Abstract
A capillary zone electrophoresis method was developed for the determination of NiII(3-OMe-salophene), a substance with anticancer activity in vitro. A fused silica capillary (56 cm × 100 µm) was used for this purpose. The method was optimized in terms of parameters affecting the electrophoretic conditions in order to optimize separation efficiency and total time of migration. The analysis was best performed using an operating buffer of 50 mM borate, adjusted to pH 9.3, mixed with acetonitrile (50%, v/v) as organic modifier. Injections were performed hydrodynamically by applying a pressure of 50 mbar for 8 s, and a 30 kV separation voltage was selected at 25 °C. Detection was carried out at 250 nm using diode array detector (DAD). The method allowed the separation of NiII(3-OMe-salophene) from four other structurally related impurities in a total migration time (tm) of 8 min. Peak identification was achieved using the standard reference of individual impurities. The purity of the migrated NiII(3-OMe-salophene) was confirmed by Ultra-violet (UV) scan overlay depending on DAD. The linear ranges for the determination of NiII(3-OMe-salophene) was 400–20,000 ng mL−1 with limit of detection (LOD) of 120 ng mL−1. Acceptable intra-day and inter-day precisions were achieved (%relative standard deviation (RSD) results were less than 0.76% and 0.30%, respectively). The proposed method was assessed for greenness and compared to reported methodologies to prove superiority.
1. Introduction
Cancer remains the most seriously undefeated illness of recent decades. Despite the eager efforts of clinical researchers to find new therapies, cancer morbidity and mortality rates remain worrying. In 2009, Hille et al. developed a metal chelating ligand known as salophene (N,N′-bis(salicylidene)-1,2-phenylenediamine), which can form metal complexes with transition metals such as (FeII&III, CoII, NiII, MnII&III, and CuII) [1]. Such complexes exhibited dose dependent anti-proliferative activity against breast cancer cell line MCF-7, where its cytotoxicity depended on the complexed metal [1]. In 2010, a research study concluded the activity of methoxy-substituted nickel-II (Salophene) derivatives [2]. NiII(3-OMe-salophene) demonstrated the highest antitumor activity and induced apoptosis in a pattern dependent on the dose. NiII(3-OMe-salophene) exhibited no necrotic effects on Burkitt-like lymphoma cells (BJAB) and human B-cell precursor cells (Nalm-6) [2]. Metal complexes with salophene-type ligands have also been recently studied and investigated for their antimicrobial effects against resistant pathogens [3].
Since full characterization of drug molecules is absolutely essential for their biological approval, several analytical methods need to be developed. Impurity profiling is a crucial step in the analyte’s characterization and the developed methodologies must be very selective in order to separate those process impurities that are, structurally, closely related. Process related impurities (PRIs) may arise during the manufacturing process of the active pharmaceutical ingredient (API) or as unwanted remains originating from the starting precursors. PRIs might be toxic, pharmacologically inactive, or even might have much lower potency than the API (as here in the present case) [2].
