Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Official Methods
- Alcohol determination was performed as follows: 50 mL of the wine sample was distilled, and the alcohol was calculated from the density of the distillate by an oscillation-type density meter (DMA 48, Anton Paar GmbH, Graz, Austria).
- Total acids were calculated as tartaric acid by titrating to pH 7.0 with 0.33 mol/L NaOH with a pH-meter (Crison Basic 20 Crison Strumenti S.p.A. Carpi (MO), Italy).
- Total SO2 was measured by iodometric titration. Sodium hydroxide solution 1 M (25 mL) was pipetted into a conical flask containing a wine sample (50 mL). The flask was shaken well and left (15 min). The 25% sulfuric acid (10 mL) and the starch indicator (2–3 drops) were added. The solution was titrated with iodine solution (0.01 M) until blue color appeared.
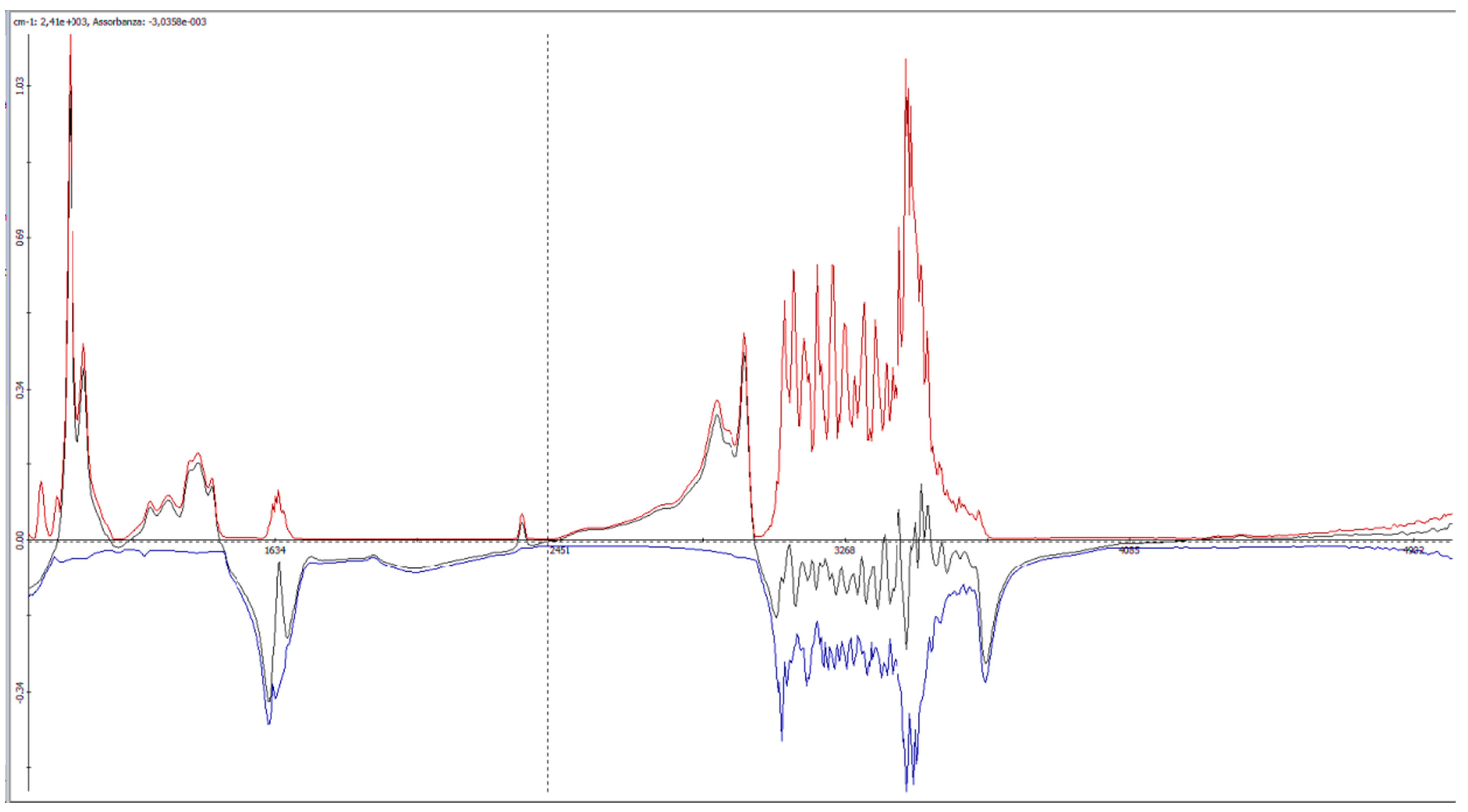
2.4. Winescan Technology
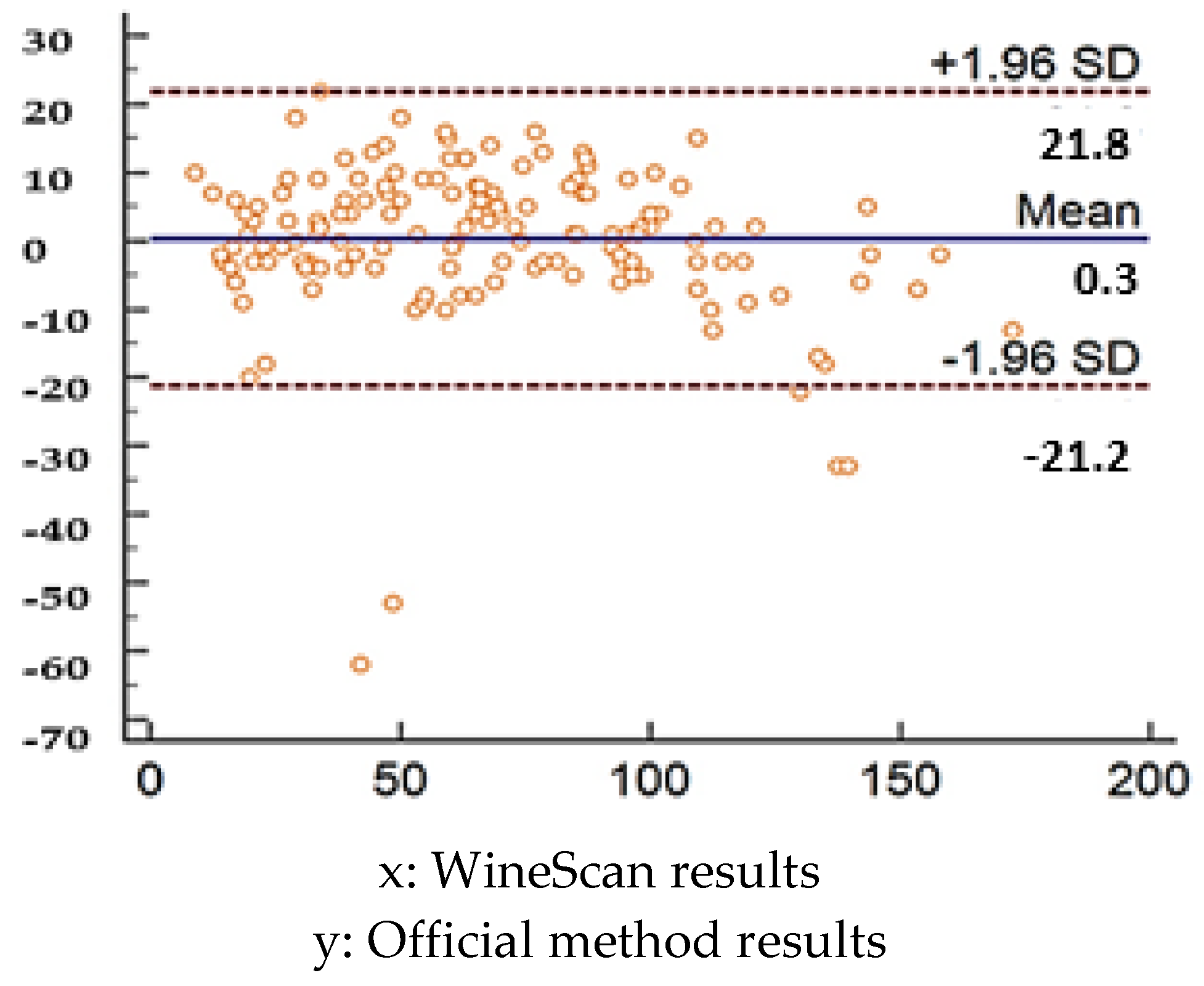
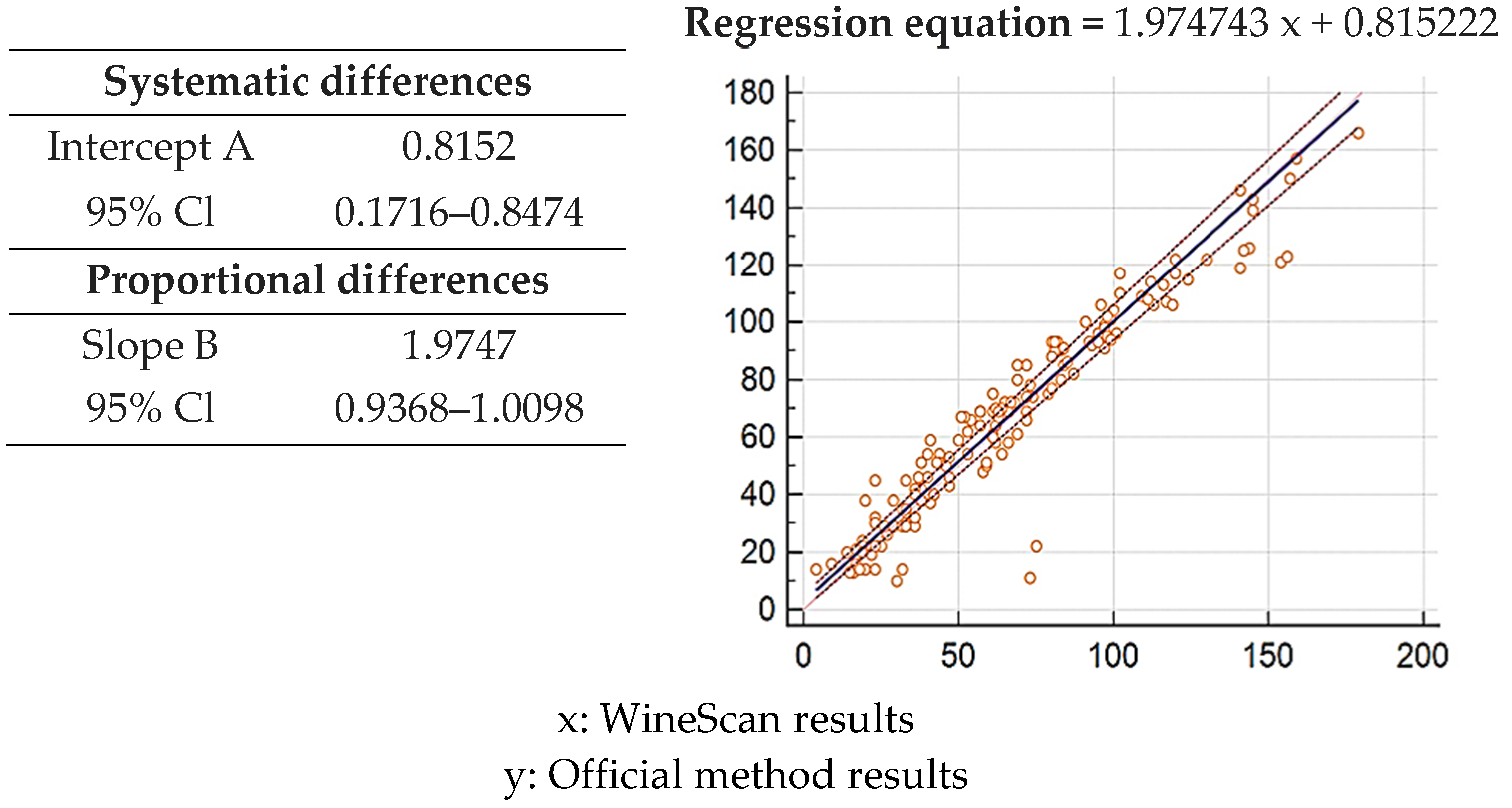
