magnetic resonance imaging of neuroinflammation in chronic pain: A role for astrogliosis? Pain 2020, 161, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Pederzolli, C.D.; Sgaravatti, A.M.; Braum, C.A.; Prestes, C.C.; Zorzi, G.K.; Sgarbi, M.B.; Wyse, A.T.S.; Wannmacher, C.M.D.; Wajner, M.; Dutra-Filho, C.S. 5-oxoproline reduces non-enzymatic antioxidant defenses in vitro in rat brain. Metab. Brain Dis. 2007, 22, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Pederzolli, C.D.; Mescka, C.P.; Zandoná, B.R.; Coelho, D.M.; Sgaravatti, A.M.; Sgarbi, M.B.; Wyse, A.T.S.; Wannmacher, C.M.D.; Wajner, M.; Vargas, C.R.; et al. Acute administration of 5-oxoproline induces oxidative damage to lipids and proteins and impairs antioxidant defense in cerebral cortex and cerebellum of young rats. Metab. Brain Dis. 2010, 25, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kincses, Z.T.; Toldi, J.; Vécsei, L. Kynurenines, neurodegeneration and Alzheimer’s disease. J. Cell. Mol. Med. 2010, 14, 2045–2054. [Google Scholar] [CrossRef] [Green Version]
- Heyes, M.P.; Saito, K.; Major, E.O.; Milsien, S.; Markey, S.P.; Vickers, J.H. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain 1993, 116, 1425–1450. [Google Scholar] [CrossRef] [PubMed]
- Naristin, D.B.; Saito, K.; Markey, S.P.; Chen, C.Y.; Heyes, M.P. Metabolism of L-tryptophan to kynurenate and quinolinate in the central nervous system: Effects of 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. J. Neurochem. 1995, 65, 2217–2226. [Google Scholar]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [Green Version]

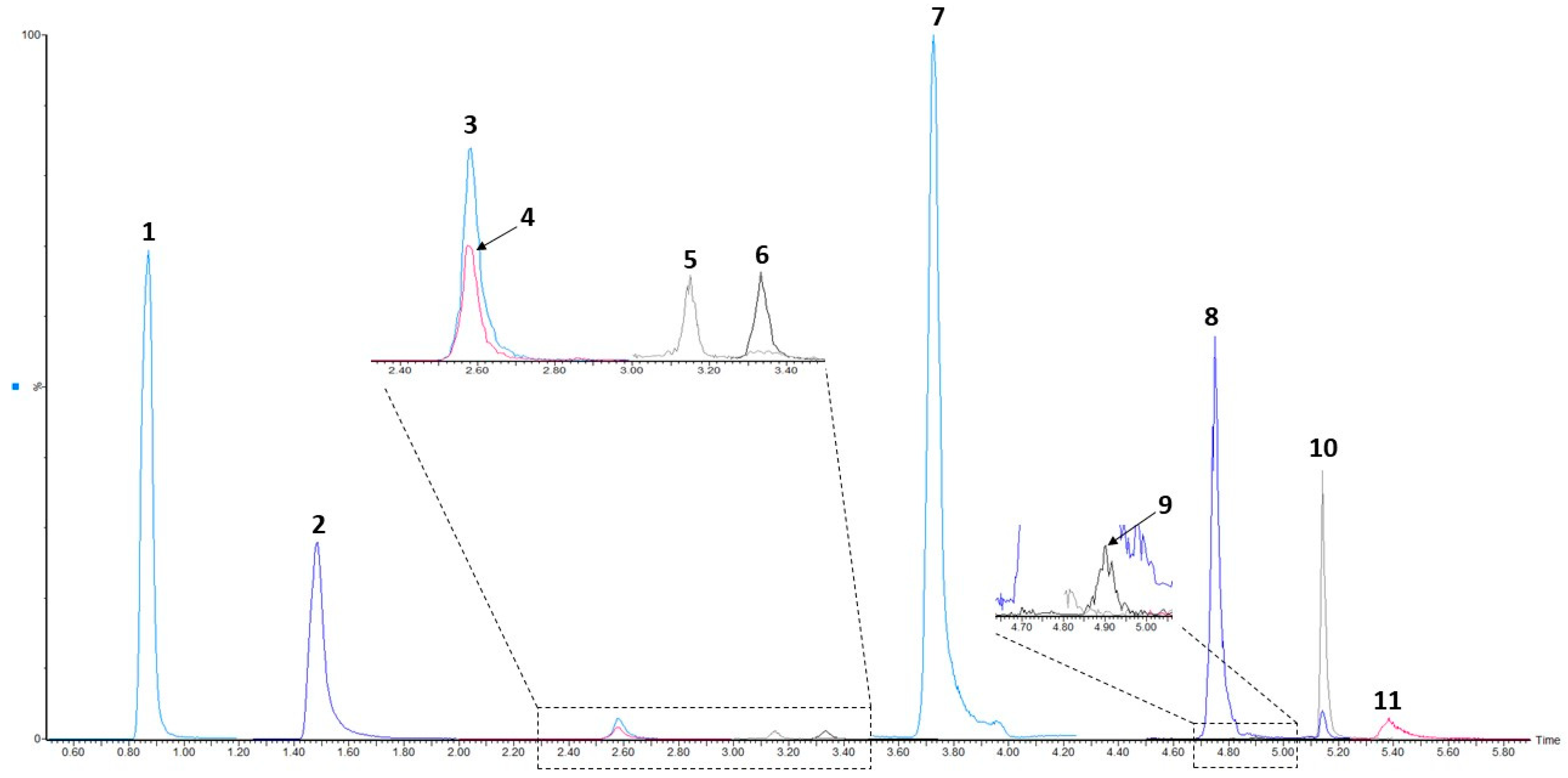
| Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | |
|---|---|---|---|---|
| Ach | 146.1 | 60.0 | 16 | 14 |
| D9–Ach | 155.1 | 87.0 | 16 | 14 |
| Chol | 104.1 | 44.9 | 41 | 30 |
| D9–Chol | 113.1 | 69.0 | 41 | 30 |
| Trp | 204.9 | 146.0 | 21 | 20 |
| D5–Trp | 209.9 | 150.0 | 21 | 20 |
| Kyn | 209.6 | 94.1 | 26 | 12 |
| D5–Kyn | 213.1 | 98.1 | 26 | 12 |
| PyroGlu | 130.1 | 84.0 | 20 | 13 |
| D5–PyroGlu | 135.1 | 89.0 | 20 | 13 |
| NAA | 176.2 | 134.1 | 16 | 10 |
| D3–NAA | 179.1 | 137.1 | 16 | 10 |
| GABA | 104.0 | 86.9 | 20 | 11 |
| D6–GABA | 110.1 | 92.1 | 20 | 11 |