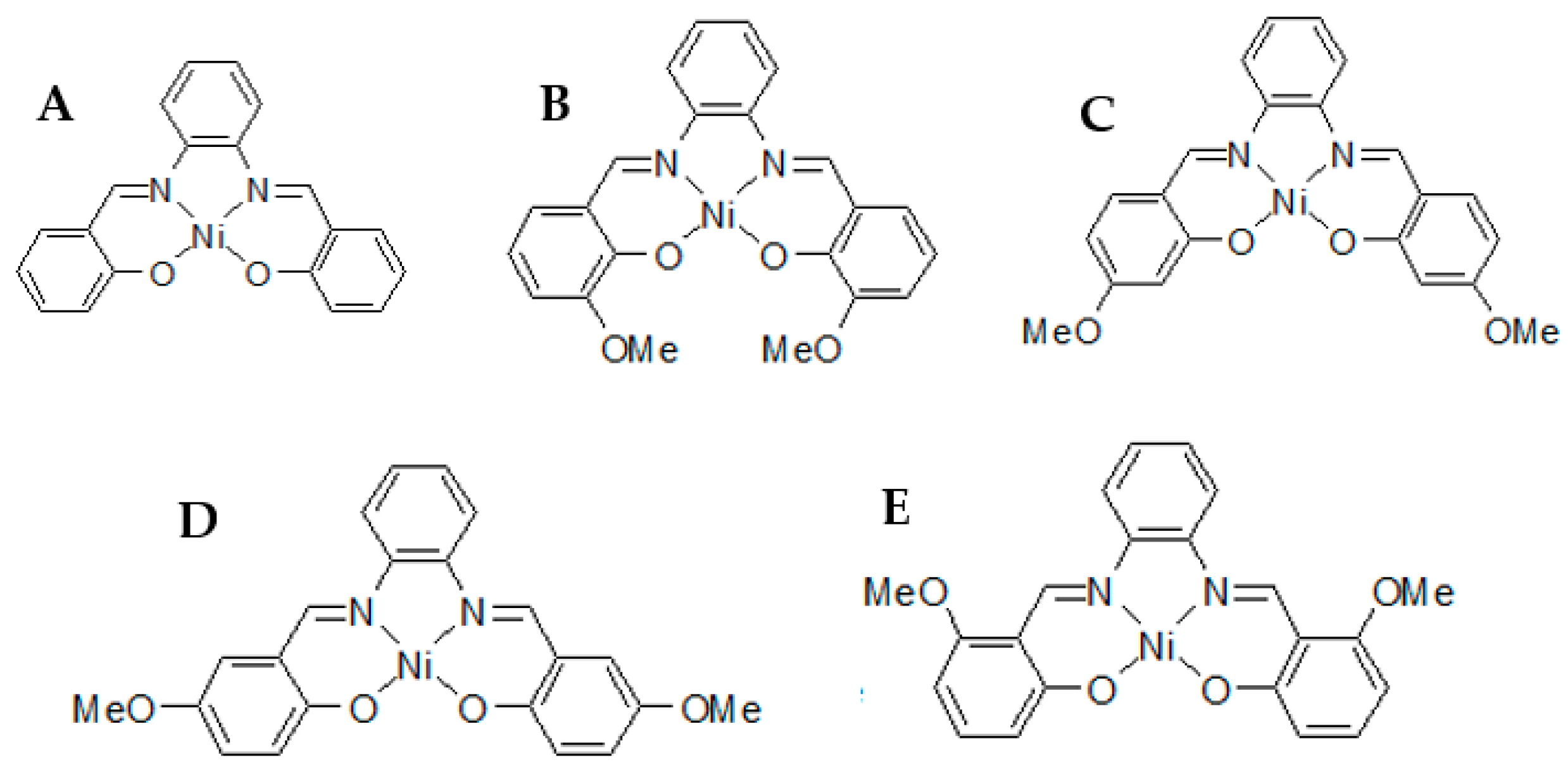
In the proposed research, four different PRIs were studied; namely, NiII(salophene), NiII(4-OMe-salophene), NiII(5-OMe-salophene), and NiII(6-OMe-salophene) (Chemical structures Figure 1). Information about these impurities remains in a state of indigence, therefore, new analytical characterization methodologies have to be introduced to help with API characterization when it reaches the API’s clinical trial phase.
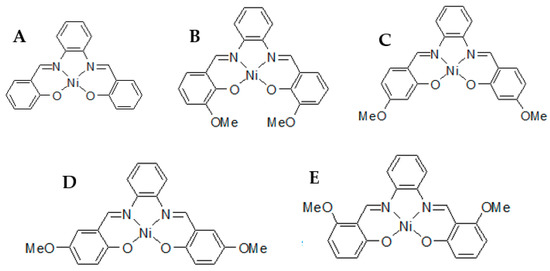
Figure 1.
Chemical structures of (A) NiII(salophene), (B) NiII(3-OMe-salophene), (C) NiII(4-OMe-salophene), (D) NiII(5-OMe-salophene), and (E) NiII(6-OMe-salophene).
