Abstract
Ionic liquids (ILs) are a group of non-conventional salts with melting points below 100 °C. Apart from their negligible vapor pressure at room temperature, high thermal stability, and impressive solvation properties, ILs are characterized by their tunability. Given such nearly infinite combinations of cations and anions, and the easy modification of their structures, ILs with specific properties can be synthesized. These characteristics have attracted attention regarding their use as extraction phases in analytical sample preparation methods, particularly in liquid-phase extraction methods. Given the liquid nature of most common ILs, their incorporation in analytical sample preparation methods using solid sorbents requires the preparation of solid derivatives, such as polymeric ILs, or the combination of ILs with other materials to prepare solid IL-based composites. In this sense, many solid composites based on ILs have been prepared with improved features, including magnetic particles, carbonaceous materials, polymers, silica materials, and metal-organic frameworks, as additional materials forming the composites. This review aims to give an overview on the preparation and applications of IL-based composites in analytical sample preparation in the period 2017–2020, paying attention to the role of the IL material in those composites to understand the effect of the individual components in the sorbent.
1. Introduction
Ionic liquids (ILs) are molten salts formed by bulky organic cations in combination with inorganic or organic anions [1], with melting points below 100 °C. The most common cation used for the preparation of ILs is imidazolium, containing one or two alkyl chains, abbreviated as [CnIm+] or [CnCmIm+], where n or m is the number of carbon atoms in the substituent, with the following exceptions for the abbreviations: M for methyl, Vi for vinyl, and A for allyl. ILs with 1-alkyl-3-methylimidazolium cations ([CnMIm+]) are the most frequently used due to their easy preparation by nucleophilic reactions using methylimidazole. Other cations used to prepare ILs include tetra-alkylammonium ([Nn,n,n,n+]), phosphonium ([Pn,n,n,n+]), pyridinium ([CnPy+]), pyrrolidinium ([CnPyrr+]), and guanidinium ([CnGu+]). Regarding the anionic moiety, a wide variety of anions are used, including chloride ([Cl−]), bromide ([Br−]), tetrafluoroborate ([BF4−]), hexafluorophosphate ([PF6−]), and more complex structures, such as bis(trifluoromethanesulfonyl)imide ([NTf2−]). Moreover, multicationic ILs can be prepared by linking two or more cations with an alkyl chain, as for example di(3-butylimidazolium)dodecane chloride ([(C4Im)2C122+]2[Cl−]) [2,3]. In turn, there is a broad spectrum of ILs due to the nearly infinite combinations of cations and anions.
These materials present a wide liquid range and negligible vapor pressure at room temperature. Therefore, they do not generate volatile organic compounds in comparison with conventional organic solvents [4]. Furthermore, they exhibit impressive solvation properties, a high conductivity and thermal stability, an outstanding tunability, while being able to interact with substances by different co-existing interactions within the same IL. Their synthetic versatility allows the preparation of tailored ILs with specific properties and physicochemical characteristics, with the nature and structure of the cation and anion moieties playing a key role in this tunability [5]. For example, the water-solubility of the ILs can be controlled by selecting the adequate anion since halides and ([BF4−]) generate mostly water-soluble ILs, while other anions containing fluorine atoms yield generally hydrophobic ILs. Despite their relatively low toxicity in comparison with halogenated solvents, there is an increasing concern on the real grade of greenness and the toxicological impact of ILs [1]. Therefore, it is important to consider toxicology, biodegradability and recyclability factors when using ILs to ensure the development of sustainable procedures. In this sense, ILs containing moieties coming from natural sources with safer toxicological profiles have become the most promising research line to improve the greenness of ILs [5].
This versatility also permits the incorporation of more complex features to ILs only requiring small changes in their structures, which leads to the preparation of interesting IL derivatives. Thus, ILs with active surface properties can be prepared by incorporating long alkyl chains in the cation or anion structure, forming IL-based surfactants [6]. It is also possible the synthesis of magnetic ILs by including a paramagnetic component in any of their moieties, leading to the fabrication of ILs that can be manipulated by the application of an external magnetic field [7]. ILs can be also polymerized to obtain polymeric ILs (PILs), which not only retain main characteristics of ILs, but also present the inherent properties of polymers with enhanced mechanical stability [8]. Moreover, the inclusion of specific functional groups within the IL structure makes possible the preparation of task-specific ILs [9].
Given these interesting properties, ILs and their derivatives have been used in many applications from different scientific fields [5], such as synthesis and catalysis [10], engineering and energy [11,12], electrochemistry. [13], biosensing [14], and separation science [15,16], among others. Regarding separation science, ILs have been particularly successful as extraction phases in analytical sample preparation methods [17,18]. In this field, taking into account the intrinsic liquid nature of most ILs, these materials have been mainly explored in liquid-phase extraction methods, and particularly in microextraction (LPME) approaches, such as single-drop microextraction, dispersive liquid-liquid microextraction (DLLME), or hollow-fiber LPME [19,20]. The success of ILs in these methods lies in the abovementioned tunability, their solvation properties and the diversity of interactions that can be promoted towards the target analytes, together with their low toxicity in comparison with the organic solvents conventionally used in these extraction strategies.
Due to the success of ILs in LPME, and with the aim of taking advantage of their exceptional features in sorbent-based extraction and microextraction methods, different strategies have been followed: (i) the use of solid IL derivatives [18,21]; and (ii) their combination with other materials to prepare IL-based solid composites [17,22]. The use of solid derivatives of ILs is mainly restricted to PILs, which limits exploiting other ILs in these methods. In this sense, the development of IL-based solid composites is the most extended approach to apply ILs in sorbent-based strategies: solid-phase extraction and its miniaturized version (SPE and µ-SPE), miniaturized dispersive solid-phase extraction (µ-dSPE) and its magnetic-assisted mode (m-µ-dSPE), and solid-phase microextraction (SPME).
The physicochemical characteristics of ILs and their derivatives facilitate their combination with many other solid materials to prepare attractive sorbents. Thus, ILs have been mainly combined by physical or chemical immobilization methods with magnetic particles (MPs), carbonaceous materials, polymers, silica-based materials, and metal-organic frameworks (MOFs) [23,24,25,26,27], among other materials. In general, these IL-based solid composites exhibit the properties of both materials, but depending on the way they are combined and the intended application, the role both materials play in the resulting material may change in comparison with the initial characteristics of the individual components. Moreover, the preparation of some ILs is characterized by high costs, while their structure may be modified when they are combined with other materials or put in contact liquid matrix samples, which can affect the performance and reproducibility of the composite for target applications. Thus, it is important to pay attention to the design of the IL-based solid composite material to obtain a non-expensive and stable extraction sorbent in which each component is providing an added value to the final composite.
In 2019, Yavir et al. reviewed the analytical applications of composites based on supported ILs on solid materials and included a thorough outlook of the physicochemical properties of ILs and how their structures are crucial for the preparation of composites, taking advantage of the multiple interactions that they may exhibit with other materials [25]. Authors also discussed the incorporation of IL-based composites in different sorbent-based extraction methods and biosensors by describing selected studies in the field. Despite the a priori similarities with the present review, in this case we overview the preparation and analytical applications of these composites but considering the different materials used to prepare the composites regardless the extraction method, with the aim of understanding in depth the benefits of combining all materials included in the IL-based solid composite.
Therefore, this review article covers the preparation of IL-based solid composites containing MPs, carbonaceous materials, polymers, silica-based materials, and/or MOFs, for the analytical sample preparation applications published in the period 2017–2020 (until March 2020). As shown in Figure 1, most applications deal with the use of IL-based solid composites with carbon materials, followed by magnetic and polymeric composites. The main features of the solid materials will be described, while the role of the IL in the resulting solid composite material will be highlighted according to the analytical performances reported. To compile a comprehensive description, composites will be described in the following sections as function of the type of material combined with the IL.
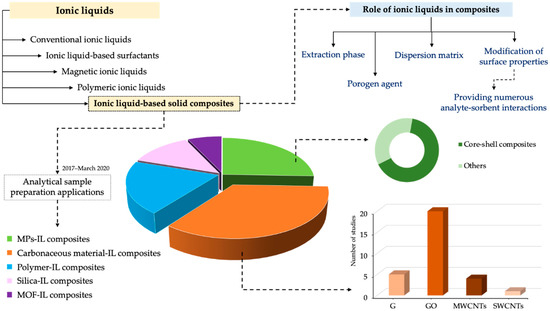
Figure 1.
Number of studies reporting the use of ILs-based solid composites in analytical sample preparation methods in the period 2017–2020 (until March 2020), together with the main roles of ILs in the composites according to the studies reported. For the definition of the abbreviations, please refer to the list of abbreviations at the end of the article.
2. Magnetic Particles-Based Ionic-Liquid Solid Composites (MP-ILs)
Magnetic particles (MPs) are a group of particles widely employed in analytical sample preparation characterized for exhibiting magnetic properties. They are mostly made from transition metals like iron (Fe), cobalt (Co) and nickel (Ni), or from their oxides and sulfides, such as cobalt ferrite (CoFe2O4), magnetite (α-Fe3O4), and greigite (Fe3S4) [23,28,29]. These materials can be synthesized by different methods, being the microwave-assisted method, the solvothermal preparation, and the chemical co-precipitation of two inorganic salts in basic medium, the most well-known and applied approaches [30,31,32]. Additionally, they can be obtained with different particle size, which can be either nano or micrometrical [28,32,33].
MPs are characterized for their high specific surface area, small particle size, and their superparamagnetic properties, which permit their easy and quick isolation when applying an external magnetic field [23,34]. Despite the numerous advantages that this kind of particles offers for the development of m-µ-dSPE methods, their easy oxidation in air, aggregation and instability in hydrophilic and acidic conditions cause their degeneration and loss of their magnetic and dispersion capacities.
