Abstract
This study systematically investigated the oxidative treatment of five selected pesticides, alachlor (ALA), carbendazim (CAR), diuron (DIU), pyrimethanil (PYR), and tebuconazole (TEB), by comparing their relative reactivities as a function of three different oxidative treatment processes (i.e., chlorine (HOCl), ozone (O3), and ozone/hydrogen peroxide (O3/H2O2)) under various oxidant dosages, reaction times, and pH conditions. For oxidative treatment, pesticide standards were spiked into rainwater. The removal efficiency of the selected pesticides varied considerably depending on the oxidative treatment processes. HOCl, O3, and O3/H2O2 treatments were highly effective at eliminating CAR (>80%) and PYR (>99%), while they were not significantly effective in removing TEB (<20%). In the case of DIU, HOCl (81%) was shown to be more effective than O3 (24%) and O3/H2O2 (49%). The removal efficiency of ALA was in the order of O3/H2O2 (49%) > O3 (20%) > HOCl (8.5%). The effect of increasing the solution pH from 5.0 to 9.0 on pesticide degradation varied between the oxidative treatment processes. Additionally, NH4+, NO2−, and humic acid in rainwater significantly inhibited pesticide degradation.
1. Introduction
Pesticides are commonly used to enhance crop production and prevent plant diseases, such as fungal infections [1]. The United States Environmental Protection Agency reported that the global application of pesticides totaled more than 2 million tons and over USD 39 billion in 2007 [2]. Although most pesticide contamination occurs due to pesticide use in agricultural fields, the use of pesticides in urban areas has gained further attention owing to their potential risk to the environment and human health (e.g., mutagenicity, toxicity, and carcinogenicity) [3]. Several studies have demonstrated the presence of pesticides in urban rainwater globally [4,5]. Alachlor (ALA) and diuron (DIU) were detected in rainwater samples in Strasbourg, France, with maximum concentrations of 5590 and 1025 ng/L, respectively [4]. Tebuconazole (TEB) was detected in Brunswick Land (Germany) in concentrations ranging between 12 and 187 ng/L [5] (Supplementary information, Table S1).
Water shortage has become a significant global problem due to climate change, rapid population growth, and urbanization. There is an increasing demand for alternative water sources, such as rainwater, to secure a sustainable water supply [6]. In recent years, rainwater has attracted attention as an urban water resource for plant irrigation, flushing toilets, laundry, and cleaning [7,8]. However, a wide range of micropollutants, including pesticides, pharmaceuticals, and endocrine-disrupting chemicals, have been detected in significant quantities in rainwater runoff [9]. Several studies have demonstrated that various pesticides may be detected in rainwater due to the high pesticide variability [4,10]. Although the average concentration of pesticides detected in rainwater is low (μg/L), the potential toxicity of pesticides can increase the risk associated with rainwater reuse [11]. Furthermore, rainwater can trigger the transport of pollutants, including pesticides and particulate matter, into surface water [12]. Therefore, pesticides must be eliminated during the rainwater treatment process.
Conventional treatment technologies, such as physical and biological treatments, are designed to mitigate pesticides from rainwater [13]. However, several researchers have found that the performance of these treatment technologies is not sufficient to reduce pesticides due to their variability. Although chemical oxidation processes using chlorine (HOCl), ozone (O3), and O3 with hydrogen peroxide (O3/H2O2) are relatively expensive compared to physical and biological treatments, these processes are more effective and stable for removing organic pollutants, including pesticides. Furthermore, these oxidation processes are more suitable to satisfy the acceptable pesticides concentrations in water quality regulations (U.S.EPA regulation for drinking water: ALA = 400 μg/L and DIU = 70 μg/L) to reuse rainwater for potable and irrigation waters [14]. The HOCl is the most commonly used oxidant in the water treatment process globally due to its low operating cost, high efficiency, and stability of chlorine residue in the distribution system, despite the disadvantage of producing harmful disinfection by-products, including trihalomethane (THM) and haloacetic acids (HAAs) [15]. O3 has been proven to be effective for micropollutant degradation through direct reaction with molecular O3 and indirect reaction with hydroxyl radicals produced by O3 decomposition [16]. In recent years, water and wastewater treatment plants have applied the O3/H2O2 advanced oxidation process (AOP) due to its relatively higher effectiveness compared to the corresponding conventional ozonation process [17]. However, there is a need for a comprehensive study on the effects of rainwater treatment via HOCl, O3, and O3/H2O2 on the removal behaviors of pesticides.
The primary purpose of this study was to provide valuable insights into the removal behavior of selected pesticides during the HOCl, O3, and O3/H2O2 processes. Therefore, the degradation of components in the rainwater by the oxidation process was identified and directly correlated to the decrease in the selected pesticides to evaluate the efficiency of HOCl, O3, and O3/H2O2. Furthermore, the effects of pH, humic acid (HA), and inorganic matters on the removal efficiency of the selected pesticides were systematically investigated to elucidate their degradation mechanisms in the three different oxidation processes.
2. Materials and Methods
2.1. Rainwater Sample Collection
Rainwater samples (n = 4) were collected within initial precipitation (within 4 h after rainfall) between July and September 2019 from the engineering building rooftop in Kangwon National University (Chunchen-si, Republic of Korea; latitude 37°52′05″ N, longitude 127°44′19″ E) using acid-cleaned funnels (45 cm diameter) connected to a polyethylene bottle. Chuncheon-si is located approximately 110 km to the east of Seoul (capital city), Republic of Korea. The rainwater samples were immediately filtered using cellulose nitrate membranes (Sartorius Stedim Biotech, Goettingen, Germany) with a pore size of 0.45 µm and stored in amber glass bottles (2 L) at 4 °C.