The aim of this research is to develop a new simple, selective and sensitive capillary zone electrophoresis (CZE) method for the determination of the target antitumor drug, NiII(3-OMe-salophene), in the presence of the aforementioned structurally related PRIs. The literature review revealed that only one publication had reported on the determination of NiII(3-OMe-salophene) using atomic absorption spectrometry and HPLC [4].
2. Materials and Methods
2.1. Materials
Sodium hydroxide and boric acid were analytical grades and were obtained from Sigma-Aldrich (Steinheim, Germany). HPLC grade acetonitrile (ACN) was purchased from Sigma-Aldrich (Steinheim, Germany). Reference standards of NiII(3-OMe-salophene), and its four impurities NiII(4-OMe-salophene), NiII(5-OMe-salophene), NiII(6-OMe-salophene), and NiII(salophene) were synthesized and purified in the laboratories of the Freie Universität Berlin, Berlin, Germany [1]. Distilled water was produced from arium pro UF/VF water purification system from Sartorius Weighing Technology GmbH (Goettingen, Germany).
2.2. Instrumentation
Method development and validation were performed using the Prince CZE system, model PrinCE-C760 from (Prince Technologies, Emmen, The Netherlands). CZE was equipped with a diode array detector (DAD). All separations were carried out on a bare fused silica capillary (56 cm length × 100 µm internal diameter), which was purchased from Polymicro Technologies (Phoenix, AZ, USA). Electropherograms were monitored and processed using DAx 3D software. Metrohm pH meter model 632 from Metrohm (Filderstadt, Germany) was used for pH adjustment. All acquired data were analyzed using Microsoft EXCEL® (Microsoft Corporation, Redmond, WA, USA, version 2010).
2.3. CZE Separation Conditions
The optimal separation conditions were obtained using a background electrolyte (BGE) composed of 50 mM buffer borate, adjusted to pH 9.3 and ACN (50:50, v/v) under 30 kV separation voltages. Thermostat temperature was set to 25 °C during the whole experiment. Detection of analytes under study was obtained using DAD, which was set at 250 nm. Hydrodynamic injection was performed by applying pressure of 50 mbar for 8 s. Under the mentioned CZE conditions, separation was acquired in a total migration time of 8.0 min only, where the main analyte migration time (tm) was 4.6 min. The purity of NiII(3-OMe-salophene) was confirmed by the overlaid DAD spectra and by identification of individual impurities’ peaks using the standard pure reference impurities.
2.4. Preparation of Standard and Stock Solutions
NiII(3-OMe-salophene) stock solution was prepared in pure ACN at a concentration of 20,000 ng mL−1. This solution was then used to prepare the standards used in the validation study. The other four impurities were prepared individually in the same solvent at a concentration of 40,000 ng mL−1.
NiII(3-OMe-salophene) stock solution was then used to prepare validation standards within linearity range of (400–20,000 ng mL−1) by serial dilution in ACN. For establishing linearity, six standard solutions were prepared at concentrations of 400, 2000, 5000, 10,000, 15,000, and 20,000 ng mL−1. For accuracy testing, three quality control (QC) standards were prepared at low (QCL), medium (QCM) and high (QCH) concentration levels within the linearity range at concentrations of 400, 2000, and 20,000 ng mL−1. QC standards were then used to establish repeatability and intermediate precision of the proposed methodology. Specificity was demonstrated for the assay purpose by spiking NiII(3-OMe-salophene) in individual solutions containing 400 ng mL−1 of each PRI at concentration 400 ng mL−1. The percentage recoveries of NiII(3-OMe-salophene) in these solutions were calculated with the results of accuracy.
3. Results
3.1. Method Development
During the method development phase, several factors that influence CE were studied for the separation of NiII(3-OMe-salophene) from its four PRIs. Variations in temperature, ionic concentration of the BGE, and voltage were tried around the optimal selected values. The effects of buffer type, concentration and pH were optimized. Borate buffer was chosen as BGE due the ability of its anions to bind to capillary surface silanol groups rendering them neutral walls, and thus reduce its ζ-potential, and in turn allow separations even in bare fused silica capillaries at high-resolutions [5]. Buffer ionic strength was optimized in the range of 25–100 mM. As buffer strength was increased, the interactions with capillary walls were reduced and the resolution between the main peak and its PRIs was improved. However, values above 50 mM increased the joule heating due to resistance of buffer to current, which resulted in temperature gradiant and peak broadening. Modification of pH around the optimal value was estimated. The pH values affected the migration time of analytes under study, where lower pH values resulted in peak overlaps and higher pH values caused a longer migration time.
