Automated Solid Phase Extraction of Cd(II), Co(II), Cu(II) and Pb(II) Coupled with Flame Atomic Absorption Spectrometry Utilizing a New Sol-Gel Functionalized Silica Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
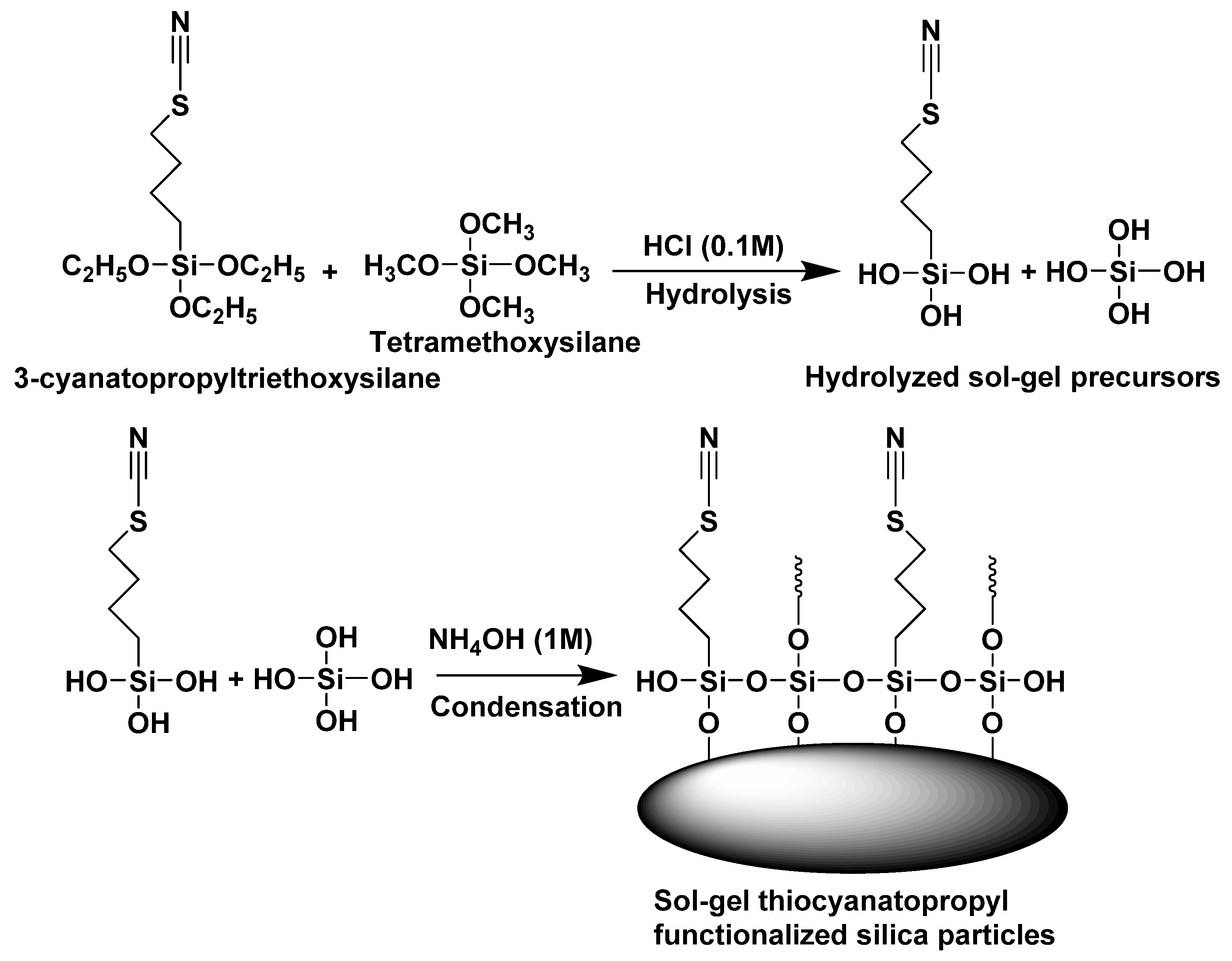
2.2. Development of the Thiocyanatopropyl Functionalized Sol-Gel Silica Sorbent
2.3. Instrumentation
2.4. Automatic Operational Procedure
3. Results and Discussion
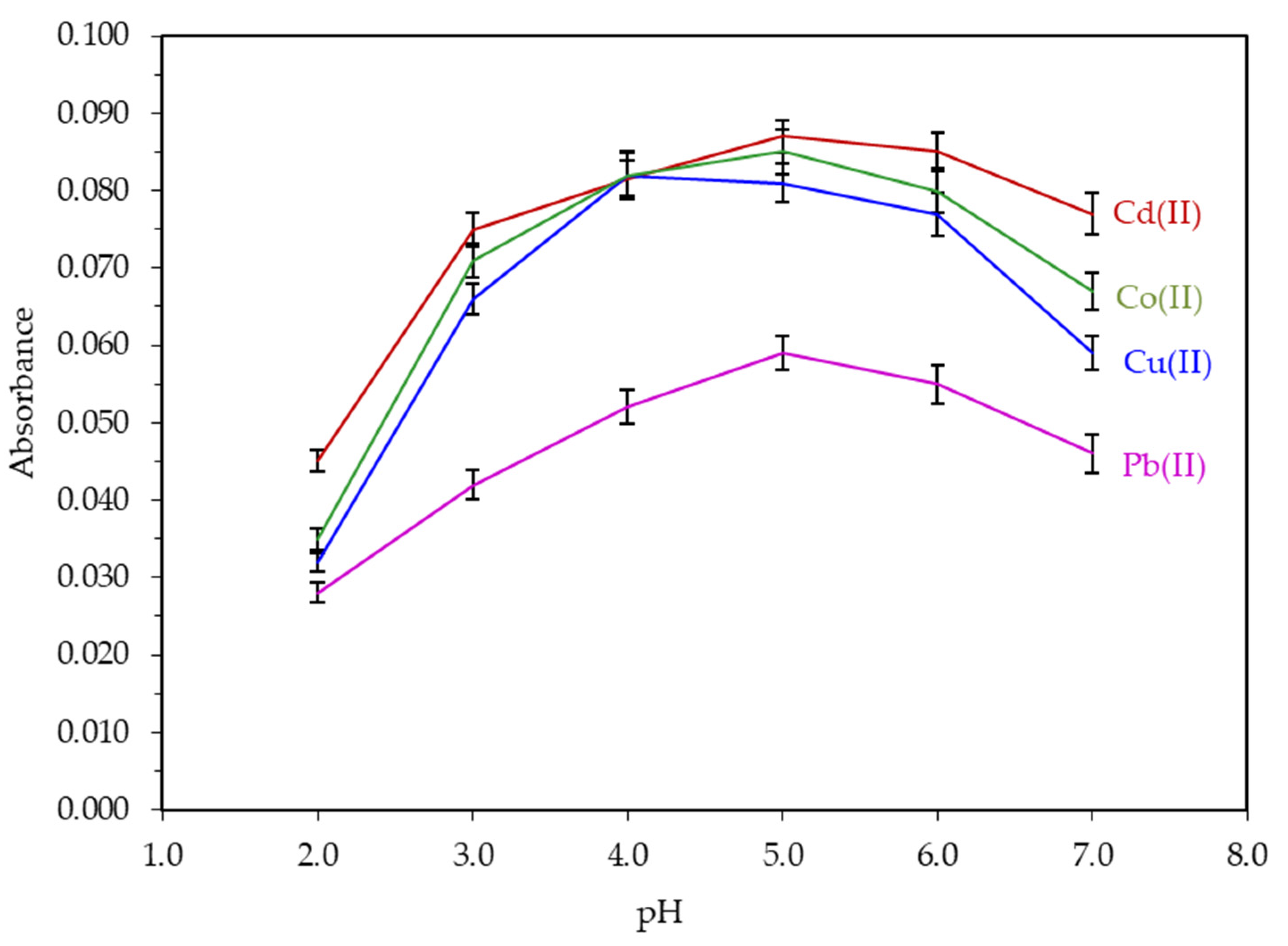
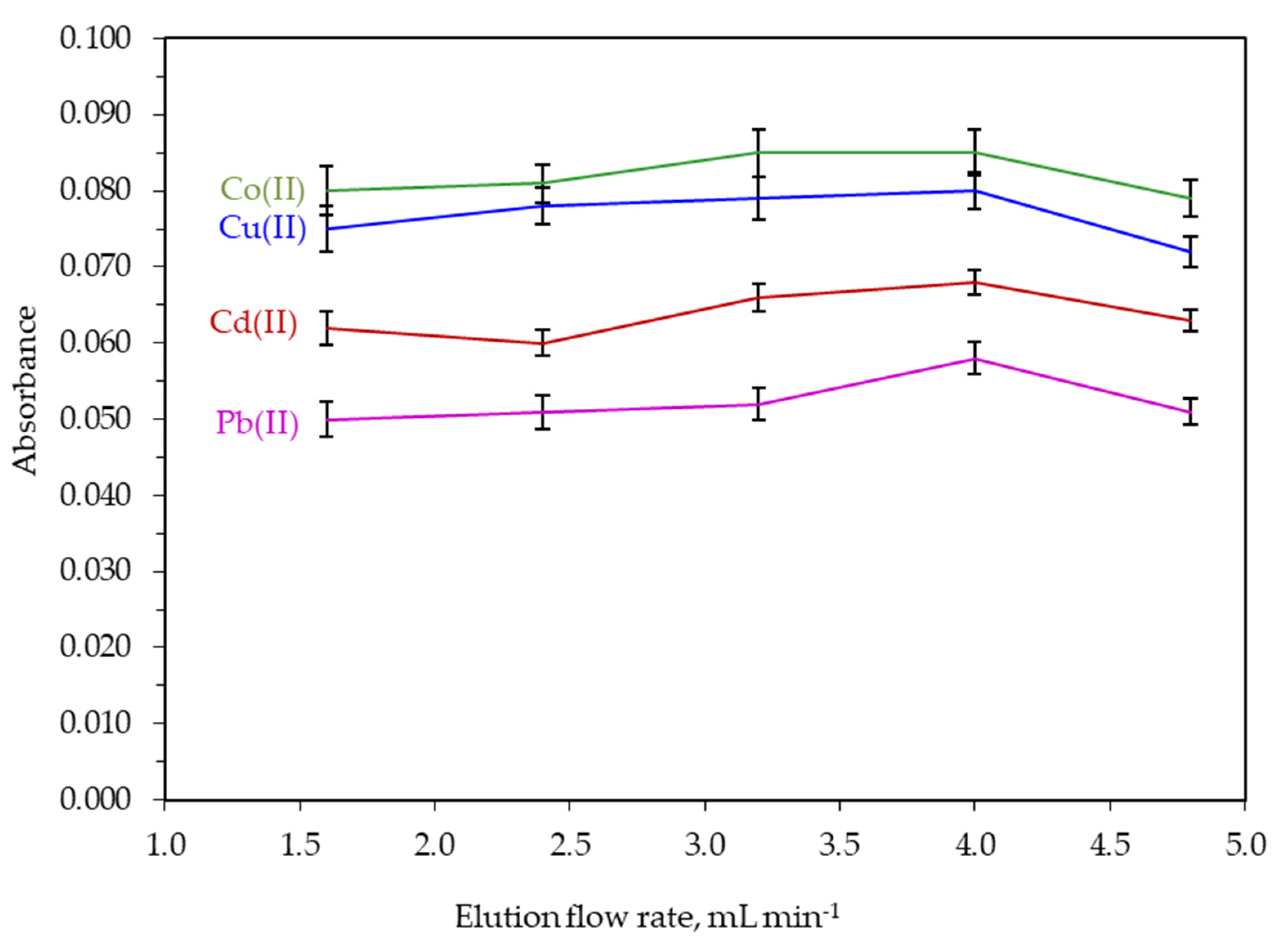
3.1. Optimization of Extraction/Preconcentration Conditions
3.2. Study of Interference Ions
3.3. Figures of Merit
3.4. Comparison of FI-FAAS Method with Other On-Line Sorbent Extraction Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, M.; Yuan, H.; Liu, B.; Peng, J.; Xu, L.; Yang, D. Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 2020, 12, 5747–5766. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.; Estela, J.M.; Cerdà, V. Application of flowing stream techniques to water analysis: Part III. Metal ions: Alkaline and alkaline-earth metals, elemental and harmful transition metals, and multielemental analysis. Talanta 2004, 63, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Hann, S.; Worsfold, P.J.; Miró, M. On-line sample treatment coupled with atomic spectrometric detection for the determination of trace elements in natural waters. J. Anal. At. Spectrom. 2020, 35, 643–670. [Google Scholar] [CrossRef]
