Abstract
Aside from the classical residues of persistent organic pollutants (POPs), the occurrence of emerging contaminants (ECs) in the environment has become a subject of increasing concern due to their harmful impact on the aquatic environment. Wastewater treatment plant (WWTP) effluents are major sources of environmental pollution. Therefore, data concerning their existence is required. In this study, twenty compounds representative of different drug groups considered ECs and belonging to antibiotics, antipsychotics, anti-inflammatory drugs plus acesulfame K were selected to be accurately detected and quantified with UHPLC–LTQ-Orbitrap MS in hospital and urban WWTP effluents. Chromatographic parameters (column efficiency, mobile phase, etc.), as well as mass spectrometry conditions concerning ionization mode and Orbitrap analysis (ESI options, mass resolving power, AGC target, tube lens, injection time), were evaluated. Moreover, a novel fabric phase sorptive extraction (FPSE) method based on fiber glass coated with PEG300 was employed as sample preparation process. Experimental parameters affecting extraction and desorption steps such as sample pH, extraction time, ionic strength, elution time and solvent have been optimized. The optimized methodology was validated providing excellent linearity (R2 > 0.99), and low detection and quantification limits up to 3.1 and 9.3 ng/L, for carbamazepine, respectively. Relative recoveries ranged from 81.1% to 114.0%, while a medium matrix effect for most of the target compounds occurred. Applying the above analytical method in effluents of WWTPs from NW Greece, nine compounds were quantified with concentrations that varied from 55.4 to 728.4 ng/L.
Keywords:
Orbitrap; UHPLC; emerging contaminants; POPs; wastewater; FPSE; pharmaceuticals; acesulfame K 1. Introduction
During the last three decades, the impact of chemical pollution has focused on persistent organic pollutants (POPs). POPs are toxic chemicals with negative effects in the environment and human health. They persist in the environment for extended periods and bioaccumulate through the food chain [1]. The criteria for chemical persistence based on their transformation half-lives and physicochemical properties have been established under many regulatory frameworks and were finally officially catalogued at the Stockholm Convention in 2009 [2]. Nevertheless, recently, it has been recognized that risks to aquatic environment and human health are not limited to chemicals fitting the classical definition of POPs. An examination of the complex mixtures of chemicals present in natural water reveals the presence of organic chemicals covering a wide range of water solubilities and environmental half-lives. For instance, in recent years, emerging contaminants (ECs) have gained increasing interest in the field of environmental research. In this study, the analytes of interest belong to ECs and are not classified as persistent pollutants. However, their continuous use and release into the environment has resulted in their description as “pseudo-persistent” compounds [3,4]. A framework for an EU action in the field of water policy by Directive 2000/60/EC was established [5]. Emerging contaminants are chemical compounds that have the potential to enter the environment and cause known or suspected adverse ecological and/or human health impacts [6,7,8,9,10] and typically are not regulated under current environmental laws. Among them are pharmaceuticals, pesticides, industrial chemicals, surfactants, sweeteners, and personal care products [10,11,12,13]. Pharmaceuticals constitute one of the most important emerging classes of environmental pollutants.
Recent studies have discovered their occurrence in environmental samples investigated worldwide, including different aqueous matrices [14,15,16]. Wastewater treatment plants (WWTP) seemed to be the main contamination discharge for pharmaceuticals from human and veterinary activities as a result of excretion and metabolism by humans and animals, and additionally disposal of unused or expired drugs [17,18]. Artificial sweeteners (ASs) on the other hand are widely used as sugar substitutes in a broad range of food, beverages, and drugs in significant quantities [19,20,21]. Over the past decade, pharmaceuticals and artificial sweeteners have been frequently detected as emerging organic contaminants at pg/L to μg/L levels in various aquatic environments such as surface water and groundwater [11,22] due to insufficient removal from WWTPs resulting in further spread through the water cycle. The presence of these micropollutants may pose adverse long-term risk to human health and aquatic ecosystems under chronic long-term exposure despite their low concentrations. In addition, an extra concern is focused on antibiotics, not only due to their extensive use, but also to their ability to alter the microbial community structure, facilitating the development of antibiotic-resistant human pathogens [23,24]. For this reason, the Watch List (WL), published in 2015 (Decision 2015/495), included, among other compounds, the macrolide antibiotic of erythromycin [25]. The forementioned Watch List also encompasses the studied anti-inflammatory drug diclofenac, which was previously introduced in the first WL of Directive 2013/39/UE [26]. In this context, it is important to set up fast, sensitive, and reliable analytical methods that enable the determination of a wide range of ECs residues in environmental waters, such as hospital and urban wastewater at the low concentration levels that they are found. One approach to attain this is to acquire a chromatographic system with a higher resolution by employing more efficient stationary phases and more sensitive mass analyzers. This has been well documented in the literature, and recently, Fornstedt et al. have provided a comprehensive description of the basic theory of liquid chromatography and its recent trends [27]. Currently, the proposed analytical procedures for determining pharmaceuticals and sweeteners in environmental waters are mainly based on UHPLC–MS/MS, due to its high selectivity and low detection limits [15,28,29].
With respect to liquid chromatography coupled to mass spectrometry technique, the recent trend involves the use of high-resolution mass analyzers such as Q-TOF or Orbitrap which allow to obtain mass accuracies lower than 5 ppm. Over recent years, interest has been steadily growing in the application of Orbitrap to environmental samples and the field of food safety. Bade et al. [30] employed high mass resolution approach technique for screening of pharmaceutical and illicit drugs in samples of wastewater and surface water from Spain and Italy. Later, Pugajeva et al. investigated effluent wastewater for the presence of 24 emerging pharmaceutical residues in wastewater [31]. Kosma et al. studied the occurrence of antipsychotic drugs with the implementation of an Orbitrap analyzer in a hospital and urban wastewater treatment plant (WWTP) in Ioannina city, in northwestern Greece [32].
Due to the increasing interest in the determination of organic micro-pollutants in complex matrices at low concentrations, several liquid or solid-phase extraction techniques were developed [33,34,35]. Research trends are increasingly promoting more environmentally friendly analytical procedures, simplifying the extraction process, and developing micro-extraction methods [36,37,38,39]. As a response to the global call for green approaches of sample preparation technologies, Kabir and Furton [40] have recently developed fabric phase sorptive extraction (FPSE), which is considered a micro-extraction sorbent-based technique [41]. This novel technique consists of a synthetic fabric coated with high-efficiency inorganic-organic sorbents using sol–gel technology [42,43]. The modified fabric is directly submerged into the sample containing the target analytes and, once equilibrium is reached, the analytes retained on the extraction medium can be eluted with appropriate organic solvent [44]. This technique ensures high retention capacity through higher sorbent loading, fast extraction interaction, relatively short extraction time and reduced consumption of organic solvents in the sample preparation [45,46,47].
FPSE has been applied to extract a wide range of analytes from different samples, such as UV stabilizers in sewage samples [48], anti-inflammatory drugs [49], alkyl phenols in aqueous and soil samples [50], triazine herbicides [35], estrogens in urine and environmental water samples [51], and polar antibiotic in raw milk [52]. Our research group has recently used FPSE for the extraction of selected antipsychotic drugs from environmental waters followed by high performance liquid chromatography-UV-DAD with satisfactory results [53].
The present study is aimed at the optimization, validation, and application of the UHPLC-LTQ-Orbitrap-HRMS method for the quantification of twenty selected pharmaceuticals of different therapeutic classes and one sweetener, namely acesulfame K. Chromatographic conditions and parameters affecting signal response, resolution, mass accuracy was evaluated to obtain the most suitable performance characteristics of UHPLC-LTQ-Orbitrap. Furthermore, the optimized analytical chromatographic technique is combined for the first time (according to our knowledge) with the application of an easy, green, and fast sample preparation process, namely fabric-phase sorptive extraction (FPSE), for the determination of these emerging contaminants. Finally, method suitability was tested successfully by its application in real water samples collected at the main wastewater and hospital effluent water of Ioannina city in Greece.