Variations in the applied voltage between 10–30 kV were optimized. Higher values shortened the migration times, however kept the current intensity below 100 µA and avoided joule heating at a borate buffer concentration of 50 mM. The best results in terms of shorter migration time and good separation efficiency were selected while maintaining good precision, avoiding capillary heating and band-broadening under the optimized CZE conditions.
3.2. Method Validation
Method validation was performed according to International Conference for harmonization (ICH) guidelines [6]. Linearity was established by plotting the DAD response obtained versus the corresponding standard solution concentration of NiII(3-OMe-salophene). Table 1 shows linearity and regression data obtained by the proposed CZE method. Good linearity results were obtained within the range proven by calibration curve regression. Limits of detection (LOD) and quantification (LOQ) were calculated from the calibration curve using the standard deviations (σ) and the slope (S). LOD is that obtained equivalent to (3.3 σ/S), while LOQ is that equivalent to (10 σ/S).

Table 1.
Linearity and regression results for determination of NiII(3-OMe-salophene) using the proposed CZE method.
Accuracy of the proposed method was established by injecting QC standards in triplicates and calculating recovery percentages. Table 2 shows recovery results of QC standards, which prove the close agreement between the proposed method results and true values. According to ICH guidelines [6], the accuracy of methodologies for assay of any analyte can be determined by several methods. In the proposed study, since the drug molecule is new and no pharmaceutical dosage form has been marketed yet, the accuracy was determined by applying the developed method on laboratory synthesized pure analyte, and, furthermore, the accuracy was inferred once precision, linearity, and specificity were proven [6].

Table 2.
Accuracy and precision results of NiII(3-OMe-salophene) determination under the proposed CZE method.