With the aim of overcoming these drawbacks, magnetic-based extraction sorbents are prepared (and indeed widely explored) by the modification of bare MPs, mainly by their surface functionalization or by their protection with a coating formed using another material [29,30,31,35,36]. Among the materials used for their modification and preparation of magnetic composites, ILs and their derivatives merit to be highlighted [23]. In all these studies, the IL or the IL derivative is the material responsible of the extraction of the resulting sorbent, while the incorporation of MPs in the composite is accomplished with the aim of setting up a m-µ-dSPE method. In other words, the inclusion of MPs in the final solid composite simplifies the operational procedure of the extraction method by speeding up and facilitating the separation of the extraction phase from the sample: filtration and centrifugation steps are avoided, because the magnetic composite containing extracted analytes is simply separated from the remaining components of the sample (and main matrix) using an external magnet. Given the wide variety of physicochemical characteristics of ILs and their derivatives, different types of magnetic composites based on ILs and MPs (MP-ILs) have been proposed, including core-shell structures and magnetic effervescent tablets, among others.
2.1. MP-ILs Composites with Core-Shell Structures
Most of the reported MP-ILs composites entail core-shell structures (IL@MPs), in which a layer of the IL or IL derivative is coating (shell) a MP (core). The immobilization of the IL (as extraction phase) on the surface of the MPs in a core-shell structure leads to an increase of its surface area in comparison with that of the neat IL. Therefore, not only the magnetic properties but also the improved surface area is an attractive characteristic of these composites. Regarding their synthesis, it has been accomplished following any of these three different strategies: (i) by simply mixing both components at room temperature [37], (ii) by the dispersion of the MPs in a solution of the IL followed by letting the organic solvent to evaporate [38,39], or (iii) by dispersing the MPs in an organic solvent containing the IL and keeping the mixture under reflux conditions to allow them to react [40,41,42]. In all cases, the functionalization of the MP surface is the key point for their preparation, since it guarantees the proper linkage between the individual components and even allows the reusability of the sorbent. This process is usually characterized for being time consuming and implying multistage procedures, this timing also considering (in several cases) the previous preparation of MPs and the IL material.
In the reported IL@MPs composites, MPs are synthesized by co-precipitation or solvothermal methods, being Fe3O4 the MPs most commonly used [38,40,41,43,44,45,46,47,48,49,50,51,52,53,54], but others, such as a mixture of Fe (II) and Co (II) salts [37], have been also reported.
Due to their high surface area and good thermal and mechanical stability, mesoporous silica coatings (SiO2) are the most common option used to reduce the main issues related to the stability of MPs and to functionalize their surface for the further immobilization of the IL or IL derivative layer. This modification step is usually accomplished by sol-gel approaches [38,39,42,44,45,46,47,49,50,52,53,54] or modifications of the a Stöber method [40,41,48]. In some cases, an amino or chlorine functionalization of the silica layer [40,41] or a double silica coupling [55] have been reported to ensure a chemical linkage with the IL material. Apart from silica, polymer coatings have also been used to protect the MP and improve the subsequent binding of the IL [37,51]. In this sense, it is interesting to mention the study reported by Abujaber et al. [37], which use magnetic cellulose nanoparticles as core for the preparation of the IL@MP composite. This material, obtained using a renewable and ecofriendly polymer, allowed a fast and straightforward combination with ILs thanks to the electrostatic interactions between both components.
Regarding the nature of the ILs, imidazolium-based ILs have been the most widely used due to their easy preparation and commercial availability. However, some alternatives have been developed in the recent years with the aim of obtaining less toxic ILs. This is the case of the 1-4-diazabicyclo[2.2.2] octane (DABCO)-based IL proposed by Sahebi et al., characterized for being less toxic than more conventional ILs [40]. In general, hydrophobic ILs are employed in the synthesis of the ILs@MPs solid composites due to their insolubility in the aqueous sample. Only a few works have reported the use of hydrophilic ILs in the preparation of these materials but performing their conversion into a water-insoluble through a metathesis reaction in the final stage of the synthetic procedure [48]. It is interesting to point out that hydrophilic ILs have been used to prepare ILs@MPs sorbents without this transformation step [40,41]. In these cases, several authors have demonstrated the loss of mass and active sites in the sorbent once it is reused [40].
Among the IL derivatives that have been explored in the preparation on these magnetic core-shell materials, PILs have attracted significant attention. This may be due to their high mechanical stability and stronger adhesion to the MPs. As it happened with neat ILs, the coating of MPs with PILs not only provides paramagnetic properties to the PIL to facilitate its separation from the sample when used in magnetic-based approaches, but also increases its availability as sorbent by increasing the number of sorption sites (due to the surface area of MPs as support), which leads to better extraction capacity of the composite in comparison with the use of the individual components [44,46,48].
Regarding the preparation of PILs-based core-shell structures (PIL@MPs), unlike ILs, PILs have been directly synthesized onto the modified MP by free radical copolymerization methods [46,48,50,52,53]. However, other approaches have been reported, such as a redox polymerization using two different ILs [43], a precipitation polymerization [47], or the in situ polymerization taking advantage of the chemical groups anchored to the surface of the MP (to ensure the binding between the magnetic core and the PIL) [44,45,51]. For example, Llaver et al. [45] coated MPs with amino-functionalized silica to promote the in situ synthesis and simultaneous linkage of the PIL on the surface of the MP by the Radziszewski reaction, which consists in a diamine polycondensation. In another approach, He et al. proposed the preparation of a PIL@MP composite through the layer-by-layer assembly of a polycationic PIL (poly(1-vinyl-3-hezylimidazolium) bromide (poly[ViC6Im+][Br−]) and the polystyrenesulfonate sodium (PSS) polyanion as shown in Figure 2A [54]. As a result, they obtain a wide variety of PIL@MPs with different thicknesses depending on the number of bilayers. As it can be observed in Figure 2B, the extraction efficiency of the magnetic composite towards pesticides increased as the number of PIL and PSS bilayers increased, 10 being the optimum number of bilayers. This preparation method allowed obtaining more homogeneous spherical particles in comparison with those obtained by the free radical polymerization method, as it can be observed in Figure 2C [54].
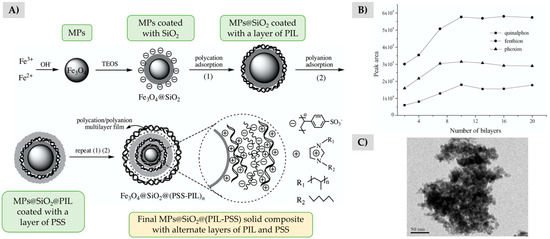
Figure 2.
(A) Scheme of the synthesis procedure of a PIL-based magnetic composite by alternating layers of poly[VMim+][Br−] and PSS. (B) Study of the extraction efficiency of the composite based on the number of deposited bilayers. (C) TEM image of the optimum composite MPs@SiO2@(PSS-poly[VMim+][Br−]) prepared with 10 bilayers of the polymers. Adapted from [54], with permission from Elsevier, 2017.
The combination of MPs with ILs or IL derivatives to prepare these solid composites represents an important improvement of the characteristics of the individual components, which makes possible their application in m-µ-dSPE methods for a the determination of a wide variety of substances in samples of different nature, such as food, environmental water, human fluids, or personal care products. Table 1 lists some representative examples of the analytical applications using this type of magnetic composites [37,40,43,45,49,54,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].

Table 1.
Representative examples of IL-based composites utilized in analytical sample preparation methods.
They have been successfully used in the determination of organic compounds such as phthalate acid esters (PAEs) [41,47,51], drugs [37,48], parabens [43,52], penicillins [40], beta blockers [38], pyrethroid residues [44], and organic UV filters [49], among others. In general, amounts between 3 and 160 mg of the magnetic composite are used, while only a few seconds are required for the magnetic isolation of the sorbent from the sample (and similar timing in the further desorption step), thus ensuring a fast method with low sources of error. Regarding the determination of metals [42,45], it is interesting to mention the study reported by Llaver et al. [45] for the determination of tellurium (Te) species in environmental samples without the requirement of a chelating agent. They take advantage of the ionic nature of PIL to establish selective electrostatic interactions with the Te species at different pH values. Thus, the sorbent is able to fully extract Te(IV) at pH lower than 4, while Te(VI) is extracted at higher pH values.
Despite the most common applications of IL@MPs and PIL@MPs relate to their use in m-µ-dSPE approaches, it is also possible to find some other interesting applications of these composites. For instance, Mei and Huang described a new method termed magnetism-enhanced monolith-based in-tube SPME [49]. A (PIL)-based monolithic capillary column doped with MPs was prepared by an in situ thermal polymerization method using [AMIm+][NTf2−] as functional monomer. In this composite, the PIL is used as extraction phase, while the MPs are used to enhance the retention of the analytes in the PIL sorbent due to the response of the diamagnetic analytes after applying different magnetic field gradients to the in-tube SPME device. The application was successful for the online determination of organic UV filters in environmental waters. A similar approach was described by Chen et al. [78]. In this case, instead of using a monolithic PIL, authors packed the IL@MPs composite in a gas-liquid-solid magnetically stabilized bed and applied an external magnetic field to enhance the extraction efficiency of flavonoids, in a similar procedure to conventional SPE.
2.2. Magnetic Effervescent Tablets and Other Strategies Based on ILs and MPs
Magnetic effervescent tablet-assisted microextraction (METM) recently emerged as an alternative to DLLME. Despite excellences of performance in DLLME, the technique always requires proper dispersion of one hydrophobic extraction solvent to ensure efficiency, while the further collection of the extraction solvent containing trapped analytes is a time consuming and, in some cases, difficult step [79]. METM does not require physical energy (vortex, ultrasound, etc.) or dispersive solvents as in DLLME to ensure dispersion of the extraction solvent. The novel technique takes advantage of the CO2 bubbles produced by the reaction of the acidic and alkaline components of the tablets (METs) in the aqueous sample. The use of METs not only helps in the dispersion of the extraction solvent thanks to the effervescence effect, but also improve the sampling of the solvent with the assistance of the MPs forming part of the tablets.