- Butler, O.T.; Cairns, W.R.L.; Cook, J.M.; Davidson, C.M.; Mertz-Kraus, R. Atomic spectrometry update-a review of advances in environmental analysis. J. Anal. At. Spectrom. 2018, 33, 8–56. [Google Scholar] [CrossRef]
- Liska, I. On-line versus off-line solid-phase extraction in the determination of organic contaminants in water. Advantages and limitations. J. Chromatogr. A 1993, 655, 163–176. [Google Scholar] [CrossRef]
- Karadaş, C.; Turhan, O.; Kara, D. Synthesis and application of a new functionalized resin for use in an on-line, solid phase extraction system for the determination of trace elements in waters and reference cereal materials by flame atomic absorption spectrometry. Food Chem. 2013, 141, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.N.; Giakisikli, G.; Xidia, S.; Miró, M. On-line sorptive preconcentration platform incorporating a readily exchangeable Oasis HLB extraction micro-cartridge for trace cadmium and lead determination by flow injection-flame atomic absorption spectrometry. Microchem. J. 2011, 98, 66–71. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Mitani, C. Flow injection dual-syringe sorbent extraction platform for metal determination in environmental matrices utilizing a new strong cation exchange sorbent micro-cartridge and flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2012, 92, 1276–1288. [Google Scholar] [CrossRef]
- Giakisikli, G.; Zachariadis, P.; Kila, I.; Teshima, N.; Anthemidis, A. Flow Injection Solid Phase Extraction for Trace Metal Determination Using a Chelating Resin and Flame Atomic Absorption Spectrometry Detection. Anal. Lett. 2016, 49, 929–942. [Google Scholar] [CrossRef]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. TrAC-Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC-Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Bitas, D.; Samanidou, V. Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk. Molecules 2018, 23, 316. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC-Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Kazantzi, V.; Anthemidis, A. Fabric sol-gel phase sorptive extraction technique: A review. Separations 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.; Samanidou, V. Fabric Phase Sorptive Extraction: A Paradigm Shift Approach in Analytical and Bioanalytical Sample Preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef]
- Castro, G.R.; Cristante, V.M.; Padilha, C.C.F.; Jorge, S.M.A.; Florentino, A.O.; Prado, A.G.S.; Padilha, P.M. Determination of Cd(II), Cu(II) and Ni(II) in aqueous samples by ICP-OES after on-line preconcentration in column packed with silica modified with 2-aminothiazole. Microchim. Acta 2008, 160, 203–209. [Google Scholar] [CrossRef]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef]
- Kazantzi, V.; Kabir, A.; Furton, K.G.; Anthemidis, A. Fabric fiber sorbent extraction for on-line toxic metal determination by atomic absorption spectrometry: Determination of lead and cadmium in energy and soft drinks. Microchem. J. 2018, 137, 285–291. [Google Scholar] [CrossRef]
- Lazaridou, E.; Kabir, A.; Furton, K.G.; Anthemidis, A. A Novel Glass Fiber Coated with Sol-Gel Poly-Diphenylsiloxane Sorbent for the On-Line Determination of Toxic Metals Using Flow Injection Column Preconcentration Platform Coupled with Flame Atomic Absorption Spectrometry. Molecules 2020, 26, 9. [Google Scholar] [CrossRef]
- Matveichuk, Y.V.; Rakhman’Ko, E.M.; Yasinetskii, V.V. Thiocyanate complexes of d metals: Study of aqueous solutions by UV, visible, and IR spectrometry. Russ. J. Inorg. Chem. 2015, 60, 100–104. [Google Scholar] [CrossRef]
- Sui, D.P.; Chen, H.X.; Li, D.W. Sol-gel-derived thiocyanato-functionalized silica gel sorbents for adsorption of Fe(III) ions from aqueous solution: Kinetics, isotherms and thermodynamics. J. Sol-Gel Sci. Technol. 2016, 80, 504–513. [Google Scholar] [CrossRef]
- Karbalaie, B.; Rajabi, M.; Fahimirad, B. Dopamine-modified magnetic graphene oxide as a recoverable sorbent for the preconcentration of metal ions by an effervescence-assisted dispersive micro solid-phase extraction procedure. Anal. Methods 2020, 12, 2338–2346. [Google Scholar] [CrossRef]
- Liang, Y.; Jun, M.; Liu, W. Enhanced Removal of Lead(II) and Cadmium(II) from Water in Alum Coagulation by Ferrate(VI) Pretreatment. Water Environ. Res. 2007, 79, 2420–2426. [Google Scholar] [CrossRef]
- Sheha, R.R.; Moussa, S.I.; Attia, M.A.; Sadeek, S.A.; Someda, H.H. Development and application of carbon nanotubes reinforced hydroxyapatite composite in separation of Co(II) and Eu(III) ions from aqueous solutions. Radiochim. Acta 2019, 107, 67–82. [Google Scholar] [CrossRef]
- Yang, B.; Tong, X.; Deng, Z.; Lv, X. The adsorption of Cu species onto pyrite surface and its effect on pyrite flotation. J. Chem. 2016, 2016, 4627929. [Google Scholar] [CrossRef]