Precision of the proposed method was assessed in terms of repeatability (intra-day precision) and intermediate precision (inter-day precision) using the QC standards. Precision was tested both on migration time and recovery percentage. QC standards were injected in triplicates at different times within the same day and in three successive days. Table 2 shows the results obtained for both the migration time and percentage of recovery of the studied analyte, proving rugged methodology.
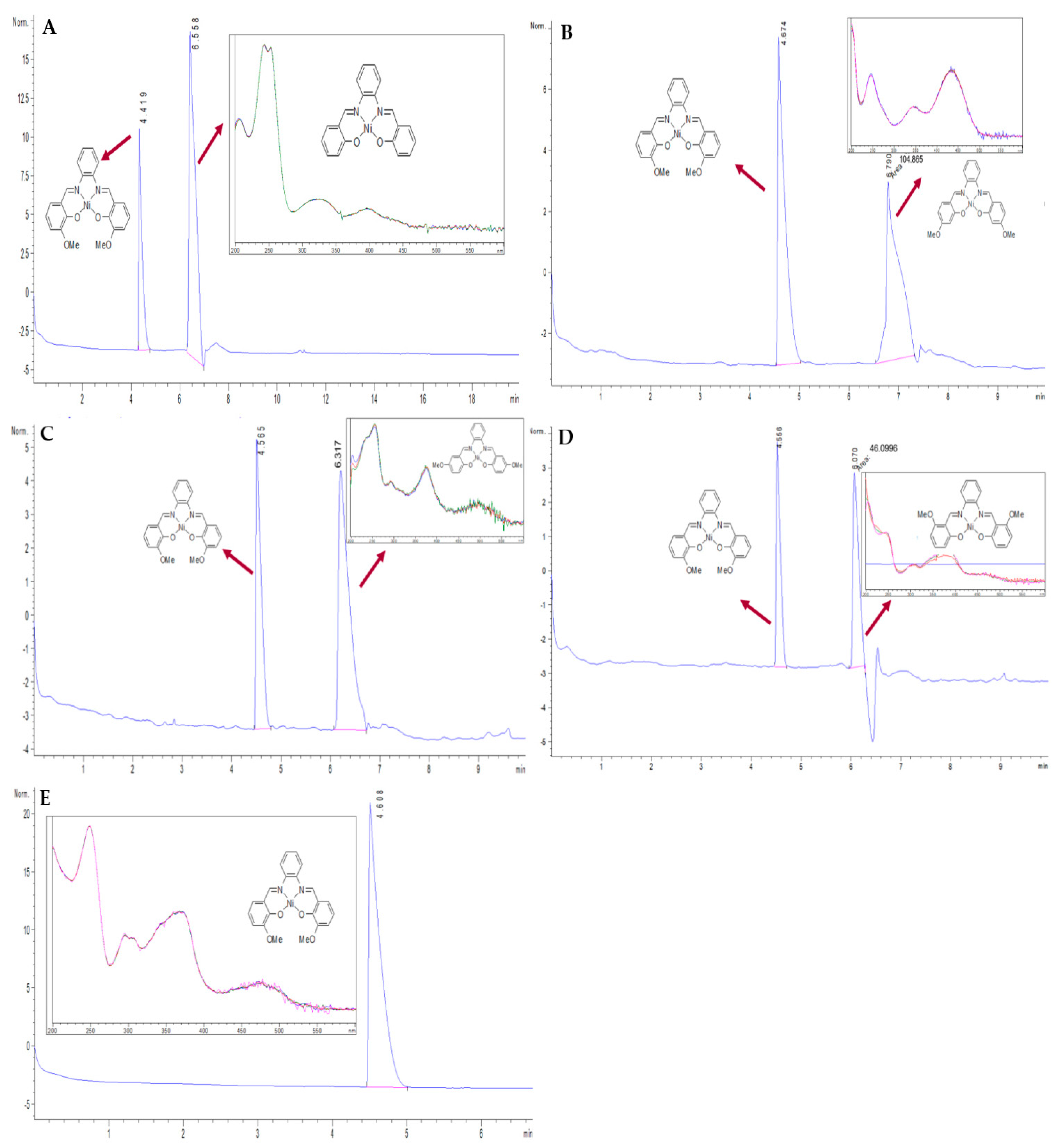
According to ICH guidelines [6], the specificity in assay methodologies can be validated where the impurities are available by demonstrating that such impurities can be resolved from the main peak during elution without affecting the main analyte’s peak. Specificity of the proposed CZE method was proven from its ability to separate NiII(3-OMe-salophene) from four of its PRIs at a good resolution. Results are shown in Figure 2. The PRIs under investigation were injeced at concentrations equal that of the API, which is unlikely to be found. In such way, the matrix effect of even high concentration levels of the impurities can be proven to have a null effect on recovery of the API under study. As shown in Figure 2A–D, all PRIs were well resolved at such high concentration levels, and the calculated recoveries were acceptable when compared to those presented in Table 2. The three spectra in each insert are presented for peak purity check by showing the UV spectra at three points of the peak at the start, at the top and at the end. Moreover, the spectral overlay indicates purity and determines the spectral homogeneity across the peak to indicate peak purity. Additionally, the method was applied on a synthetic batch of the API under study after purification (Figure 2E), where the API was injected at a high concentration (2000 ng mL−1). As shown, the absence of any peak at the migration time corresponding to PRIs indicated the high purity of API and also proved the validity of the established purification technique.
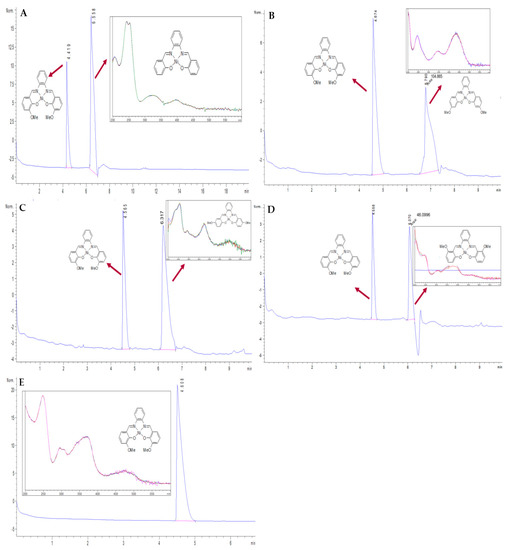
Figure 2.