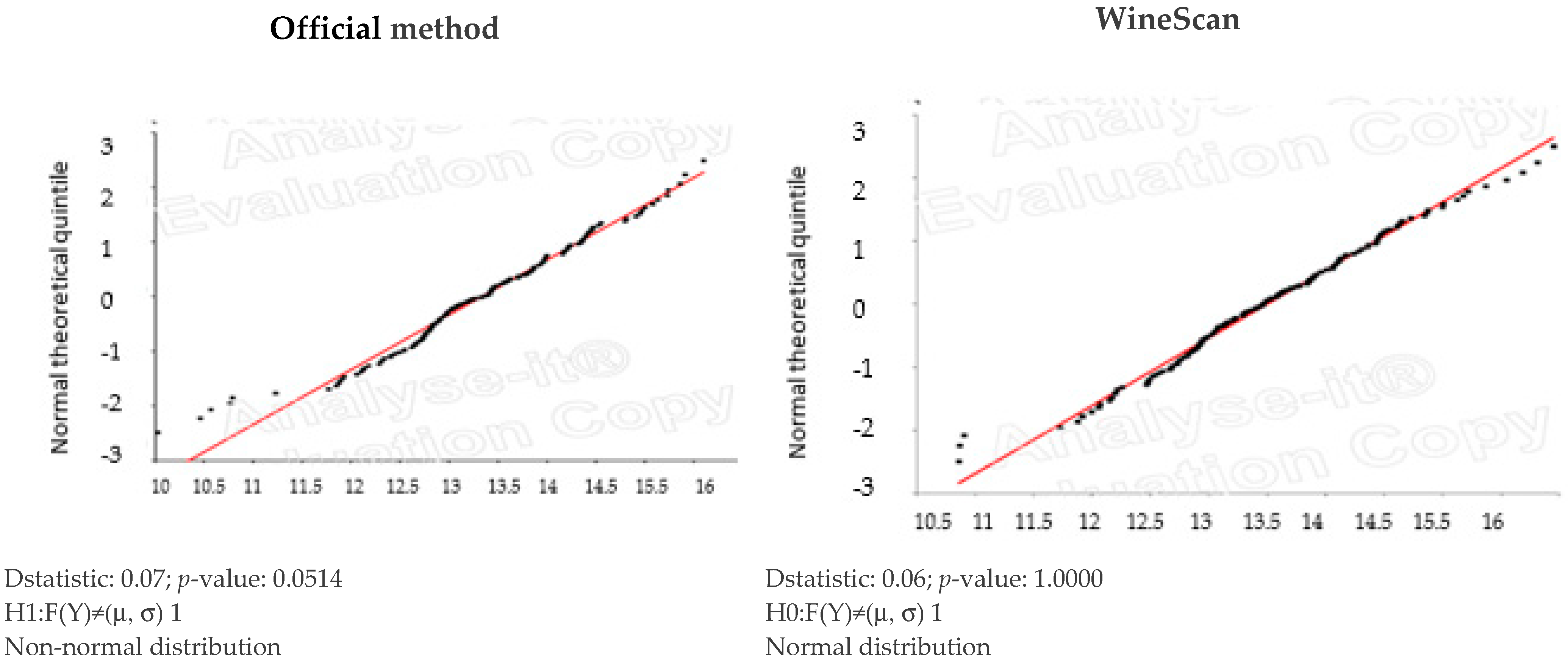
2.5. Statistical Analysis
3. Results
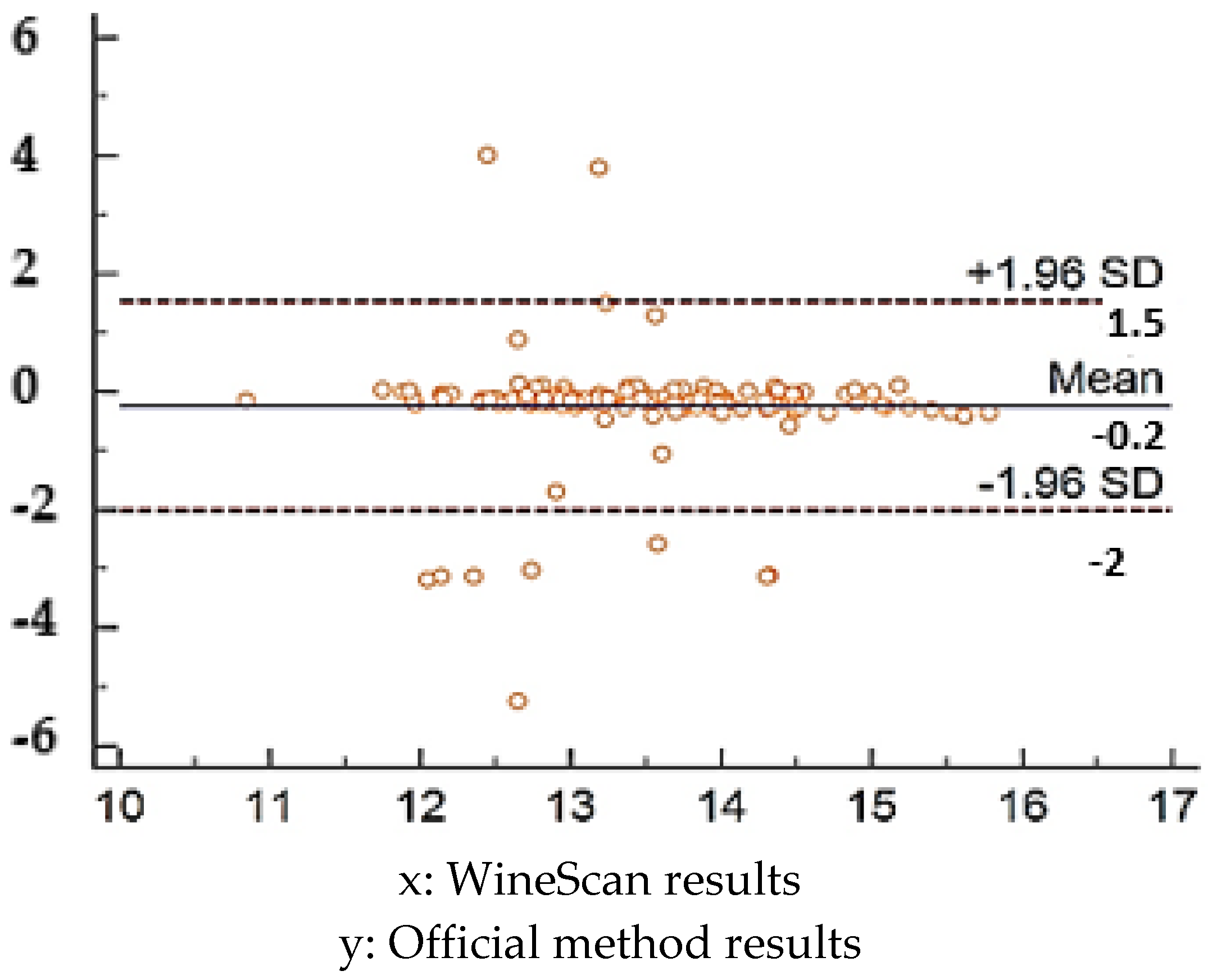
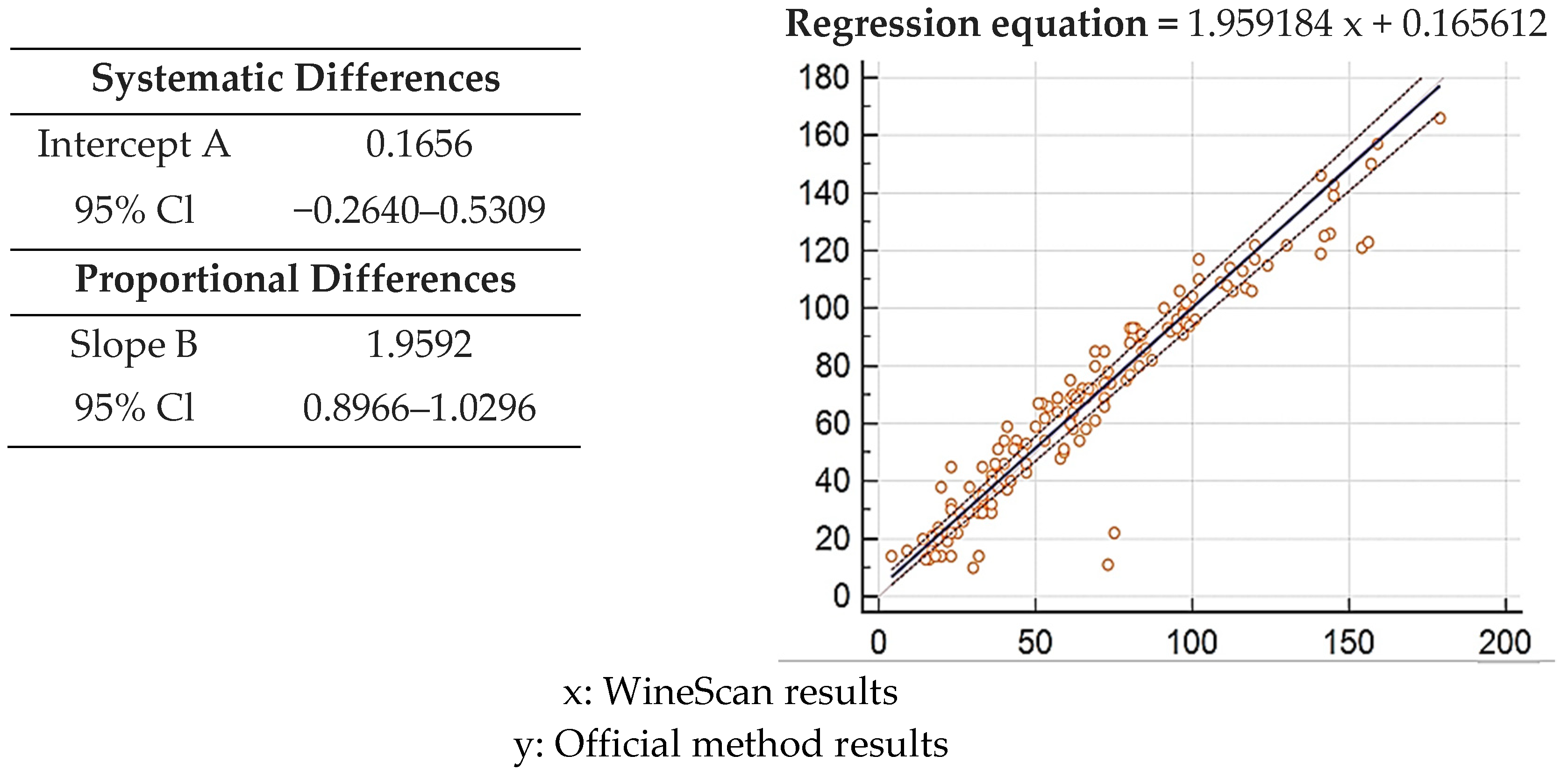
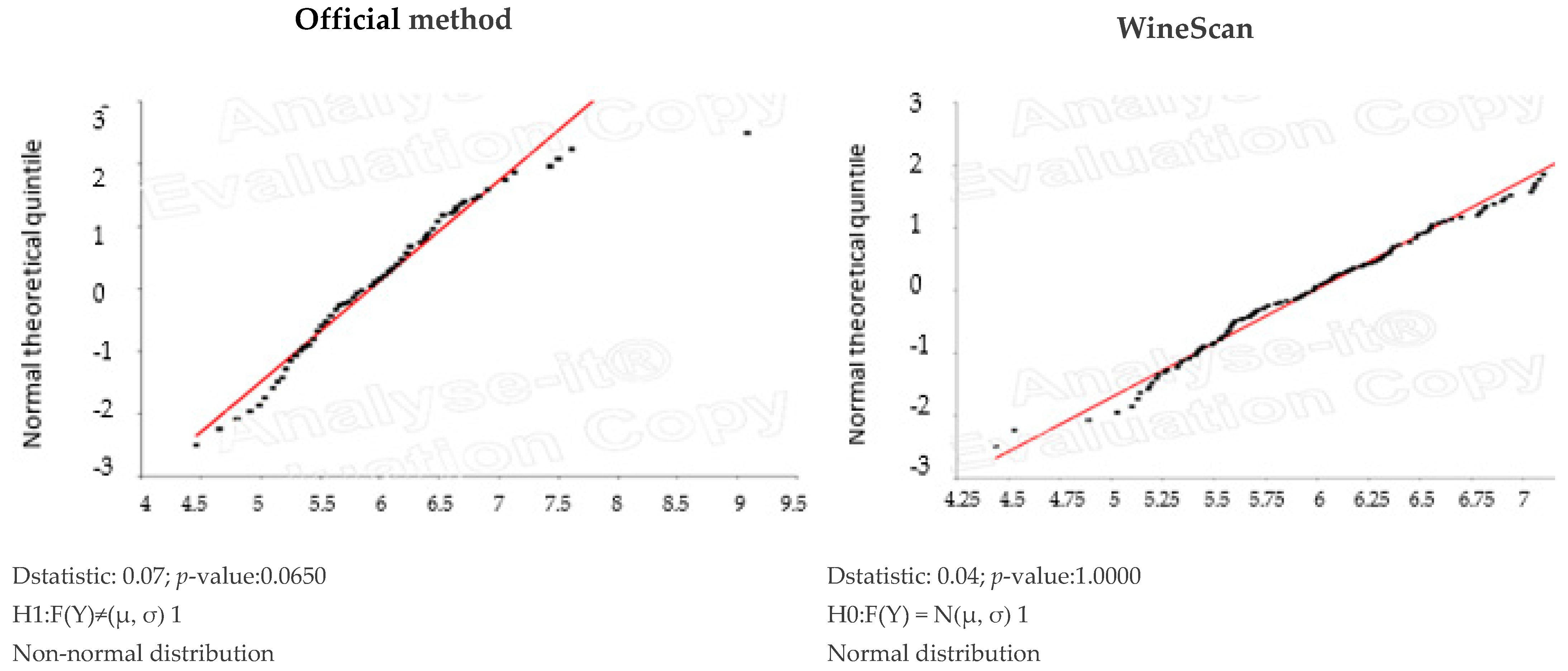
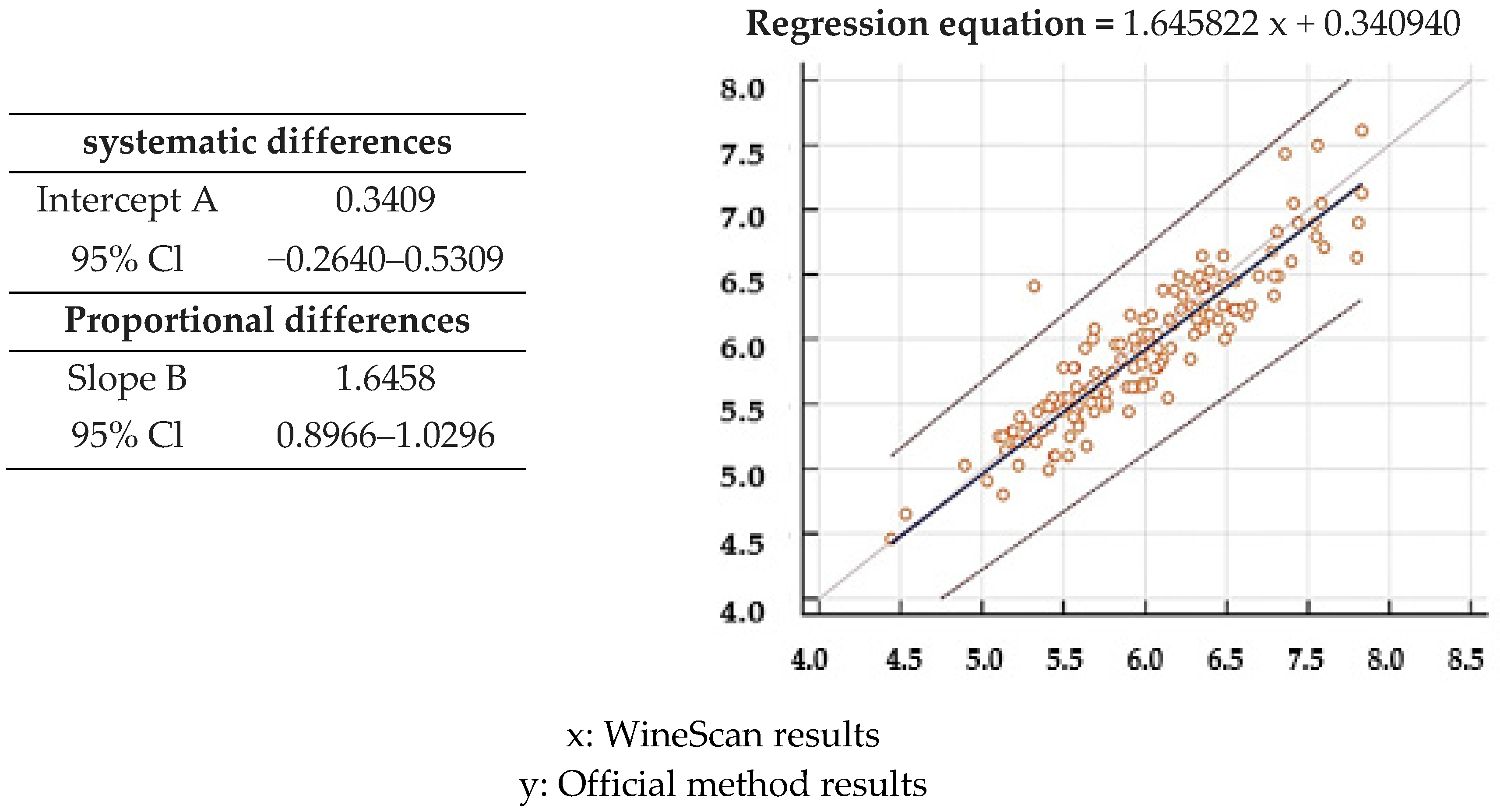
3.1. Validation of SO2 Concentration Obtained by Winescan Technology
3.2. Validation of Alcohol Concentration Obtained by WineScan Technology
3.3. Validation of Total Acid Concentration Obtained by Winescan Technology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wines of Campania. Ischia Doc. Available online: http://www.agricoltura.regione.campania.it/viticoltura/ischia.html (accessed on 20 November 2017).