The preparation of METs based on the combination of ILs and MPs has been described, using hydrophobic ILs as extraction solvents, thus being the material responsible of the extraction of the target analytes [80]. In general, the preparation of these composites is very simple and fast, as it only involves the homogeneous mixing of the MPs, the IL, sodium carbonate, and a weak acid, and their compression to form a compact tablet. Fe3O4 MPs are the most common in this approach [81,82], but other MPs with better characteristics have also been used, such as Fe3S4 MPs synthesized by the solvothermal method [56], or MFe2O4 NPs using different metals [83]. Regarding the ILs, they are mainly composed of imidazolium cations and fluorine-containing anions to obtain water-insoluble ILs [56,82,83]. However, it is interesting to mention the use of a hydrophilic IL in the MET reported by Wu et al. [81]. In this case, the use of a water-soluble IL in the tablet enhances even more the dispersion of the extraction solvent in the solution. The addition of an anion-exchange reagent after the effervescence process led to the insolubilization of the IL, which immediately interacts with the MPs thus ensuring an easy collection (magnetic-assisted) for its further analysis.
Considering the advantages of METs (simple preparation of the composite, efficient dispersion of the IL as extraction phase, and facile collection of the IL phase—containing trapped analytes—due to the MPs included in the tablet), it is not surprising the high number of applications reported. Thus, they have been employed with milk and human fluids samples (among others) for the preconcentration and determination of different organic contaminants, such as PAEs [83], polybrominated diphenyl ethers (PBDEs) [56] or pyrethroids [82], and biological compounds like endogenous steroids hormones [81].
Similar strategies do not necessarily require the preparation of a composite, since the IL or liquid IL derivative is added to the aqueous sample as extraction solvent, followed by the addition of MPs as carrier to ensure the quick and complete separation of the extraction material (the IL or its derivative) from the sample. Hydrophobic ILs can be collected after the addition of the magnetic material due to the electrostatic interactions between the IL cation and the negatively-charged surface of the MPs [84,85,86,87,88,89]. Furthermore, despite the hydrophilicity of IL-based surfactants, they form mixed hemimicelles (hemimicelles plus admicelles) on the surface of MPs due to electrostatic interactions, thus functionalizing these MPs to improve their extraction efficiency [90,91]. The formation of hemimicelles as a monolayer of IL provided hydrophobic interactions, while the bilayer of admicelles provided more electrostatic interactions between the sorbent and the target analytes. More recently, MILs have also been used in these strategies, in which the paramagnetic characteristics of both materials allow their combination and speeds up the separation step [92].
3. Carbonaceous-Based Ionic-Liquid Composites (C-ILs)
Carbon materials have attracted much attention in analytical sample preparation due to their exceptional physical and chemical properties, such as their flexibility, large surface area with delocalized π-electrons, and their easily modifiable surface [93]. They constitute a group of different hexagonal (honeycomb-like) structures of sp2 hybridized carbon atoms. A single layer of such material is called graphene (G) that, together with graphene oxide (GO) and reduced graphene oxide (rGO), are the most representative two-dimensional forms. The difference between G, GO, and rGO lies in the presence of oxygen functional groups on their surface and the water solubility of the material. G and rGO are non-polar and hydrophobic materials with high affinity towards carbon ring structures, while it is possible to prepare hydrophilic GO that forms stable colloidal suspensions in water [94]. The three-dimensional forms of G are single (SWCNTs) or multiple wall carbon nanotubes (MWCNTs). Both materials share the same exceptional properties as G and its derivatives, but with a few differences. The adsorption in case of CNTs occurs only on the outer wall of the tubes, because the inner wall is blocked by the steric hindrance. Furthermore, the synthesis itself is simpler for G and its derivatives and they can be easily prepared in the laboratory, while the preparation of CNTs requires more exhaustive protocols [94]. Although they present a lot of advantages, their difficult solubility and dispersibility and their lack of selectivity are the main limitations of these materials. Therefore, ILs emerges as a suitable solution to overcome these drawbacks by the modification of the surface of carbon materials [24].
In the C-ILs composites, the most predominant role of ILs is the modification of the characteristics of the carbon material, including hydrophilicity, π-π interactions, or reducing the aggregation of the carbon material, thus improving the sorption capacity, extraction efficiency, and selectivity of the extraction sorbent when utilized in an extraction method. Therefore, the resulting composite incorporates the benefits of both ILs and carbon materials.
The preparation of the composite material is generally carried out by dispersing each component in water or an organic solvent and stirring the mixture for up to 48 h at room temperature or heating. Some studies also use sonication to improve the dispersion of the carbon material [59,60,95,96,97]. It is interesting to mention that most of the studies use magnetic C-IL materials [61,62,63,64,65,77,96,98,99,100,101,102,103,104,105,106]. In this case, the carbon materials are used as a support for the synthesis of MPs increasing the surface area of the material, and then, the magnetic carbon material is subsequently modified with the IL. Regarding the type of carbonaceous materials used in the extraction schemes to prepare the composite, most of the studies use GO [58,62,64,65,95,97,98,99,103,104,105,106,107,108,109,110,111,112,113], followed by G [57,63,100,102,114], while a few have reported the use of SWCNTs [96] and MWCNTs [59,60,77,101]. This may be due to the easy preparation of GO and G in comparison with the three-dimensional structures.
Regarding the ILs, the vast majority of C-ILs materials use the more conventional and easily tunable imidazolium-based ILs [57,58,59,60,62,63,64,95,97,98,99,100,101,102,103,105,106,108,109,110,111,112,113,114], but guanidinium-based [65,107] and DABCO-based ILs [96] have also been used. With regards to the anions, both hydrophilic ILs with halide anions [58,61,62,63,65,77,95,96,98,100,106,110,112,113] and hydrophobic ILs with [PF6−] as an anion have been reported [57,59,60,64,97,99,101,102,103,104,105,109,114]. It is suggested that the use of hydrophilic ILs makes the surface of the sorbent more hydrophilic, which favors the sorption of the analytes [100]. However, attention should be paid to the leaching of the hydrophilic IL when the sorbent is added to the aqueous sample. The utilization of IL-based surfactants has also been described as an interesting way to modify the surface of carbon materials. In this case, imidazolium-based ILs with a long alkyl chain are used for the assembly of mixed hemimicelles on the surface of magnetic GO. As it happened with mixed hemimicelles of IL-based surfactants onto bare MPs, the IL-based surfactant is the main responsible of the extraction, while the magnetic GO only acts as support to generate a high surface area while improving the mass transfer [61,98].
Interestingly, some studies have reported the use of IL as a matrix or as a trapping phase to disperse the carbon materials, resulting in a bucky gel [57,59,60,97,99,103]. This soft viscous material is obtained by direct dispersion of G/GO in the IL using either an agate mortar or by sonication. Although the IL is playing the role of a dispersant in these gels, the resulting material shows the properties of both precursors and, indeed, the IL is also participating in the extraction process. All these studies use ILs composed of [PF6−] anion due to its hydrophobic character [59,97]. This strategy has also been used for the preparation of magnetic bucky gels or ferrofluids by the incorporation of MPs in the gel structure. Thus, Jamshidi et al. [99] and Rafouei et al. [103] have reported their use in m-µ-dSPE. Yousefi et al. prepared SPME fibers by dipping the etched stainless steel wires in the bucky gel several times until a thickness of 90 µm was obtained. The obtained fiber was then heated at high temperatures to obtain a stable and homogeneous coating, which was used in a headspace-SPME-GC-FID method [57].
PILs have also been used to modify the surface of GO with the purpose of obtaining more selective sorbents [107,108,111]. In all cases, the prepared sorbents were used to prepare packed µ-SPE devices. In the study of Hou et al. [111], GO-grafted silica (GO@SiO2) was modified with mercaptopropyl groups to prepare in situ the PIL. Authors used [ViC6Im+][Br−] as monomer to form the PIL, which was then subjected to a metathesis reaction to exchange the anion for [PF6−], thus generating a more selective coating towards acidic compounds. The obtained composite sorbent was packed inside a polypropylene cartridge for the µ-SPE device [111]. PILs have also been useful as polymeric backbone to prepare molecularly imprinted polymers (MIPILs) on the surface of GO with the purpose of highly improving the specific selectivity of the sorbent while obtaining high stable polymers due to the properties of the PILs. Such approach was reported by Yuan et al. [107], using the reversible addition-fragmentation chain radical polymerization to grow a thin layer of MIPIL on the GO surface. This composite sorbent was packed into a pipette tip and exhibited fast mass transfer rate and specific selectivity when compared with the non-imprinted PIL for the determination of β-agonists in swine urine. In the study of Ma et al., a dual-template MIPIL monolithic SPE column was prepared, using GO as the support to obtain higher surface to volume ratio. This sorbent was used for the simultaneous extraction of ciprofloxacin and levofloxacin from human urine samples with high affinity and selectivity [108].
Due to the well-known extraction capability of the materials involved in C-ILs composites, it is important to compare the extraction efficiency of the composite material with that of the single components to justify the need of them for the specific application. In general, this comparison study is not included, and the role of the individual components is not fully demonstrated. There are only a few studies that compare the sorbents. For example, Rofouei et al. [103] compared extraction recoveries using only magnetic GO, the IL and resulting magnetic bucky gel, proving both materials participate in the extraction and there was an enhancement in the extraction performance when both materials were combined. In another study using guanidinium-based IL to modify a magnetic chitosan/GO composites for DNA extraction, authors performed the comparison between each intermediate during the preparation of the final composite, showing incremental improvements in the extraction efficiency when GO and the IL were included in the sorbent [65]. In the study reported by Lotfi et al. [96], the magnetic SWCNTs were modified by covalently bonding DABCO-based IL and then the composite was used as a sorbent in m-µ-dSPE for the selective extraction of serotonin reuptake inhibitors in plasma samples. The comparison between the intermediates of the C-IL composite showed significant increase in recoveries of the analytes after the modification with the IL. From the comparison study described by Wang et al. [62] using a magnetic GO-IL composite (Figure 3A), it was demonstrated that the use of the IL was crucial for the extraction of the target polysaccharides, because GO exhibited lower sorption ability, as shown in Figure 3B. Moreover, authors compared the effect of several imidazolium-based ILs with different alkyl chains on the extraction capacity of the composite. According to the results shown in Figure 3B, the functionalization of the IL chain with an amino group led to a superior sorption ability in comparison with the other IL-modified magnetic GO sorbents. It is also interesting to mention the preparation of composites containing magnetic MWCNTs and MOFs modified with ILs [77,101], in which the carbon material and the IL are demonstrated to be important components for the extraction performance of the sorbent. However, they will be discussed in Section 6 dealing with MOF-IL composites.