2.2. Pesticides and Reagents
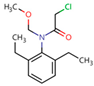
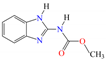
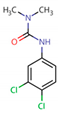
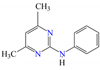
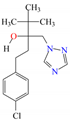
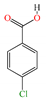
Five different pesticides, ALA (2-chloro-N-(2,6-diethylphenyl)-N-(methoxymethyl)acetamide), carbendazim (CAR, methyl N-(1H-benzimidazol-2-yl) carbamate), DIU (1-(3,4-dichlorophenyl)-3,3-dimethylurea), pyrimethanil (PYR, 4,6-dimethyl-N-phenylpyrimidin-2-amine), and TEB (1-(4-chlorophenyl)-4,4-dimethyl-3-(1,2,4-triazol-1-ylmethyl) pentan-3-ol), were selected as representative compounds with various functional groups closely related to HOCl, O3, and O3/H2O2 reactivities, and -chlorobenzoic acid (pCBA) was used to probe these compounds for hydroxyl radicals (Table 1). Sodium hypochlorite (NaOCl) containing 5% free chlorine was obtained from Junsei Chemical Co., Ltd. (Tokyo, Japan), and H2O2 (30 wt% solution) was purchased from Daejung Chemicals & Metals Co., Ltd. (Siheung-si, Republic of Korea). Sodium hydroxide (NaOH), hydrochloric acid (HCl), HA, ammonium acetate (>98%), sodium nitrite (>97%), and potassium phosphate monobasic (>99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade acetonitrile (ACN) was obtained from J.T. Baker (Deventer, The Netherlands), and phosphoric acid (H3PO4) was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Deionized (DI) water (resistivity >18.2 MΩ cm−1, Barnstead Nanopure Water System, Lake Balboa, CA, USA) was used to prepare stock solutions of the selected pesticides (concentration of each pesticide = 1 mmol/L). These stocks were stored at 4 °C in the dark before use. The physicochemical properties of the selected pesticides are summarized in Table 1.

Table 1.
Physicochemical characteristics of the selected pesticides.
2.3. Experimental Procedures
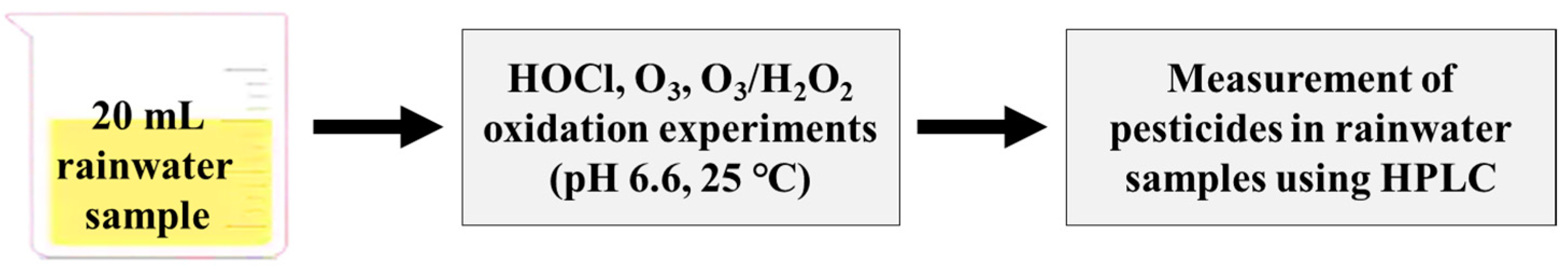
Oxidant consumption experiments for HOCl and O3 were conducted for rainwater, where each oxidant was applied at a dosage of 40 μM at pH 6.6 in 20 mL reaction volume. Samples (5 mL) were collected to measure residual oxidants at several time intervals: 0.5, 1, 2, 5, 10, and 30 min. HOCl, O3, and O3/H2O2 oxidation experiments were carried out on a bench-scale using rainwater at pH 6.6 and the range of specific O3 values (0.17–0.20 mg/s; O3 generator, LAB-II, Ozone Tech, Daejeon, Republic of Korea) and HOCl (oxidant/dissolved organic carbon (DOC) = 10, 20, 30, and 40 µM) and H2O2 (molar ratio = 0.5) dosages at room temperature (25 ± 1 °C) (Figure 1). Each pesticide was spiked into a 25 mL reaction bottle at a concentration of 2 μM. For the O3/H2O2 experiment, a stock solution of H2O2 was added before adding the O3. At the end of the reaction time (30 min), the samples were immediately quenched with 50 μL of thiosulfate solution (100 mM) and analyzed for residual pesticide concentrations using HPLC.
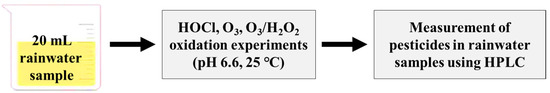
Figure 1.
The diagram for batch-scale oxidation experiments for pesticides in rainwater samples.
2.4. Analytical Methods
The concentrations of the selected pesticides (i.e., ALA, CAR, DIU, PYR, and TEB) in the rainwater samples were quantified using high-performance liquid chromatography (HPLC) fitted with an ultraviolet absorbance (UVA) detector (SPD-10AVP, Shimadzu, Kyoto, Japan) and an XDB C18 column (ZORBAX Eclipse ®, 4.6 × 150 mm, inner diameter = 5 µm, Agilent, Santa Clara, CA, USA) at a constant flow rate of 1.0 mL/min for 15 min. Acetonitrile/0.05 M phosphoric acid (50:50, v/v) at a 1.0 mL/min flow rate was used as the mobile phase for ALA, CAR, DIU, PYR, TEB, and pCBA. The wavelength of the UVA detector was set at 210 nm for the selected pesticides and 240 nm for p-CBA. A total organic carbon analyzer (TOC-VCPH, Shimadzu, Kyoto, Japan) equipped with total dissolved nitrogen (TN) measuring unit (TNM-1, Shimadzu, Kyoto, Japan) was used to quantify the DOC and TN in rainwater (detection limits for DOC and TN were 0.012 mg C/L and 0.006 mg N/L, respectively). Inorganic ions, such as ammonia (NH4+), sodium (Na+), calcium (Ca2+), magnesium (Mg2+), chlorine (Cl−), nitrite (NO2−), nitrate (NO3−), sulfate (SO42−), and phosphate (PO43−), were measured using ion chromatography (761 Compact IC, Metrohm, Herisau, Switzerland). NH4+ and NO2− ions were determined by colorimetric methods at 655 (DIN 38 406, German standard methods) and 540 nm (DIN EN 26 777, German standard methods), respectively. Several colorimetric methods have been used to measure the oxidant concentrations in rainwater. The dissolved O3 in the rainwater samples were detected using the indigo method (UV wavelength = 600 nm, Ozone Accuvac ® Amplules, Hach Company, Loveland, CO, USA), and the determination of free chlorine quantification was used by the N,N-diethyl-p-phenylenediamine (DPD) free chlorine kit and colorimeter (Hach Company, Loveland, CO, USA) [19,20].