- de Villiers, A.; Alberts, P.; Tredoux, A.G.; Nieuwoudt, H.H. Analytical techniques for wine analysis: An African perspective; a review. Anal. Chim. Acta 2012, 730, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC-Trends Anal. Chem. 2019, 120, 115630–115644. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Henschen, C.; Cantu, A.; Watrelot, A.A.; Waterhouse, A.L. Understanding microoxygenation: Effect of viable yeasts and sulfur dioxide levels on the sensory properties of a Merlot red wine. Food Res. Int. 2018, 108, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fazio, T.; Warner, C.R. A review of sulfites in foods—Analytical methodology and reported findings. Food Addit. Contam. 1990, 7, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A. New Frontiers in Wine Microbiology. Foods 2021, 10, 1077. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Gonzalo Diago, A.; Guerrero, R.F.; Ortiz, V.; Cantos-Villar, E. Effect of hydroxytyrosol on quality of sulfur dioxide-free red wine. Food Chem. 2016, 192, 25–33. [Google Scholar] [CrossRef]
- Costa, M.A.S.; Cerri, B.C.; Ceccato-Antonini, S.R. Ethanol enhances acid treatment to eliminate Lactobacillus fermentum from the fermentation process for fuel ethanol production. Lett. Appl. Microbiol. 2018, 66, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Aragón-García, F.; Ruíz-Rodríguez, A.; Palma, M. Changes in the Aromatic Compounds Content in the Muscat Wines as a Result of the Application of Ultrasound during Pre-Fermentative Maceration. Foods 2021, 10, 1462. [Google Scholar] [CrossRef]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Community Methods for Wine Analysis Commission Regulation (EEC) No 2676/90 of 17 September 1990. Available online: https://op.europa.eu/en/publication-detail/-/publication/6528497d-1ece-4355-ab08-c73b3242f7ee (accessed on 17 September 1990).
- Moros, J.; Garrigues, S.; de la Guardia, M. Vibrational spectroscopy provides a green tool for multi–component analysis. Trends Anal. Chem. 2010, 29, 578–591. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, Y.; Han, Y.; Li, Z.; Yang, Z.; Chai, Q.; Wang, W.; Zhang, Y.; Fu, C. A Review of the Discriminant Analysis Methods for Food Quality Based on Near-Infrared Spectroscopy and Pattern Recognition. Molecules 2021, 26, 749. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Mizaikoff, B. Mid-infrared spectroscopy for protein analysis: Potential and challenges. Anal. Bioanal. Chem. 2016, 408, 2875–2889. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Huber, L. Validation and Qualification in Analytical Laboratories; CRC Press: New York, NY, USA, 2007; pp. 1–288. [Google Scholar]
- General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/obp/ui/#iso:std:iso-iec:17025:ed-2:v1:en (accessed on 15 September 2020).
- Dini, I.; Seccia, S.; Senatore, A.; Coppola, D.; Morelli, E. Development and Validation of an Analytical Method for Total Polyphenols Quantification in Extra Virgin Olive Oils. Food Anal. Meth. 2019, 13, 457–464. [Google Scholar] [CrossRef]
- Dini, I.; Di Lorenzo, R.; Senatore, A.; Coppola, D.; Laneri, S. Validation of Rapid Enzymatic Quantification of Acetic Acid in Vinegar on Automated Spectrophotometric System. Foods 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives: Sulfur Dioxide. Available online: http://www.inchem.org/documents/jecfa/jeceval/jec_2215.htm (accessed on 12 November 2017).