Electropherograms for the separation of NiII(3-OMe-salophene) from (A) NiII(salophene), (B) NiII(4-OMe-salophene), (C) NiII(5-OMe-salophene), and (D) NiII(6-OMe-salophene) at concentrations 400 ng mL−1; (E) NiII(3-OMe-salophene) alone (2000 ng mL−1). CZE condition: BGE; 50 mM buffer borate, pH 9.3, and ACN (50:50, v/v); 30 kV separation voltages; Temperature 25 °C; Hydrodynamic injection under pressure of 50 mbar for 8 s.
3.3. Evaluation of the Proposed Method
As previously mentioned during the introduction, only a few methods were reported with regard to the determination of NiII(3-OMe-salophene). CZE is a simple separation technique capable of performing highly efficient separations for even large and complex molecules. Although a high-resolution continuum-source atomic absorption spectroscopy (HR-CS-AAS) method was previously developed for the determination of the analyte under study [4], HR-CS-AAS was based on the determination of the centrally bound metal atom only and is therefore unsuitable in the current case, which resolves different complex analytes. The reported LC method [4] was based on the determination of the analyte under study alone without considering the other PRIs. The proposed method was proven to be capable for the separation and determination of the target drug in the presence of such structurally related isomers. However, this method is not able to efficiently separate the mentioned potential impurities from each other. Thus, it might be difficult to determine, using the developed method, which specific impurity is present in a prepared NiII(3-OMe-salophene) sample. Therefore, there is a space for future improvements to the studied conditions in order to separate and estimate each of these PRIs.
Green chemistry philosophy is one of the most prominent approaches to have recently emerged. Green analytical chemistry (GAC) has twelve principles that were introduced and aimed at decreasing the consumption and generation of ecologically hazardous solvents [7]. Since then, scientists were motivated to develop assessment tools in order to help researchers in evaluating their work methods. The assessment of newly developed analytical methodologies became of great importance in demonstrating their ecological impact. Several assessment metrics were reported, including National Environmental Methods Index (NEMI) [8], the analytical eco-scale [9], green analytical procedure index (GAPI) [10], and Agree-tool [11]. GAPI is one of the most cited recent metrics available for greenness assessment [10]. GAPI is composed of five pentagrams representing fifteen steps of the analytical procedure, namely; method type, sample collection, preparation, reagents and instrumentation used. The greenness of each step is assessed in with three color codes, red, yellow and green. The red code indicates the highest ecological impact, while the green code indicates the least impact and most eco-friendly [12].
As seen from GAPI pictograms (Table 3), the proposed method has more green zones and less red zones than those presented by the reported liquid chromatography (LC) method [4].

Table 3.
Comparison of the proposed CZE analytical method to previously reported HPLC-DAD method.
The proposed CZE method is greener in terms of the amount of organic modifier used, and waste generated. CZE consumes a lower amount of reagents due to the small volume within the capillary column. Most of electro-kinetic separations conducted by CZE are performed using aqueous buffers. However, even if organic modifiers were used that would not represent an ecological problem due to the sub-microliter level of consumption in CZE [13]. That accounts for the small amount of waste generated, which can reach up to 98% reduction in organic solvents consumed and waste generated [13]. The volume of most of the vials containing the separation buffer contains about 600μL on each side of the capillary, and this volume can be recycled for several runs. The electrochemical reactions occuring at the positive and negative electrode ends of the capillary result in a slight change in the pH of the background electrolyte (BGE), which in turn can affect the mobility of the analyte and hence repeatability in CZE [14]. Although it is recommended to replace the buffer used for separation every other run [13], for buffered BGE, however, the maximum number of runs that can be conducted under the proposed conditions can reach up to 36 runs using the same inlet vial (Max. no. of runs = 5 ∗ capillary volume in µL/ BGE volume in mL) [14]. Under the proposed conditions, BGE vials were refreshed after each 20 runs to improve the method’s repeatability. As for the instrumentation, energy consumed by CZE is much lower than those required for the operation of HPLC and more simple in instrumentation set-up, which can be demonstrated clearly by the instrumentation lower right pentagram in the GAPI pictogram (Table 3). The proposed method is totally greener in terms of instrumentation than the reported methodology [4].