2.5. Statistical Analysis
The statistical analysis was carried out using a SigmaPlot (Version 12.5, Systat Software, Inc, CA, USA). All experiments were performed in triplicate. Furthermore, the results (i.e., mean ± standard deviation) are presented in the manuscript.
3. Results and Discussion
3.1. Rainwater Quality Analyses
Table 2 shows the physicochemical characteristics of the rainwater samples at the study site. The conductivity and pH values were not significantly different during collecting periods (conductivity: 1.9–2.0 μS/cm; pH: 6.5–6.6). The average conductivity value (2.0 μS/cm) of the rainwater was considerably lower than that of the Korean urban precipitation (Goyang = 13.9 mS/cm; Gangneung = 30 μS/cm). In contrast, the mean pH value of 6.6 was slightly higher than that of other urban areas (Goyang = 5.6; Gangneung = 5.3) [21,22]. Moreover, the rainwater has fewer inhibition factors for oxidation treatments due to low DOC (0.4 mgC/L) and inorganic nitrogen species (TN = 70.2 μgN/L, NO3− = 51.3 μgN/L). Na+ and Ca2+ were the most abundant cations, and Cl− and SO42− were the most abundant anions. Other rainwater contents, such as NO3−, NH4+, and Mg2+, were found in μg/L levels.

Table 2.
Rainwater quality parameters (n = 3).
3.2. Consumption Kinetics of HOCl and O3 in Rainwater Sample
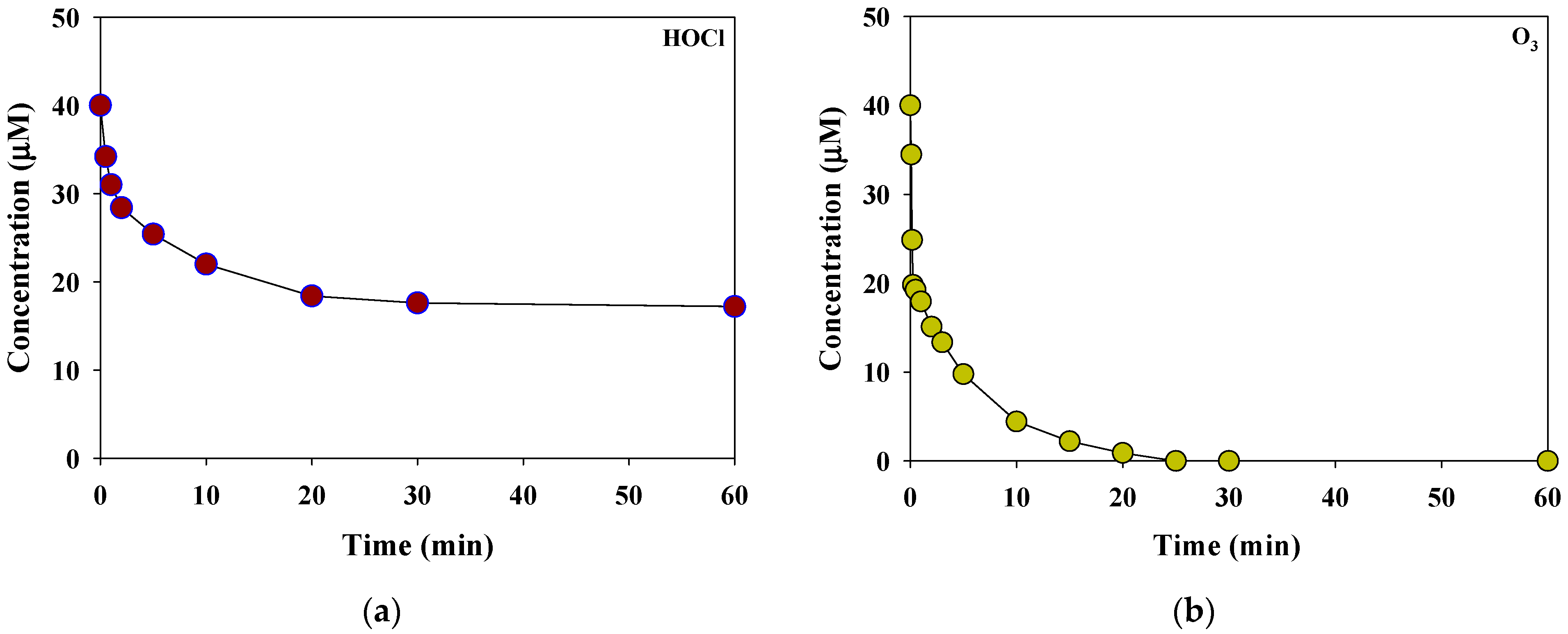
Figure 2 presents the HOCl and O3 consumption kinetics in a rainwater sample at pH 6.6 for each oxidant dose of 40 μM. The rainwater sample had a low concentration of inorganic nitrogen species, such as NO3− = 0.83 μM (51.3 μg N/L) and NH4+ = 1.03 μM (18.6 μg N/L). Therefore, the consumption of HOCl and O3 was predominantly affected by DOC (0.4 mgC/L (13.32 μM)). HOCl showed rapid consumption (29%) within 2 min from the initial oxidant dose (in terms of the initial phase), followed by a slow decrease over the next 60 min of reaction time (in terms of the second phase, the consumption rate = 70%). In contrast, O3 depleted in less than 30 min (the consumption rate during the initial phases = 49%). Similar decay behaviors have been observed in the removal of effluent organic matter in secondary wastewater effluent by HOCl and O3 [23].