2. Materials and Methods
2.1. Standard Solutions and Reagents
For the present study, a set of 20 multiclass pharmaceuticals and one artificial sweetener was selected. All standards were of high purity (>95%), and purity grade of the standard was considered for the preparation of standard solutions. Specifically, acesulfame (>95%), amitriptyline (>98%), carbamazepine (>99%), clomipramine HCl (>98%), cyclobenzaprine HCL (>98%), diclofenac (>98%), erythromycin (>99%), fluoxetine HCl (>98%), indomethacin (>97%), mefenamic acid (>99%), paroxetine (98%), salicylic acid (>97%), sulfacetamide (>98%), sulfamethazine (>98%), sulfamethoxazole (>98%), sulfamethoxy-pyridazine (>97%), sulfapyridine (>98%), sulfaquinoxaline (>98%), tolfenamic acid (>99%), triclosan (>99%), and trimethoprim (>98%). Their physicochemical characteristics and chemical structures are listed in Supplementary Table S1. The preparation of stock standard solutions as well as reagents and solvents are described in detail in the Supplementary Material (Section S2.1).
2.2. Sample Collection
Composite effluent wastewater samples were collected from the wastewater treatment plant of Ioannina city located in Epirus region, NW Greece, (WWTP-U) as well as from Ioannina hospital of the University (WWTP-H). The University hospital of Ioannina city has a capacity of 800 beds and provides a broad range of clinical services and medical specialties since it is also a center of research. It serves a population of approximately 130,000 inhabitants since it is a reference hospital for Epirus region and suburbs. Wastewater effluents of the hospital are discharged into public sewer network, being co-treated with domestic wastewaters in municipal WWTPs. WWTP-U has a traditional three-stage treatment technology that includes mechanical screening, biological treatment, and sewage sludge treatment. WWTP-U serves Ioannina, a city, with population of almost 120,000 people. Domestic, industrial wastes, and rain waters are obtained from the WWTP-U, often surpassing the WWTP’s capacity to deal with this amount. WWTP-U also receives hospital effluents, therefore the occurrence of pharmaceutical compounds in both plants has a significant interest [54].
The sampling took place during three different days on 13–15 February 2020. Samples were collected every 120 min, from 8:00 to 16:00 h, and then combined to provide a final representative composite sample. Final volumes of 1 L (n = 3) wastewater effluents were collected at the final stage after the secondary treatment plant for each sampling site (WWTP-U and WWTP-H). One aliquot of sample was used for BOD and COD analysis, while the other was pooled for occurrence analysis of target compounds. BOD and COD analysis was performed for three effluents in every plant, one for each sampling day. All samples were collected in amber glass bottles pre-rinsed with deionized water. Up on their arrival in the laboratory, were centrifuged (4000 rpm, 25 °C, 10 min) and filtered with 0.2-μm polypropylene (PP) filters. Afterwards, the samples were stored in the dark at 4 °C before sample pretreatment. The samples were extracted within 48 h in all the cases. Biochemical oxygen demand (BOD5) ranged from 2 to 5 mg/L for WWTP-H samples and 7–10 mg/L for WWTP-U effluent samples, while Chemical oxygen demand (COD) was 10–20 mg/L 50–60 mg/L for WWTP-H and WWTP-U samples, respectively. Hospital WWTP is a smaller unit compared to Ioannina city WWTP as it receives lower loads, and therefore the differentiations in BOD and COD analyses were expected.
2.3. UHPLC–LTQ Orbitrap MS Analysis
Chromatographic conditions were evaluated for positive (PI) and negative (NI) ionization. Chromatographic separation was conducted using an Accela UHPLC system (Thermo Fisher Scientific, Bremen, Germany) consisting of an Accela autosampler (model 2.1.1) and an Accela quaternary gradient UHPLC pump (model 1.05.0900). Separation of target analytes was carried out on a reversed-phase Hypersil Gold C18 analytical column (100 mm, 2.1 mm, 1.9 μm) maintained at 35 °C. The mobile phase consisted of water (A) and methanol (B), both containing 0.1% v/v formic acid (f.a). The gradient program for the elution of target compounds in positive and negative ionization is described in Supplementary Material (Section S2.3).
The LC system was coupled to a hybrid LTQ Orbitrap XL Fourier transform mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). The linear ion trap (LTQ) part of the hybrid MS system was equipped with an Ion Max electrospray ionization probe, operating in the positive and negative ionization mode. The qualification and quantification analyses were performed in full scan accurate mass spectra at high resolution as profile data mode in two separate runs for negative and positive ionization. For PI mode the following ionization parameters were applied: tube lens voltage, 90 V, spray voltage, 4.0 kV, capillary temperature, 320 °C, capillary voltage, 50 V, flow rates for the sheath (N2) and auxiliary (N2) gas, 35 arbitrary units (au) and 10 (au), respectively. In full-scan MS mode, the following parameters were used: resolution was set at 60,000; mass range, 120–1000, automatic gain control target (AGC), 5 × 105, and the maximum injection time (IT) was set to 100 ms, and the number of microscans to be performed was set at 1 scan s−1.
In negative ionization mode (NI) the following operational parameters were used: tube lens voltage, −90 V, spray voltage, 2.7 kV, capillary temperature, 320 °C, capillary voltage, −30 V, flow rates for the sheath (N2) and auxiliary(N2) gas, 10 arbitrary units (au) and 7 (au) respectively. In full-scan MS mode, resolution was set at 60,000 and the m/z scan range was 120–600, automatic gain control target (AGC) was set at target value of 4 × 104, and maximum injection time (IT) at 80 ms. The number of microscans to be performed was set at 1 scan s−1.
In data-dependent MS/MS mode, the precursor quadrupole isolation window was set to 1 m/z, the default charge state was set to 1 and −1 for PI and NI, respectively. The resolution was lower (15,000) both in the positive and negative modes. The ion fragmentation technique used was collision-induced dissociation with normalized collision energies (NCE) specified in the inclusion list of the software. The NCE energies were optimized for each target compound by injecting the working mix standard solution at a concentration of 10 μg/L.
Furthermore, the MS/MS scans were applied by targeting the automatic gain control (AGC) at 2 × 105 and 2 × 104 ions for PI and NI, respectively, while maximum injection time (IT) was set at 50 ms for both polarity modes. The mass tolerance window was set to 5 ppm.
The total instrument control and data processing was done with Xcalibur 2.1 (Thermo Electron, San Jose, CA, USA). Parameters for full MS/dd-MS2 analysis are listed in Supplementary Tables S2 and S3.
2.4. Fabric Phase Sorptive Extraction (FPSE)
The primary material that was used for the extraction of target analytes was a Whatman microfiber glass filter (FG) coated with short-chain poly (ethylene glycol) (PEG) by sol-gel process. Details about the sol-gel coating technique, the synthesis and characterization of the FPSE media are described in previous publication of our research group [53]. Based on the aforementioned publication with some modifications the procedure was applied for the extraction of 21 analytes.
Prior to the extraction two circle-shaped (FG)@ PEG300 with a diameter of 1 cm materials were soaked using tweezers (avoiding possibility of contamination) in 5 mL of methanol: acetonitrile (50:50 v/v) solution for 5 min. This is necessary to activate the FPSE media and remove any unwanted residue deposited during the storage. Afterwards, a conditioning step is followed by soaking the material into 5 mL of deionized water for 5 min, disposing in this way the previous organic solvents. Next, the FPSE media was transferred to a 12 mL screw-capped glass tube vial with 10 mL of aqueous sample along with a clean PTFE magnetic stir bar. The magnetic stirrer was set at medium level (350 rpm) for 30 min to achieve an adequate transfer of target analytes within the aqueous sample. After that time, the FPSE media was removed from the water sample, and let it dry to remove residual water. Then the FPSE media was inserted in a clean vial with 1 mL MeOH (acidic/alkaline) and the analytes were eluted with the aid of stirring (350 rpm) for 10 min. The extracts were collected and evaporated to dryness under gentle stream of nitrogen. Finally, they were reconstituted to the initial conditions of mobile phase (H2O:MeOH, 90:10 v/v acidified with 0.1%f. a v/v) for further UHPLC-Orbitrap-MS analysis.
3. Results and Discussion
3.1. Method Optimization
3.1.1. Chromatographic Separation
First, several experiments were performed on different mobile phases consisting of acetonitrile (AcN) or methanol (MeOH) as organic phase and water as polar phase with different concentrations of acetic and formic acid (from 0.05 to 0.5 v/v%), ammonium formate, ammonium acetate (from 1 mM to 10 mM) [31,55,56]. Methanol was chosen due to the observed overall reduction of ESI signal intensity when using AcN. Moreover, MeOH achieved better resolution and sensitivity. The addition of formic acid enhanced the formation of [M + H]+ and [M − H]− as dominant molecular ions for polar and negative ionization, respectively. Finally, the best results were obtained when MeOH and water were used with the addition of 0.1% f.a v/v in both phases.
Three chromatographic columns were surveyed to optimize suitable chromatographic conditions, Speed core-Diphenyl (50 mm × 2.1, 2.6 µm), Speed Core C18 (100 mm × 2.1, 2.6 μm) from Fortis and Hypersil Gold C18 (100 mm × 2.1, 1.9 μm) from Thermo Fischer Scientific. Between all the mobile phase combinations mentioned, and the three examined columns, the best peak shapes and responses in both positive and negative ionization modes were achieved using the Speed core- Diphenyl (50 mm × 2.1, 2.6 µm) and Hypersil GOLD (1.9 μm) columns with acidified water and methanol as mobile phases, while the Speed Core C18 (100 mm × 2.1, 2.6 μm) was overruled because of the absence of chromatographic peaks especially in the sulfonamide group. Finally, Hypersil GOLD C18 yielded the best results by means of peak shape and area of chromatographic peaks. Characteristic examples of the obtained chromatograms and response are depicted in Figure S1.
Hypersil Gold Column provided narrow symmetrical chromatographic peaks that ensure the optimum resolution. Obtaining narrow peak widths is especially challenging for basic pharmaceutical compounds. Recently, Samuelsson et al. comprehensively explained that the tremendous different peak deformation and peak splitting effects are dependent on the solute species form and how band distortions due to pH mismatch can be effectively avoided by careful control of the protolytic species form in the sample preparation [57]. Moreover, such splitting effects get worse in complex matrices where sample solvent interferes with eluent analytes [58]. The reduced silanol activity on Hypersil Gold columns reduces tailing for basic analytes, improving resolution. The influence of the injection solution composition on the quality of LC–MS methods, in terms of column efficiency and peak shape, was investigated. Taking into consideration the efficiency of chromatographic separation as well as the stability of the analytes in the solvent, the injection solvent selected was 90:10 water: methanol with 0.1% v/v formic acid (f.a).
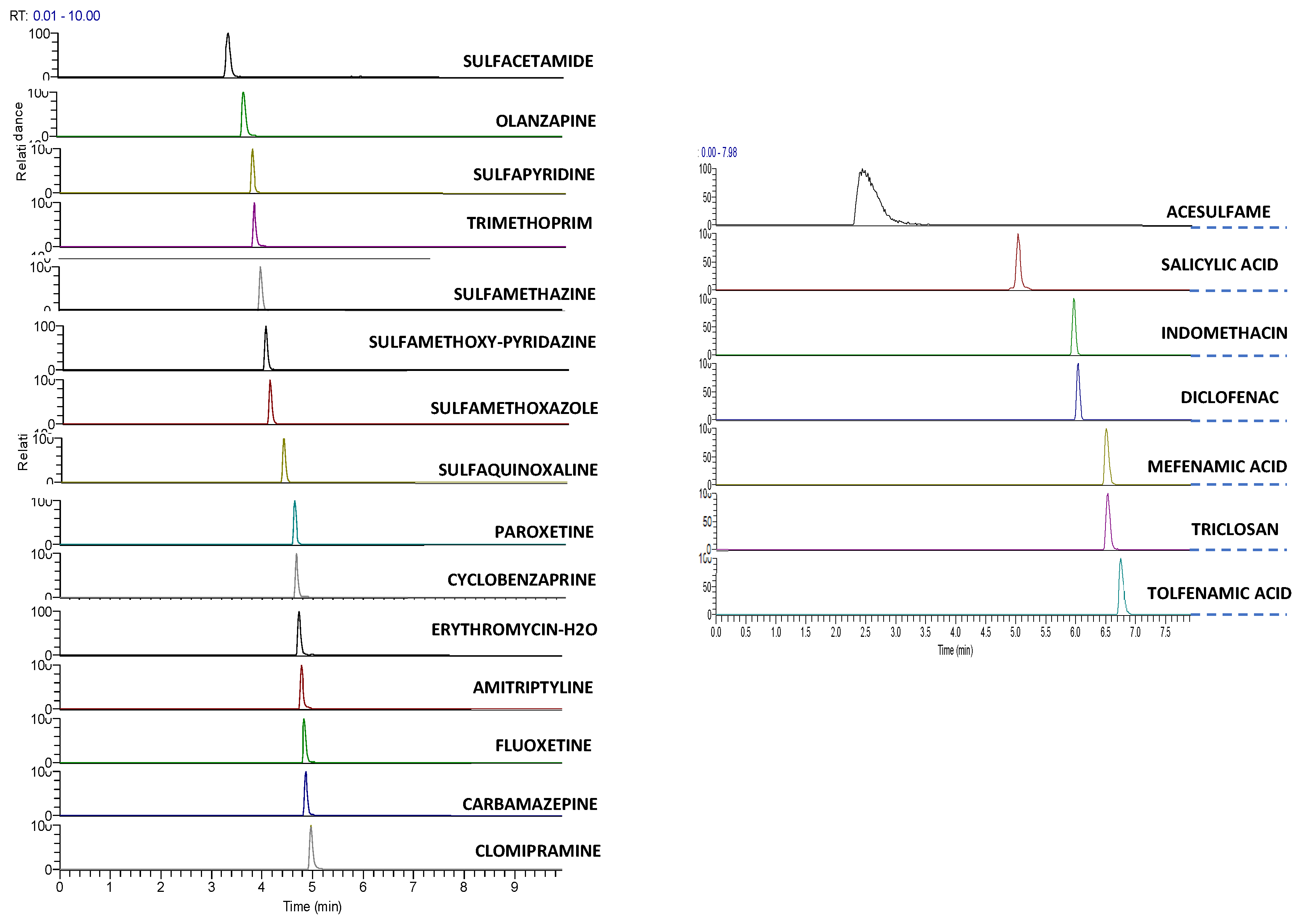
A representative extracted ion chromatogram (XIC) of all analytes of interest at a concentration of 5 μg/L is illustrated (Figure 1).
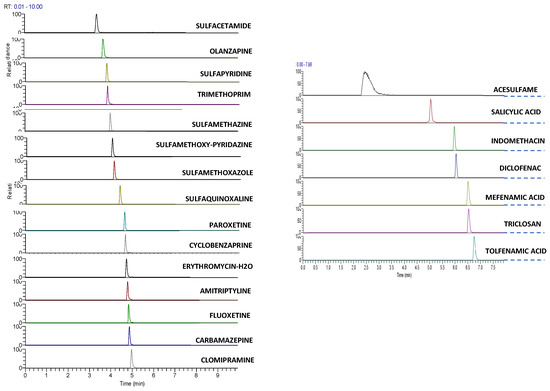
Figure 1.
Extracted Ion Chromatogram, XIC of a standard solution of 5 μg L−1 in UHPLC–LTQ/Orbitrap of target analytes from left to right positive and negative ionization.
3.1.2. Mass Spectrometry-LTQ Orbitrap
ESI Conditions
For the evaluation of instrumental conditions in mass spectrometry, it is interesting to also study the parameters that can favor the ionization of target analytes and their further detection. Three ESI parameters that can affect the ionization of the analytes were evaluated: tube-lens, sheath gas flow and capillary temperature.
The sheath gas flow assists in the drying of the drop emitted by the capillary, to which it was assigned moderate relevance and its values were ranged between 5 and 40 au for both polarity modes. The sheath gas flow executed best results by means of signal intensity in the range of 25 and 35 au, and specifically the value of 35 and 30 au was chosen as optimal for PI and NI, respectively. A capillary temperature, that helps the analytes to be emitted in solution form, was also tested and varied between 260, 280, 300, 320, 340, 360, and 380 °C. Capillary temperatures between 280–320 °C provided similar and satisfactory results with some differentiations for more acidic compounds which exhibited higher sensitivity in high capillary temperatures. This observation is in accordance with another published work [59]. There is no ideal capillary temperature that fits equally for all the analytes of interest, so 320 °C was selected as optimal.
Finally, tube lens values evaluated were 70, 90, and 110 V, with the best results accomplished with 90 V and −90 V for positive and negative polarity, respectively. Variations of tube lens voltage for target analytes seem to be influenced by the molecular mass (Supplementary Figure S2). For example, a tube lens value of 110 V in the case of Erythromycin-H2O (MW 715.93) provided a higher response compared to tube lens value of 90 for the same compound. However, the majority of target analytes molecular masses ranged from 200–350.
Orbitrap Mass Analyzer
The last step of the optimization was the investigation of the parameters affecting the detection region of Orbitrap analyzer on the signal intensity of target compounds. Thus, the AGC target and the maximum injection time values of the Orbitrap (IT) were evaluated.
The automatic gain control (AGC) target value refers to the ion population Orbitrap mass analyzer. The context behind the AGC is to regulate the number of ions in the mass analyzer to avoid or minimize space charge effects to improve mass accuracy. The objective of the optimization has been set to maximize the response concerning the ECs in both polarity modes. Four AGC target settings were investigated for their effects on quantitation of 3 × 105, 5 × 105, 106, and 3 × 106 for positive ionization while for negative ionization the corresponding test values were 2 × 104, 4 × 104, 105, and 5 × 105. The effects of AGC target are presented in Supplementary Figure S3.
It is important to notice that the AGC target value and maximum ion injection time are dependent parameters. Either the maximum ion injection or AGC target value is responsible for the scan events of MS or MS/MS depending on which parameter is reached first. As an example, if maximum ion injection time is 100 ms while the AGC target value is set at 1 × 104 but it needs more time than 100 ms to accumulate 1 × 104 ions, therefore the MS events will be performed anyway within 100 ms regardless of the set of AGC target value. If it takes less time (<100 ms) to accumulate 1 × 104 ions, then the MS events will be executed with the already set AGC target. Increasing the AGC target values should follow an increase of the maximum ion injection time since it takes longer to accumulate more ions set [60]. Optimized injection time for AGC target value 5 × 105 was 100 ms in positive method ionization, and in negative method 80 ms for AGC target value 4 × 104 was selected.
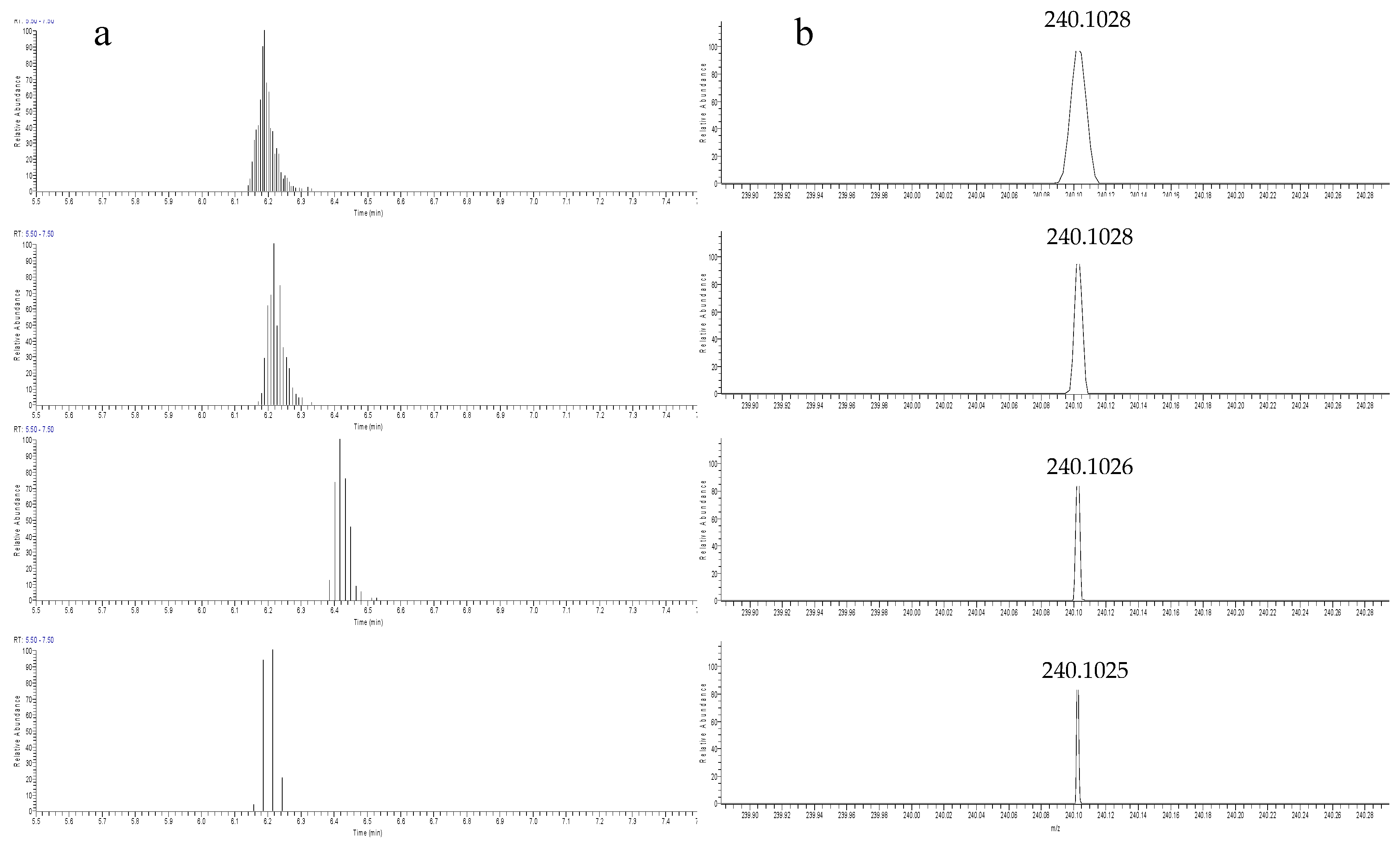
Resolving power is a parameter that affects the total acquisition time in the Orbitrap mass analyzer. The higher the resolution setting, the longer the time required for performing a scan cycle and the less the possibility to obtain the points acquired to form a chromatographic peak or a mass spectrum. To optimize the resolving power (R), the system was operated in full-scan mode (120–1000 and 120–600 m/z) and the studied R values varied between 15,000, 30,000, 60,000, and 100,000 FWHM. Six (6) blank effluent wastewaters spiked after FPSE extraction with a mixed standard solution (50 ng/L) of 21 target analytes in total for positive and negative ionization were analyzed. The resolving power was evaluated measuring the peak area (signal response). High mass resolution provides higher mass accuracy and increases the selectivity, in multi-residue analyses, in complex matrices, allowing the increase in the number of screened compounds. However, an excessively high resolution (such as 100,000 FWHM) has an impact in the sensitivity due to the increased scan duration and limited data points (Figure 2). A possible explanation could be the loss of ion energy during the travel time and distance, which is more enabled at high resolution settings [61]. To eliminate matrix interferences, but also to accurately quantify a chromatographic peak, a sufficient number of data points is required across the peak width. Therefore, the optimum resolving power was evaluated and the value of 60,000 FWHM was found to be optimal for both polarity modes.
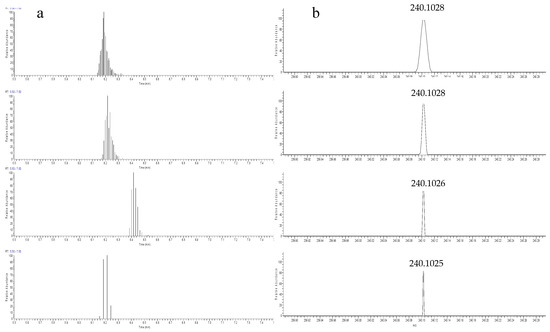
Figure 2.
(a) Peak chromatogram (presented as data points) and (b) mass spectrum of Mefenamic acid (post-spiked sample at concentration 50 ng/L) with the resolution of 15,000 FWHM, 30,000 FWHM, 60,000 FWHM, and 100,000 FWHM (from top to the bottom).
Concerning the confirmatory experiments assessed with dd-MS2, a lower resolution setting was selected for measurements of fragment ions after the application of the normalized collision energy (NCE). The R value of 15,000 FWHM proved to be sufficient in this mode and fulfills the requirement of an excellent selectivity.
Employing the optimized chromatographic conditions and the selected mass spectrometric parameters, accurate mass measurements were recorded for all precursor ([M + H]+, [M + H]−) and fragment ions of the compounds of interest and optimum normalized collision energy (NCE) was selected by several optimization experiments. UHPLC-Orbitrap MS/MS data including fragment ions for PI and NI are summarized (Supplementary Tables S2 and S3). It is important to highlight high mass accuracy results for all studied analytes, below 2 ppm and 5 ppm for PI and NI, respectively.
3.2. Optimization of Fabric Phase Sorptive Extraction
In this study, a one-variable-at-a-time optimization approach was used in the optimization of the FPSE. Adsorption studies were conducted with ultrapure water spiked with level 10 μg/L of the target analytes. In all samples, prior to extraction, a chelating agent such as Na2EDTA was added, since among target analytes there exist antibiotics of the sulfonamides and macrolides classes which, according to literature, have the tendency to form complexes with multivalent metal ions that are already soluble in water [31,62], This resultantly affects the recovery of the procedure. Na2EDTA is a strong chelating agent which act as a competent agent for multivalent cations improving the extraction efficiency. Therefore, 0.1% final concentration (g solute/g solution) of Na2EDTA was achieved.
To obtain high extraction efficiencies for the FPSE, several parameters were optimized including sample volume, extraction time, pH, ionic strength. Desorption conditions such as elution solvent, and its volume were evaluated too. In our previous study, FPSE technique was used for the extraction of antipsychotic drugs from environmental water samples [63]. Following that data, initial conditions of 1 mL of aqueous sample with a fixed elution volume of 1 mL MeOH solvent was chosen for the evaluation of pH effect. pH values of 3.0, 7.0 and 11.0 (taking into consideration pKa of basic compounds) were tested. The results of the pH evaluation are presented in Supplementary Figure S4a. The target analytes have different pKa and belong in different therapeutic and chemical classes, so they follow different adsorption rules according to pH variations. Antibiotics classified in sulfonamide category, NSAIDs and artificial sweetener (acesulfame) displayed higher adsorption in pH 3.0. In this pH the specific compounds exist in their neutral form according to pKa and their speciation charts [64], enabling in this way the extraction On the other hand, when pH increases to 7.0 and 11.0 the adsorption decreases significantly since the mostly ionizable charged form of these compounds exists in aqueous solution. In alkaline region (pH 11.0), the highest adsorptions were obtained for antipsychotic drugs with basic pKa. However, the adsorption of antipsychotic drugs was also satisfactory in other pH values. Carbamazepine provided high adsorption in all pH values. Different pKa values of analytes of interest do not allow to apply a pH value that would be equally efficient for all, therefore pH 3.0 was selected as a satisfactory value for most analytes.
After the evaluation of pH, the next step was to determine the volume of the aqueous sample that could be loaded in the FPSE device accomplishing at the same time high enrichment factor. To address this issue, two circles of 1 cm FG@PEG were used and three volumes of 5, 10, 20 mL of ultrapure water spiked with the target analytes were tested. The lowest adsorption rates were achieved with 20 mL of sample, while 5 and 10 mL provided satisfactory rates, respectively. When the sample volume was increased from 5 mL to 10 mL, adsorption slightly increased for most analytes. Taking into consideration the preconcentration factor and making a compromise between the adsorption efficiency and the sensitivity of the method, 10 mL of sample was selected for further analysis.
A series of extraction times of 10, 20, 25, 30, 35, and 40 min was studied under two stirring speeds (250 and 350 rpm). The scale of 350 rpm was by far more effective in all cases. From Supplementary Figure S4b, it is obvious that the equilibrium of target analytes onto the sorbent is accomplished within 35 min, while further stirring time did not significantly improve the adsorption rates. For this reason, 35 min was chosen as the optimal extraction time and employed for the subsequent tests.
Generally, increasing the ionic strength of a solution results in an improvement in the extraction efficiency when salting-out effect plays a crucial role in the procedure. The effect of ionic strength on the adsorption of target analytes was examined using NaCl at concentrations ranging between 0%, 5%, and 10% (w/v). With the addition of 5% w/v of NaCl no significant improvement in the extraction efficiency was assessed. On the other hand, higher salt content of 10% w/v decreases the adsorption of several analytes and has a negative impact especially for non-polar ones (Supplementary Figure S4c). Target compounds with logP > 3.5 like the studied NSAIDs present the lowest adsorption rate with the highest salt content. This finding is in accordance with other reports [65,66,67].
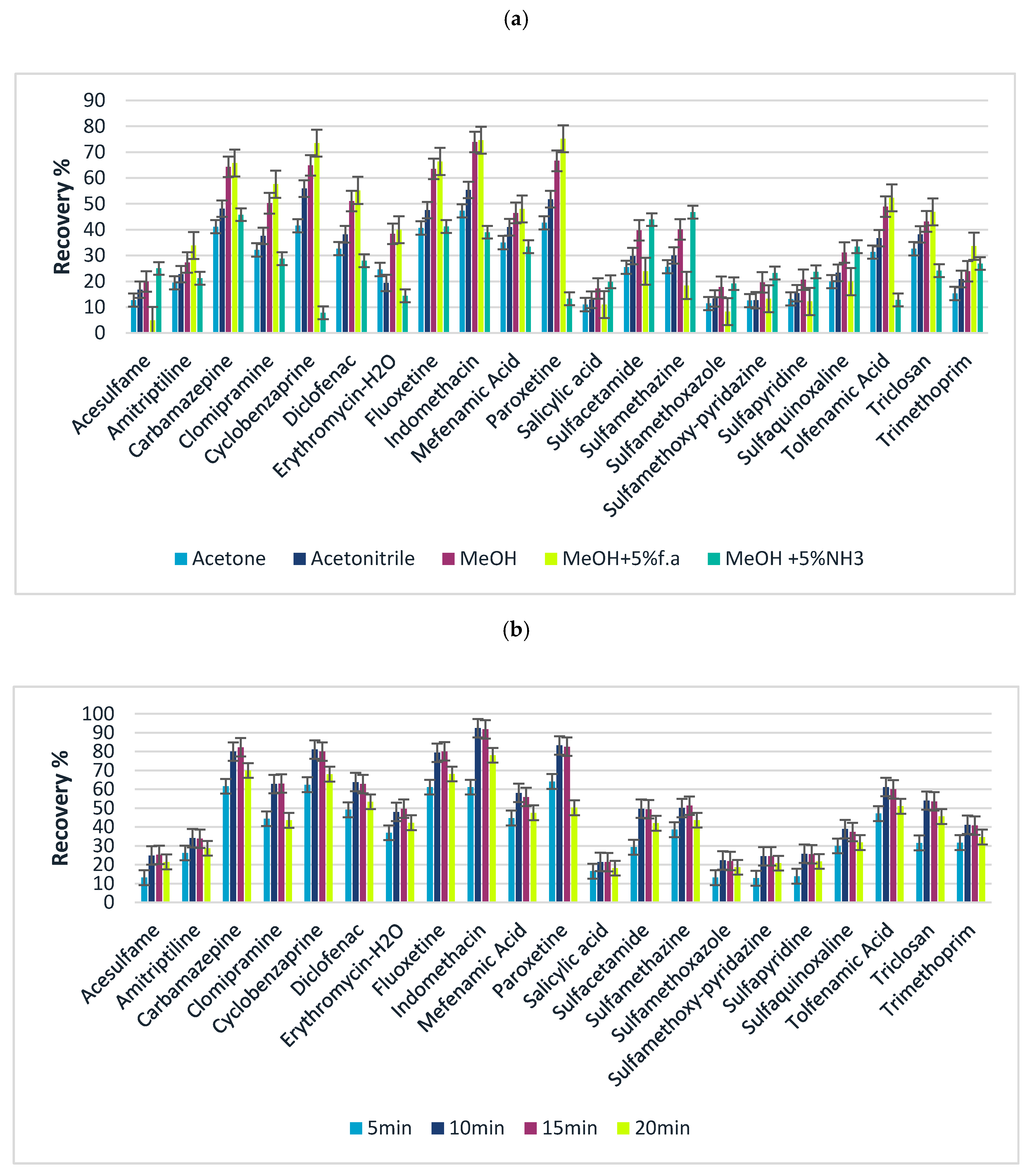
Following the optimization of the adsorption step of FPSE, desorption conditions of extraction technique such as elution solvent, volume as well as elution time were evaluated. Different solvents, such as acetone, acetonitrile, and methanol, were evaluated under the same conditions: extraction time 35 min, 10 mL of sample, 1 mL of elution solvent, stirring for 15 min–350 rpm. Acetone presented the lowest recoveries especially for polar analytes (logP < 3), followed by acetonitrile while methanol exhibited the higher elution efficiencies. To increase the desorption yield, taking into consideration the pH dependency on sorbent, formic acid or ammonia was added at various percentages (1–5% v/v) to increase the acidity or alkalinity. The results (Figure 3a) showed that the extraction efficiency reached the maximum when two consecutive elution systems of MeOH with 5% f. a v/v and MeOH with 5% v/v NH3 were employed. Analytes with maximum recovery in acidified methanol showed the lowest recovery in alkaline methanol and vice versa. This may be attributed to the different physicochemical properties of the analytes and the variation in pKa. During desorption process, the ionic state of each compound should be promoted, reducing in this way the adsorption onto the sorbent and facilitating the transfer of the analytes in elution solvent [68]. As an example, considering the pKa of fluoxetine, in alkaline pH, the compound exists in its neutral form, maintaining its adsorption onto the FPSE media, thus an acidified solvent would be more suitable for the successful desorption. For sulfonamide compounds, the same vice versa phenomenon occurs. In low pH values sulfonamides exist in their neutral form which enables the retention onto the sorbent, indicating that a desorption solvent in alkaline media would be more efficient. To overcome this observation, the elution of the target analytes was accomplished with consecutive elution steps by adding aliquots of MeOH 5% NH3 v/v and MeOH 5% f. a v/v. In this way, we take advantage of the optimal elution conditions for all target analytes by avoiding the performance of two different extraction processes, simplifying FPSE.
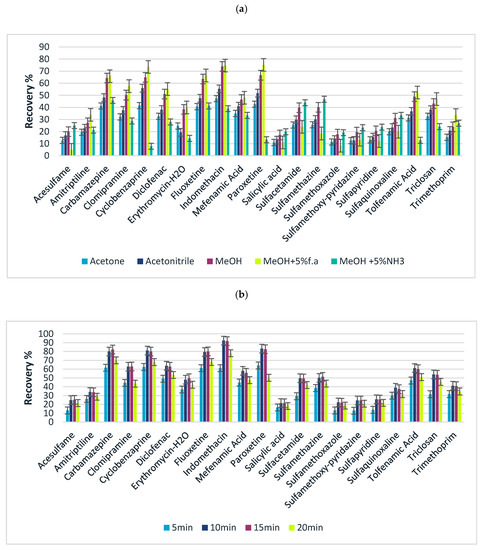
Figure 3.
Effects on the desorption of target analytes (a) elution solvent and (b) elution time.
For determining the volume of the elution solvent two fractions of 1 mL and 5 mL were investigated. The results demonstrated similar recoveries for both selected volumes, thus 1 mL was selected for further studies. In addition, to increase the preconcentration factor, the 1 mL acidified/alkaline methanol elution extracts were evaporated until dryness and reconstituted in 150 μL of mobile phase initial conditions.
Finally, regarding the effect of desorption time 5, 10, 15, and 20 min of stirring (350 rpm) was investigated. From Figure 3b it is noticed that recoveries increase with time of exposure of the FPSE media. Specifically, the maximum recoveries were achieved within 10 and 15 min, while 5 min of stirring was not long enough to achieve complete desorption. On the other hand, 20 min of stirring provided lower desorption efficiencies, probably due to the back re-sorption of analytes onto the coated media [48,52].
In accordance with the obtained results, the optimum conditions for FPSE procedure were as follows: 10 mL of aqueous sample adjusted to pH 3 (with no addition of NaCl) was extracted for 35 min under continuous stirring at 350 rpm. Desorption occurred with 1 mL of methanol (acidified/alkaline) within 10 min of stirring at 350 rpm. The optimal conditions were applied to wastewater effluents for the determination of target analytes.
3.3. Analytical Performance
The FPSE method developed for the determination of the selected analytes in aqueous media was validated. The validation procedure was conducted in pooled samples of effluent wastewater of Ioannina city as well as effluent wastewater from University hospital of Ioannina, providing excellent performance criteria, such as sensitivity, linearity, precision, reproducibility, and accuracy. Matrix effect studies were also evaluated for the investigated aqueous matrices.
Accuracy of the developed method was expressed as the percentage of relative recovery (RR %). They were estimated from absolute recoveries of ultra-pure spiked water samples. Relative recovery is defined as the % concentration of target analytes recovered from the wastewater effluent with reference to the concentration found at spiked ultra-pure water. For this purpose, three replicates of spiked effluent sample at three concentration levels of LOQ, 10 times LOQ and 100 times LOQ (low, medium, high) were analyzed under the optimum conditions. Blank samples (non-spiked) were analyzed as well. Due to the fact that non-spiked effluent samples already contained some of the compounds, the concentration of the respective non-spiked sample (blank) was subtracted from the concentration in the spiked sample and then divided by the spiked level. For the medium spiking level relative recoveries ranged from 83.7% to 114.0%, as presented in Supplementary Table S4. Intra-day precision (n = 5) and inter-day precision also referred to as reproducibility (n = 15) expressed as relative standard deviation percentage (RSDr and RSDR) were lower than 8% and 11%, respectively, for all target compounds (Supplementary Table S4).
The limit of detection (LOD) was calculated as the lowest concentration of analyte that provides a signal-to-noise ratio equal to 3 (S/N = 3.) Similarly, the limit of quantification (LOQ), was determined as the concentration that generates a S/N = 10. LODs and LOQs were ranged from 3.1–149.4 ng/L and 9.3–447.7 ng/L, respectively. Linearity of the method was investigated by triplicate analysis in effluent wastewater by constructing an 11-point method calibration curve covering the range of LOQ to approximately 100 times LOQ for each target analyte. Coefficients of determination (R2) were greater than 0.99 indicating that linearity is satisfactory for all target analytes. The aforementioned analytical parameters as well as matrix effect values (ME %) of effluent water are presented in Table 1.

Table 1.
Linearity as correlation coefficient (R2), Detection and Quantification Limits and Matrix Effect values (ME %).
Matrix effect studies (ME) were performed for pooled sample of effluent waters, from University hospital of Ioannina and Ioannina city to evaluate the contribution of the matrix to signal enhancement or suppression. For this purpose, the slopes of the respective matrix-matched calibration curves were compared with the slope of the calibration curve prepared in solvent and calculated according to Equation (1).
A value of zero indicates that there is no ME, while for a positive value there is an ion enhancement signal and for a negative value an ion suppression signal. Low matrix occurs when values range between +20% and −20%, while values between −50% and +50% indicate medium matrix effect. Finally, higher values of +50% and less than −50% are considered as strong matrix effect [9,12,13]. From the results depicted in Supplementary Figure S5, significant matrix effects were observed. ECs analyzed in PI mode were noticed to be subjected to ion suppression while those in NI mode showed ion enhancement. Most of the target compounds in WWTP effluent displayed medium matrix effect, with only exceptions sulfacetamide, trimethoprim, sulfamethoxazole, sulfaquinoxaline, carbamazepine which presented low ME (−20 < ME < +20). On the other hand, diclofenac and triclosan were the only compounds that displayed high matrix effect expressed as signal enhancement 54.7% and 52.8%, respectively. The results were expected taking into consideration the complex matrix of effluent water and the high content of organic matter. In any case, matrix matched-calibration curves were used for the quantification of target analytes to avoid inaccurate results.
3.4. Application to Real Samples
To investigate the applicability of the method in real water samples, two pooled effluent waters of three different sampling days collected from Ioannina city and University hospital WWTPs were analyzed (triplicate analysis). The mean concentrations determined with FPSE method are summarized in Table 2. Out of 21 target compounds 11 were detected but two were found below LOQ (fluoxetine and indomethacin). The mean concentrations of the detected compounds varied from 55.4 to 1135.4 ng/L and 63.9 to 728.4 ng/L for effluent urban water and hospital effluent, respectively. Maximum concentrations were detected for acesulfame in urban effluent water followed by salicylic acid which presented high concentration in both effluents. In addition, a high concentration of the anti-inflammatory drug diclofenac in effluent hospital water substantiates the decision of European Union to establish regulations within the framework of EU-wide water monitoring in the adopted Directive, 2013/39/EU that concerns priority substances in the field of water policy [26]. Salicylic acid on the other hand is ubiquitous in wastewater effluents of Greece [56,69] since it is the metabolite of aspirin (acetylsalicylic), a popular first line anti-inflammatory drug which can be purchased without prescription. Similarly, high concentrations of acesulfame were expected due to its many applications as food additives, as sugar substitute in beverages, sanitary products, pharmaceuticals, and personal care products [70].

Table 2.
Mean concentrations (n = 3) with Standard Deviation (SD) of target analytes in urban (WWTP-U) and hospital (WWTP-H) effluent waters.
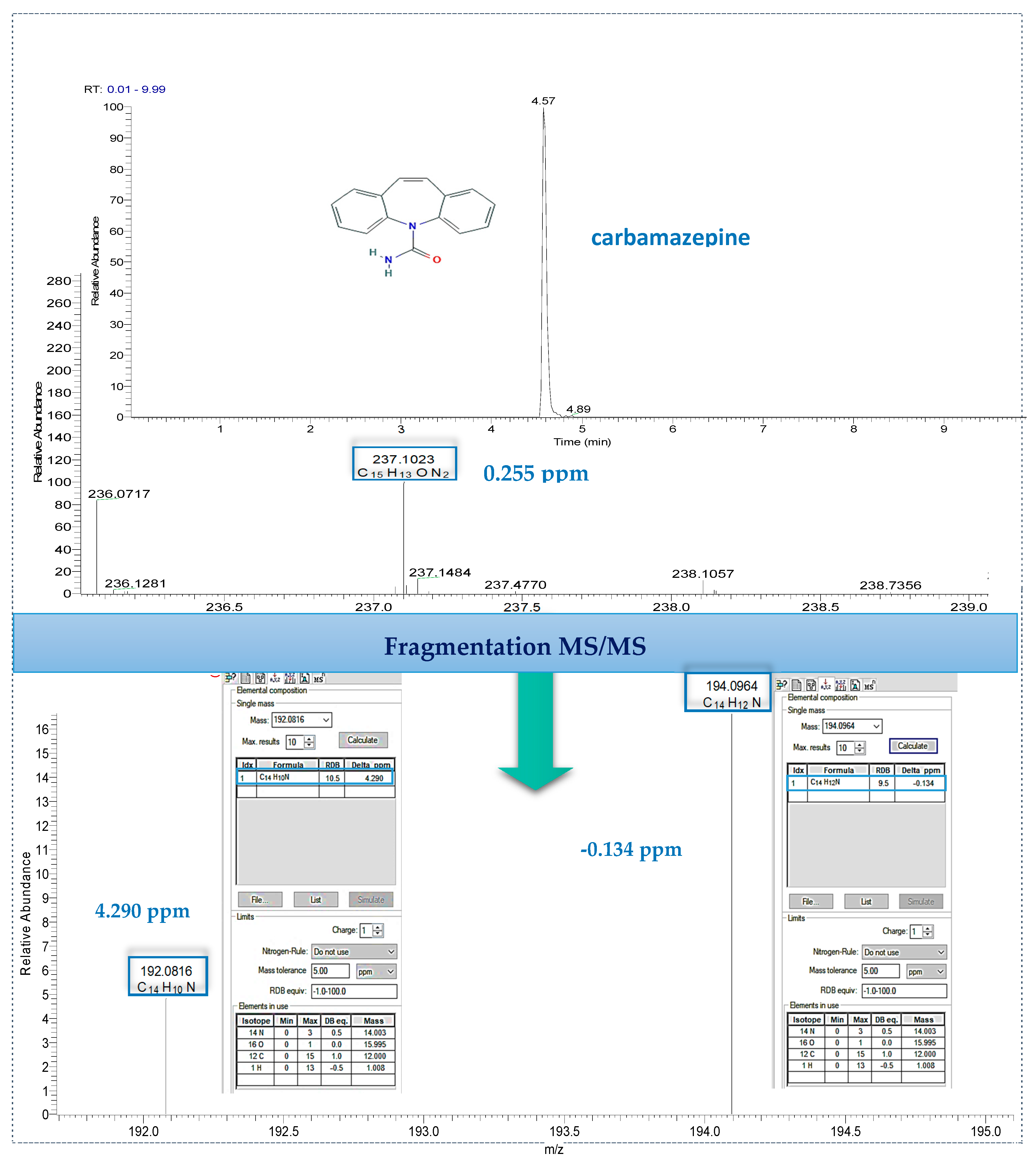
Finally, the identification of detected target compounds was employed with data-dependent MS/MS by using the predominant advantage of Orbitrap mass spectrometry, of high mass accuracy both for precursor ion and fragment ion as well. The main process was based on the criteria for both screening and confirmatory analytical methods for pharmaceutical residues according to the identification points proposed by EU Commission Decision 2002/657/EC in combination with FDA guidelines that take full advantage of the capabilities of modern HRMS instruments [71,72], and also the last update exploring the means of identification of small molecules [72]. An example of identification with MS/MS fragmentation is illustrated (Figure 4) for carbamazepine. The full scan MS spectrum of the chromatographic peak detected at 4.57 min (on the top of Figure 4), showed an abundant signal at m/z 237.1023 which corresponds within 0.255 ppm to the theoretical exact mass of carbamazepine. Additional MS/MS data shows two intense fragment ions which correspond to protonated molecules of C14H12N+ (194.096 m/z) and C14H10N+ (192.0810 m/z) with mass errors in relation to an exact mass below 5 ppm in both cases. High mass accuracy results for precursor ion as well as for its fragments confirm the presence of carbamazepine in real effluent water.
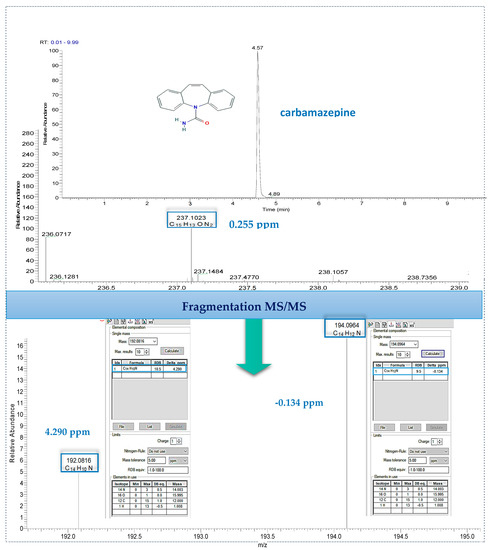
Figure 4.
Identification of carbamazepine in effluent wastewater by performing UHPLC–LTQ Orbitrap tandem mass spectrometry fragmentation.
4. Conclusions
In this study, a step by step optimization of operational parameters of Orbitrap MS was assessed to take full advantage of the utilities of this high-resolution and mass accuracy analyzer for the determination of selected classes of ECs in effluent water. Chromatographic conditions taking into consideration the effects of distorted peaks as well as parameters that influence the ionization were also evaluated. UHPLC-LTQ Orbitrap MS proved to be a powerful technique for the quantitation and identification of analytes of interest in effluent matrix with excellent mass accuracies below 2 ppm and 5 ppm for positive and negative ionization, respectively. Sample pretreatment of effluent water was performed with the aid of FPSE which was optimized and validated, providing excellent analytical performance. Finally, application to real samples revealed the presence of eleven target compounds which were successfully identified by MS/MS fragmentation, providing high mass accuracy as well.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/7/3/46/s1, Table S1: Physicochemical properties and chemical structures of target analytes, S2.1: Standard Solutions and reagents, S2.3: UHPLC–LTQ Orbitrap MS analysis, Table S2: Parameters for full MS/dd-MS2 analysis in positive ionization mode, Table S3: Parameters for full MS/dd-MS2 analysis in negative ionization mode, Figure S1: Chromatograms of selected pharmaceuticals of standard solution at concentration of 5 μg/L. (A) Hypersil Gold C18 (100 mm × 2.1, 1.9 μm), (B) Speedcore- Diphenyl (50 mm × 2.1, 2.6 µm), Figure S2: Response variance with different voltage of Tube Lens (a) Positive Ionization, (b) Negative Ionization, Figure S3: Effect of AGC target values on the response of studied analytes (a) positive and (b) negative ionization mode, Figure S4: Optimization of FPSE extraction: (a) sample pH, (b) extraction time, (c) ionic strength, Table S4: Recoveries and precision results expressed as RSDr and RSDR: within each spiking level, Figure S5: Matrix effects for the target analytes in the effluent water.
Author Contributions
Conceptualization, C.J.-H.; Data curation, M.K.; Formal analysis, M.K. and V.B.; Funding acquisition, V.S.; Investigation, M.K.; Methodology, M.K., C.C., C.J.-H. and V.S.; Project administration, V.S.; Resources, V.B. and T.A.; Supervision, V.S.; Validation, M.K.; Writing—original draft, M.K.; Writing—review & editing, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (MIS-5000432), implemented by the State Scholarships Foundation (ΙΚΥ). Part of the work was also financially supported from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 765860.
Acknowledgments
The authors would like to thank the Unit of Environmental, Organic and Biochemical High-Resolution Analysis-Orbitrap-LC-MS of the University of Ioannina for providing access to the facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, K.C.; De Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Stockholm Convention on Persistent Organic Pollutants (POPs); Secretariat of the Stockholm Convention on Persistent Organic Pollutants: Geneva, Switzerland, 2009. [Google Scholar]
- Daughton, C.G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ. Health Perspect. 2003, 111, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- The European Parlament and the Council of the European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Parliam. 2000, L327, 1–82. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals as environmental pollutants: The ramifications for human exposure. In International Encyclopedia of Public Health; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 66–102. ISBN 9780123739605. [Google Scholar]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of root vegetables with treated wastewater: Evaluating uptake of pharmaceuticals and the associated human health risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci. Total Environ. 2019, 650, 2188–2198. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Huen, K.; Bradman, A.; Harley, K.; Yousefi, P.; Boyd Barr, D.; Eskenazi, B.; Holland, N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ. Res. 2012, 117, 8–16. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Khan, E.; Chen, H.; Nguyen, V.T.; Li, Y.; Goh, S.G.; Nguyen, Q.B.; Saeidi, N.; Gin, K.Y.H. Emerging contaminants in wastewater, stormwater runoff, and surface water: Application as chemical markers for diffuse sources. Sci. Total Environ. 2019, 676, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Miossec, C.; Lanceleur, L.; Monperrus, M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019, 42, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a multi-residue method for the determination of human and veterinary pharmaceuticals and some of their metabolites in aqueous environmental matrices by SPE-UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhu, Q.; Sun, S.; Peng, Y.; Shuai, Q. Suspect screening and target quantification of human pharmaceutical residues in the surface water of Wuhan, China, using UHPLC-Q-Orbitrap HRMS. Sci. Total Environ. 2018, 635, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Pacheco Ferreira, A. Trace Analysis of Pharmaceutical Residues in Wastewater Treatment Plants in Rio de Janeiro, Brazil; Islamic Azad University-Damghan Branch: Damghan, Iran, 2018; Volume 4. [Google Scholar]
- Lange, F.T.; Scheurer, M.; Brauch, H.J. Artificial sweeteners-A recently recognized class of emerging environmental contaminants: A review. Anal. Bioanal. Chem. 2012, 403, 2503–2518. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. eSPen 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Carniel Beltrami, M.; Döring, T.; De Dea Lindner, J. Sweeteners and sweet taste enhancers in the food industry. Food Sci. Technol. 2018, 38, 181–187. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef]
- Meyer, M.T.; Bumgarner, J.E.; Varns, J.L.; Daughtridge, J.V.; Thurman, E.M.; Hostetler, K.A. Use of radioimmunoassay as a screen for antibiotics in confined animal feeding operations and confirmation by liquid chromatography/mass spectrometry. Sci. Total Environ. 2000, 248, 181–187. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU). 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L78/40, 20–30. [Google Scholar]
- The European Parlament and the Council of the European Union. Directives of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, 2013, 1–17. [Google Scholar]
- Fornstedt, T.; Forssén, P.; Westerlund, D. Basic HPLC Theory and Definitions: Retention, Thermodynamics, Selectivity, Zone Spreading, Kinetics, and Resolution. Anal. Sep. Sci. 2015, 1–24. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Bade, R.; Rousis, N.I.; Bijlsma, L.; Gracia-Lor, E.; Castiglioni, S.; Sancho, J.V.; Hernandez, F. Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS. Anal. Bioanal. Chem. 2015, 407, 8979–8988. [Google Scholar] [CrossRef]
- Pugajeva, I.; Rusko, J.; Perkons, I.; Lundanes, E.; Bartkevics, V. Determination of pharmaceutical residues in wastewater using high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2017. [Google Scholar] [CrossRef]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewaters: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Liquid-liquid-liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999, 71, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.E.; Pedersen-Bjergaard, S.; Krogh, M.; Grefslie Ugland, H.; Grønhaug, T. Development of a simple in-vial liquid-phase microextraction device for drug analysis compatible with capillary gas chromatography, capillary electrophoresis and high-performance liquid chromatography. J. Chromatogr. A 2000, 873, 3–11. [Google Scholar] [CrossRef]
- Valcárcel, M.; Cárdenas, S.; Lucena, R. Microextraction techniques Microextraction Techniques. Anal. Bioanal. Chem. 2014, 406, 1999–2000. [Google Scholar] [CrossRef]
- Jeannot, M.A.; Cantwell, F.F. Solvent microextraction into a single drop. Anal. Chem. 1996, 68, 2236–2240. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T. Dispersive liquid-liquid microextraction. TrAC Trends Anal. Chem. 2011, 30, 1382–1399. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent 20140274660A1, 18 September 2014. [Google Scholar]
- Kabir, A.; Furton, K.G. Novel Sol-Gel Sorbents in Sorptive Microextraction. In Analytical Microextraction Techniques; Valcarcel, S.M., Cardenas, R.L., Eds.; Bentham Science Publishers: Miami, FL, USA, 2017; pp. 28–69. [Google Scholar]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric phase sorptive extraction explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent trends in microextraction techniques employed in analytical and bioanalytical sample preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1084, 53–63. [Google Scholar] [CrossRef]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marcé, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav, H.; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef]
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138. [Google Scholar] [CrossRef]
- Jiménez-Holgado, C.; Chrimatopoulos, C.; Stathopoulos, V.; Sakkas, V. Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. [Google Scholar] [CrossRef]
- Konstas, P.S.; Kosma, C.; Konstantinou, I.; Albanis, T. Photocatalytic treatment of pharmaceuticals in real hospital wastewaters for effluent quality amelioration. Water 2019, 11, 2165. [Google Scholar] [CrossRef]
- Rusko, J.; Jansons, M.; Pugajeva, I.; Zacs, D.; Bartkevics, V. Development and optimization of confirmatory liquid chromatography—Orbitrap mass spectrometry method for the determination of 17 anticoccidials in poultry and eggs. J. Pharm. Biomed. Anal. 2019, 164, 402–412. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multianalyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4229–4245. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, J.; Forssén, P.; Fornstedt, T. Sample conditions to avoid pH distortion in RP-LC. J. Sep. Sci. 2013, 36, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Fornstedt, T.; Forssén, P.; Westerlund, D. System peaks and their impact in liquid chromatography. TrAC Trends Anal. Chem. 2016, 81, 42–50. [Google Scholar] [CrossRef]
- Roiffé, R.R.; Ribeiro, W.D.; Sardela, V.F.; de la Cruz, M.N.S.; de Souza, K.R.; Pereira, H.M.G.; Aquino Neto, F.R. Development of a sensitive and fast method for detection of catecholamines and metabolites by HRMS. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Kalli, A.; Smith, G.T.; Sweredoski, M.J.; Hess, S. Evaluation and optimization of mass spectrometric mode: Focus on LTQ-Orbitrap Mass analyzers. J. Proteome Res. 2014, 12, 3071–3086. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.G.; Dineen, B.A.; Stack, M.A.; James, K.J. A critical evaluation of liquid chromatography with hybrid linear ion trap-Orbitrap mass spectrometry for the determination of acidic contaminants in wastewater effluents. J. Chromatogr. A 2012, 1270, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon. ChemAxon—Software Solutions and Services for Chemistry and Biology. Available online: https://chemaxon.com/ (accessed on 3 August 2020).
- Carpinteiro, I.; Ramil, M.; Rodríguez, I.; Nogueira, J.M.F. Combining stir-bar sorptive extraction and large volume injection-gas chromatography-mass spectrometry for the determination of benzotriazole UV stabilizers in wastewater matrices. J. Sep. Sci. 2012, 35, 459–467. [Google Scholar] [CrossRef]
- Serôdio, P.; Nogueira, J.M.F. Multi-residue screening of endocrine disrupters chemicals in water samples by stir bar sorptive extraction-liquid desorption-capillary gas chromatography-mass spectrometry detection. Anal. Chim. Acta 2004, 517, 21–32. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.A.; Etxebarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Thomaidis, N.S. Determination of eight artificial sweeteners in wastewater by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 3825–3833. [Google Scholar] [CrossRef]
- Eurpean Commission. Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. [Google Scholar]
- Food and Drug Administration. Acceptance Criteria for Confirmation of Identity of Chemical Residues using Exact Mass Data within the Office of Foods and Veterinary Medicine; Food and Drug Administration: Silver Spring, MD, USA, 2015; pp. 1–16.
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).