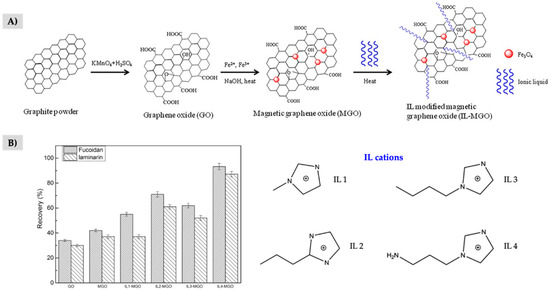
Figure 3.
(A) Synthesis of magnetic GO-IL composite. (B) Extraction recoveries using the individual components and the resulting composite with different ILs for the extraction of polysaccharides, together with the structures of the IL cations tested for the preparation of the composite. Adapted from [62], with permission from Wiley, 2017.
Table 1 includes representative examples of the analytical applications using C-ILs composites. Amounts of the sorbents ranging between 2 and 80 mg have been employed in µ-dSPE and m-µ-dSPE methods, while the SPE cartridges were prepared using packing 200 mg of the composites. They have been mainly used for the determination of heavy metals [59,60,97,99,103,104,105,112], pollutants [57,58,61,106,109,110,113], pesticides [63,95,101,102], drugs [96,98,107,108], hormones [64,114], microcystins [100], and phenolic acids [111], in a wide variety of samples, including water [60,61,63,95,99,100,101,103,105,109,110,112,113], food [64,99,102,104,106,110,111,114], and body fluids [57,58,59,96,97,98,107,108,111]. It is worth to point out interesting and challenging applications using these sorbents, such as the determination of digestive enzyme in porcine pancreas [77], polysaccharides in brown alga [62], and DNA in human whole blood [65].
4. Polymer-Based Ionic-Liquid Composites (Pol-ILs)
Polymers are large molecules composed of more than a hundred repeating units (monomers) connected by covalent bonds while forming a high mass macromolecular substance [115]. Polymeric materials have demonstrated good mechanical stability properties and are considered versatile due to the high number of different combinations of monomers, crosslinkers, and other additives [116]. For that reason, they have been widely used in different applications such as fabrication of membranes [117], gas separation [118], sensors [119], in biomedicine [120], and also in sample preparation [121,122].
In analytical sample preparation, one of the most common and interesting polymeric materials are MIPs due to their high selectivity [123]. They contain specific spots for target analytes in the polymeric backbone, promoting the recognition of these analytes against other interferent substances in the sample matrix. This is possible due to the synthesis of the polymer by the common polymerization reactions using a functional monomer, a crosslinker complex, and a porogen agent, but also a template molecule to create the recognition sites. Once the polymer is formed around the template molecule, the template is removed from the material leaving a cavity for the extraction of analytes with the same or similar structure of the template [123]. Thus, the selection of the different components of MIPs highly affects the template-monomer interactions and is crucial for the preparation of MIPs with specific properties.
Although MIPs have great extraction capability, especially in terms of selectivity, their application for the extraction of analytes from aqueous samples is sometimes limited due to their swelling and shrinking properties, which leads to changes in the polymeric network and the consequent reduction of its recognition ability and extraction capability. Therefore, it is necessary to reduce their hydrophobicity to improve the analytical performance in aqueous media. In this sense, hydrophilic ILs have attracted significant attention as functional monomer to prepare MIPILs [67,68,69,124,125,126], and as porogen agents to improve the characteristics of the MIPs [66,127,128,129].
In addition, the incorporation of ILs in the MIP structure also enhances even more the selectivity of the material providing electrostatic, ion-exchange, and π-π interactions, towards the target analytes. Indeed, this has been demonstrated when the IL is used as functional monomer: the extraction capability of the MIP improves due to the (stronger) multiple interactions between the target analytes and the IL in comparison with conventional functional monomers, such as methacrylic acid. Therefore, the use of ILs in the preparation of MIPs improves the imprinting of the resulting polymer [126]. Regarding the use of ILs as porogen agent, the length of the alkyl chain and the nature of the counter anion of the IL play an important role on the polymer permeability [125,127]. Sun et al. studied the use of different lengths for the alkyl chain (C4, C6, and C8) of the [CnMIm+] IL used, showing better permeability for the MIP prepared with the C4 chain. On the other hand, [BF4−], [Br−], [PF6−], and [HSO4−] were tested as anions, demonstrating that the highest permeability for the material was obtained when using [BF4−] since the other anions yielded denser structures that reduced the permeability of the material [127].
Although the use of the IL is reported for a specific purpose, such as porogen agent or as functional monomer, the IL usually plays both roles at the same time in the resulting material. Indeed, in general, another conventional functional monomer is also included in the synthesis procedure, being the ratio between the amount of IL and this conventional monomer an important parameter to determine whether if it is main role is as a porogen or as a functional monomer. For example, Han et al. used [ViPTMSIm+][Cl−] (propyltrimethoxysilane abbreviated as PTMS) IL in combination with methacrylic acid as functional monomers to form the complex polymeric structure shown in Figure 4A. The adsorption capacity studies of the resulting MIPIL showed that the surface area of the material was higher when introducing the IL than for the MIP free of the IL, and the non- imprinted polymer (NIP) also free of IL, as shown in Figure 4B. This is due to the additional role of the IL as a porogen agent, resulting in the preparation of a more porous material with a rougher surface [126].
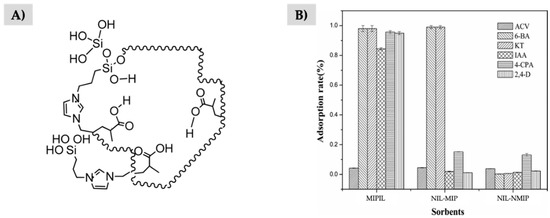
Figure 4.
(A) Scheme of the polymeric structure formed using [ViPTMSIm+][Cl−] in combination with methacrylic acid, and (B) comparative study of the adsorption capacity when using MIPIL, the MIP non-modified with the IL (NIL-MIP) and the non-imprinted polymer non-modified with the IL (NIL-NIP, termed by authors as NIL-NMIP). Adapted from [126], with permission from ACS Publications, 2017.
[CnMIm+]-based ILs with alkyl chains with different lengths is the main type of ILs used to prepare monolithic MIPILs [66,127,128,130], being [C4MIm+]-based ILs the most frequently used [66,127,128,130] The synthesis of these materials is mainly accomplished by filling a column-shaped device with the mixture containing the IL, the conventional monomer (or silica monomer if they are prepared by sol-gel approaches), the template molecule, the crosslinker, and the initiator to form the monolith by an in situ polymerization reaction [66,67,68,69,70,124,125,126,127,128,130]. The resulting monoliths have been mainly used as sorbent in SPE using cartridges [66,68,69,70,124,125,126,127,128,130], and in one application they have also been included as coatings in SPME [67].
It is also quite common the formation of a coating all over the surface of a supporting material creating a core-shell structure. The support materials that have been used are silica or mesoporous silica [68,69] and MPs [55,131,132,133]. MIPIL@SiO2 composites have been packed to prepare µ-SPE cartridges using just ~50 mg of the material [68,69], while the magnetic MPIL@MPs have been used in m-µ-dSPE methods [55,131,132,133]. As it was previously described in Section 2.1, the supported MIPILs frequently requires the functionalization of the support surface prior to the polymerization to ensure the formation of a chemically bonded coating.
MIPIL-based sorbents have been mainly used for the selective determination of antibiotics [68,69,124], drugs [125], herbicides [126], phenolic acids [67], and phthalate esters [66], among others [127,128,129]; and in different matrixes, such as water [68,69,124,129], soils [68,69,124], and food and drinks [66,67,126].
Table 1 shows some representative examples of analytical applications using MPILs composites. Among these applications, it is interesting to mention the study of Zhu et al. where a MIPIL was used for the determination of sulfonamides by SPE-LC-DAD. Authors demonstrated the higher selectivity and extraction capability of the MIPIL in comparison with the simple MIP, reaching values of relative recoveries around 100% at a concentration level of 12 μg·L−1 in complex samples, such as soils and sediments [68].
It is also important to mention another type of polymeric materials used in combination with ILs: poly-cyclodextrins (polyCDs) [71,134,135]. These polymers present functional cavities prepared from cyclodextrins (CDs), which are non-reduced sugars made up of macrocyclic oligosaccharides highly utilized in chiral analyses. They are considered supramolecular polymers with hydrophilic properties on the outer surface and hydrophobic characteristics in the inner cavity, so they can encapsulate guest molecules into their cavities without forming chemical bonds [136]. The polymerization of CDs is crucial for their use in the analysis of aqueous samples due to the water-solubility of CDs. This polymerization affects the properties of the inner cavity of CDs and reduce their sorption capability. ILs are excellent materials to compensate such loss of the extraction efficiency due to the incorporation of new possible analyte-sorbent interactions [71,135]. In this sense, β-cyclodextrins (β-CDs) have been used in combination with MPs to prepare magnetic sorbents for m-µ-dSPE methods. Two approaches have been described for the preparation of these composites: (i) the modification of the previously modified CD with 1-vinylimidazole to form the IL, in which the CD is the alkyl chain of the IL, followed by the polymerization of the IL-modified CD on the surface of the MPs [71,135]; and (ii) the preparation of Fe3O4 MPs by the co-precipitation method in presence of the polyCD, followed by grafting the [C4MIm+][Cl−] IL on the surface of the magnetic material [134]. These materials have been used for the determination of PAHs in water, rice and tea [71,135], and parabens in water and cosmetics samples [134]. The amount of material used on these applications ranged between 10 and 25 mg [134,135].