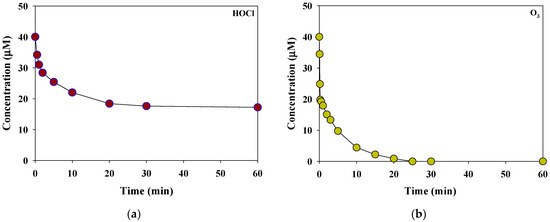
Figure 2.
Consumption kinetics of the selective oxidants, (a) HOCl and (b) O3, in the rainwater sample (initial concentration of each oxidants = 40 μM, reaction time = 1 h, temperature = 25 °C, pH = 6.6).
3.3. Effects of Oxidant Dosages
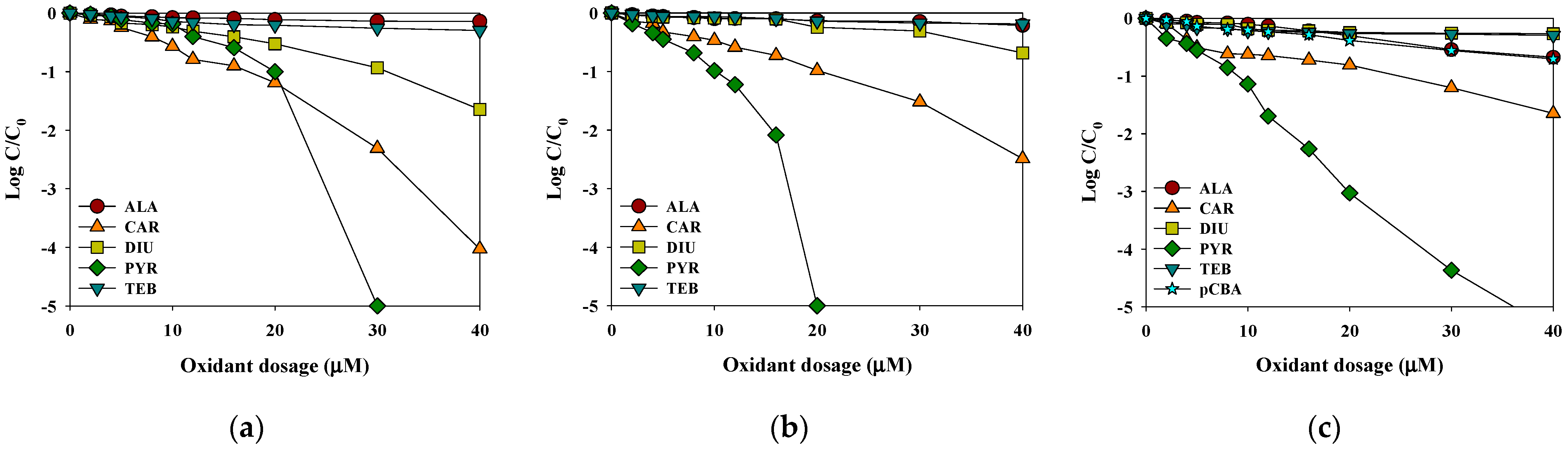
The removal efficiencies of the selected pesticides were compared in the rainwater samples using selective (HOCl and O3) and non-selective (hydroxyl radicals: O3/H2O2) oxidants. With increasing oxidant dosage, the degradation of the selected pesticide’s removal efficiency increased. Figure 3 shows that the removal efficiencies of CAR, PYR, and DIU by HOCl and O3 were between 15% and 50% for 10 µM oxidant concentration after 30 min, while the removal efficiency of ALA and TEB was only 9.5% under the same experimental conditions. After increasing the oxidant concentration to 40 µM, the removal efficiency of CAR and DIU reached between 40% and 90% after 30 min, while the efficiency of ALA and TEB was enhanced to 21%. PYR was fully degraded with all oxidants. For HOCl and O3 (the selective oxidants), there was a lag phase at lower oxidant dosages, where the pesticide concentration decreased slightly with increasing oxidant dosage (Figure 2a,b). The low removal efficiency in the lag phase was attributed to the high competition for the selective oxidants between the CAR and PYR and the DOC [23]. Additionally, with an increase in the selective oxidant dosages higher than the initial consumption by DOC, the residual concentrations of CAR and PYR started to decrease significantly. However, the removal rates of ALA and TEB were less than 21% for HOCl and O3 processes. In contrast, in the case of O3/H2O2, the removal efficiencies of the selected pesticides were linearly proportional to the applied hydroxyl radical dosage (Figure 3c). These observations indicate that the magnitude of the competition for hydroxyl radicals between pesticides and rainwater matrix remained constant during the entire oxidation process. Based on these experiments on the removal efficiency of the selected pesticides according to the HOCl, O3, and O3/H2O2 dosages, 40 μM was selected as the optimal dosage and used in subsequent experiments.
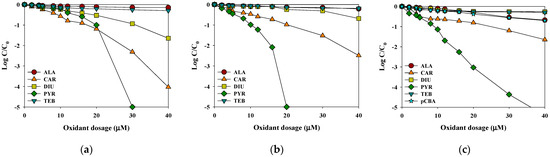
Figure 3.
The effects of the oxidant dosage on the removal efficiencies of the selected pesticides by (a) HOCl, (b) O3 and (c) O3/H2O2 (initial concentration of each pesticide and pCBA = 2 μM, reaction time = 0.5 h, temperature = 25 °C, pH = 6.6).