- Zachariadis, G.A.; Anthemidis, A.N.; Bettas, P.G.; Stratis, J.A. Determination of lead by on-line solid phase extraction using a PTFE micro-column and flame atomic absorption spectrometry. Talanta 2002, 57, 919–927. [Google Scholar] [CrossRef]
- Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G.; Anthemidis, A. On-line fabric disk sorptive extraction via a flow preconcentration platform coupled with atomic absorption spectrometry for the determination of essential and toxic elements in biological samples. Separations 2018, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.D.; Han, D.M. Multi-walled carbon nanotubes as sorbent for flow injection on-line microcolumn preconcentration coupled with flame atomic absorption spectrometry for determination of cadmium and copper. Anal. Lett. 2006, 39, 2285–2295. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Segatelli, M.G.; Pereira, A.C.; de Santana Santos, A.; Kubota, L.T.; Luccas, P.O.; Tarley, C.R.T. Solid-phase extraction system for Pb (II) ions enrichment based on multiwall carbon nanotubes coupled on-line to flame atomic absorption spectrometry. Talanta 2007, 71, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
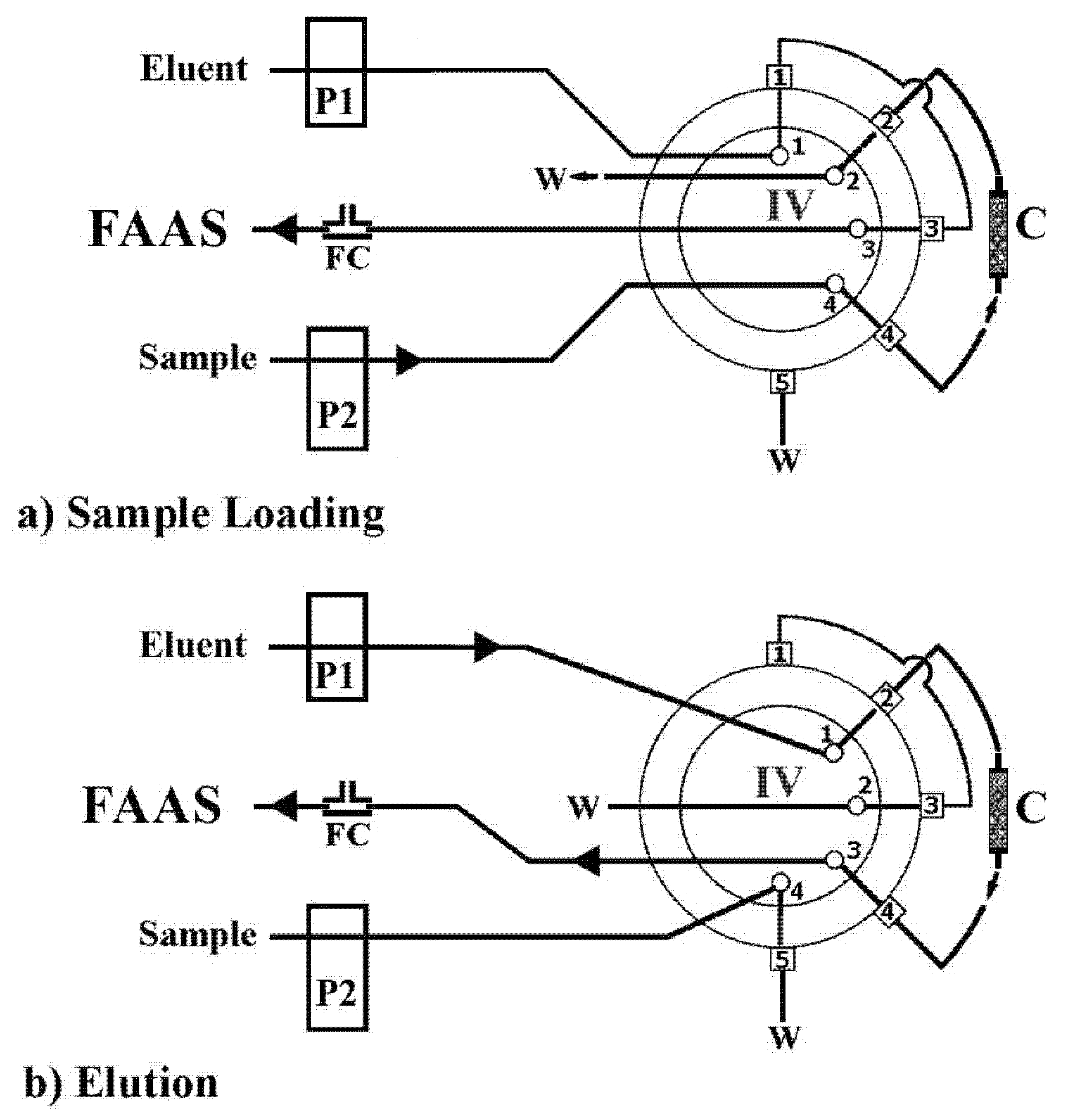


| Step | IV | P1 | P2 | Transported Medium | Flow Rate (mL min−1) | Time (s) | Operation |
|---|---|---|---|---|---|---|---|
| 1 | Load | off | on | Sample/standard solution | 10.0 | 120 | Preconcentration |
