Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation and Methods
2.3. Preparation and Characterization of Photocatalyst Materials
2.3.1. B/NaF-TiO2
2.3.2. B/NaF–TiO2–SiPc Photocatalyst
2.4. Photocatalytic Experiments
3. Results and Discussion
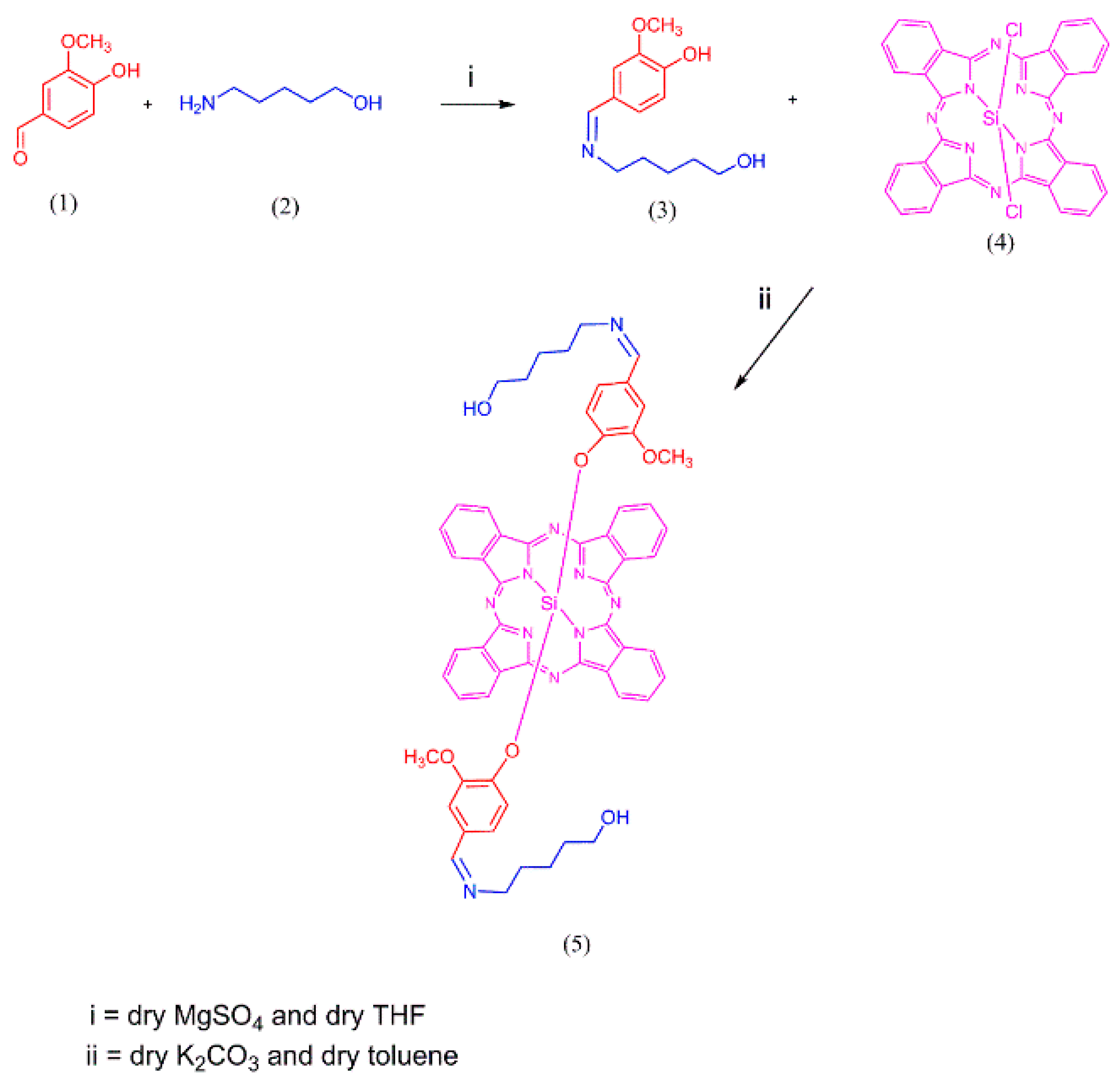
3.1. Synthesis and Characterization of the Methoxy-Substituted Silicon Phthalocyanine (SiPc)
3.1.1. Synthesis of the Methoxy-Substituted Silicon Phthalocyanine (SiPc)
3.1.2. Characterization of SiPc
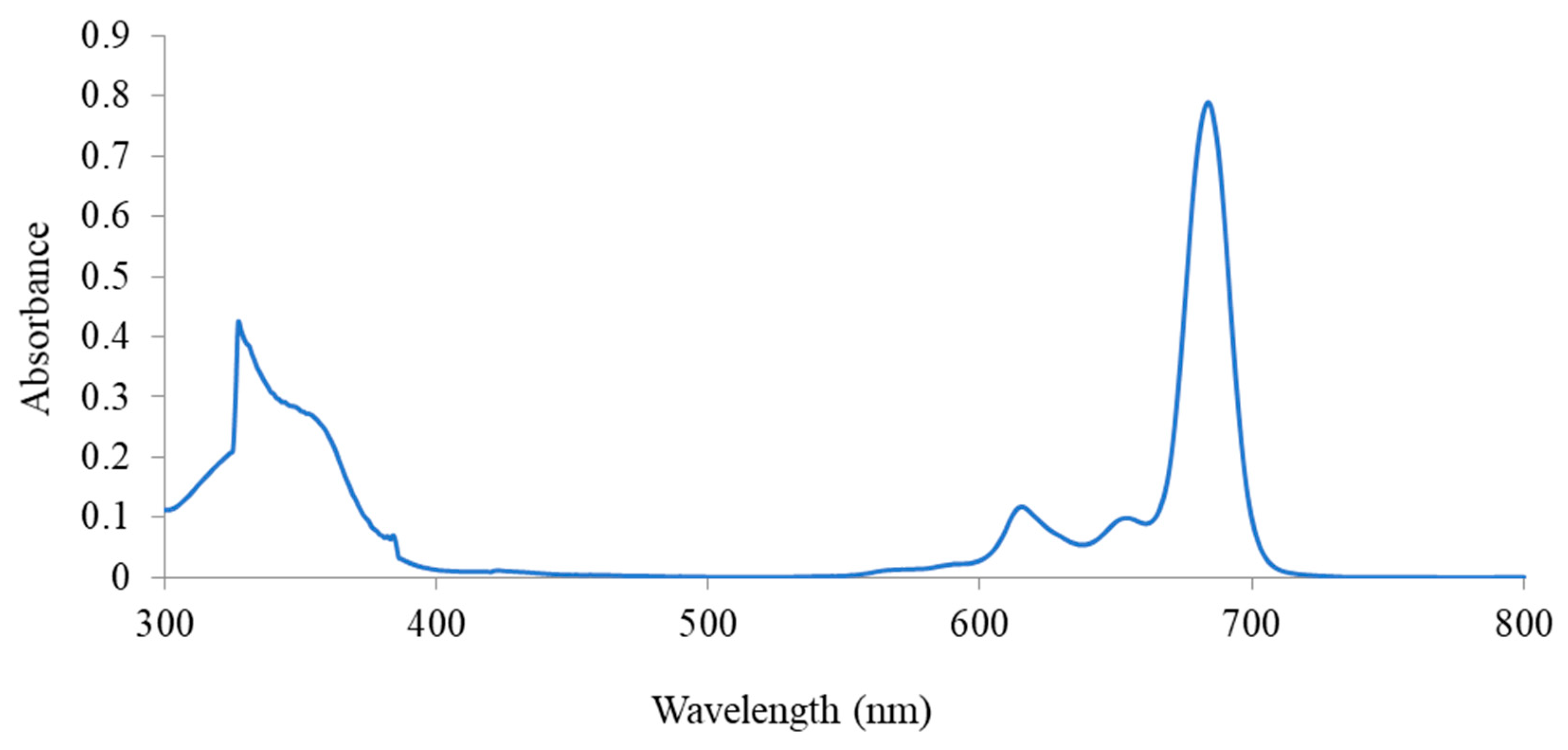
3.1.3. UV–Vis Absorption of the Methoxy-Substituted Silicon Phthalocyanine
3.2. Photocatalyst Characterization
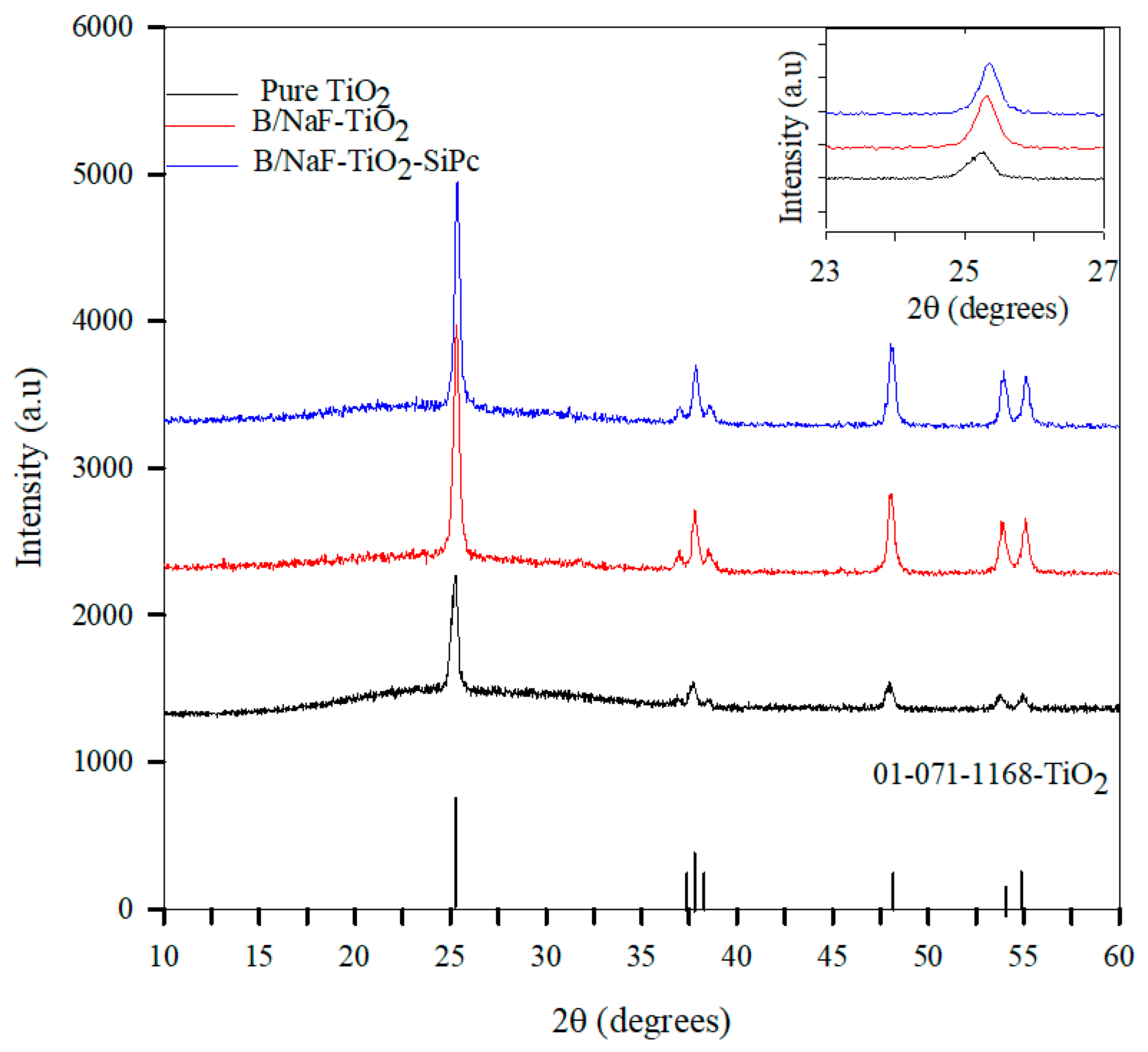
3.2.1. X-Ray Diffraction (XRD) Analysis
3.2.2. N2 Adsorption–Desorption at 77K (BET and BJH Model Application)
3.2.3. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.2.4. Transmission Electron Microscopy (TEM)
3.2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.2.6. Light Absorption and Energy Band Gap Structural Analysis
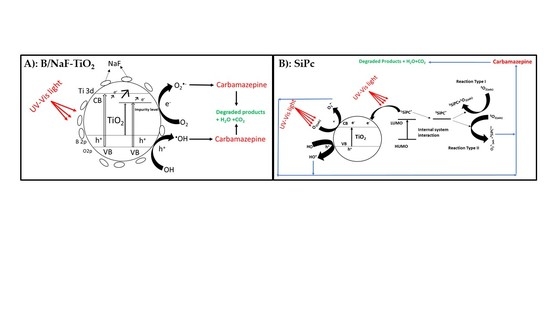
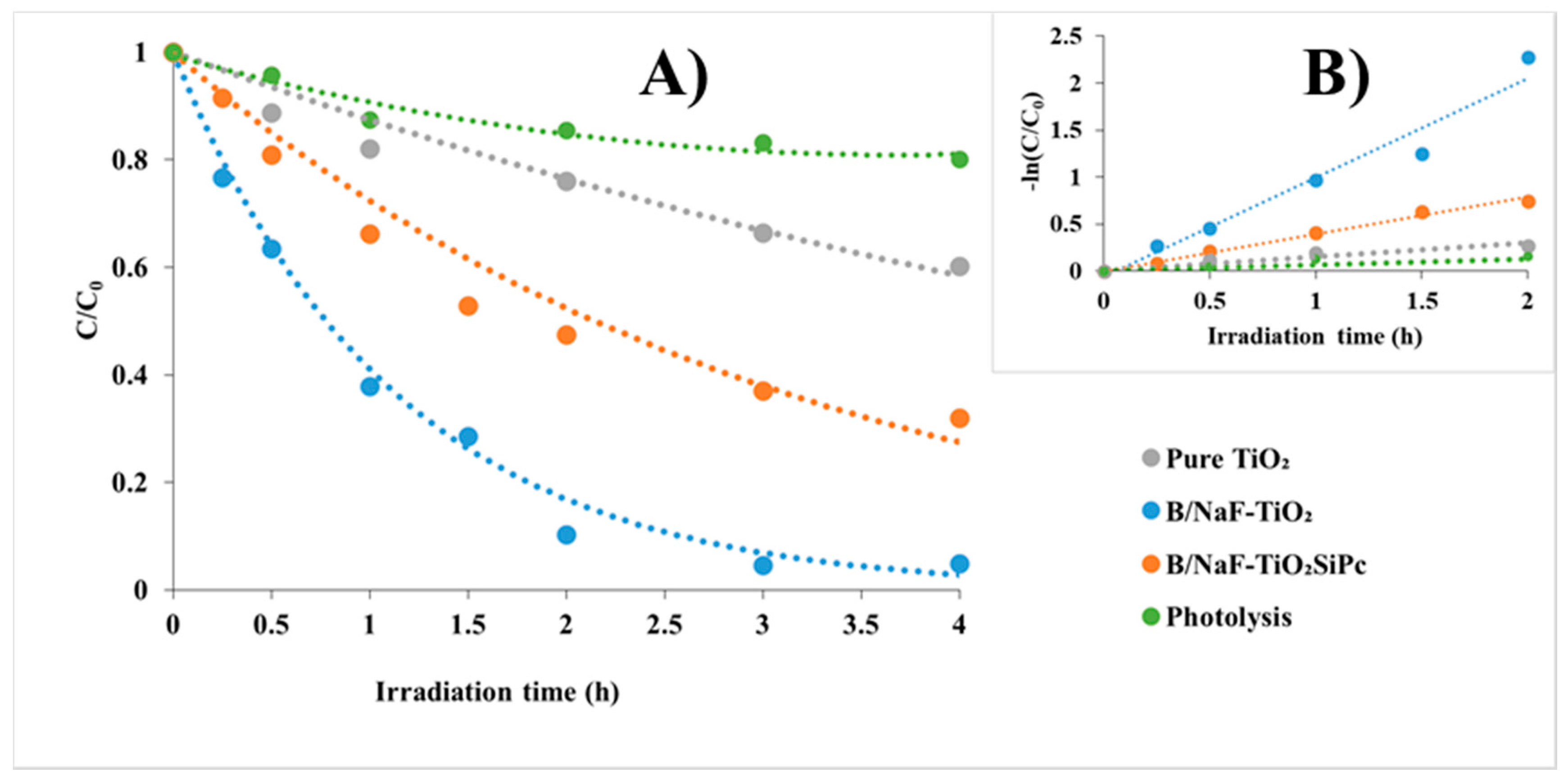
3.3. Photocatalytic Activity
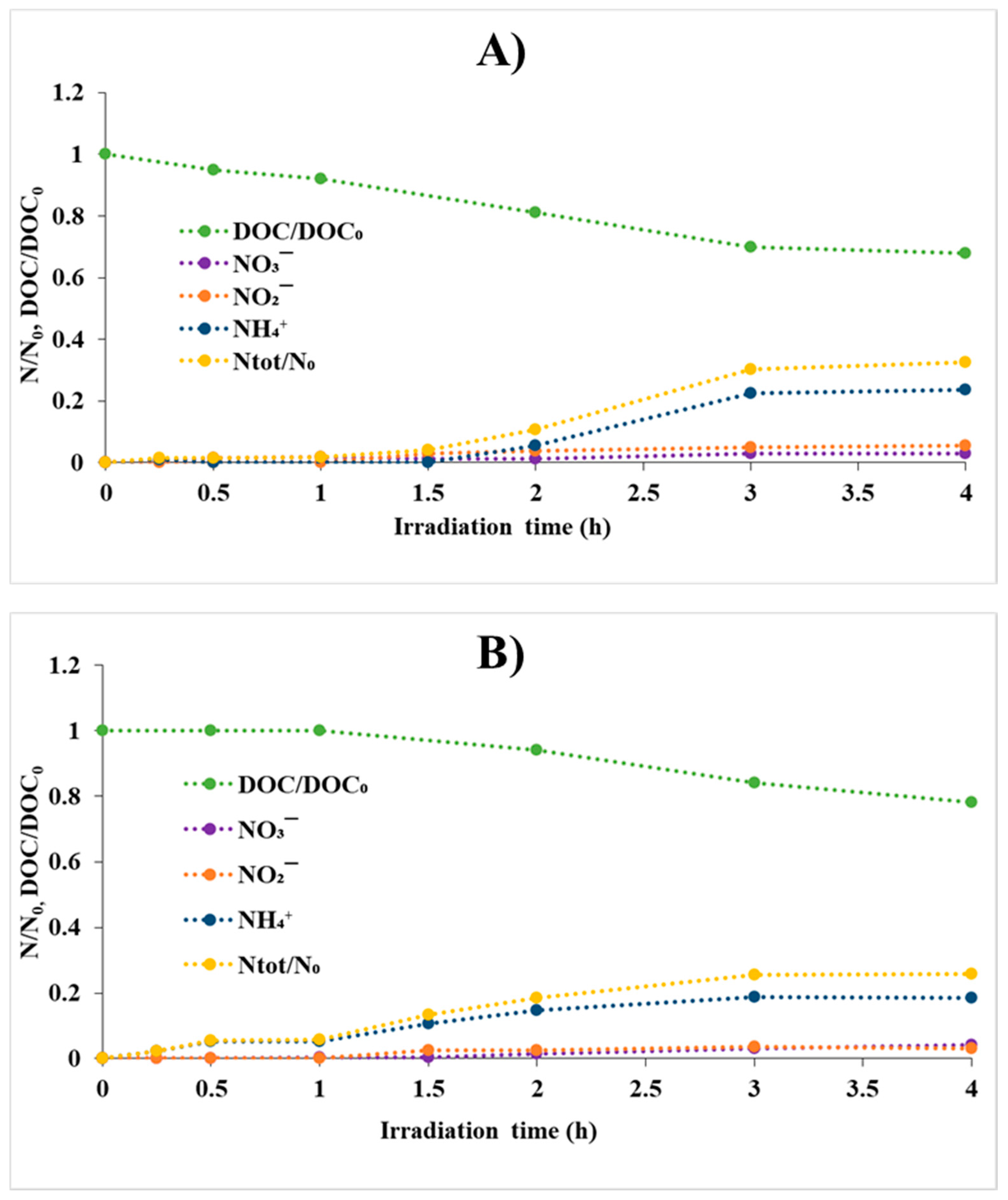
3.4. Transformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saracino, M.; (Institute for Organic Synthesis and Photoreactivity (ISOF), Italian National Research Council (CNR), Bologna, Emilia Romagna, Italy); Salvatore, S.E.; (Institute for Organic Synthesis and Photoreactivity (ISOF), Italian National Research Council (CNR), Bologna, Emilia Romagna, Italy); Zanelli, A.; (Institute for Organic Synthesis and Photoreactivity (ISOF), Italian National Research Council (CNR), Bologna, E. Romagna, Italy). Chemistry and Industry. Scientific Communication, 1997.
- Jimenez-Hogaldo, C.; Chrimatopoulos, C.; Stathopoulos, V.; Sakkas, V. Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. [Google Scholar] [CrossRef]
- Ganesh, I. Surface Structural Energy Band Gap and Photocatalytic Features of an Emulsion –Driven Boron-Doped TiO2 Nano Powder. Mol. Catal. 2018, 451, 51–65. [Google Scholar] [CrossRef]
- Dal Santo, V.; Naldoni, A. Titanium Dioxide Photocatalysis. Catalysts 2018, 8, 591. [Google Scholar] [CrossRef]
- Subramanian, A.; Wang, H.W. Effects of Boron Doping in TiO2 Nanotubes and the Performance of Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2012, 258, 6479–6484. [Google Scholar] [CrossRef]
- Dozzi, M.A.; Artiglia, L.; Granozzi, G.; Ohtani, B.; Elena, S. Photocatalytic Activity vs Structural Features of Titanium Dioxide Materials Singly Doped or Co-doped with Fluorine and Boron. J. Phys. Chem. C 2004, 118, 25579–25589. [Google Scholar] [CrossRef]
- Palmas, D.; Pozzo, A.D.; Mascia, M.; Vacca, A.; Ricci, P.C. Sensitization of TiO2 Nanostructures with Coumarin 343. Chem. Eng. J. 2012, 211–212, 285–292. [Google Scholar] [CrossRef]
- Murphy, S.; Saurel, C.; Morrissey, A.; Tobin, J.; Oelgemoller, M.; Nojan, K. Photocatalytic Activity of a Porphyrin/TiO2 Composite in the Degradation of Pharmaceuticals. Appl. Catal. B. Environ. 2012, 119–120, 156–165. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Zanjanchi, M.A.; Noei, H.; Arvand, M.; Wang, Y. TiO2 Nanoparticles Containing Sulphonated Cobalt Phthalocyanine: Preparation, Characterization and Photocatalytic Performance. J. Environ. Chem. Eng. 2014, 2, 484–494. [Google Scholar] [CrossRef]
- Giuseppe, M.; Elisa, G.; Leonardo, P.; Gabriela, D.; Rudolf, S. Photocatalytic Degradation of 4-Nitrophenol in Aqueous Suspension by Using Polycrystalline TiO2 Impregnated with Lanthanide Double-Decker Phthalocyanine Complexes. J. Phys. Chem. C. 2017, 111, 6581–6588. [Google Scholar]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Photocatalytic Degradation of Erythromycin under Visible Light by Zinc Phthalocyanine Modified Titania Nanoparticles. Mater. Sci. Semi. Proc. 2014, 23, 98–103. [Google Scholar] [CrossRef]
- Vargas, E.; Vargas, R.; Nunez, O. A TiO2 Surface Modified with Copper (II) Phthalocyanine Tetrasulfonic Acid Tetrasodium Salt as a Catalyst During Photoinduced Dichlorvos Mineralization by Visible Solar Light. Appl. Catal. B Environ. 2014, 156–157, 8–14. [Google Scholar] [CrossRef]
- Albay, C.; Koc, M.; Altin, I.; Bayrak, R.; Degirmencioglu, I. New Dye Sensitized Photocatalysts: Copper (II)-Phthalocyanine/TiO2 Nanocomposite for Water Remediation. J. Photochem. Biol. A Chem. 2016, 324, 117–125. [Google Scholar] [CrossRef]
- Szkoda, M.; Lisowska-Oleksiak, A.; Suizdak, K. Optimization of Boron -Doping Process of Titania Nanotubes Via Electrochemical Method Toward Enhanced Photoactivity. J. Solid State Electrochem. 2016, 20, 1765–1774. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, M.; Zhang, J.; Chen, F. Effective Visible Light-Active Boron and Carbon Modified TiO2 Photocatalyst for Degradation of Organic Pollutant. Appl. Catal. B Environ. 2010, 97, 182–189. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Biyiklioglu, Z.; Bacaksiz, E.; Polat, I.; Stathopoulos, V.N. Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation Under UV Irradiation. Nanomaterials 2020, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.T.; Gohel, M.C.; Patel, L.D. Studies to Enhance Dissolution Properties of Carbamazepine. Indian J. Pharm. Sci. 2007, 69, 427–430. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Nunes, C.O.; Pereira, M.F.R. Environ Int. An overview on the Advanced Oxidation Processes Applied for the Treatment of Water Pollutants Defined in the Recently Launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Removal of Selected Persistent Organic Pollutants by Heterogeneous Photocatalysis in Water. Catal. Today 2005, 101, 195–202. [Google Scholar] [CrossRef]
- Miao, X.; Metcalfe, C. Determination of Carbamazepine and its Metabolites in Aqueous Samples using Liquid Chromatography –Electrospray Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 3731–3738. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, G.; Viruthagiri, N.; Shanmugam, R.; Gobi, P. Synthesis and Characterization of Undoped and Cobalt-Doped TiO2 Nanoparticles via Sol-gel Technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef]
- Altin, I.; Sokmen, M.; Biyiklioglu, Z. Sol gel Synthesis of Cobalt Doped TiO2 and Its Dye Sensitization for Efficient Pollutant Removal. Mater. Sci. Semicon. Proc. 2016, 45, 36–44. [Google Scholar] [CrossRef]
- Bayrak, R.; Bekircan, O.; Durmus, M.; Degirmencioglu, I. The Synthesis and Structural Characterization of New Metallo Phthalocyanines with 1,2,4-triazole Fragments. J. Organomet. Chem. 2014, 767, 101–107. [Google Scholar] [CrossRef]
- Yalcin, I.; Yanik, H.; Akcay, H.T.; Dergimencioglu, I.; Durmus, M. Photophysical and Photochemical Study on the Tetra 4- Isopropylbenzyloxy Substituted Phthalocyanines. J. Lumin. Source 2017, 192, 739–744. [Google Scholar] [CrossRef]
- Keles, T.; Barut, B.; Ozel, A.; Biyiklioglu, Z. Synthesis of Water-Soluble Silicon Phthalocyanine, Napthalocyanine Bearing Pyridine Groups and Investigation of their DNA Interaction, Topoisomerase Inhibition, Cytotoxic Effects and Cell Cycle Arrest Properties. Dyes Pigm. 2019, 164, 372–383. [Google Scholar] [CrossRef]
- Avetta, P.; Prevot, A.B.; Fabbri, D.; Montoneri, E.; Tomasso, L. Photodegradation of Naphthalene Sulphonic Compounds in the Presence of a Bio-Waste Derived Sensitizer. Chem. J. Eng. 2012, 197, 193–198. [Google Scholar] [CrossRef]
- Manuela, D.; Fabbri, D.; Bianco Prevot, A.; Pramauro, E. Removal of Alkylphenols from Polluted Sites Using Surfactant-Assisted Soil Washing and Photocatalysis. Environ. Sci. Pollut Res. 2011, 18, 783–789. [Google Scholar]
- Carlos, L.; Fabbri, D.; Capparelli, A.L.; Bianco Prevot, A.; Pramauro, E.; Einschlag, F.G. Effect of Simulated Solar Light on the Autocatalytic Degradation of Nitrobenzene Using Fe3+ and Hydrogen Peroxide. J. Photochem. Photobiol. C 2009, 201, 32–38. [Google Scholar] [CrossRef]
- Bas, H.; Biyiklioglu, Z. Non -aggregated Axially Napthoxacin Group Substituted Silicon Phthalocyanines: Synthesis and Electrochemistry. J. Organomet. Chem. 2015, 791, 238–243. [Google Scholar] [CrossRef]
- Biyiklioglu, Z. Electrochemical and Aggregation Properties of Newly Synthesized Dendritic Axially Morpholine -Disubstituted Silicon Phthalocyanine, Mono-substituted Sub phthalocyanine and their Quanternized Derivatives. Inorg. Chem. Commun. 2015, 55, 60–64. [Google Scholar] [CrossRef]
- Demirkapi, D.; Sirin, A.; Yildiz, B.T.; Cakar, Z.P.; Sesalan, B.S. The Synthesis of New Silicon Phthalocyanines and Analysis of their Photochemical and Biological Properties. Synth. Met. 2014, 187, 152–159. [Google Scholar] [CrossRef]
- Sahin, M.G.; Biyiklioglu, Z.; Durmus, M. The Water Soluble Axially Disubstituted Silicon Phthalocyanines: Photophysicochemical Properties and Invitro Studies. J. Biol. Inorg. Chem. 2017, 22, 953–967. [Google Scholar]
- Bayrak, R.; Akcay, F.S.; Sahin, E.; Bayrak, H.; Dermibas, U. Synthesis, Aggregation and Spectroscopic Studies of Novel Water-Soluble Metal Free Zinc, Copper and Magnesium Phthalocyanines and Investigation of their Anti-Bacterial Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Klung, H.P.; Alexander, L.E.; Langford, J.I. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials. J. Appl. Crystallogr. 1975, 8, 573–574. [Google Scholar]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effects of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties of the CO Oxidation Performance of Nanostructured CeO2/TiO2. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Optical and Morphological Analysis of Pristine Titanium di oxide Nanoparticles via Sol-gel Route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible Light Active Titanium Oxide Photocatalyst Realized by an Oxygen –Deficient Structure and by Nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Herrera-Alonso, M.; Abdala, A.A.; McAllister, M.J.; Aksay, I.A.; Prud’homme, R.K. Intercalation and Stitching of Graphite Oxide with Diamino alkanes. Langmuir 2007, 23, 10644–10649. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Zhou, F.F.; Shi, J.S.; Huang, Y.M. Strong Photo-Oxidative Capability of ZnWO4 Nanoplates with Highly Exposed {0 -1 1} Facets. Catalysts 2019, 9, 178. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Wang, Q.; Jiang, E. Effects of Boron Doping on Photocatalytic Activity and Microstructure of Titanium Dioxide Nanoparticles. Ind. Eng. Chem. Res. 2006, 45, 4110–4116. [Google Scholar] [CrossRef]
- Li, D.; Xing, Z.; Yu, X.; Cheng, X. One-Step Hydrothermal Synthesis of C-N-S Tri-doped TiO2 Based Nanosheets Photoelectrode for Enhanced Photo electrocatalytic Performance and Mechanism. Electrochim. Acta 2015, 170, 182–190. [Google Scholar] [CrossRef]
- Appavoo, I.A.; Hu, J.; Huang, Y.; Li, S.F.Y.; Ong, S.L. Response Surface Modelling of Carbamazepine (CBZ) Removal by Graphene P-25 Nanocomposites/UVA Process using Central Composite Design. Water Res. 2014, 57, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Ho, W.; Zhang, L.; Zhou, Z.; Lee, S. Biomolecule Controlled Hydrothermal Synthesis of C-N-S Tri-doped TiO2 Nanocrystalline Photocatalyst for NO Removal under Simulated Solar Light Irradiation. J. Hazard. Mater. 2009, 169, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Wantabe, Y.; Hashimoto, K. Nitrogen Concentration Dependence on the Photocatalytic Activity of TiO2-XNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Photocatalytic Oxidation of Phenolic Compounds by Using a Carbon- Nanotube Titanium Dioxide Composite Catalyst. Chem. SUS Chem. 2010, 3, 609–618. [Google Scholar] [CrossRef]
- Wang, W.; Serp, P.; Kack, P.; Faria, J.L. Photocatalytic Degradation of Phenol on MWNT and Titania Composite Catalyst Prepared by a Modified Sol Gel Method. Appl. Catal. B. 2005, 56, 305–312. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Baiocchi, C. Identification of the Unknown Transformation Products derived from Clarithromycin and Carbamazepine Using Liquid Chromatography/High-Resolution Mass Spectrometry. Rapid Comm. Mass Spec. 2012, 1687–1704. [Google Scholar] [CrossRef]
- Liu, K.; Che-Chin Yu, J.; Dong, H.; Wu, J.C.S.; Hoffmann, M.R. Degradation and Mineralization of Carbamazepine Using an Electro Fenton Reaction Catalysed by Magnetite Nanoparticles Fixed on an Electrocatalytic Carbon Fibre Textile Cathode. Environ. Sci. Techol. 2018, 52, 12667–12674. [Google Scholar] [CrossRef]
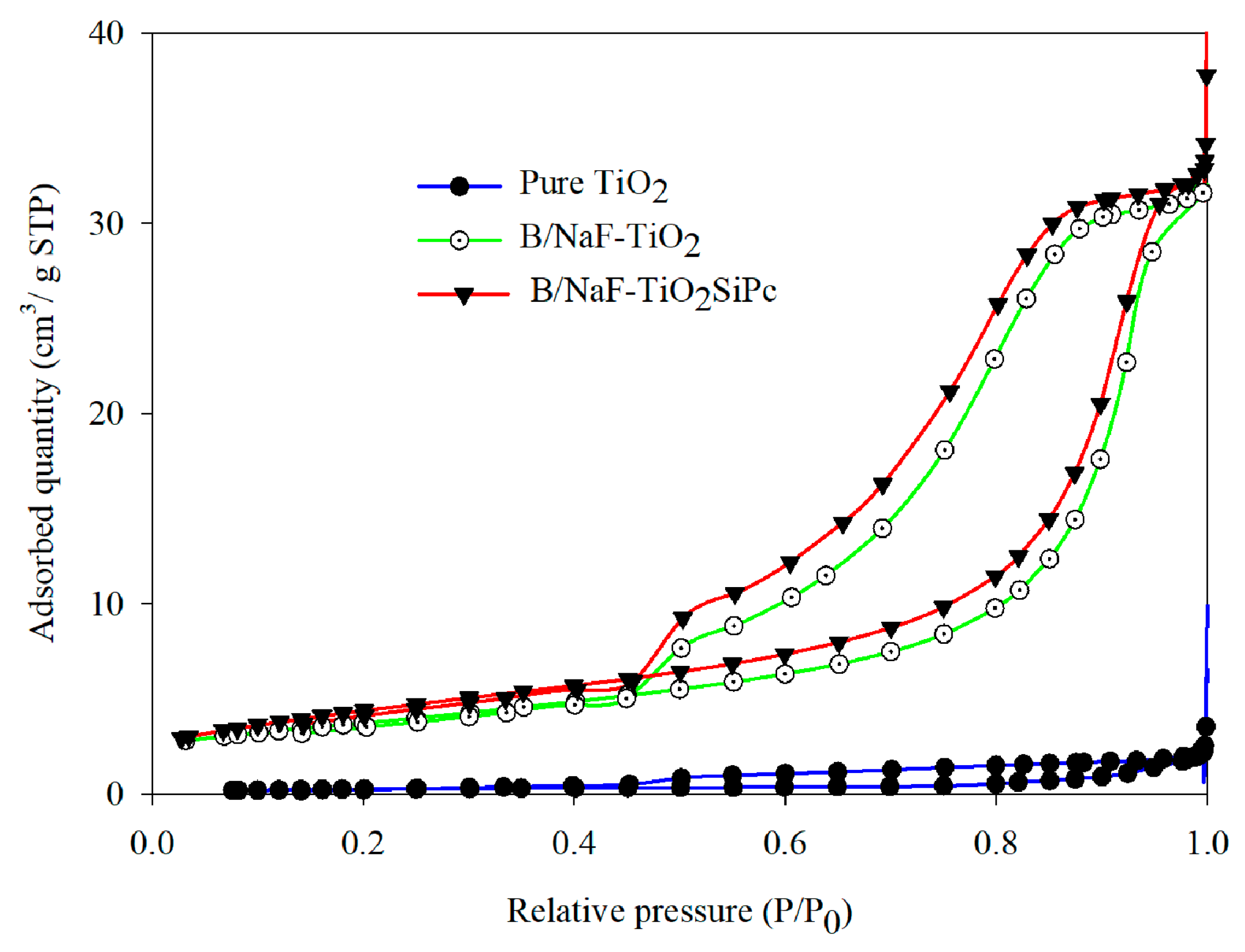
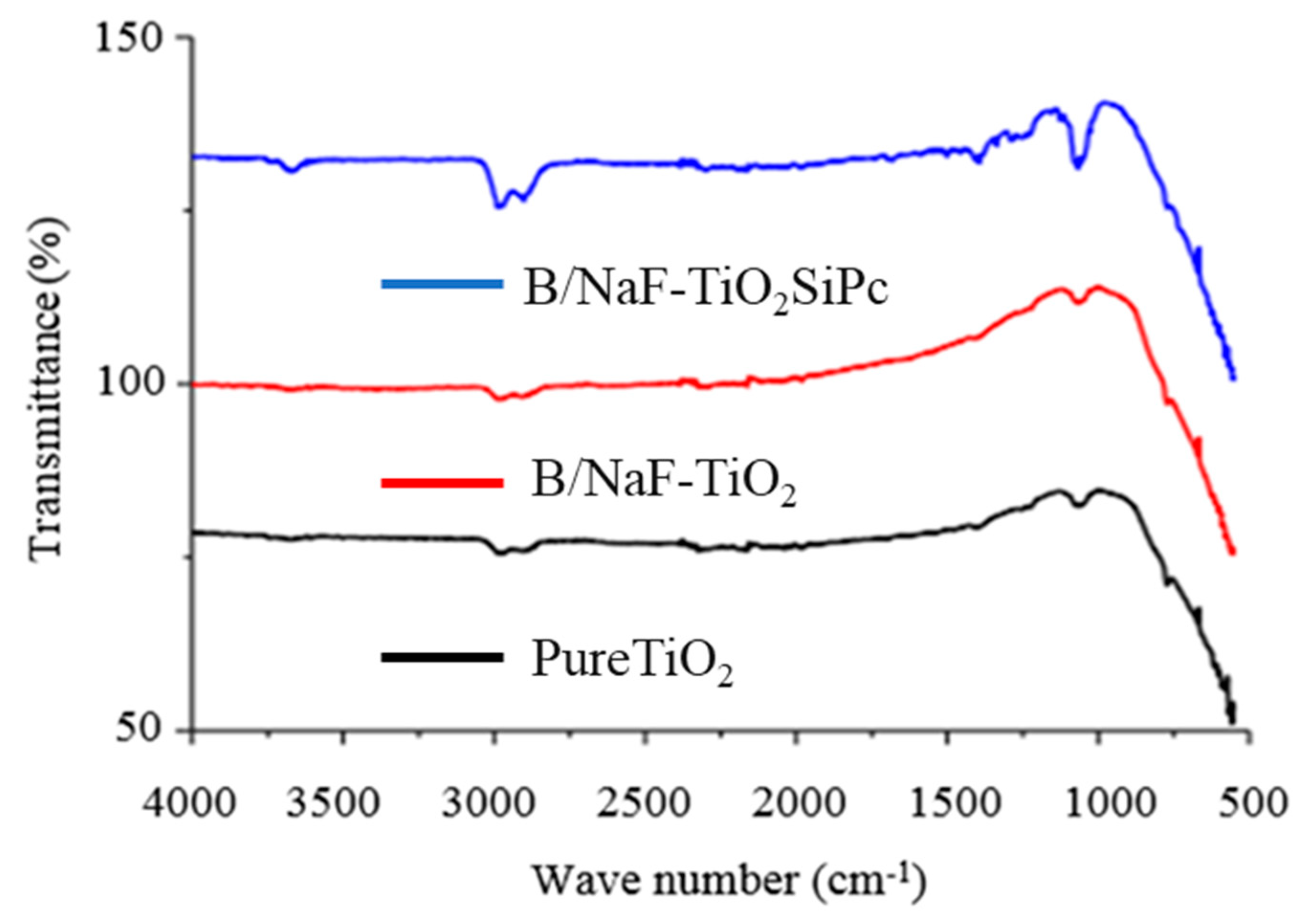
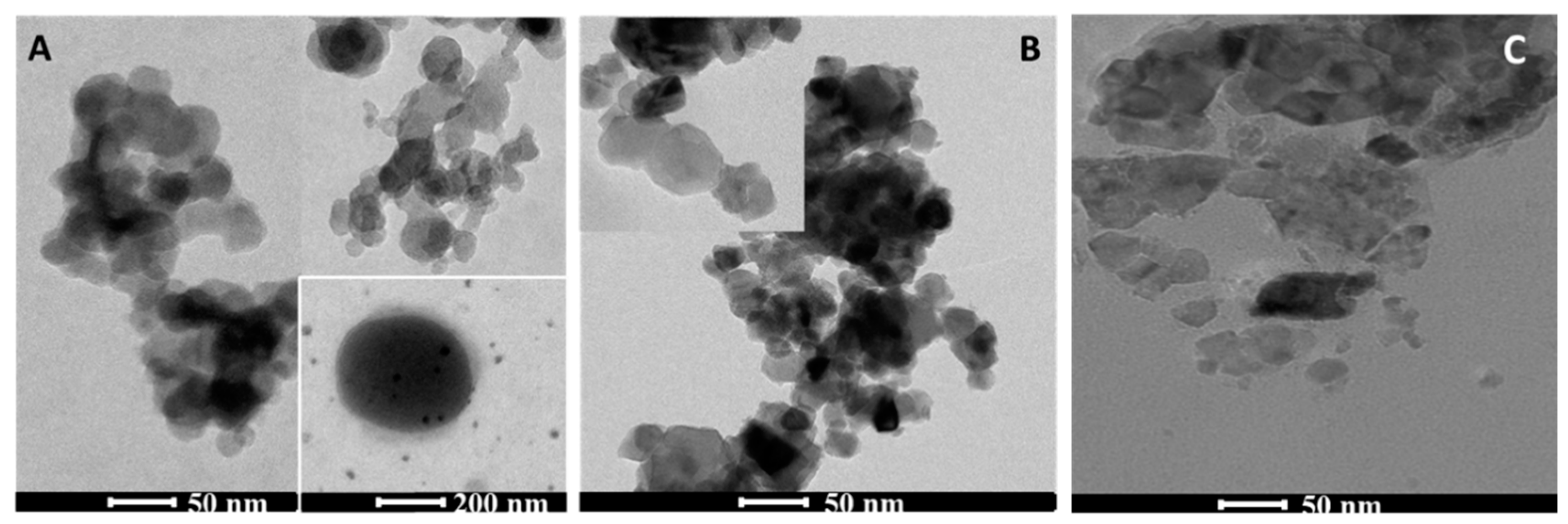

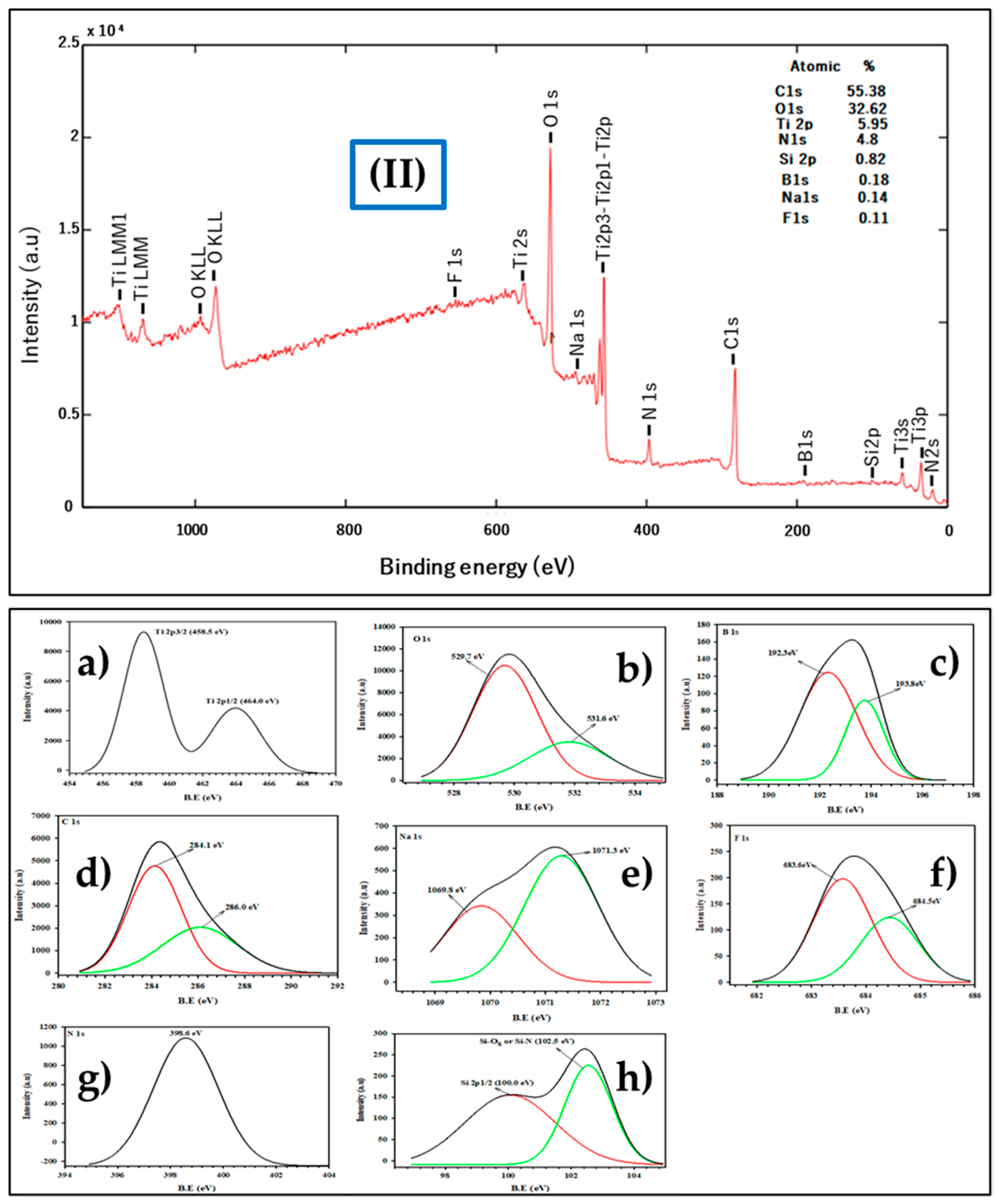

| Catalyst | SBET (m2/g) | a Vpore (cm3/g) | b CS, Crystallite Size (nm) | c dTEM, Particle Size (nm) |
|---|---|---|---|---|
| Pure TiO2 | ˜1 | ˂0.01 | 24 | 25 |
| B/NaF-TiO2 | 13 | 0.05 | 24 | 28 |
| B/NaFTiO2SiPc | 13 | 0.05 | 29 | 31 |
| [M-H]+ | Label | Retention Time (min) | Chemical Structure |
|---|---|---|---|
| 237 | 237 | 5.96 | 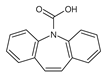 |
| 251 | 251_A | 3.72 | 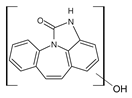 |
| 251 | 251_B | 4.43 |  |
| 251_C | 4.69 | 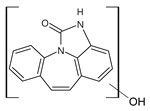 | |
| 253 | 253_A | 4.29 | 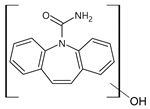 |
| 253_B | 4.90 | ||
| 253_C | 5.20 | ||
| 253_D | 5.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anucha, C.B.; Altin, I.; Fabbri, D.; Degirmencioglu, I.; Calza, P.; Magnacca, G.; Stathopoulos, V.N.; Bacaksiz, E. Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations 2020, 7, 71. https://doi.org/10.3390/separations7040071
Anucha CB, Altin I, Fabbri D, Degirmencioglu I, Calza P, Magnacca G, Stathopoulos VN, Bacaksiz E. Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations. 2020; 7(4):71. https://doi.org/10.3390/separations7040071
Chicago/Turabian StyleAnucha, Chukwuka B., IIknur Altin, Debora Fabbri, Ismail Degirmencioglu, Paola Calza, Giuliana Magnacca, Vassilis N. Stathopoulos, and Emin Bacaksiz. 2020. "Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine" Separations 7, no. 4: 71. https://doi.org/10.3390/separations7040071
APA StyleAnucha, C. B., Altin, I., Fabbri, D., Degirmencioglu, I., Calza, P., Magnacca, G., Stathopoulos, V. N., & Bacaksiz, E. (2020). Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations, 7(4), 71. https://doi.org/10.3390/separations7040071