3.4. Removal Efficiency of the Selected Pesticides in Rainwater Samples by HOCl, O3 and O3/H2O2
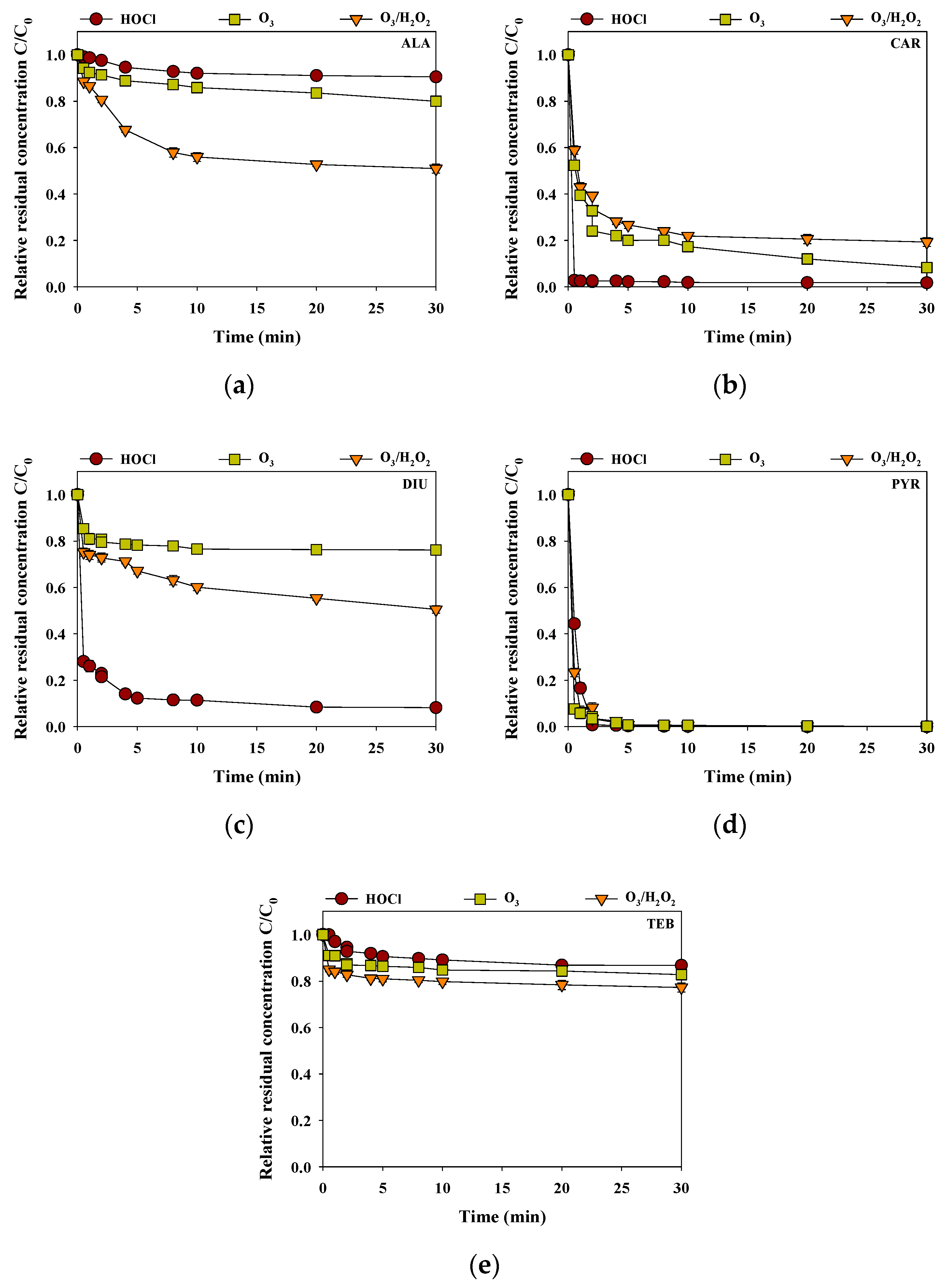
Figure 4 presents the removal efficiencies of the selected pesticides in the rainwater sample during the HOCl, O3, and O3/H2O2 processes at pH 6.6 for 0.5 h (each oxidant dose of 40 μM). For ALA and TEB, the removal efficiencies of HOCl, O3, and O3/H2O2 were in the order of O3/H2O2 (ALA = 49% and TEB = 23%) > O3 (ALA = 20% and TEB = 17%) > HOCl (ALA = 9% and TEB = 13%) (Figure 4a,e). ALA was reported to be lowly reactive with HOCl due to the inhibitive effect of the acetanilide functional group in the ALA. In addition, the ethyl groups of the ortho positions in the aromatic ring on the ALA reduced the reactivity toward the electrophilic attack of O3 to the aromatic moieties [24]. The relatively higher reactivity of TEB with O3/H2O2 compared with HOCl and O3 might be caused by the reaction between the aromatic C6-cycle or the C5 aromatic N-heterocycle and hydroxyl radicals [25]. The removal efficiencies of CAR, DIU, and PYR by HOCl (CAR = 98%, DIU = 92% and PYR = 100%) were more efficient than those of O3 (CAR = 81%, DIU = 49%, and PYR = 99%) and O3/H2O2 (CAR = 92%, DIU = 24%, and PYR = 98%) (Figure 4b–d). CAR and PYR were highly reactive to HOCl and O3, and O3/H2O2 due to the reaction sites of the CAR (C=O double bond) and PYR (the nitrogen bridge between the two rings, the phenyl ring, and the pyrimidyl ring) [26,27]. DIU was lowly reactive with O3 due to the nitrogens at the urea function groups and the aromatic ring with the two chlorine atoms [28]. These findings suggest that choosing an appropriate oxidation process is important as the reactivity of the oxidant varies with the type of pesticide.
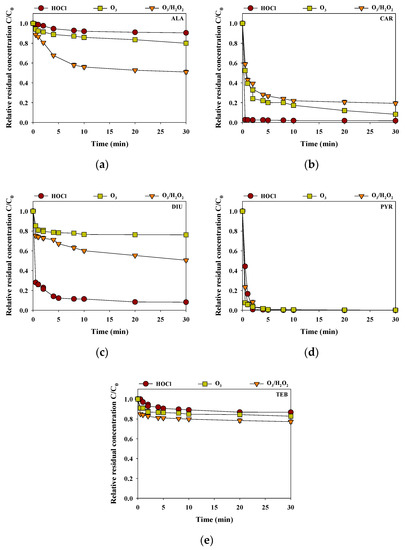
Figure 4.
Removal efficiencies of (a) ALA, (b) CAR, (c) DIU, (d) PYR and (e) TEB by HOCl, O3, and O3/H2O2 in the rainwater (initial concentration of each pesticide = 2 μM, reaction time = 0.5 h, temperature = 25 °C, pH = 6.6).