| 2 | Elute | on | off | 1.0 mol L−1 HNO3 | 4.0 | 30 | Elution/Measurement |
| Parameter | Cd(II) | Co(II) | Cu(II) | Pb(II) |
|---|---|---|---|---|
| Preconcentration time (s) | 120 | 120 | 120 | 120 |
| Sampling frequency (h−1) | 25 | 25 | 25 | 25 |
| Regression equation | A = 0.0017 + 0.0154 [Cd(II)] | A = 0.0026 + 0.0043 [Co(II)] | A = 0.0024 + 0.0045 [Cu(II)] | A = 0.0032 + 0.0012 [Pb(II)] |
| Enhancement factor | 152 | 105 | 73 | 126 |
| Detection limit (3 s, μg L−1) | 0.15 | 0.5 | 0.5 | 1.9 |
| Quantification limit (10 s, μg L−1) | 0.5 | 1.7 | 1.7 | 6.4 |
| Linear range (μg L−1) | 0.5–25 | 1.7–80 | 1.7–80 | 6.4–300 |
| Precision (RSD, n = 10) (%) | 2.6 | 3.8 | 2.8 | 3.2 |
| Correlation coefficient (r) | 0.9994 | 0.9985 | 0.9995 | 0.9989 |
| Certified Reference Material | Certified Value (μg L−1) | Found * | texp. | Recovery (%) |
|---|---|---|---|---|
| CRM 1643e | ||||
| Cd | 6.568 ± 0.073 | 6.3 ± 0.2 | 2.321 | 95.9 |
| Co | 27.06 ± 0.32 | 25.6 ± 0.7 | 3.613 | 94.6 |
| Cu | 22.76 ± 0.31 | 22.5 ± 0.5 | 0.901 | 98.9 |
| Pb | 19.63 ± 0.21 | 19.1 ± 0.5 | 1.836 | 97.3 |
| BCR 278-R | ||||
| Cd | 0.348 ± 0.007 | 0.33 ± 0.02 | 3.118 | 94.8 |
| Co | - | - | - | - |
| Cu | 9.45 ± 0.13 | 9.2 ± 0.4 | 1.083 | 97.4 |
| Pb | 2.00 ± 0.04 | 1.96 ± 0.06 | 1.155 | 98.0 |
| Sample | Analyte (μg L−1) | Added * | Found * | Recovery (%) |
|---|---|---|---|---|
| Strymon River | Cd(II) | - | ND | - |
| 5.0 | 4.7 ± 0.2 | 94.0 | ||
| Co(II) | - | 2.2 ± 0.1 | - | |
| 10.0 | 11.9 ± 0.4 | 97.0 | ||
| Cu(II) | - | 5.2 ± 0.2 | - | |
| 10.0 | 15.0 ± 0.4 | 98.0 | ||