5. Silica-Based Ionic Liquid Composites (Si-ILs)
Silica-based materials have been widely used in many scientific fields due to their astonishing properties, such as biomedicine [137], catalysis [138], sensing [139], for the removal of pollutants [140], and as sorbent material [141]. There are different kinds of silica-based materials, including SiO2 nanoparticles and ordered mesoporous silica (OMS), among others. OMS is well-known for its large specific surface area, well-defined pore volume, low toxicity, robustness, and low cost [142,143]. Regarding its use in analytical sample preparation, the skeletal structure of OMS allows the interaction of analytes with the sorbent not only on their outer surface, but also through their inner pore system. However, neat silica sorbents have problems due to their low number of surface functional groups able to undergo efficient interactions with target compounds [143]. In this sense, their chemical and physical properties can be easily modified by performing a post-functionalization of the material pores [143,144]. Given these reasons, it is not a surprise that the preparation of functionalized silica sorbents such as silica-carbonaceous materials [145], silica-MOF [146], silica-polymers [147], and silica-ILs composites [148], is a hot trend nowadays.
The modification of silica sorbents using ILs is mainly carried out to increase the sorption efficiency and selectivity taking advantage of the ILs properties. The incorporation of ILs on the surface and the inner pores of the silica skeleton makes possible a higher number of interactions. Therefore, in these Si-ILs composites, the IL material is playing an important role on the extraction efficiency of the resulting sorbent, which is increased thanks to the high surface area of silica materials.
Most common silica materials functionalized with ILs (mainly imidazolium-based ILs) used in analytical sample preparation strategies include SiO2 nanoparticles [72,74,149,150], mesoporous silica [75,151,152], nanosilica [153,154], and silica polymers prepared by sol-gel approaches [73,155]. The synthesis of these composites can be done by chemical bonding (grafting) or by physical interactions. Chemical bonding procedures allow controlling the coating process. It also allows the incorporation of a high number of different ILs immobilized (indeed, only a few ILs are able to form stable composites through physical immobilization). Nevertheless, these protocols require several time-consuming and non-sustainable reaction steps [72,73,74,75,149,150,151,152,153,155]. In most cases, the first step is the activation of the silanol groups (located all over the surface of the silica material), which are the main active groups that permit the further functionalization of the silica [72,74,153]. The surface can be also modified with silanization agents [149] to obtain silica materials with more reactive groups, such as amino or chlorine groups. Then, two strategies have been followed to obtain the Si-ILs composites: (i) the IL or PIL is in situ synthesized on the surface of the silica material [72,74,75,149,150], or (ii) the previously synthesized IL is chemically immobilized by its reaction with the functional groups on the surface of the silica particles [152,153]. In the first approach, when the silica material is modified with an IL, the imidazole precursor of the IL cation is immobilized, and the IL is in situ synthetized by refluxing the modified silica material with the reagents required for the IL preparation [74]. In the case of such modification with PILs, crosslinker reagents are used to ensure the chemical linkage between the PIL formed and the silica surface [75,149,150].
In some cases, to avoid the multiple steps of chemical functionalization while maintaining the silica-IL chemical bonding, the composite is synthetized by sol-gel methodologies and, thus, the IL is embedded in the silica polymeric structure [73,151,155]. In the preparation procedure, known as the Stöber process, the IL is mixed with a solution of tetraethyl orthosilicate (TEOS) and the Si-IL composite is formed by adding an ammonia solution dropwise.
Regarding other polymeric structures involving Si-IL, it is also interesting to mention the study reported by Zhou et al., in which an IL-modified monolithic polymer containing silica particles is prepared in a capillary tube for the fabrication of an in-tube SPME device [129]. In this case, stellated mesoporous silica nanoparticles, which are the main extraction material, are embedded in the monolithic polymer using butyl methacrylate as functional monomer and a mixture of the [C6MIm+][BF4−] IL and a deep eutectic solvent as porogen agents to improve the homogeneity and permeability of the resulting polymer.
On the other hand, the physical adhesion methods are simpler and faster, but the stability and reusability of the material are lower [72,154]. Indeed, Tian et al. compared the extraction capability and the stability of Si-ILs composites in which the IL was physically or chemically immobilized on SiO2 particles. Moreover, authors evaluated nine different ILs with different alkyl chains and counter anions [72]. In the Si-ILs prepared by physical bonding, the ILs are retained on the surface of the silica by weak hydrogen bonds. This leads to leaching of the IL from the sorbent composite during the elution step after the extraction procedure. Thus, the reuse of these composites is not recommended. On the contrary, chemically bonded ILs keep retained on the silica, being possible their reuse without noticing considerable loses on the extraction efficiency. In addition, it was also observed different extraction efficiencies depending on the alkyl chain length of the IL and the counter anion. Larger chains were associated with an increment of the non-polar properties of the IL, while electron-withdrawing anions improved the interactions between the IL and analytes containing electro-donating groups such as -OH [72]. This assessment regarding the influence of the IL structure in Si-ILs materials has also been performed in other studies, confirming this behavior for the sorbent-analyte interactions [74,154,155].
Due to the versatility on the preparation of these composites, they have been used in different solid-based extraction methods. Table 1 includes some representative examples.
They have been mainly applied in SPE in its off-line [72,74,149] and on-line versions [75,150,155], for the determination of several analytes, including phenols [72], flavonoids [149], aristocholic acid [74], antibiotics [150,155], and hydroxybenzoic acid [75]. The amount of sorbent required in packed on columns or cartridges in the reported applications vary between 0.04 and 3 g [72,75], utilized in different matrices, such as water [72], plants [74,75], and milk [150,155].
Si-ILs composites have also been used as sorbent in µ-dSPE for the determination of growth regulators in ginseng samples [151] and metals in different water samples, and soils and sediments [151,153,154]. In this case, the amount of sorbent required is much lower in comparison with the amount required to prepare SPE cartridges above mentioned. Among the applications, it is interesting to mention the study reported by Llaver et al. for the extraction of inorganic Se species (in the complexed form with a chelating agent) from water samples using a Si-IL composite prepared by physical immobilization [154]. Authors studied the effect of the solubility and hydrophobicity of different ILs on the extraction capability of the resulting material, demonstrating that the type of IL selected for the modification of the silica material plays an important role on the analytical performance. The highest extraction efficiency was achieved using the nanosilica modified with the IL [C12MIm+][Br−], which was the IL completely soluble in water that has the longest alkyl chain. The long chain of the IL allowed the formation of hemimicelles and admicelles on the surface of silica particles when high concentrations of the IL were used, which improved the extraction efficiency towards the low-polar target analytes. Although more hydrophobic ILs with longer alkyl chains were tested, the extraction efficiency decreased due to their lower solubility, which reduces the amount of IL available during the composite preparation in aqueous media.
In a less extended way, Si-ILs composites have also been used in the form of silica-IL aerogel [73] and mesoporous silica-IL [152], both types as SPME coatings. For the fabrication of the device, the composite is immobilized on the stainless-steel fiber by using an adhesive and dipping the support in the dry sorbent [73,152]. Regarding the aerogel-based coating, the Si-IL composite was prepared by the Stöber method forming a gel, and then it was freeze-dried to obtain the aerogel with high porosity as it can be observed in Figure 5A [73]. A bipyridine-based dicationic IL containing PTMS groups was used to improve the chemical linkage between the IL and the silica monomers to form the polymer. Aerogels present highly porous, amorphous, and light structures, being obtained by crosslinking colloidal particles, such as silica particles in this case. Authors demonstrated that the modification of the silica aerogel surface using ILs not only improved the extraction performance in comparison with the neat aerogel as shown in Figure 5B, but also the mechanical strength of the material. Thus, the SPME fiber could be reused at least 120 times in a direct-immersion SPME method for the extraction of PAHs, BTEX, and alkanes in cigarettes smoke and cigarettes ashes.
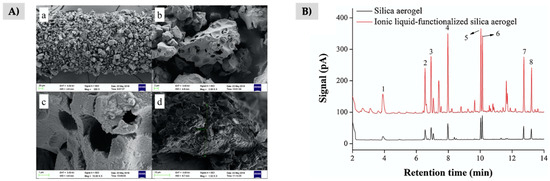
Figure 5.
(A) SEM of the SPME fibers prepared using a Si-IL aerogel, and (B) chromatograms comparing the extraction performance of the Si-IL aerogel and the neat silica aerogel-based SPME fibers for the extraction of 8 PAHs. Reproduced from [73], with permission from Elsevier, 2019.
6. Metal-Organic Framework Based-Ionic Liquid Composites (MOF-ILs)
Metal-organic frameworks (MOFs) are a class of highly ordered three dimensional porous crystalline materials formed by inorganic secondary buildings (SBUs) and organic SBUs connected through strong coordination bonds [156]. The inorganic SBUs are formed by metal ions or metallic clusters with a defined coordination sphere, which sets the geometrical disposition of the organic SBUs while acting as nodes of the network. On the other hand, organic SBUs are molecules that connect the metallic centers to form the ordered network and are so called linkers. The metal center, the linker and the synthesis conditions used for the preparation of the material, determine the properties of the MOF, including porosity and stabilities [157,158].
MOFs have attracted an increasing attention due to the fascinating properties associated with their porous structure that makes them the material with the highest surface areas known so far. This property is associated with high sorption capacity and large number of active sites where different molecules can be hosted [158]. In addition, MOFs have high thermal stability, good chemical stability, and they are easily tunable. The number of different possible combinations of metal nodes and organic linkers make these materials highly versatile for their use in diverse applications including sensing [159], gas storage [160], fuel cells [161], biomedical applications [162], and as sorbents in sample preparation [163,164].
Regarding the use of MOFs in analytical sample preparation strategies, its application is mainly due to their porosity and topological diversity since it is an ideal environment to confine the analytes of interest [163,164]. In this sense, they have been successfully applied as sorbents in different sorbent-based extraction methods [165,166,167,168]. However, it has been claimed that water and moisture stability are one of the main drawbacks of MOFs, but in the vast library of MOFs there is an impressive number of these structures stable enough to be used for the analysis of aqueous samples. The water stability of a MOF is influenced by the hydrophobicity of the framework, the inertness of the coordinative metal-linker bond against hydrolysis, and the lability of the coordination bonds. There are two main strategies to improve the stability of MOFs: (i) the synthesis of thermodynamic stable MOFs by controlling the designing step, and (ii) performing a post-synthesis modification of the surface and the cavities of the crystal by introducing a material that improves their water stability [169].