3.5. Effects of pH on the Removal of the Selected Pesticides
The changes in removal efficiencies of the selected pesticides during the HOCl, O3, and O3/H2O2 processes as a function of the pH of the rainwater samples are presented in Table 3. In general, the reactivities of the selected pesticides with HOCl, O3, and O3/H2O2 increased as the pH increased. However, in CAR, DIU, and PYR, their removal efficiencies in the chlorinated rainwater samples were strongly dependent on the pH condition. These results could be attributed to the accelerated reaction of HOCl in the chlorinated rainwater at pH 7, which was closely related to the removal of CAR, DIU and PYR [29].

Table 3.
The effects of pH on the removal efficiencies (%) of the selected pesticides by HOCl, O3 and O3/H2O2 processes (initial concentration of each pesticide = 2 μM, reaction time = 0.5 h, temperature = 25 °C).
3.6. Effects of Humic Acids on the Removal of the Selected Pesticides
The consumption of oxidants by HA is a significant factor in determining the removal efficiency of the selected pesticides. In the presence of HA (Table 4), a significant abatement in the removal efficiency (reaction time = 0.5 h) of the selected pesticides was found for the HOCl processes. Despite the higher removal efficiency of CAR, DIU, and PYR during the HOCl, O3, and O3/H2O2 processes compared to other pesticides, the influence of HA on the decrease in the selected pesticides was more pronounced in the O3/H2O2 treated rainwater samples (the difference between the relative residual concentrations (∆C/C0) of CAR, DIU, and PYR without and with 4 mg/L HA = 0.96, 0.75 and 0.41, respectively) than that for the chlorinated rainwater samples (∆C/C0 of CAR, DIU, and PYR without and with 4 mg/L HA = 0.31, 0.42 and 0.018, respectively) due to the phenolic group in HA, which could easily react with dissolved O3 and hydroxyl radicals during the O3/H2O2 processes [23]. Similar behaviors were observed for removal efficiencies of micropollutants by microbubble ozone in the HA presence condition [19].

Table 4.
The effects of HA on the removal efficiencies (%) of the selected pesticides by HOCl, O3 and O3/H2O2 processes (initial concentration of each pesticide = 2 μM, the concentrations of HA = 0, 1, and 4 mg/L, reaction time = 0.5 h, temperature = 25 °C).
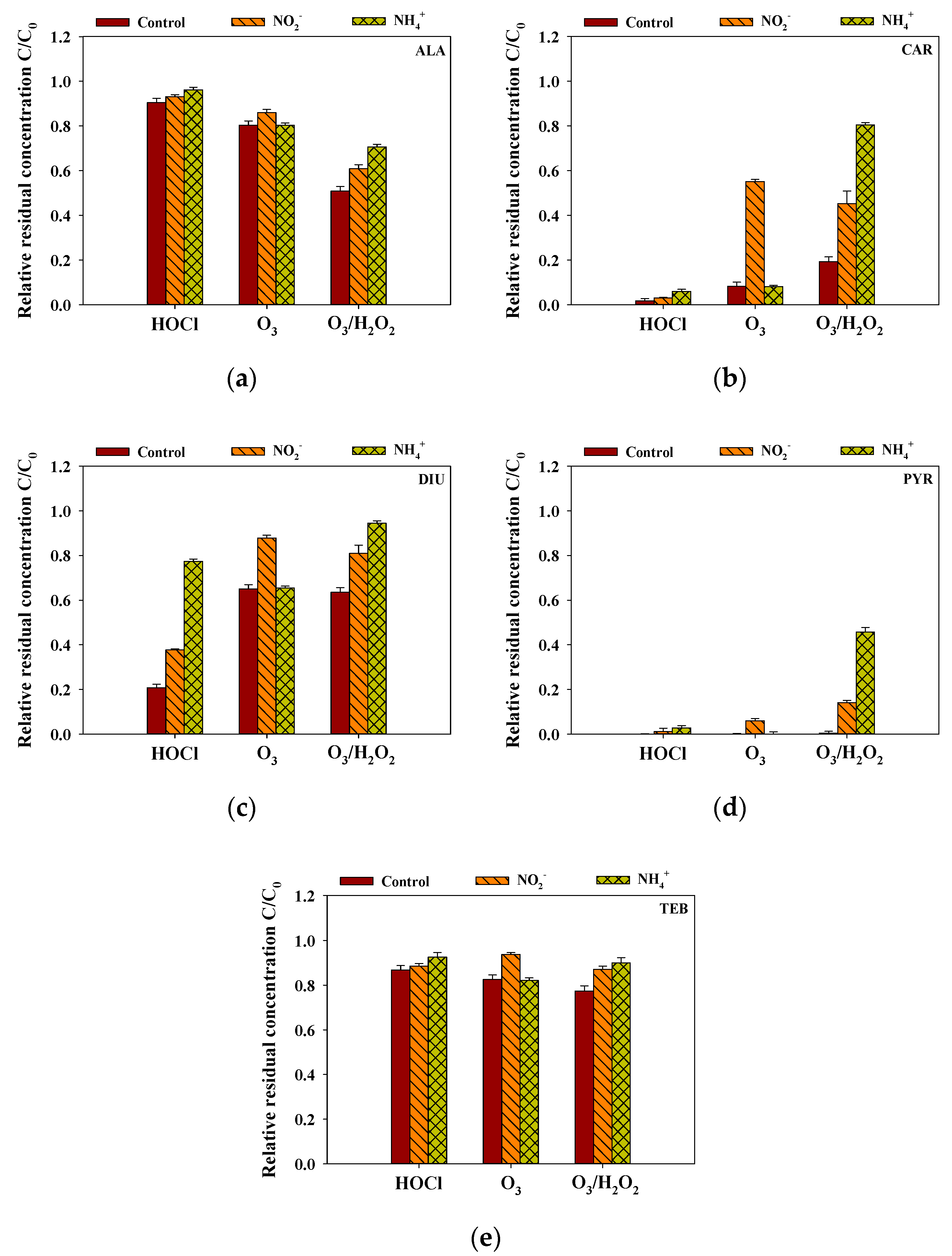
3.7. Effects of Inorganic Matters on the Removal of the Selected Pesticides
Inorganic matters, including NO2− and NH4+, generally exist in surface water and can rapidly consume the selective and non-selective oxidants and affect the removal efficiency of the selected pesticides. The rainwater samples spiked (4 mg/L) with additional NO2− and NH4+ was treated with the predetermined oxidant dosage. Figure 5 shows that, in the case of HOCl and O3/H2O2, NH4+ considerably decreased the removal efficiencies of ALA, CAR, DIU, PYR, and TEB. Remarkably, the DIU removal efficiency decreased by more than 55% for the HOCl-treated rainwater samples in the presence of NH4+ as HOCl rapidly reacted with NH4+ [30]. NH4+ significantly reduced the removal efficiencies of CAR and PYR during the O3/H2O2 treatment process (C/C0 of CAR without NH4+: 0.19; C/C0 of CAR with NH4+: 0.81; C/C0 of PYR without NH4+: 0.004; C/C0 of PYR with NH4+: 0.46). In the case of O3, the interference effects on selected pesticides were affected by NO2−, which was consistent with the relatively high k-value (3.7 × 105 M−1 S−1) of O3 with NO2− (C/C0 without NO2−: ALA = 0.80, CAR = 0.083, DIU = 0.65, PYR = 0.001, TEB = 0.82; C/C0 with NO2−: ALA = 0.86, CAR = 0.55, DIU = 0.88, PYR = 0.060, TEB = 0.94) [31]. Therefore, these observations suggest that suitable pretreatment processes are needed to enhance the removal efficiency of the selected pesticides in rainwater during the oxidation process.
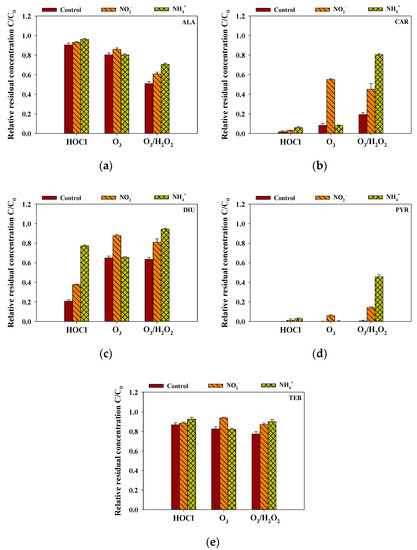
Figure 5.
The effects of inorganic matter on the removal efficiencies of (a) ALA, (b) CAR, (c) DIU, (d) PYR and (e) TEB by HOCl, O3, and O3/H2O2 (initial concentration of each pesticide = 2 μM, the concentration of each inorganic matter = 4 mg/L, reaction time = 0.5 h, temperature = 25 °C, pH = 6.6).
4. Conclusions
In this study, the potential of the various oxidant processes for the abatement of selected pesticides was evaluated and compared to the HOCl, O3 (i.e., the selective oxidants), and O3/H2O2 (i.e., hydroxyl radical as the non-selective oxidant) processes to provide deeper insights into the removal behavior of the selected pesticides. The primary outcomes were as follows:
- DOC is a major rainwater component and has a more significant influence on the consumption kinetics of the oxidants in rainwater than inorganic nitrogen species;
- The dosage of oxidants for the removal of 90% CAR was in the order of HOCl (18 μM) > O3 (21 μM) > O3/H2O2 (25 μM), and the dosage of oxidants for removal of 90% PYR was in the order of O3/H2O2 (9 μM) > O3 (11 μM) > HOCl (20 μM);
- The removal efficiencies of CAR, DIU, and PYR by HOCl were more efficient than those of O3 and O3/H2O2. In contrast, O3/H2O2 was the most effective oxidant for removing ALA and TEB;
- In general, the reactivities of the selected pesticides toward the HOCl, O3, and O3/H2O2 increased due to deprotonation when the pH of the rainwater sample was higher than the pKa values of the selected pesticides;
- The interference effects of HA and inorganic matter in the rainwater on removing the selected pesticides were more significant during the O3/H2O2 process than those of the other oxidation processes;
- These findings suggest that the oxidation processes (i.e., HOCl, O3, and O3/H2O2) might be a promising method to enhance the removal efficiencies of organic pollutants, including pesticides, practically applicable for the wastewater treatment process.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations8070101/s1, Table S1: Concentrations of pesticides in rainwater.
Author Contributions
Conceptualization, D.O. and Y.L.; methodology, D.O. and Y.L.; validation, J.S., J.K. and S.K.; formal analysis, D.O. and Y.L.; investigation, D.O. and Y.L.; data curation, D.O. and Y.L.; writing—original draft preparation, D.O. and Y.L.; writing—review and editing, K.C.; supervision, K.C.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by 2019 Research Grant (PoINT) from Kangwon National University and was supported by Korea Environment Industry & Technology Institute (KEITI) through Aquatic Ecosystem Conservation Research Program, funded by Korea Ministry of Environment (MOE) (RE202001312).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALA | Alachlor |
| C | Concentration of the selected pesticide after oxidation (μmol/L) |
| C0 | Initial concentration of the selected pesticide (μmol/L) |
| CAR | Carbendazim |
| DIU | Diuron |
| DOC | Dissolved organic carbon |
| HA | Humic acids |
| HOCl | Chlorine |
| NO2− | Nitrite |
| NH4+ | Ammonia |
| O3 | Ozone |
| O3/H2O2 | Ozone/hydrogen peroxide |
| pCBA | -chlorobenzoic acid |
| PYR | Pyrimethanil |
| TEB | Tebuconazole |
| TN | Total nitrogen |
References
- Lee, Y.-G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Kim, G.-Y.; Lee, C.-H.; Chon, K. Enhanced Adsorption Capacities of Fungicides Using Peanut Shell Biochar via Successive Chemical Modification with KMnO4 and KOH. Separations 2021, 8, 52. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage; US EPA: Washington, DC, USA, 2011. [Google Scholar]
- Rippy, M.A.; Deletic, A.; Black, J.; Aryal, R.; Lampard, J.-L.; Tang, J.Y.-M.; McCarthy, D.; Kolotelo, P.; Sidhu, J.; Gernjak, W. Pesticide occurrence and spatio-temporal variability in urban run-off across Australia. Water Res. 2017, 115, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. Pesticides analysed in rainwater in Alsace region (Eastern France): Comparison between urban and rural sites. Atmos. Environ. 2007, 41, 7241–7252. [Google Scholar] [CrossRef]
- De Rossi, C.; Bierl, R.; Riefstahl, J. Organic pollutants in precipitation: Monitoring of pesticides and polycyclic aromatic hydrocarbons in the region of Trier (Germany). Phys. Chem. Earth Parts ABC 2003, 28, 307–314. [Google Scholar] [CrossRef]
- Schang, C.; Schmitt, J.; Gao, L.; Bergman, D.; McCormak, T.; Henry, R.; McCarthy, D. Rainwater for residential hot water supply: Managing microbial risks. Sci. Total Environ. 2021, 782, 146889. [Google Scholar] [CrossRef]
- Tran, S.H.; Dang, H.T.; Dao, D.A.; Nguyen, V.-A.; Nguyen, L.T.; Han, M. On-site rainwater harvesting and treatment for drinking water supply: Assessment of cost and technical issues. Environ. Sci. Pollut. Res. 2020, 28, 11928–11941. [Google Scholar] [CrossRef]
- Wang, C.-H.; Blackmore, J.M. Supply–Demand Risk and Resilience Assessment for Household Rainwater Harvesting in Melbourne, Australia. Water Resour. Manag. 2012, 26, 4381–4396. [Google Scholar] [CrossRef]
- Vezzaro, L.; Mikkelsen, P.S. Application of global sensitivity analysis and uncertainty quantification in dynamic modelling of micropollutants in stormwater runoff. Environ. Model. Softw. 2012, 27–28, 40–51. [Google Scholar] [CrossRef]
- Hamers, T.; van den Brink, P.J.; Mos, L.; van der Linden, S.C.; Legler, J.; Koeman, J.H.; Murk, A.J. Estrogenic and esterase-inhibiting potency in rainwater in relation to pesticide concentrations, sampling season and location. Environ. Pollut. 2003, 123, 47–65. [Google Scholar] [CrossRef]
- Nowell, L.H.; Norman, J.E.; Moran, P.W.; Martin, J.D.; Stone, W.W. Pesticide Toxicity Index—A tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. Sci. Total Environ. 2014, 476–477, 144–157. [Google Scholar] [CrossRef] [Green Version]
- Delpla, I.; Jung, A.V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef]
- Gerecke, A.C.; Schärer, M.; Singer, H.P.; Müller, S.R.; Schwarzenbach, R.P.; Sägesser, M.; Ochsenbein, U.; Popow, G. Sources of pesticides in surface waters in Switzerland: Pesticide load through waste water treatment plants––current situation and reduction potential. Chemosphere 2002, 48, 307–315. [Google Scholar] [CrossRef]
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Holland, P.; Katayama, A.; Kurihara, N.; Linders, J.; Unsworth, J. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1123–1155. [Google Scholar] [CrossRef]
- Kudlek, E. Identification of Degradation By-Products of Selected Pesticides During Oxidation and Chlorination Processes. Ecol. Chem. Eng. S 2019, 26, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Wacławek, S. Do We Still Need a Laboratory to Study Advanced Oxidation Processes? A Review of the Modelling of Radical Reactions used for Water Treatment. Ecol. Chem. Eng. S 2021, 28, 11–28. [Google Scholar] [CrossRef]
- Acero, J.L.; Von Gunten, U. Characterization of oxidation processes: Ozonation and the AOPO3/H2O2. J. Am. Water Work. Assoc. 2001, 93, 90–100. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Cho, K.H.; Chon, K. Effects of NaOH Activation on Adsorptive Removal of Herbicides by Biochars Prepared from Ground Coffee Residues. Energies 2021, 14, 1297. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Park, Y.; Lee, G.; Kim, Y.; Chon, K. Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids. Energies 2019, 12, 4373. [Google Scholar] [CrossRef] [Green Version]
- Gibson, K.E.; Schwab, K.J. Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Appl. Environ. Microbiol. 2011, 77, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.H.; Lee, S.; Kim, Y.M.; Lee, J.H.; Kim, S.K.; Kim, S.G. Pollutants in Rainwater Runoff in Korea: Their Impacts on Rainwater Utilization. Environ. Technol. 2005, 26, 411–420. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, J.-S.; Han, M.; Choi, J. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci. Total Environ. 2010, 408, 896–905. [Google Scholar] [CrossRef]
- Lee, Y.; Von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Liu, C.; Dong, B.; Zhang, Y. Degradation mechanism of alachlor during direct ozonation and O3/H2O2 advanced oxidation process. Chemosphere 2010, 78, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Rokbani, O.; Fattouch, S.; Chakir, A.; Roth, E. Heterogeneous oxidation of two triazole pesticides (diniconazole and tebuconazole) by OH-radicals and ozone. Sci. Total Environ. 2019, 694, 133745. [Google Scholar] [CrossRef] [PubMed]
- Mazellier, P.; Leroy, É.; De Laat, J.; Legube, B. Transformation of carbendazim induced by the H2O2/UV system in the presence of hydrogenocarbonate ions: Involvement of the carbonate radical. New J. Chem. 2002, 26, 1784–1790. [Google Scholar] [CrossRef]
- Karaca, H.; Walse, S.S.; Smilanick, J.L. Effect of continuous 0.3μL/L gaseous ozone exposure on fungicide residues on table grape berries. Postharvest Biol. Technol. 2012, 64, 154–159. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012. [Google Scholar]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Gonzalez, M. Kinetics of reactions between chlorine or bromine and the herbicides diuron and isoproturon. J. Chem. Technol. Biotechnol. 2007, 82, 214–222. [Google Scholar] [CrossRef]
- Deborde, M.; Von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).