| Pb(II) | - | ND | - | |
| 30.0 | 29.1 ± 0.9 | 97.0 | ||
| Prespa Lake | Cd(II) | - | ND | - |
| 5.0 | 5.1 ± 0.2 | 102.0 | ||
| Co(II) | - | ND | - | |
| 10.0 | 9.6 ± 0.3 | 96.0 | ||
| Cu(II) | - | 4.2 ± 0.1 | - | |
| 10.0 | 13.6 ± 0.4 | 94.0 | ||
| Pb(II) | - | ND | - | |
| 30.0 | 28.5 ± 1.1 | 95.0 | ||
| Urine | Cd(II) | - | ND | - |
| 5.0 | 4.6 ± 0.15 | 92.0 | ||
| Co(II) | - | ND | - | |
| 10.0 | 10.5 ± 0.4 | 105.0 | ||
| Cu(II) | - | 6.5 ± 0.2 | - | |
| 10.0 | 16.23 ± 0.4 | 98.0 | ||
| Pb(II) | - | ND | - | |
| 30.0 | 29.5 ± 0.9 | 98.3 |
| Analyte | Sorbent/Support | Ligand | SC (mL) | PT (s) | LOD (μg L−1) | RSD% | EF | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cd(II), Co(II), Cu(II), Mn(II), Pb(II) | Amberlite XAD-4 2,6-pyridinedicarboxaldehyde functionalized | - | 10 | 660 | 0.13–2.19 | - | 23.6–28.9 | [6] |
| Cd(II), Pb(II) | Oasis HLB© | DDTP | 12 | 90 | 0.09–0.9 | <2.9 | 155–180 | [7] |
| Cd(II), Co(II), Cu(II), Pb(II) | HypersepSCX | - | 15 | 150 | 0.14–2.1 | <3.3 | 77–99 | [8] |
| Cd(II), Cu(II), Pb(II) | Nobias chelate PA-1 | - | 20 | 120 | 0.1–1.0 | <3.3 | 98–106 | [9] |
| Cd(II), Pb(II) | polyester fabric fibers coated sol-gel PDMS | DDTC | 18 | 90 | 0.3–1.6 | <2.9 | 40–167 | [19] |
| Pb(II) | Glass fiber coated sol-gel PDPS | APDC | 16 | 120 | 1.1 | 3.0 | 215 | [20] |
| Cd(II), Cu(II), Pb(II) | Polyester fabric disks coated sol-gel PCL-DMS-CL | APDC | 18 | 90 | 0.15–1.62 | <3.5 | 36–250 | [28] |
| Cd(II), Cu(II) | MWCNTs | - | 4.3 | 60 | 0.11–0.30 | <2.4 | 24–25 | [29] |