The properties associated to ILs make these materials highly desirable to perform post-synthesis modification of MOFs to increase the interactions between the sorbent and the guest molecules and even to improve stability in several cases [76,77,101,170,171,172,173]. The combination of ILs and MOFs can be done by physical interactions or by chemical bonding, depending on the preparation procedure. In any case, it is generally accepted that there is a strong interaction between the anion moiety of ILs and metal clusters of MOFs. However, it is important to point out that the hybridization of MOFs and ILs is a field within material science that is starting to be addressed from a critical point of view to prepare of materials with an added value [26].
The preparation of MOF-ILs composites by physical interactions is mainly done by the impregnation method. The MOF and the IL are prepared independently and then, they are mixed in a determined ratio under continuous stirring or applying ultrasounds. Next, the material is washed several times to remove the non-retained IL in the MOF [76,77,101,173]. Despite the introduction of the ILs on the MOF cavities reduce the sorption capability of the initial solid material, at the same time it increases the extraction efficiency due to the stronger and more selective interactions analyte-sorbent [76,77,173]. During the preparation of these materials, it is important to control the MOF/IL ratio since a high content of IL can produce a low dispersibility of the MOF during the material preparation, leading to a lower content of IL in the composite and lower analytical efficiencies [76,101].
The MOFs that have been used to form these IL composites include MIL-100(Fe) [173], ZIF-8(Zn) [76,101], and ZIF-67(Co) [77], in combination with the ILs [C4MIm+][Cys−] (with Cys for L-cysteine), [C8MIm+][NTf2−], [C6Mim+][Cl−], and [C6Mim+][PF6−].
Huang et al. synthetized and evaluated the effect of hydrophobic [CnMIm+] ILs with different lengths of the alkyl chain and different anions for the extraction of insecticides from tea infusions. It was observed that these factors did not exert a great influence on the extraction efficiency of the analytes, but the extraction efficiency of the composite was higher in comparison with the sorbent without the IL [76].
In addition, MOFs-ILs have been combined with magnetic materials, such as Fe3O4 MPs [76] and magnetic MWCNTs [77,101]. In the last case, the combination of the three materials resulted in an increase on the extraction capability since both the carbon material and the MOF provide a high surface area, while the IL enhanced the interaction forces between the analytes and the sorbent [77].
Table 1 shows some representative examples of analytical applications using MOF-ILs sorbents. These composites prepared by physical interactions have been applied in µ-dSPE [76,173] and its magnetic-assisted version (m-µ-dSPE) [77,101] for the determination of insecticides, pesticides, PAHs, and a serine protease, in tea infusions, animal organs, water, vegetables, and fruit juice. Although the excellent analytical performance offered by MOFs-ILs prepared by physical interactions, they suffer from leaching of the IL after several uses due to the MOF-IL bonds lacking hampering the homogeneity of the material [77].
Regarding the immobilization of ILs by chemical bonds, it can be carried out by several approaches to form stable bonds between the MOF and the IL. The encapsulation of ILs in MOF is the main procedure followed for the preparation of MOF-ILs composites as alternative to those based on physical interactions [170,171,172]. In the encapsulation approach, the MOF acts as a host and the IL as a guest. However, this procedure is challenging since, in general, the geometrical size of the ILs is larger than the MOF windows for getting trapped inside the pores, which hinder the encapsulation efficiency [26,174]. For this reason, the ship-in-bottle (SIB) strategy is the ideal procedure to achieve the preparation of these composites. In this case, the smaller reagents required to synthesize the IL diffuse into the pores of the MOF by stirring and/or heating. Then, the synthesis of the IL takes place by dispersing the modified MOF in a solution containing the remaining reagents required to form the IL [26,174]. The amount of IL loaded inside the MOF cavities plays an important role on the material efficiency since an excess of IL can dramatically reduce the extraction capability due to the saturation of the pores of the MOF [170]. The MOFs used in this strategy were ZIF-8(Zn) [172] and MIL-101(Cr) [170] in combination with [C4MIm+][Br−] and [C3Tr+][PF6−] (with Tr for tropine), and used for the determination of alkaloids and herbicides in tablets and waters, respectively. In the case of the ZIF-8(Zn)-[C4MIm+][Br−] composite, a final step of pyrolysis is performed to get a highly porous carbonaceous derived material. Therefore, the MOF structure and the IL are destroyed during the carbonization step, thus losing the main structure and properties of both individual materials [172]. The SIB protocol has also been used for the preparation of a MIPIL in combination with the MOF ZIF-67(Co) (Figure 6A), which was packed in a SPE cartridge [171]. In this case, authors demonstrated the presence of the IL in the composite is the main responsible of the extraction of aristolochic acid from herbal plants, while the incorporation of the recognition sites with the molecular imprinting leads to quantitative extractions, as shown in Figure 6B. Despite the MOF could extract small amounts of the target analyte, it seems it was mainly acting as support to provide a high surface area for the final composite material.
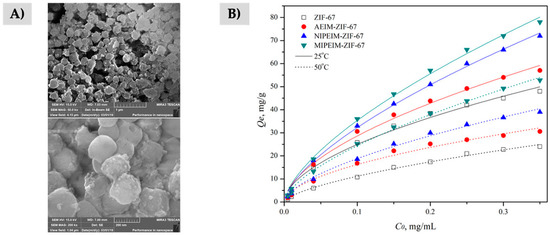
Figure 6.
(A) Scanning electron microscopy images of the synthetized MIPIL-ZIF-67(Co), and (B) Comparison study of the relationship between the initial concentration of analytes and the amount adsorbed when using neat ZIF-67(Co) (white), modified IL-ZIF-67(Co) (red), non-imprinted PIL-ZIF-67(Co) (blue), and MIPIL-ZIF-67(Co) (green). Adapted from [171], with permissions of John Wiley and Sons, Ltd., 2019.
7. Concluding Remarks
ILs are one of the most used materials in analytical sample preparation strategies due to their solvation properties, high extraction capacity, tunable characteristics, and adaptability, which makes their combination with different materials easy. They have been mainly used as modifiers of the surface properties of solid materials to enhance the extraction efficiency and selectivity by incorporating new interactions towards the target analytes, such as electrostatic, π-π, hydrogen bonding, and hydrophilic and hydrophobic interactions. The immobilization of ILs and derivatives on the surface of solid materials also enhances the mass transfer of the analytes and speeds up the extraction procedure due to the increased surface area of the resulting composite material. In this sense, the IL is usually the main component responsible for the extraction in these solid composites.
Apart from surface modification, ILs have also been used with other materials to prepare more complex and interesting structures, including the formation of MIPs using IL as monomers, polymers with silica materials, or bucky gels with carbon materials. In these cases, the IL material not only participates in the extraction of the substances of interest, but also play an important role on the resulting characteristics of the composite material. Thus, the use of ILs also improves the dispersion of solid materials to prepare more homogeneous sorbents and enhances the porosity and permeability of the composites.
However, it is important to highlight that ongoing and future studies should include a comparison of the extraction performance of the individual components and the final composite to clarify the necessity and usefulness of all the materials incorporated in the sorbent. This study is particularly interesting when dealing with composites prepared with materials that have an important extraction capacity by themselves, such as carbonaceous materials or MOFs in combination with ILs. In general, this type of studies is scarcely reported in the literature.
Moreover, practically all the applications described in the last years use conventional imidazolium-based ILs with different alkyl chains, mainly methyl and alkyl chains with 4–16 carbon atoms. Despite some authors have studied the influence of the IL structure on the characteristics or extraction performance of the resulting composite, these studies limit to the effect of different lengths of the alkyl chain and different anions. Therefore, the tunability of ILs is barely explored in this field. Given the great variety of ILs that are possible to prepare and the easily functionalization of ILs, it is important to move in this direction to prepare selective sorbents with specific properties for certain applications. We believe it is important to find a rationale behind the selection of specific ILs and exploit the potential of ILs for the preparation of the composite considering the limitations of both individual materials and the intended application.
Author Contributions
Conceptualization: A.G.-S., I.P.-F. and V.P.; investigation: A.G.-S., P.I.N.-T., J.Š. and I.P.-F.; resources: V.P.; writing—original draft preparation: A.G.-S., P.I.N.-T., J.Š. and I.P.-F.; writing—review and editing: V.P.; supervision: V.P.; project administrator: V.P.; funding acquisition: V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy, Industry and Competitiveness, grant number MAT2017-89207-R.