| Gln | 147.1 | 84.0 | 20 | 18 |
| D5–Gln | 152.1 | 89.1 | 20 | 18 |
| Asn | 133.1 | 87.0 | 16 | 12 |
| D5–Asn | 137.9 | 97.0 | 16 | 12 |
| Glu | 148.2 | 84.0 | 20 | 18 |
| D3–Glu | 151.1 | 86.1 | 20 | 18 |
| Asp | 134.1 | 74.0 | 16 | 12 |
| D3–Asp | 137.0 | 91.0 | 16 | 12 |
| Ach | Chol | Trp | Kyn | PyroGlu | NAA | GABA | Gln | Asn | Glu | Asp | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| tR (min) | 0.87 | 1.49 | 2.60 | 2.61 | 3.16 | 3.33 | 3.73 | 4.74 | 4.91 | 5.18 | 5.38 |
| a (counts) | −0.0001 | 0.0115 | −0.0829 | −0.0752 | −0.0001 | −0.0017 | −0.0167 | −0.0145 | −0.0015 | −0.0062 | −0.0137 |
| RSDa (%), n = 6 | 6.9 | 2.0 | 8.5 | 7.4 | 3.8 | 6.8 | 2.6 | 7.0 | 4.7 | 7.8 | 1.3 |
| b (counts × µg−1 × mL) | 0.0548 | 0.0617 | 0.0948 | 0.0826 | 0.0073 | 0.0081 | 0.0363 | 0.1962 | 0.0067 | 0.2074 | 0.1314 |
| RSDb (%), n = 6 | 0.3 | 0.3 | 0.5 | 0.7 | 0.8 | 0.8 | 0.3 | 0.4 | 0.4 | 0.9 | 0.8 |
| r2 | 0.9998 | 0.9999 | 0.9952 | 0.9946 | 0.9992 | 0.9994 | 0.9993 | 0.9998 | 0.9997 | 0.9980 | 0.9985 |
| Linear range (µg/mL) | 0.025–2.5 | 2.5–250 | 0.1–25 | 0.1–25 | 0.25–25 | 0.25–25 | 2.5–250 | 2.5–250 | 0.25–25 | 2.5–250 | 2.5–250 |
| LOD (µg/mL) | 0.01 | 0.91 | 0.07 | 0.07 | 0.05 | 0.24 | 0.97 | 1.22 | 0.12 | 1.68 | 1.70 |
| LLOQ (µg/mL) | 0.025 | 2.5 | 0.1 | 0.1 | 0.25 | 0.25 | 2.5 | 2.5 | 0.25 | 2.5 | 2.5 |
| Intra-Day, n = 6 | Inter-Day, n = 12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | QC Level | Nominal (µg/mL) | Found (µg/mL) | CV (%) | Accuracy (%) | Found (µg/mL) | CV (%) | Accuracy (%) | Recovery (%) |
| Ach | Low | 0.025 | 0.025 | 11.3 | 101.6 | 0.02 | 14.3 | 99.3 | 91.8 |
| Medium | 0.25 | 0.25 | 4.2 | 100.2 | 0.26 | 3.9 | 102.2 | 88.2 | |
| High | 2.5 | 2.51 | 0.7 | 100.2 | 2.53 | 1.4 | 101.1 | 90.1 | |
| Chol | Low | 2.5 | 2.64 | 7.2 | 105.6 | 2.48 | 9.3 | 99.3 | 87.0 |
| Medium | 10 | 10.15 | 0.7 | 101.5 | 10.16 | 0.5 | 101.6 | 94.3 | |
| High | 250 | 250.80 | 0.9 | 100.3 | 250.56 | 1.6 | 100.2 | 94.6 | |
| Trp | Low | 0.1 | 0.10 | 11.9 | 101.3 | 0.11 | 14.5 | 111.4 | 87.3 |
| Medium | 5 | 4.95 | 5.5 | 99.0 | 4.61 | 5.4 | 92.1 | 90.2 | |
| High | 25 | 26.03 | 3.1 | 104.1 | 24.83 | 3.9 | 99.3 | 90.0 | |
| Kyn | Low | 0.1 | 0.11 | 9.5 | 107.1 | 0.12 | 12.4 | 119.6 | 90.5 |
| Medium | 5 | 4.38 | 4.8 | 87.6 | 4.52 | 7.4 | 90.4 | 81.6 | |
| High | 25 | 24.31 | 2.7 | 97.3 | 23.45 | 4.7 | 93.8 | 92.6 | |
| PyroGlu | Low | 0.25 | 0.23 | 10.7 | 92.2 | 0.22 | 14.4 | 88.9 | 74.8 |
| Medium | 2.5 | 2.54 | 2.0 | 101.6 | 2.55 | 3.6 | 102.0 | 75.2 | |
| High | 25 | 25.33 | 2.8 | 101.3 | 25.04 | 3.6 | 100.2 | 87.8 | |
| NAA | Low | 0.25 | 2.61 | 10.1 | 104.5 | 2.18 | 14.4 | 87.2 | 85.0 |
| Medium | 2.5 | 23.98 | 6.3 | 95.9 | 10.05 | 4.5 | 100.5 | 88.8 | |
| High | 25 | 249.79 | 3.2 | 99.9 | 257.72 | 5.0 | 103.1 | 88.1 | |
| GABA | Low | 2.5 | 2.36 | 4.9 | 94.3 | 2.55 | 3.5 | 101.9 | 79.4 |
| Medium | 10 | 10.24 | 1.1 | 102.4 | 10.27 | 1.1 | 102.7 | 99.3 | |
| High | 250 | 250.56 | 0.8 | 100.2 | 252.09 | 1.1 | 100.8 | 103.3 | |
| Gln | Low | 2.5 | 2.27 | 6.6 | 90.6 | 2.50 | 8.7 | 100.1 | 87.4 |
| Medium | 10 | 10.16 | 0.7 | 101.6 | 10.15 | 0.6 | 101.5 | 94.3 | |
| High | 250 | 250.40 | 0.6 | 100.2 | 249.70 | 0.8 | 99.9 | 93.3 | |
| Asn | Low | 0.25 | 0.25 | 7.2 | 98.7 | 0.24 | 9.2 | 97.1 | 73.4 |
| Medium | 2.5 | 2.55 | 2.3 | 102.0 | 2.51 | 3.2 | 100.5 | 97.5 | |
| High | 25 | 24.91 | 1.1 | 99.7 | 24.87 | 1.1 | 99.5 | 103.7 | |
| Glu | Low | 2.5 | 2.40 | 5.8 | 96.1 | 2.41 | 6.3 | 96.3 | 92.5 |
| Medium | 10 | 10.33 | 4.6 | 103.3 | 10.16 | 5.0 | 101.6 | 91.1 | |
| High | 250 | 250.74 | 2.7 | 100.3 | 249.64 | 3.1 | 99.9 | 89.9 | |
| Asp | Low | 2.5 | 2.36 | 6.4 | 94.4 | 2.38 | 6.1 | 95.3 | 85.3 |
| Medium | 10 | 9.70 | 2.3 | 97.0 | 9.61 | 3.9 | 96.1 | 89.7 | |
| High | 250 | 250.57 | 2.8 | 100.2 | 252.04 | 2.2 | 100.8 | 94.1 | |
| Autosampler Stability (24 h), n = 6 | Freeze-to-Thaw Stability, n = 6 | |||||
|---|---|---|---|---|---|---|
| Analyte | QC Level | Nominal (µg/mL) | Found (µg/mL) | Accuracy (%) | Found (µg/mL) | Accuracy (%) |
| Ach | Low | 0.025 | 0.03 | 101.9 | 0.02 | 96.3 |
| Medium | 0.25 | 0.24 | 95.5 | 0.24 | 97.4 | |
| High | 2.5 | 2.64 | 105.7 | 2.62 | 104.6 | |
| Chol | Low | 2.5 | 2.50 | 100.1 | 2.47 | 98.8 |
| Medium | 10 | 10.25 | 102.5 | 10.25 | 102.5 | |
| High | 250 | 244.11 | 97.6 | 243.64 | 97.5 | |
| Trp | Low | 0.1 | 0.09 | 86.6 | 0.09 | 86.1 |
| Medium | 10 | 9.39 | 93.9 | 9.29 | 92.9 | |
| High | 25 | 25.33 | 101.3 | 23.30 | 93.2 | |
| Kyn | Low | 0.1 | 0.08 | 80.0 | 0.08 | 83.9 |
| Medium | 10 | 9.43 | 94.3 | 9.33 | 93.3 | |
| High | 25 | 22.93 | 90.7 | 23.25 | 93.0 | |
| PyroGlu | Low | 0.25 | 0.25 | 98.8 | 0.21 | 83.0 |
| Medium | 2.5 | 2.46 | 98.4 | 2.58 | 103.0 | |
| High | 25 | 23.79 | 95.2 | 253.04 | 101.2 | |
| NAA | Low | 0.25 | 0.25 | 100.2 | 0.29 | 116.0 |
| Medium | 2.5 | 2.71 | 108.2 | 2.80 | 111.9 | |
| High | 25 | 24.34 | 97.3 | 26.08 | 104.3 | |
| GABA | Low | 2.5 | 2.41 | 96.4 | 2.52 | 100.7 |
| Medium | 10 | 10.23 | 102.3 | 9.72 | 97.2 | |
| High | 250 | 243.18 | 97.3 | 248.50 | 99.4 | |
| Gln | Low | 2.5 | 2.50 | 99.9 | 2.54 | 101.5 |
| Medium | 10 | 9.72 | 97.2 | 9.55 | 95.5 | |
| High | 250 | 246.51 | 98.6 | 246.60 | 98.6 | |
| Asn | Low | 0.25 | 0.21 | 85.0 | 0.26 | 104.2 |
| Medium | 2.5 | 2.51 | 100.3 | 2.53 | 101.2 | |
| High | 25 | 25.17 | 100.7 | 24.44 | 97.7 | |
| Glu | Low | 2.5 | 2.47 | 98.8 | 2.69 | 107.7 |
| Medium | 10 | 8.78 | 87.8 | 9.78 | 97.8 | |
| High | 250 | 241.51 | 96.6 | 252.23 | 100.9 | |
| Asp | Low | 2.5 | 2.37 | 94.7 | 2.30 | 92.1 |
| Medium | 10 | 9.35 | 93.5 | 10.24 | 102.4 | |
| High | 250 | 242.74 | 97.1 | 252.4 | 101.0 | |
| Medulla Oblongata | Pons | Frontal Cortex | Parietal Cortex | |||||
|---|---|---|---|---|---|---|---|---|
| SHR 24 (µg/mg) | Control (µg/mg) | SHR 24 (µg/mg) | Control (µg/mg) | SHR 24 (µg/mg) | Control (µg/mg) | SHR 24 (µg/mg) | Control (µg/mg) | |
| Ach | 16.120 ± 4.671 | 14.154 ± 3.729 | 12.559 ± 3.694 | 12.942 ± 4.529 | 0.024 ± 0.013 | 0.018 ± 0.005 | 0.014 ± 0.007 | 0.012 ± 0.006 |
| Chol | 0.075 ± 0.029 | 0.043 ± 0.017 | 0.203 ± 0.063 | 0.119 ± 0.031 | 0.229 ± 0.035 | 0.107 ± 0.031 | 0.087 ± 0.053 | 0.077 ± 0.047 |
| Trp | 0.012 ± 0.002 | 0.011 ± 0.002 | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.007 ± 0.011 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Kyn | 0.014 ± 0.003 | 0.015 ± 0.003 | 0.025 ± 0.037 | 0.044 ± 0.044 | 0.114 ± 0.011 | 0.121 ± 0.045 | 0.142 ± 0.024 | 0.123 ± 0.025 |
| PyroGlu | 0.527 ± 0.466 | 1.010 ± 0.412 | 0.175 ± 0.039 | 0.166 ± 0.033 | 0.458 ± 0.247 | 0.441 ± 0.327 | 0.436 ± 0.208 | 0.482 ± 0.190 |
| NAA | 3.062 ± 0.264 | 3.002 ± 0.196 | 1.770 ± 0.119 | 1.986 ± 0.099 | 2.672 ± 0.628 | 2.647 ± 0.344 | 2.621 ± 0.509 | 2.200 ± 0.507 |
| GABA | 0.247 ± 0.044 | 0.215 ± 0.032 | 0.301 ± 0.031 | 0.216 ± 0.023 | 0.378 ± 0.137 | 0.319 ± 0.077 | 0.380 ± 0.120 | 0.300 ± 0.089 |
| Gln | 0.980 ± 0.067 | 1.117 ± 0.487 | 0.859 ± 0.051 | 1.018 ± 0.495 | 1.928 ± 0.509 | 2.105 ± 0.957 | 1.818 ± 0.336 | 1.774 ± 0.520 |
| Asn | 0.021 ± 0.003 | 0.022 ± 0.005 | 0.020 ± 0.002 | 0.020 ± 0.003 | 0.032 ± 0.008 | 0.031 ± 0.007 | 0.025 ± 0.007 | 0.023 ± 0.006 |
| Glu | 1.227 ± 0.109 | 1.276 ± 0.059 | 1.285 ± 0.098 | 1.365 ± 0.074 | 2.634 ± 0.650 | 2.516 ± 0.297 | 2.774 ± 0.555 | 2.429 ± 0.599 |
| Asp | 0.632 ± 0.081 | 0.582 ± 0.064 | 0.746 ± 0.084 | 0.615 ± 0.075 | 0.885 ± 0.264 | 0.815 ± 0.137 | 0.739 ± 0.116 | 0.592 ± 0.118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piestansky, J.; Forgacsova, A.; Olesova, D.; Galba, J.; Mikus, P.; Majerova, P.; Kovac, A. Targeted UHPLC-ESI-MS/MS Analysis of Selected Neurotransmitters, Tryptophan and Its Metabolite Kynurenine in Tau Transgenic Rat Brain Tissue: A Pivotal Study. Separations 2022, 9, 16. https://doi.org/10.3390/separations9010016
Piestansky J, Forgacsova A, Olesova D, Galba J, Mikus P, Majerova P, Kovac A. Targeted UHPLC-ESI-MS/MS Analysis of Selected Neurotransmitters, Tryptophan and Its Metabolite Kynurenine in Tau Transgenic Rat Brain Tissue: A Pivotal Study. Separations. 2022; 9(1):16. https://doi.org/10.3390/separations9010016
Chicago/Turabian StylePiestansky, Juraj, Andrea Forgacsova, Dominika Olesova, Jaroslav Galba, Peter Mikus, Petra Majerova, and Andrej Kovac. 2022. "Targeted UHPLC-ESI-MS/MS Analysis of Selected Neurotransmitters, Tryptophan and Its Metabolite Kynurenine in Tau Transgenic Rat Brain Tissue: A Pivotal Study" Separations 9, no. 1: 16. https://doi.org/10.3390/separations9010016
APA StylePiestansky, J., Forgacsova, A., Olesova, D., Galba, J., Mikus, P., Majerova, P., & Kovac, A. (2022). Targeted UHPLC-ESI-MS/MS Analysis of Selected Neurotransmitters, Tryptophan and Its Metabolite Kynurenine in Tau Transgenic Rat Brain Tissue: A Pivotal Study. Separations, 9(1), 16. https://doi.org/10.3390/separations9010016