| Pb(II) | MWCNTs | - | 5 | 240 | 2.6 | <7.7 | 44 | [30] |
| Cd(II), Co(II), Cu(II), Pb(II) | Sol-gel Silica thiocyanatopropyl functionalized | 20 | 120 | 0.15–1.9 | <3.8 | 73–152 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Kabir, A.; Furton, K.G.; Zachariadis, G.A.; Anthemidis, A. Automated Solid Phase Extraction of Cd(II), Co(II), Cu(II) and Pb(II) Coupled with Flame Atomic Absorption Spectrometry Utilizing a New Sol-Gel Functionalized Silica Sorbent. Separations 2021, 8, 100. https://doi.org/10.3390/separations8070100
Manousi N, Kabir A, Furton KG, Zachariadis GA, Anthemidis A. Automated Solid Phase Extraction of Cd(II), Co(II), Cu(II) and Pb(II) Coupled with Flame Atomic Absorption Spectrometry Utilizing a New Sol-Gel Functionalized Silica Sorbent. Separations. 2021; 8(7):100. https://doi.org/10.3390/separations8070100
Chicago/Turabian StyleManousi, Natalia, Abuzar Kabir, Kenneth G. Furton, George A. Zachariadis, and Aristidis Anthemidis. 2021. "Automated Solid Phase Extraction of Cd(II), Co(II), Cu(II) and Pb(II) Coupled with Flame Atomic Absorption Spectrometry Utilizing a New Sol-Gel Functionalized Silica Sorbent" Separations 8, no. 7: 100. https://doi.org/10.3390/separations8070100
APA StyleManousi, N., Kabir, A., Furton, K. G., Zachariadis, G. A., & Anthemidis, A. (2021). Automated Solid Phase Extraction of Cd(II), Co(II), Cu(II) and Pb(II) Coupled with Flame Atomic Absorption Spectrometry Utilizing a New Sol-Gel Functionalized Silica Sorbent. Separations, 8(7), 100. https://doi.org/10.3390/separations8070100