Acknowledgments
A.G.-S. and I.P.-F. thank the Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI), co-funded by the European Social Fund, for their FPI PhD fellowships. P.I.N.-T. thanks her Collaboration Fellowship with the Spanish Ministry of Education and Vocational Training (MEFP). J.Š. thanks the Erasmus + Programme. V.P. thanks the Spanish Ministry of Economy and Competitiveness (MINECO) for the Project Ref. MAT2017-89207-R. This article is based upon work from the Sample Preparation Task Force and Network supported by the Division of Analytical Chemistry of the European Chemical Society.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| [Ac−] | Acetate |
| [AQSO3−] | 1-Anthraquinonesulfonate |
| [BF4−] | Tetrafluoroborate |
| [CnCmIm+] | Di-alkylimidazolium, being n and m the number of carbon atoms in the chains |
| [CnGu+] | Alkylguanidinium, being n the number of carbon atoms in the chain |
| [CnIm+] | Alkylimidazolium, being n the number of carbon atoms in the chain |
| [NTf2−] | Bis(trifluoromethanesulfonyl)imide |
| [PF6−] | Hexafluorophosphate |
| [Tos−] | Tosylate |
| A | Allyl chain |
| AT-FAAS | Atom trapping flame atomic absorption spectroscopy |
| BTEX | Benzene, toluene, ethylbenzene and xylene |
| C-IL | Composite of carbon materials and ionic liquids |
| CD | Cyclodextrin |
| CNTs | Carbon nanotubes |
| DABCO | 1-4-Diazabicyclo[2.2.2] octane |
| DAD | Diode array detector |
| DLLME | Dispersive liquid-liquid microextraction |
| ED | Ethylene dimethacrylate |
| ET-AAS | Electrothermal atomic absorption spectroscopy |
| FD | Fluorescence detector |
| FI-HG-AFS | Flow-injection hydride generation atomic fluorescence spectroscopy |
| FID | FLAME ionization detector |
| G | Graphene |
| GC | Gas chromatography |
| GO | Graphene oxide |
| IL | Ionic liquid |
| LC | Liquid chromatography |
| LOQ | Limit of quantification |
| LPME | Liquid-phase microextraction |
| M | Methyl chain |
| m-µ-dSPE | Magnetic-assisted miniaturized dispersive solid-phase extraction |
| MCM-48 | Mobil Composition of Matter No. 48 (a type of mesoporous silica) |
| MCNPs | Magnetic cellulose nanoparticles |
| MEM in tube SPME | Magnetism enhanced monolith-based in-tube solid-phase microextraction |
| MET | Magnetic effervescent tablet |
| METM | Magnetic effervescent tablet-assisted microextraction |
| mGO | Magnetic graphene oxide |
| MIL | Materials Institute of Lavoisier (a type of metal-organic framework) |
| MIP | Molecularly imprinted polymer |
| MIPIL | Molecularly imprinted polymeric ionic liquid |
| MOF | Metal-organic frameworks |
| MOF-IL | Composite of metal-organic framework and ionic liquids |
| MP | Magnetic particle |
| MP-IL | Composite of magnetic particles and ionic liquids |
| MPE | Multi-phase extraction |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| MWCNTs | Multi-walled carbon nanotubes |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OMS | Ordered mesoporous silica |
| PAEs | Phthalic acid esters |
| PAHs | Polycyclic aromatic hydrocarbons |
| PBDEs | Polybrominated diphenyl ethers |
| PIL | Polymeric ionic liquid |
| Pol-IL | Composite of polymers and ionic liquids |
| polyCD | Poly-cyclodextrin |
| PSS | Polystyrenesulfonate sodium |
| PTMS | Propyltrimethoxysilane |
| rGO | Reduced graphene oxide |
| RSD | Relative standard deviation |
| SBU | Secondary building unit |
| SEM | Scanning electron microscopy |
| Si-IL | Composite of silica materials and ionic liquids |
| SIB | Ship-in-bottle |
| SLTPE | Solid-liquid trap phase extraction |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
| SWCNTs | Single-walled carbon nanotubes |
| TDI | Toluene-2,4-diisocyanate |
| TEM | Transmission electron microscopy |
| TEOS | Tetraethyl ortosilicate |
| UV | Ultra-violet detector |
| Vi | Vinyl chain |
| VWD | Variable wavelength detector |
| ZIF | Zeolitic imidazolate framework (a type of metal-organic framework) |
| µ-dSPE | Miniaturized dispersive solid-phase extraction |
| µ-SPE | Miniaturized solid-phase extraction |
References
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.; Khashei, F. Density, surface tension and glass transition temperature of series of mono-, di-, and tri-cationic imidazolium-based ionic liquids-A predictive approach. Fluid Phase Equilib. 2018, 460, 135–145. [Google Scholar] [CrossRef]
- Nacham, O.; Martín-Pérez, A.; Steyer, D.J.; Trujillo-Rodríguez, M.J.; Anderson, J.L.; Pino, V.; Afonso, A.M. Interfacial and aggregation behavior of dicationic and tricationic ionic liquid-based surfactants in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 224–234. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Pino, V.; Ayala, J.H.; Afonso, A.M. Ionic liquid-based surfactants: A step forward. In Ionic Liquid Devices, Smart Materials No. 28, 1st ed.; Eftekhari, A., Ed.; The Royal Society of Chemistry: London, UK, 2018; pp. 53–78. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem.-Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Zia ul Mustafa, M.; bin Mukhtar, H.; Md Nordin, N.A.H.; Mannan, H.A.; Nasir, R.; Fazil, N. Recent Developments and Applications of Ionic Liquids in Gas Separation Membranes. Chem. Eng. Technol. 2019, 42, 2580–2593. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.S. Ionic liquid-based electrolytes for energy storage devices: A brief review on their limits and applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: A review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef]
- Nogueira, A.P.P.C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, M. Recent advances of ionic liquids in sample preparation. Trends Anal. Chem. 2020, 125, 115833. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Fernández, I.; Pino, V. Extraction with ionic liquids-Organic Compounds. In Liquid-Phase Extraction from Handbooks in Separation Science, 1st ed.; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 499–537. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X.; Chen, L. Recent development and applications of poly (ionic liquid)s in microextraction techniques. Trends Anal. Chem. 2019, 112, 123–134. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Pino, V.; Miró, M. High-throughput microscale extraction using ionic liquids and derivatives: A review. J. Sep. Sci. 2020, 43. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Ning, T.; Yu, J.; Chen, P.; Yu, H.; Wang, J.; Yang, H.; Zhu, S. Recent advances and applications of magnetic nanomaterials in environmental sample analysis. Trends Anal. Chem. 2020, 126, 115864. [Google Scholar] [CrossRef]
- Jon, C.S.; Meng, L.Y.; Li, D. Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. Trends Anal. Chem. 2019, 120, 115641. [Google Scholar] [CrossRef]
- Yavir, K.; Marcinkowski, Ł.; Marcinkowska, R.; Namieśnik, J.; Kloskowski, A. Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: A review. Anal. Chim. Acta 2019, 1054, 1–16. [Google Scholar] [CrossRef]
- Luo, Q.X.; An, B.W.; Ji, M.; Zhang, J. Hybridization of metal-organic frameworks and task-specific ionic liquids: Fundamentals and challenges. Mater. Chem. Front. 2018, 2, 219–234. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pino, V. Extraction methods facilitated by the use of magnetic nanoparticles. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino, V., Stalcup, A.M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 5, pp. 1681–1723. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Hu, L.; Li, Y.; Luo, D.; Mei, S. Recent applications of magnetic composites as extraction adsorbents for determination of environmental pollutants. Trends Anal. Chem. 2019, 119, 115611. [Google Scholar] [CrossRef]
- Baker, I. Magnetic nanoparticle synthesis. In Nanobiomaterials: Nanostructured Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Elsevier: Duxford, UK, 2018; pp. 197–229. [Google Scholar] [CrossRef]
- Xiao, D.; Lu, T.; Zeng, R.; Bi, Y. Preparation and highlighted applications of magnetic microparticles and nanoparticles: A review on recent advances. Microchim. Acta 2016, 183, 2655–2675. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Yan, Y.; Dong, Y.; Shen, X.; Huang, C. Application of magnetic particles in forensic science. Trends Anal. Chem. 2019, 121, 115674. [Google Scholar] [CrossRef]
- Alves, M.N.; Miró, M.; Breadmore, M.C.; Macka, M. Trends in analytical separations of magnetic (nano)particles. Trends Anal. Chem. 2019, 114, 89–97. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Wang, M.; Xiong, C.; Xie, D.; Chen, Q.; Chu, X.; Qiu, X.; Li, Y.; Xiao, X. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Ríos, Á.; Guzmán Bernardo, F.J.; Rodríguez Martín-Doimeadios, R.C. Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem. J. 2018, 137, 490–495. [Google Scholar] [CrossRef]
- Jamshidi, S.; Rofouei, M.K.; Thorsen, G. Using magnetic core-shell nanoparticles coated with an ionic liquid dispersion assisted by effervescence powder for the micro-solid-phase extraction of four beta blockers from human plasma by ultra high performance liquid chromatography with mass spectrom. J. Sep. Sci. 2019, 42, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hou, X.; Huang, P.; Jiang, C.; Chen, L.; Zhao, L. Ionic liquid-based dispersive liquid-liquid microextraction combined with functionalized magnetic nanoparticle solid-phase extraction for determination of industrial dyes in water. Sci. Rep. 2017, 7, 13844. [Google Scholar] [CrossRef]
- Sahebi, H.; Konoz, E.; Ezabadi, A.; Niazi, A.; Ahmadi, S.H. Simultaneous determination of five penicillins in milk using a new ionic liquid-modified magnetic nanoparticle based dispersive micro-solid phase extraction followed by ultra-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2020, 154, 104605. [Google Scholar] [CrossRef]