- Vahl, J.M.; Converse, J.E. Ripper procedure for determining sulfur dioxide in wine: Collaborative study. J. Assoc. Off. Anal. Chem. 1980, 63, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hu, J.; Jiang, X.; Hou, X.; Tian, Y. Point discharge microplasma reactor for high efficiency conversion of H2S to SO2 for speciation analysis of sulfide and sulfite using molecular fluorescence spectrometry. Anal. Chim. Acta 2018, 1042, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Aberl, A.; Coelhan, M. Determination of sulfur dioxide in wine using headspace gas chromatography and electron capture detection. Food Addit. Contam. Part A 2013, 30, 226–233. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; Hernández-Artiga, M.P.; Palacios-Ponce de León, L.; Bellido-Milla, D. Selective methods for polyphenols and sulphur dioxide determination in wines. Food Chem. 2015, 182, 47–54. [Google Scholar] [CrossRef]
- Migneault, D.R. Enhanced Detection of Sulfite by Inductively Coupled Plasma Atomic Emission Spectroscopy with High-Performance Liquid Chromatography. Anal. Chem. 1989, 61, 272–275. [Google Scholar] [CrossRef]
- Nakahara, T.; Mori, T.; Morimoto, S.; Ishikawa, H. Continuous-flow determination of aqueous sulfur by atmospheric-pressure helium microwave-induced plasma atomic emission spectrometry with gas-phase sample introduction. Spectrochim. Acta Part B 1995, 50, 393–403. [Google Scholar] [CrossRef]
- Robbins, K.S.; Shah, K.S.; MacMahon, R.S.; de Jager, L.S.J. Development of a Liquid Chromatography–Tandem Mass Spectrometry Method for the Determination of Sulfite in Food. J. Agric. Food Chem. 2015, 63, 5126–5132. [Google Scholar] [CrossRef] [Green Version]
- Carrascon, V.; Ontañón, I.; Bueno, M.; Ferreira, V. Gas chromatography-mass spectrometry strategies for the accurate and sensitive speciation of sulfur dioxide in wine. J. Chromatogr. A 2017, 1504, 27–34. [Google Scholar] [CrossRef]
- Silva, E.M.; Takeuchi, R.M.; Santos, A.L. Carbon nanotubes for voltammetric determination of sulphite in some beverages. Food Chem. 2015, 17, 763–769. [Google Scholar] [CrossRef]
- Endecott, B.R.; Sanders, D.C.; Chaturvedi, A.K. Simultaneous gas chromatographic determination of four toxic gases generally present in combustion atmospheres. J. Anal. Toxicol. 1996, 20, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, C.S.; Zhou, L.; Shi, S.; Wang, Y.; Sun, J. Effective and inexpensive gas chromatography method for detection of trace level SO2 in H2 fuel. J. Shanghai Univ. Eng. Ed. 2010, 14, 322–325. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Q.; Li, X.; Fang, S.; Duan, M. Total sulfur dioxide determination in red wine by suppressed ion chromatography with in-sample oxidation and SPE. Chromatographia 2018, 81, 1003–1011. [Google Scholar] [CrossRef]
- Araujo, C.S.T.; de Carvalho, J.L.; Mota, D.R.; Coelho, N.M.M. Determination of sulphite and acetic acid in foods by gas permeation flow injection analysis. Food Chem. 2005, 92, 765–770. [Google Scholar] [CrossRef]
- Leinweber, F.J.; Monty, K.J. Sulfite determination: Fuchsin method. Methods Enzymol. 1987, 143, 15–17. [Google Scholar] [PubMed]
- Brychkova, G.; Yarmolinsky, D.; Fluhr, R.; Sagi, M. The determination of sulfite levels and its oxidation in plant leaves. Plant Sci. 2012, 190, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Shekho, N.H.; Al-Hadi, B.A.A. Spectrophotometric determination of sulphite in various water samples via chromium-1,5-diphenylcarbazide complex. Baghdad Sci. J. 2018, 15, 181–188. [Google Scholar]
- Lv, H.M.; Wang, Z.Y.; Gao, N. A fluorescence method of detecting sulfur dioxide derivatives. In Proceedings of the 4th International Conference on Water Resource and Environment (WRE 2018), Kaohsiung City, Taiwan, 17–21 July 2018; IOP Conference Series: Earth and Environmental Science; Li, P., Ed.; IOP publishing: Bristol, UK, 2018; Volume 191, p. UNSP 012107. [Google Scholar] [CrossRef]
- Chen, H.; Wu, X.; Wang, J.; Wang, H.; Tao, F.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. A fluorescent probe for colorimetric detection of bisulfite and application in sugar and red wine. Food Sci. Biotechnol. 2019, 28, 983–990. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Guo, H.; Li, X.; Tang, H.; Li, J.; Guo, Y. A novel colorimetric and ratiometric fluorescent probe for selective detection of bisulfite in real samples and living cells. Dyes Pigment. 2019, 163, 285–290. [Google Scholar] [CrossRef]
- Li, X.; Zeng, R.; Xi, C.; Tang, D.; Li, Q.; Zhang, B.; Huang, T. Silica nanoparticles doped with a benzo[e]indolium-tethered iridium(III) complex for reversible detection of HSO3−and Hg2+/Cu2+ in water. Dyes Pigment. 2019, 165, 128–136. [Google Scholar] [CrossRef]
- Hlavay, J.; Guilbault, G.G. Determination of sulfite by use of a fiber-optic biosensor based on a chemiluminescent reaction. Anal. Chim. Acta 1994, 299, 91–96. [Google Scholar] [CrossRef]
- Culbert, J.; Cozzolino, D.; Ristic, R.; Wilkinson, K. Classification of Sparkling Wine Style and Quality by MIR Spectroscopy. Molecules 2015, 20, 8341–8356. [Google Scholar] [CrossRef]
- Lilliefors, H.W. On the Kolmogorov-Smirnov Test for normality with mean and variance unknown. J. Am Stat Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar] [CrossRef]
- Brereton, P.; Hasnip, S.; Bertrand, A.; Wittkowski, R.; Guillou, C. Analytical methods for the determination of spirit drinks. Trends Anal Chem. 2003, 22, 19–25. [Google Scholar] [CrossRef]
- Strunk, D.H.; Hamman, J.W.; Timmel, B.M. Determination of proof of distilled alcoholic beverages, using an oscillating U-tube density meter. J. AOAC 1979, 62, 653–658. [Google Scholar] [CrossRef]
- Mark, F.G.; Vaughn, T.E. Determination of proof of alcoholic beverages using oscillating U-tube density meter. J. AOAC 1980, 63, 970–972. [Google Scholar] [CrossRef]
- Kovár, J. Oscillating U-tube density meter determination of alcoholic strength: Analysis of paramter errors. J. AOAC 1981, 64, 1424–1430. [Google Scholar] [CrossRef]
- Rebelein, H. Schnellmethode zur Bestimmung des Alkoholgehaltes in Likören und Branntweinen. Alkohol-Industrie 1995, 16, 376–378. [Google Scholar]
- Beutler, H.-O.; Michal, G. Neue Methode zur enzymatischen Bestimmung von Äthanol in Lebensmitteln. Z. Anal. Chem. 1977, 284, 113–117. [Google Scholar] [CrossRef]
- Tomassetti, M.; Angeloni, R.; Marchiandi, S.; Castrucci, M.; Sammartino, M.P.; Campanella, L. Direct Methanol (or Ethanol) Fuel Cell as Enzymatic or Non-Enzymatic Device, Used to Check Ethanol in Several Pharmaceutical and Forensic Samples. Sensors 2018, 18, 3596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Lin, N.B.; Chai, X.S.; Zhong, L.; Barnes, D.G. A rapid method for simultaneously determining ethanol and methanol content in wines by full evaporation headspace gas chromatography. Food Chem. 2015, 183, 169–172. [Google Scholar] [CrossRef]
- Yaritaa, T.; Nakajima, R.; Otsuka, S.; Ihara, T.; Takatsu, A.; Shibukawa, M. Determination of ethanol in alcoholic beverages by high-performance liquid chromatography-flame ionization detection using pure water as mobile phase. J. Chromatogr. A 2002, 976, 387–391. [Google Scholar] [CrossRef]
- Abreu, T.; Perestrelo, R.; Bordiga, M.; Locatelli, M.; Daniel Coïsson, J.; Câmara, J.S. The Flavor Chemistry of Fortified Wines—A Comprehensive Approach. Foods 2021, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Zaky, A.S.; Pensupa, N.; Andrade-Eiroa, Á.; Tucker, G.A.; Du, C.Y. A new HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in food samples. J. Food Compos. Anal. 2017, 56, 25–33. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.; Tonin, F.; Tavares, M.F.M.; Rodriguez-Amaya, D.B. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Pereira, A.C.; Reis, M.S.; Saraiva, P.M.; Marques, J.C. Madeira wine ageing prediction based on different analytical techniques: UV-vis, GC-MS, HPLC-DAD. Chemom. Intell. Lab. Syst. 2011, 105, 43–55. [Google Scholar] [CrossRef]
- Kim, D.Y.; Cho, B.K. Rapid monitoring of the fermentation process for Korean traditional rice wine ‘Makgeolli’ using FT-NIR spectroscopy. Infrared Phys. Technol. 2015, 73, 95–102. [Google Scholar] [CrossRef]
- Egidio, V.D.; Sinelli, N.; Giovanelli, G.; Moles, A.; Casiraghi, E. NIR and MIR spectroscopy as rapid methods to monitor red wine fermentation. Eur. Food Res. Technol. 2010, 230, 947–955. [Google Scholar] [CrossRef]
- Lourenço, A.S.; Nascimento, R.F.; Silva, A.C.; Ribeiro, W.F.; Araujo, M.C.U.; Oliveira, S.C.B.; Nascimento, V.B. Voltammetric determination of tartaric acid in wines by electrocatalytic oxidation on a cobalt(II)-phthalocyanine-modified electrode associated with multiway calibration. Anal. Chim. Acta 2018, 1008, 29–37. [Google Scholar] [CrossRef]
- Pigani, L.; Simone, G.V.; Foca, G.; Ulrici, A.; Masino, F.; Cubillana-Aguilera, L.; Calvini, R.; Seeber, R. Prediction of parameters related to grape ripening by multivariate calibration of voltammetric signals acquired by an electronic tongue. Talanta 2018, 178, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Laneri, S.; Di Lorenzo, R.; Sacchi, A.; Dini, I. Dosage of Bioactive Molecules in the Nutricosmeceutical Helix aspersa Muller Mucus and Formulation of New Cosmetic Cream with Moisturizing Effect. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New Strategies in the Cultivation of Olive Trees and Repercussions on the Nutritional Value of the Extra Virgin Olive Oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. An Environmentally Friendly Practice Used in Olive Cultivation Capable of Increasing Commercial Interest in Waste Products from Oil Processing. Antioxidants 2020, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Taverniers, I.; de Loose, M.; van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- BIPM Bureau International des Poids et Mesures (2017). What Is Metrology? Available online: https://www.bipm.org/en/worldwide-metrology/ (accessed on 28 September 2008).
- Belouafa, S.; Habti, F.; Benhar, S.; Belafkih, B.; Tayane, S.; Hamdouch, S.; Bennamara, A.; Abourriche, A. Statistical tools and approaches to validate analytical methods: Methodology and practical examples. Int. J. Metrol. Qual. Eng. 2017, 8, 9. [Google Scholar] [CrossRef]
- Parampreet, K.; Stoltzfus, J. Type I, II, and III statistical errors: A brief overview. Biostatistics 2017, 3, 268–270. [Google Scholar]
- Robin, E.D.; Lewiston, N.J. Type 3 and type 4 errors in the statistical evaluation of clinical trials. Chest 1990, 98, 463–465. [Google Scholar] [CrossRef]
- Patz, C.D.; David, A.; Hente, K.T. Wine Analysis using FTIR. Focus 2000, 24, 16–18. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilic-Zulle, L. Comparison of methods: Passing and Bablok regression. Biochem. Med. 2011, 21, 49–52. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Di Lorenzo, R.; Senatore, A.; Coppola, D.; Laneri, S. Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine. Separations 2021, 8, 191. https://doi.org/10.3390/separations8100191
Dini I, Di Lorenzo R, Senatore A, Coppola D, Laneri S. Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine. Separations. 2021; 8(10):191. https://doi.org/10.3390/separations8100191
Chicago/Turabian StyleDini, Irene, Ritamaria Di Lorenzo, Antonello Senatore, Daniele Coppola, and Sonia Laneri. 2021. "Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine" Separations 8, no. 10: 191. https://doi.org/10.3390/separations8100191
APA StyleDini, I., Di Lorenzo, R., Senatore, A., Coppola, D., & Laneri, S. (2021). Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine. Separations, 8(10), 191. https://doi.org/10.3390/separations8100191