4. Conclusions
A validated CZE method was developed and validated for the determination of the anticancer compound, NiII(3-OMe-salophene), with acceptable sensitivity and precision. Using the proposed separation conditions of CZE, impurity profiling of NiII(3-OMe-salophene) was feasible. The method can resolve the target drug from its main four process related impurities, especially as all the impurities are structurally related to the target drug and have very similar chemical properties (making their separation a challenging issue). Moreover, the developed methodology is more ecologically friendly than previously reported methodologies, as demonstrated by the greenness assessment using recently developed metrics.
Author Contributions
Conceptualization, S.E.D., G.W. and R.G.; methodology, S.E.D., G.W. and R.G.; software, S.E.D., G.W. and R.G.; validation, S.E.D., A.E.I. and A.A.-H.; formal analysis, S.E.D. and A.E.I.; investigation, S.E.D.; resources, S.E.D., G.W. and R.G.; data curation, S.E.D. and A.E.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hille, A.; Ott, I.; Kitanovic, A.; Kitanovic, I.; Alborzinia, H.; Lederer, E.; Wölfl, S.; Metzler-Nolte, N.; Schäfer, S.; Sheldrick, W.S.; et al. [N,N′-Bis(salicylidene)-1,2-phenylenediamine]metal complexes with cell death promoting properties. JBIC J. Biol. Inorg. Chem. 2009, 14, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Hille, A.; Frias, C.; Kater, B.; Bonitzki, B.; Wölfl, S.; Scheffler, H.; Prokop, A.; Gust, R. [NiII(3-OMe-salophene)]: A Potent Agent with Antitumor Activity. J. Med. Chem. 2010, 53, 6064–6070. [Google Scholar] [CrossRef] [PubMed]
- Baecker, D.; Sesli, Ö.; Knabl, L.; Huber, S.; Orth-Höller, D.; Gust, R. Investigating the antibacterial activity of salen/salophene metal complexes: Induction of ferroptosis as part of the mode of action. Eur. J. Med. Chem. 2021, 209, 112907. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, S.; Ma, B.N.; Gust, R. Determination of NiII(3-OMe-salophene) in MCF7 and HT29 cancer cell lines using HR-CS-AAS and in serum albumin using LC with monolithic silica. Microchem. J. 2012, 101, 24–29. [Google Scholar] [CrossRef]
- Dolnik, V. Borate-containing background electrolytes to improve CE separation in bare capillaries. Electrophoresis 2020, 41, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- ICH-Guidelines. Validation of Analytical Procedures: Text and Methodology Q2 (R1); International Conference on Harmonization: Geneva, Switzerland, 2005. [Google Scholar]
- Ibrahim, A.E.; Elmaaty, A.A.; El-Sayed, H.M. Determination of six drugs used for treatment of common cold by micellar liquid chromatography. Anal. Bioanal. Chem. 2021, 413, 5051–5065. [Google Scholar] [CrossRef] [PubMed]
- KeithL, H.; Gron, L.U.; Young, J.L. Green Analytical Methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Konieczka, P.; Namiesnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; El Deeb, S.; Abdelhalim, E.M.; Al-Harrasi, A.; Sayed, R.A. Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms. Separations 2021, 8, 243. [Google Scholar] [CrossRef]
- Wuethrich, A.; Quirino, J.P. The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 517–532. [Google Scholar]
- Kok, W. (Ed.) Capillary Electrophoresis: Instrumentation and Operation; Vieweg+Teubner Verlag: Wiesbaden, Germany, 2000; pp. 20–43. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).