- Bahrololoomi Fard, S.M.; Ahmadi, S.H.; Hajimahmodi, M.; Fazaeli, R.; Amini, M. Preparation of magnetic iron oxide nanoparticles modified with imidazolium-based ionic liquids as a sorbent for the extraction of eight phthalate acid esters in water samples followed by UPLC-MS/MS analysis: An experimental design methodology. Anal. Methods 2019, 12, 73–84. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Hassanlouei, S.; Zamani-Kalajahi, M. Preparation of ionic liquid-modified SiO2@Fe3O4 nanocomposite as a magnetic sorbent for use in solid-phase extraction of zinc(II) ions from milk and water samples. RSC Adv. 2017, 7, 23293–23300. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Yan, Y.; Tang, K.; Ding, C.F. Self-assembly of poly(ionic liquid) functionalized mesoporous magnetic microspheres for the solid-phase extraction of preservatives from milk samples. J. Sep. Sci. 2020, 43, 766–773. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, D.; Chu, H.; Wang, C.; Wei, Y. Magnetic mesoporous nanoparticles modified with poly(ionic liquids) with multi-functional groups for enrichment and determination of pyrethroid residues in apples. J. Sep. Sci. 2019, 42, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Llaver, M.; Casado-Carmona, F.A.; Lucena, R.; Cárdenas, S.; Wuilloud, R.G. Ultra-trace tellurium preconcentration and speciation analysis in environmental samples with a novel magnetic polymeric ionic liquid nanocomposite and magnetic dispersive micro-solid phase extraction with flow-injection hydride generation atomic fluoresce. Spectrochim. Acta B 2019, 162, 105705. [Google Scholar] [CrossRef]
- Aflatouni, F.; Soleimani, M. Preparation of a New Polymerized Ionic Liquid-Modified Magnetic Nano Adsorbent for the Extraction and Preconcentration of Nitrate and Nitrite Anions from Environmental Water Samples. Chromatographia 2018, 81, 1475–1486. [Google Scholar] [CrossRef]
- Zhou, S.; Song, N.; Lv, X.; Jia, Q. Preparation of carboxylatocalix[4]arene functionalized magnetic polyionic liquid hybrid material for the pre-concentration of phthalate esters. J. Chromatogr. A 2018, 1565, 19–28. [Google Scholar] [CrossRef]
- Badragheh, S.; Zeeb, M.; Talei Bavil Olyai, M.R. Silica-coated magnetic iron oxide functionalized with hydrophobic polymeric ionic liquid: A promising nanoscale sorbent for simultaneous extraction of antidiabetic drugs from human plasma prior to their quantitation by HPLC. RSC Adv. 2018, 8, 30550–30561. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X. Online analysis of five organic ultraviolet filters in environmental water samples using magnetism-enhanced monolith-based in-tube solid phase microextraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2017, 1525, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, A.A.A.A.; Zhu, X.S. Poly(ionic liquid) immobilized magnetic nanoparticles as sorbent coupled with fluorescence spectrophotometry for separation/analysis of Allura red. J. Mol. Liq. 2017, 242, 900–906. [Google Scholar] [CrossRef]
- Liu, G.; Su, P.; Zhou, L.; Yang, Y. Microwave-assisted preparation of poly(ionic liquids)-modified polystyrene magnetic nanospheres for phthalate esters extraction from beverages. J. Sep. Sci. 2017, 40, 2603–2611. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Huang, X. Fabrication of polymeric ionic liquid-modified magnetic adsorbent for extraction of apolar and polar pollutants in complicated samples. Talanta 2017, 172, 23–30. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Huang, X. Extraction of triazole fungicides in environmental waters utilizing poly (ionic liquid)-functionalized magnetic adsorbent. J. Chromatogr. A 2017, 1524, 13–20. [Google Scholar] [CrossRef]
- He, L.; Cui, W.; Wang, Y.; Zhao, W.; Xiang, G.; Jiang, X.; Mao, P.; He, J.; Zhang, S. Polymeric ionic liquid based on magnetic materials fabricated through layer-by-layer assembly as adsorbents for extraction of pesticides. J. Chromatogr. A 2017, 1522, 9–15. [Google Scholar] [CrossRef]
- Chen, S.; Fu, J.; Fu, Z.; Li, Y.; Su, X.; Zou, L.; He, L.; Liu, S.; Ao, X.; Yang, Y. Preparation and characterization of magnetic molecular imprinted polymers with ionic liquid for the extraction of carbaryl in food. Anal. Bioanal. Chem. 2020, 412, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, J.; Liu, W.; Jin, L.; Zhou, P.; Zhang, Y.; Zhang, B.; Dahlgren, R.A.; Wang, X.; Zhou, Y. Magnetic effervescent tablet-assisted ionic liquid-based dispersive liquid-liquid microextraction of polybrominated diphenyl ethers in liquid matrix samples. Talanta 2019, 195, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Modification of a steel fiber with a graphene based bucky gel for headspace solid-phase microextraction of volatile aromatic hydrocarbons prior to their quantification by GC. Microchim. Acta 2018, 185, 509. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.G.; Chen, W.S.; Cheng, H.L.; Zeng, X.Q.; Zhu, Y. Three-dimensional ionic liquid-ferrite functionalized graphene oxide nanocomposite for pipette-tip solid phase extraction of 16 polycyclic aromatic hydrocarbons in human blood sample. J. Chromatogr. A 2018, 1552, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanloo, H.; Karamzadeh, Z.; Rakhtshah, J.; Kazemi, N.M. A novel biostructure sorbent based on CysSB/MetSB@MWCNTs for separation of nickel and cobalt in biological samples by ultrasound assisted-dispersive ionic liquid-suspension solid phase micro extraction. J. Pharm. Biomed. Anal. 2019, 172, 285–294. [Google Scholar] [CrossRef]
- Hamid Shirkhanloo; Kheirolnesa Merchant; Mostafa Dehghani Mobarake Ultrasound-assisted Solid-liquid Trap Phase Extraction based on Functionalized Multi Wall Carbon Nanotubes for Preconcentration and Separation of Nickel in Petrochemical Waste Water. J. Anal. Chem. 2019, 74, 865–876. [CrossRef]
- Liu, W.; Zha, J.; Meng, X.; Wu, J.; Sun, C.; Qiu, P.; Fizir, M.; He, H. Efficient removal and trace determination of chlorophenols from water using mixed hemi-/ad-micelle ionic liquid-coated magnetic graphene oxide and the adsorption mechanism. Anal. Methods 2017, 9, 4581–4591. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Row, K.H. Magnetic graphene oxide modified by imidazole-based ionic liquids for the magnetic-based solid-phase extraction of polysaccharides from brown alga. J. Sep. Sci. 2017, 40, 3301–3310. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Sun, Y.; Niu, C.; Qiao, F.; Yan, H. An ionic liquid-magnetic graphene composite for magnet dispersive solid-phase extraction of triazine herbicides in surface water followed by high performance liquid chromatography. Analyst 2018, 143, 175–181. [Google Scholar] [CrossRef]
- Cao, S.; Chen, J.; Lai, G.; Xi, C.; Li, X.; Zhang, L.; Wang, G.; Chen, Z. A high efficient adsorbent for plant growth regulators based on ionic liquid and β-cyclodextrin functionalized magnetic graphene oxide. Talanta 2019, 194, 14–25. [Google Scholar] [CrossRef]
- Liu, M.; Ding, X.; Wang, X.; Li, J.; Yang, H.; Yin, Y. Extraction of DNA from complex biological sample matrices using guanidinium ionic liquid modified magnetic nanocomposites. RSC Adv. 2019, 9, 23119–23128. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Wang, N.; Ni, X.; Wang, H.; Gu, Z.; Wu, X.; Xu, W. Synthesis and Evaluation of Ionic Liquid–Mediated Molecularly Imprinted Polymer for Highly Selective Recognition of Dibutyl Phthalate from Liquor Samples. Adv. Polym. Technol. 2017, 36, 220–229. [Google Scholar] [CrossRef]
- Chen, L.; Huang, X. Preparation and application of a poly (ionic liquid)-based molecularly imprinted polymer for multiple monolithic fiber solid-phase microextraction of phenolic acids in fruit juice and beer samples. Analyst 2017, 142, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, G.; Wang, L.; Yu, W.; Wang, P.; Fan, J. A new ionic liquid surface-imprinted polymer for selective solid-phase-extraction and determination of sulfonamides in environmental samples. J. Sep. Sci. 2019, 42, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, W.; Wang, L.; Wang, P.; Shi, D.; Wang, J.; Fan, J. Using ionic liquid monomer to improve the selective recognition performance of surface imprinted polymer for sulfamonomethoxine in strong polar medium. J. Chromatogr. A 2019, 1592, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xie, J.; Lin, L.; Tian, M.; Row, K.H. Multi-phase extraction of ephedrine from Pinellia ternata and herbal medicine using molecular imprinted polymer coated ionic liquid-based silica. Phytochem. Anal. 2020, 31, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Boon, Y.H.; Mohamad Zain, N.N.; Mohamad, S.; Osman, H.; Raoov, M. Magnetic poly(β-cyclodextrin-ionic liquid) nanocomposites for micro-solid phase extraction of selected polycyclic aromatic hydrocarbons in rice samples prior to GC-FID analysis. Food Chem. 2019, 278, 322–332. [Google Scholar] [CrossRef]
- Tian, M.; Yan, X.; Xiao, W.; Lin, L. The investigation of adsorption behaviors of nine ionic liquid-immobilized silicas on two phenols in water and soil samples. Mater. Sci. 2020, 26, 10–19. [Google Scholar] [CrossRef]
- Tian, Y.; Feng, J.; Wang, X.; Luo, C.; Sun, M. Ionic liquid-functionalized silica aerogel as coating for solid-phase microextraction. J. Chromatogr. A 2019, 1583, 48–54. [Google Scholar] [CrossRef]
- Fang, L.; Tian, M.; Yan, X.; Xiao, W.; Row, K.H. Dual ionic liquid-immobilized silicas for multi-phase extraction of aristolochic acid from plants and herbal medicines. J. Chromatogr. A 2019, 1592, 31–37. [Google Scholar] [CrossRef]
- Dai, X.; Wang, D.; Li, H.; Chen, Y.; Gong, Z.; Xiang, H.; Shi, S.; Chen, X. Hollow porous ionic liquids composite polymers based solid phase extraction coupled online with high performance liquid chromatography for selective analysis of hydrophilic hydroxybenzoic acids from complex samples. J. Chromatogr. A 2017, 1484, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Liu, H.; Liu, G.; Xu, X.; Li, L.; Lv, J.; Gao, H.; Xu, D. Magnetic solid-phase extraction of pyrethroid insecticides from tea infusions using ionic liquid-modified magnetic zeolitic imidazolate framework-8 as an adsorbent. RSC Adv. 2019, 9, 39272–39281. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Chen, J.; Xu, P.; Zhou, Y. Preparation of ionic liquid modified magnetic metal-organic frameworks composites for the solid-phase extraction of α–chymotrypsin. Talanta 2018, 182, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, Y.; Zhang, B.; Chang, R.; Luo, D.; Yang, L.; Yang, Y.; Liu, D. Magnetically stabilized bed packed with synthesized magnetic silicone loaded with ionic liquid particles for efficient enrichment of flavonoids from tree peony petals. J. Chromatogr. A 2020, 1613, 460671. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; Jia, Y.; Xi, X.; Yang, X.; Lu, R.; Zhang, S.; Gao, H.; Zhou, W. Use of magnetic effervescent tablet-assisted ionic liquid dispersive liquid-liquid microextraction to extract fungicides from environmental waters with the aid of experimental design methodology. Anal. Chim. Acta 2016, 906, 118–127. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Z.; Pan, Y.; Shi, Y.; Bao, X.; Li, J.; