Influence of Harvest Time, Method of Preparation and Method of Distillation on the Qualitative Properties of Organically Grown and Wild Helichrysum italicum Immortelle Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Used in the Investigation
2.2. Immortelle Preparation Methods
2.3. Methods of Distillation
2.4. Chromatographic Analysis of the Essential Oil
2.5. Statistical Analysis
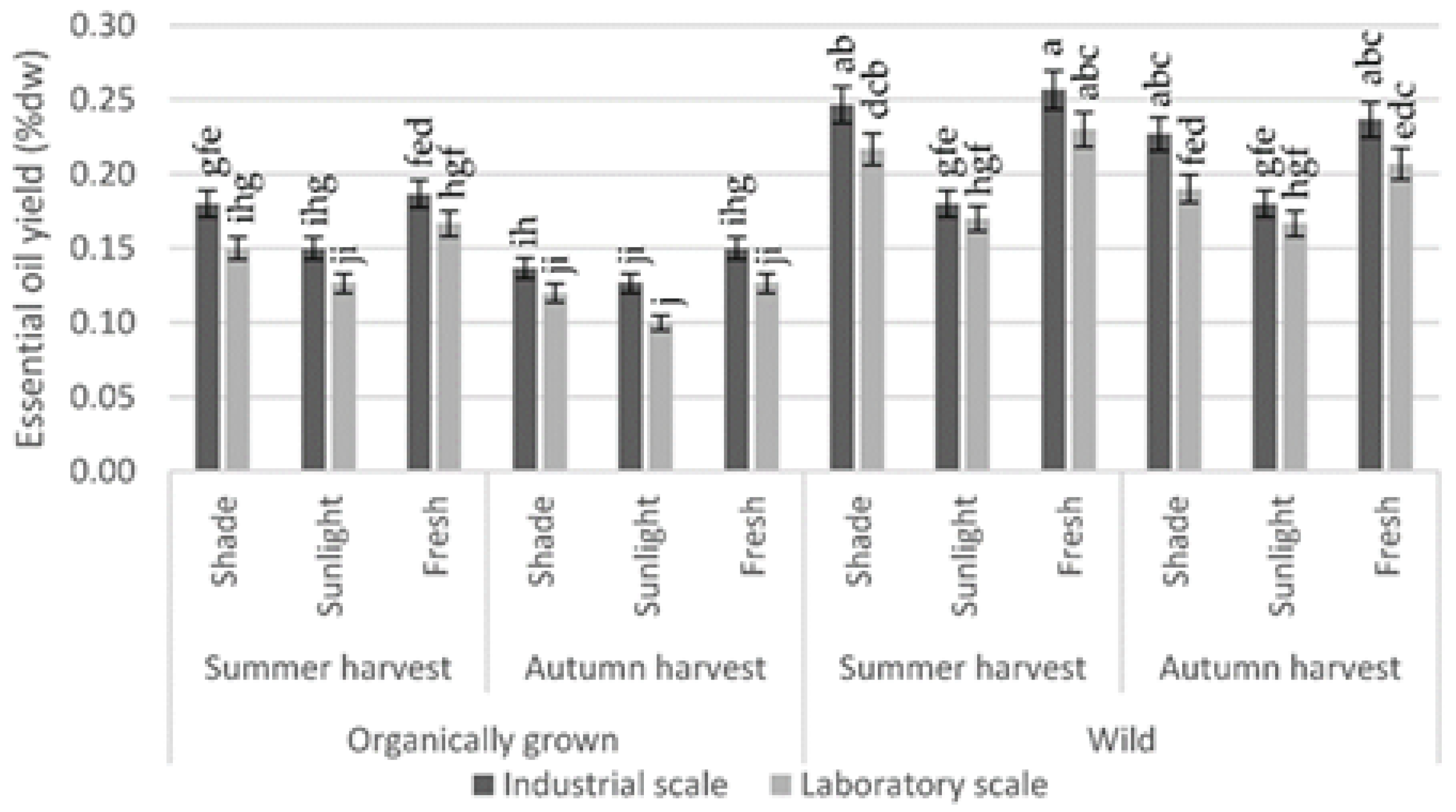
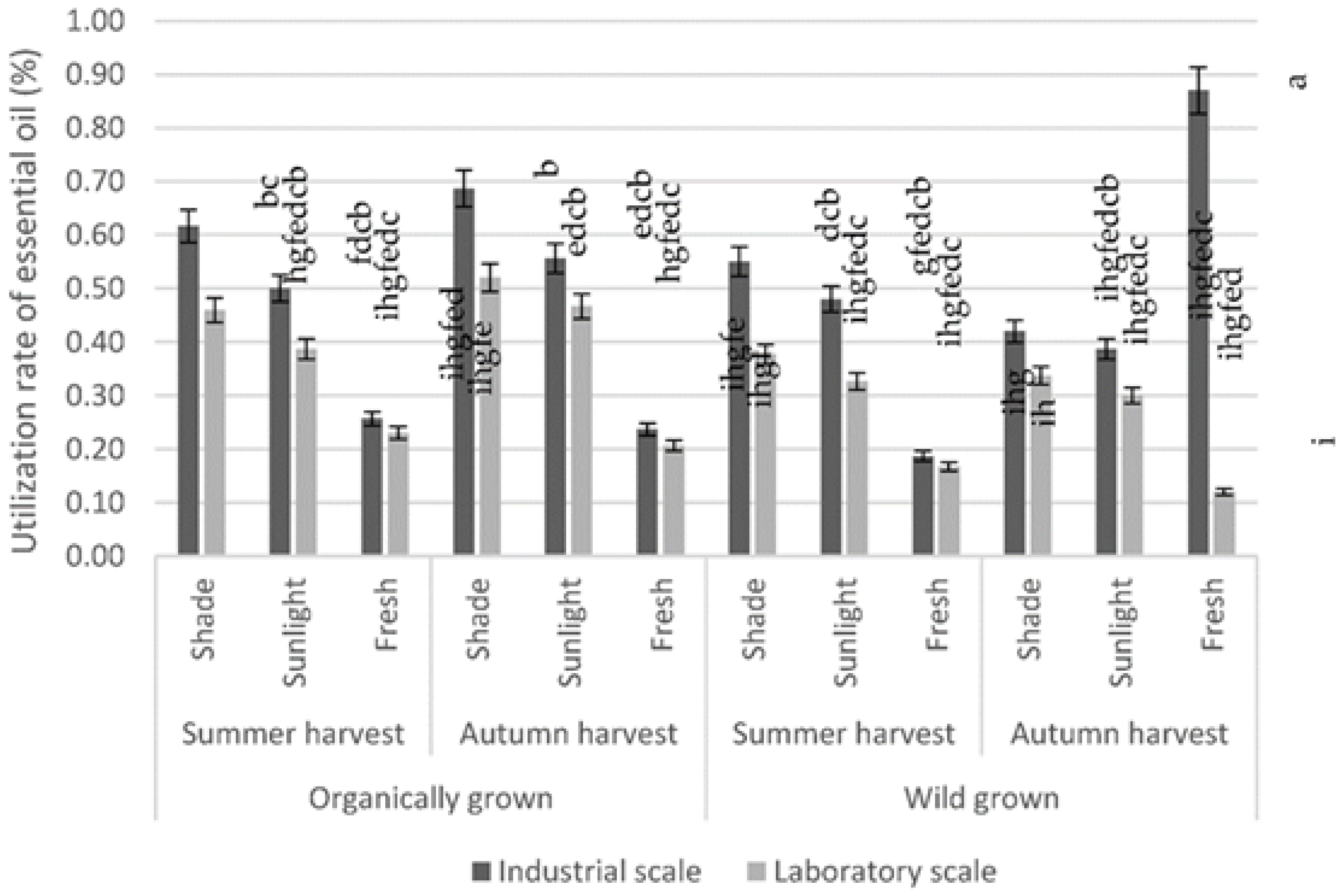
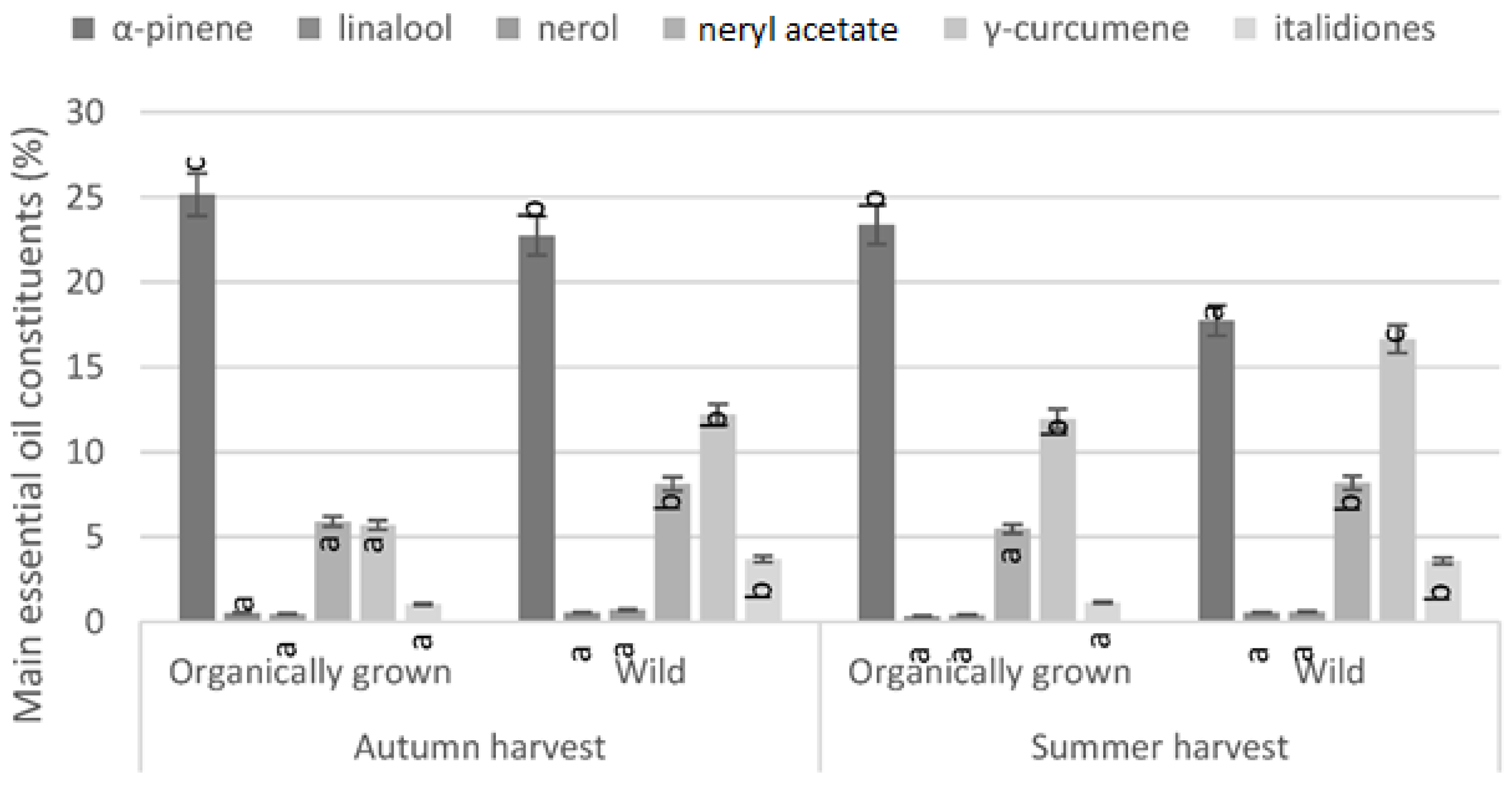
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrini, R.; Alba, V.; Ruta, C.; Morone-Fortunato, I.; Blanco, A.; Montemurro, C. An evaluation of a new approach to the regeneration of Helichrysum italicum (Roth) G. Don, and the molecular characterization of the variation among sets of differently derived regenerants. Cell Mol. Biol. Lett. 2009, 14, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Biondi, E. Thoughts on the ecology and syntaxonomy of some vegetation typologies of the Mediterranean coast. Fitosociologia 2007, 44, 3–10. [Google Scholar]
- Galbany-Casals, M.; Blanco-Moreno, J.M.; Garcia-Jacas, N.; Breitwieser, I.; Smissen, R.D. Genetic variation in Mediterranean Helichrysum italicum (Asteraceae; Gnaphalieae): Do disjunct populations of subsp. microphyllum have a common origin? Plant Biol. 2011, 13, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Pohajda, I.; Dragun, G.; Puharić Visković, L. Smilje. Handbook, Hrvatska Savjetodavna Služba; Hrvatska Savjetodavna Služba: Zagreb, Croatia, 2015. [Google Scholar]
- Bianchini, A.; Tomi, P.; Bernardini, A.F.; Morelli, I.; Flamini, G. A comparative study of volatile constituents of two Helichrysum italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour. Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Stepanović, B.; Radanović, D.; Turšić, I.; Nemčević, N.; Ivanec, J. Uzgoj ljekovitog i aromatičnog bilja. In Handbook; Jan-Spider: Pitomača, Croatia, 2009. [Google Scholar]
- Sala, A.; Recio, M.D.C.; Giner, R.M.; Máñez, S.; Tournier, H. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef]
- Sala, A.; del Carmen Recio, M.; Schinella, G.; Máñez, S.; Giner, R.M. New acetophenone glucosides isolated from extracts of Helichrysum italicum with antiinflammatory activity. J. Nat. Prod. 2003, 64, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Maffei Facino, R.; Carini, M.; Franzoi, L.; Pirola, O.; Bosisio, E. Phytochemical characterization and radical scavenger activity of flavonoids from Helichrysum italicum G. Don (Compositae). Pharmacol. Res. 1990, 22, 709–721. [Google Scholar] [CrossRef]
- Chinou, I.B.; Roussis, V.; Perdetzoglou, D.; Loukis, A. Chemical and biological studies on two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.A.; Musolino, A.D.; Procopio, F.; Alonzo, V. Helichrysum italicum extract interferes with the production of enterotoxins by Staphylococcus aureus. Lett. Appl. Microbiol. 2002, 35, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Mastelić, J.; Politeo, O.; Jerkovic, I.; Radosevic, N. Composition and antimicrobial activity of Helichrysum italicum essential oil and its terpene and terpenoid fractions. Chem. Nat. Compd. 2005, 41, 35–40. [Google Scholar] [CrossRef]
- Politeo, O.; Jukić, M.; Miloš, M. Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croat. Chem. Acta 2006, 79, 545–552. [Google Scholar]
- Schinella, G.R.; Tournier, H.A.; Máñez, S.; de Buschiazzo, P.M.; del Carmen Recio, M. Tiliroside and gnaphaliin inhibit human low density lipoprotein oxidation. Fitoterapia 2007, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J. Antibacterial action of essential oils from Corsica. J. Essent.Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Mastelić, J.; Politeo, O.; Jerković, I. Contribution to the analysis of the essential oil of Helichrysum italicum (Roth) G. Don.–determination of ester bonded acids and phenols. Molecules 2008, 13, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef]
- Barber, A.; Redero, S.; Corbi, M.; Alba, B.; Molina, D. Aproximación al Conocimiento Etnobiológico y Etnoecológico de Ibi. (Foia de Castalla-L‘Alcoià, Alicante); Identia Institute: Alicante, Spain, 2005. [Google Scholar]
- Rivera, D.; Alcaraz, F.; Verde, A.; Fajardo, J.; Obón, C. Las Plantas en la Cultura Popular. Enciclopedia Divulgativa de la Historia Natural de Jumilla-Yecla; Sociedad Mediterránea de Historia Natural: Murcia, Spain, 2008; Volume 9. (In Spanish) [Google Scholar]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinezde-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientifi c data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Rajić, M.; Bilić, M.; Aladić, K.; Šimunović, D.; Pavković, T. Od tradicionalne uporabe do znanstvenog značaja: Cvijet smilja. Glas. Zaštite Bilja 2015, 38, 16–26, (In Croatian with an abstract in English). [Google Scholar]
- Paolini, J.; Desjobert, J.M.; Costa, J.; Bernardini, A.F.; Castellini, C.B. Composition of essential oils of Helichrysum italicum (Roth) G. Don fil subsp. italicum from Tuscan archipelago islands. Flavour. Fragr. J. 2006, 21, 805–808. [Google Scholar] [CrossRef]
- Politeo, O. Season’s Variation of Chemical Content and Biological Activity of Everlasting Essential Oil, Helychrysum Italicum (Roth) G. Don. Ph.D. Thesis, Faculty of Science of the University of Zagreb, Zagreb, Croatia, 2003. (In Croatian with an abstract in English). [Google Scholar]
- Cristofari, G.; Znini, M.; Majidi, L.; Costa, J.; Hammouti, B.; Ponlini, J. Helichrysum italicum subsp. italicum essential oil as environmentally friendly inhibitor on the corrosion of mil steel in hydrochloric acid. Int. J. Electrochem. Sci. 2012, 7, 9024–9041. [Google Scholar]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N. Biochemical characterization of Helichrysum italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers 2015, 12, 419–431. [Google Scholar] [CrossRef]
- Houdret, J. Practical Herb Garden: A Comprehensive AZ Directory and Gardener’s Guide to Growing Herbs Successfully; Hermes House: London, UK, 2002. [Google Scholar]
- Radanović, D.; Nastovski, T. Proizvodnja lekovitog i aromatičnog bilja po principima organske poljoprivrede. Lekovite Sirovine 2002, 22, 83–99. (In Serbian) [Google Scholar]
- Pan, Y.K.; Zhao, L.J.; Dong, Z.X.; Mujumdar, A.S.; Kudra, T. Intermittent drying of carrot in a vibrated fluid bed: Effect on product quality. Dry Technol. 1999, 17, 2323–2340. [Google Scholar] [CrossRef]
- Pereira, N.R.; Marsaioli, A., Jr.; Ahrné, L.M. Effect of microwave power, air velocity and temperature on the final drying of osmotically dehydrated bananas. J. Food Eng. 2007, 81, 79–87. [Google Scholar] [CrossRef]
- Matin, A.; Krička, T.; Majdak, T.; Židovec, V.; Jurišić, V.; Maričić, M.; Grubor, M. Utjecaj temperature sušenja na intenzitet obojenja cvatova nevena za potrebe hrane za životinje. Krmiva Časopis o Hranidbi Životinj. Proizv. I Tehnol. Krme 2017, 59, 69–75, (In Croatian with an abstract in English). [Google Scholar] [CrossRef]
- Matin, A.; Krička, T.; Bukal, N.; Grubor, M. Utjecaj temperature zraka sušenja na kvalitativna svojstva latica lizijantusa. In Proceedings of the 54th Croatian & 14th International Symposium on Agriculture, Vodice, Croatia, 17–22 February 2019; pp. 588–592, (In Croatian with an abstract in English). [Google Scholar]
- Ministry of Agriculture. Regulations on Sampling Methods and Quality Testing; Ministry of Agriculture: Zagreb, Croatia, 2008.
- Tzanova, M.; Grozeva, N.; Gerdzhikova, M.; Atanasov, V.; Terzieva, S.; Prodanova, R. Biochemical composition of essential oil of Corsican Helichrysum italicum (Roth) G. Don, introduced and cultivated in South Bulgaria. Bulg. J. Agric. Sci. 2018, 24, 1071–1077. [Google Scholar]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of phytochemicals from Helichrysum italicum: An analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry 2017, 138, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Mollova, S.; Fidan, H.; Antonova, D.; Bozhilov, D.; Stanev, S. Chemical composition and antimicrobial and antioxidant activity of Helichrysum italicum (Roth) G. Don subspecies essential oils. Turk. J. Agric. 2020, 44. [Google Scholar] [CrossRef]
- Satta, M.; Tuberoso, C.I.G.; Angioni, A.; Pirisi, F.M.; Cabras, P. Analysis of the essential oil of Helichrysum italicum G. Don ssp. microphyllum (Willd) Nym. J. Essent. Oil Res. 1999, 11, 711–715. [Google Scholar] [CrossRef]
- Usai, M.; Foddai, M.; Bernardini, A.F.; Muselli, A.; Costa, J. Chemical composition and variation of the essential oil of wild Sardinian Helichrysum italicum G. Don subsp. microphyllum (Willd) Nym. from vegetative period to post-blooming. J. Essent. Oil Res. 2010, 22, 373–380. [Google Scholar] [CrossRef]
- Costa, E.; Oliveira, A.P.; Almeida, J.; Filho, J.S.; Araujo, E. Chemical composition of essential oil from the leaves of jatropha mutabilis (pohl) baill (Euphorbiaceae). J. Essent. Oil-Bear Plants 2014, 17, 1156–1160. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Desogus, E.; Porcedda, S.; Ballero, M. Analysis of the volatile concentrates of the leaves and flowers of Helichrysum italicum (Roth) G. Don ssp. microphyllum (Willd) Nyman (Asteraceae) by supercritical fluid extraction and their essential oils. J. Essent. Oil Res. 2003, 15, 20–126. [Google Scholar] [CrossRef]
- Talic, S.; Odak, I.; Lukic, T.; Brkljaca, M.; Bevanda, A.M.; Lasic, A. Chemodiversity of helichrysum italicum (roth) g. Don subsp. Italicum essential oils from Bosnia and Herzegovina. Fresenius Environ. Bull. 2021, 30, 2492–2502. [Google Scholar]
- Ćavar Zeljković, S.; Šolić, M.E.; Maksimović, M. Volatiles of Helichrysum italicum (Roth) G. Don from Croatia. Nat. Prod. Res. 2015, 29, 1874–1877. [Google Scholar] [CrossRef] [PubMed]
- Staver, M.M.; Gobin, I.; Ratkaj, I.; Petrovic, M.; Vulinovic, A.; Dinarina-Sablic, M.; Broznic, D. In vitro antiproliferative and antimicrobial activity of the essential oil from the flowers and leaves of Helichrysum italicum (Roth) G. Don growing in central Dalmatia (Croatia). J. Essent. Oil Bear Plants 2018, 21, 77–91. [Google Scholar] [CrossRef]
- Peršić, M.; Leko, K.; Dudaš, S. Kriteriji kvalitete biljnog materijala i eteričnog ulja primorskog smilja (Helichrysum Italicum (roth.) g. don). Zb. Veleučilišta U Rijeci 2019, 7, 425–431. [Google Scholar] [CrossRef]
- Bouchaala, M.; Ramdani, M.; Lograda, T.; Chalard, P.; Figueredo, G. Chemical composition, antibacterial activity and chromosome number of Helichrysum lacteum, endemic from Algeria. Int. J. Pharma Res. Health. Sci. 2017, 5, 1539–1545. [Google Scholar] [CrossRef]
- Blažević, N.; Petričić, J.; Stanić, G.; Maleš, Ž. Variations in yields and composition of immortelle (Helichrysum italicum, Roth Guss.) essential oil from different locations and vegetation periods along Adriatic coast. Acta Pharm. 1995, 45, 517–522. [Google Scholar]
- Bianchini, A.; Tomi, P.; Costa, J.; Bernardini, A.F. Composition of Helichrysum italicum (Roth) G. Don fil. subsp. italicumessential oils from Corsica (France). Flavour. Fragr. J. 2001, 16, 30–34. [Google Scholar] [CrossRef]
- Brlek, I.; Ludaš, A.; Sutlović, A. Synthesis and Spectrophotometric Analysis of Microcapsules Containing Immortelle Essential Oil. Molecules 2021, 26, 2390. [Google Scholar] [CrossRef]
| Influencing Factors | Statistical Indicators | ||||
|---|---|---|---|---|---|
| Sum of Squares | Degree of Freedom | Mean of Square | F | p-Value | |
| Intercept | 2.233089 | 1 | 2.233089 | 6207.815 | 0.000000 |
| Harvest | 0.010756 | 1 | 0.010756 | 29.900 | 0.000002 |
| Type | 0.077356 | 1 | 0.077356 | 215.042 | 0.000000 |
| Preparation | 0.026178 | 2 | 0.013089 | 36.386 | 0.000000 |
| Distillation | 0.010272 | 1 | 0.010272 | 28.556 | 0.000002 |
| Harvest* Type | 0.001422 | 1 | 0.001422 | 3.954 | 0.052488 |
| Harvest* Preparation | 0.001111 | 2 | 0.000556 | 1.544 | 0.223858 |
| Type* Preparation | 0.002678 | 2 | 0.001339 | 3.722 | 0.031425 |
| Harvest* Distillation | 0.000006 | 1 | 0.000006 | 0.015 | 0.901617 |
| Type* Distillation | 0.000006 | 1 | 0.000006 | 0.015 | 0.901617 |
| Preparation* Distillation | 0.000311 | 2 | 0.000156 | 0.432 | 0.651432 |
| Harvest* Type* Preparation | 0.000078 | 2 | 0.000039 | 0.108 | 0.897748 |
| Harvest* Type* Distillation | 0.000050 | 1 | 0.000050 | 0.139 | 0.710922 |
| Harvest* Preparation* Distillation | 0.000044 | 2 | 0.000022 | 0.062 | 0.940168 |
| Type* Preparation* Distillation | 0.000478 | 2 | 0.000239 | 0.664 | 0.519405 |
| Harvest* Type* Preparation* Distillation | 0.000100 | 2 | 0.000050 | 0.139 | 0.870580 |
| Error | 0.017267 | 48 | 0.000360 | - | - |
| Influencing Factors | Statistical Indicators | ||||
|---|---|---|---|---|---|
| Sum of Squares | Degree of Freedom | Mean of Square | F | p-Value | |
| Intercept | 11.62423 | 1 | 11.62423 | 492.4944 | 0.000000 |
| Harvest | 0.04061 | 1 | 0.04061 | 1.7207 | 0.195847 |
| Type | 0.04550 | 1 | 0.04550 | 1.9278 | 0.171410 |
| Preparation | 0.55770 | 2 | 0.27885 | 11.8143 | 0.000067 |
| Distillation | 0.42781 | 1 | 0.42781 | 18.1255 | 0.000096 |
| Harvest* Type | 0.00190 | 1 | 0.00190 | 0.0806 | 0.777764 |
| Harvest* Preparation | 0.09211 | 2 | 0.04605 | 1.9512 | 0.153209 |
| Type* Preparation | 0.21867 | 2 | 0.10933 | 4.6323 | 0.014472 |
| Harvest* Distillation | 0.03967 | 1 | 0.03967 | 1.6807 | 0.201036 |
| Type* Distillation | 0.05837 | 1 | 0.05837 | 2.4729 | 0.122391 |
| Preparation* Distillation | 0.02831 | 2 | 0.01415 | 0.5997 | 0.553047 |
| Harvest* Type* Preparation | 0.22995 | 2 | 0.11498 | 4.8713 | 0.011854 |
| Harvest* Type* Distillation | 0.04253 | 1 | 0.04253 | 1.8021 | 0.185773 |
| Harvest* Preparation* Distillation | 0.16744 | 2 | 0.08372 | 3.5470 | 0.036585 |
| Type* Preparation* Distillation | 0.13462 | 2 | 0.06731 | 2.8518 | 0.067565 |
| Harvest* Type* Preparation* Distillation | 0.15994 | 2 | 0.07997 | 3.3881 | 0.042033 |
| Error | 1.13293 | 48 | 0.02360 | - | - |
| Influencing Factors | Statistical Indicators | |||||
|---|---|---|---|---|---|---|
| Constituent | Sum of Squares | Degree of Freedom | Mean of Square | F | p-Value | |
| α-Pinene | Harvest | 34.10 | 1 | 34.10 | 1099.3 | 0.000001 |
| Type | 48.68 | 1 | 48.68 | 1569.1 | 0.000001 | |
| Harvest* Type | 7.86 | 1 | 7.86 | 253.2 | 0.000002 | |
| Error | 0.25 | 8 | 0.03 | |||
| Linalool | Harvest | 0.0280 | 1 | 0.02803 | 0.478 | 0.509 |
| Type | 0.0385 | 1 | 0.03853 | 0.657 | 0.441 | |
| Harvest* Type | 0.0261 | 1 | 0.02613 | 0.445 | 0.523 | |
| Error | 0.4695 | 8 | 0.05868 | |||
| Nerol | Harvest | 0.0184 | 1 | 0.01841 | 0.252 | 0.629 |
| Type | 0.1519 | 1 | 0.15187 | 2.077 | 0.187 | |
| Harvest* Type | 0.0010 | 1 | 0.00101 | 0.014 | 0.909 | |
| Error | 0.5849 | 8 | 0.07312 | |||
| Neryl Acetate | Harvest | 0.118 | 1 | 0.118 | 2.317 | 0.1665 |
| Type | 18.229 | 1 | 18.229 | 357.893 | 0.000003 | |
| Harvest* Type | 0.180 | 1 | 0.180 | 3.536 | 0.0969 | |
| Error | 0.407 | 8 | 0.051 | |||
| γ-Curcumene | Harvest | 85.28 | 1 | 85.28 | 1412.5 | 0.000002 |
| Type | 94.58 | 1 | 94.58 | 1566.6 | 0.000003 | |
| Harvest* Type | 2.42 | 1 | 2.42 | 40.1 | 0.000225 | |
| Error | 0.48 | 8 | 0.06 | |||
| Italidiones | Harvest | 0.000 | 1 | 0.000 | 0.000 | 0.992 |
| Type | 19.431 | 1 | 19.431 | 280.931 | 0.000002 | |
| Harvest* Type | 0.025 | 1 | 0.025 | 0.364 | 0.563 | |
| Error | 0.553 | 8 | 0.069 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matin, A.; Pavkov, I.; Grubor, M.; Jurišić, V.; Kontek, M.; Jukić, F.; Krička, T. Influence of Harvest Time, Method of Preparation and Method of Distillation on the Qualitative Properties of Organically Grown and Wild Helichrysum italicum Immortelle Essential Oil. Separations 2021, 8, 167. https://doi.org/10.3390/separations8100167
Matin A, Pavkov I, Grubor M, Jurišić V, Kontek M, Jukić F, Krička T. Influence of Harvest Time, Method of Preparation and Method of Distillation on the Qualitative Properties of Organically Grown and Wild Helichrysum italicum Immortelle Essential Oil. Separations. 2021; 8(10):167. https://doi.org/10.3390/separations8100167
Chicago/Turabian StyleMatin, Ana, Ivan Pavkov, Mateja Grubor, Vanja Jurišić, Mislav Kontek, Franko Jukić, and Tajana Krička. 2021. "Influence of Harvest Time, Method of Preparation and Method of Distillation on the Qualitative Properties of Organically Grown and Wild Helichrysum italicum Immortelle Essential Oil" Separations 8, no. 10: 167. https://doi.org/10.3390/separations8100167
APA StyleMatin, A., Pavkov, I., Grubor, M., Jurišić, V., Kontek, M., Jukić, F., & Krička, T. (2021). Influence of Harvest Time, Method of Preparation and Method of Distillation on the Qualitative Properties of Organically Grown and Wild Helichrysum italicum Immortelle Essential Oil. Separations, 8(10), 167. https://doi.org/10.3390/separations8100167







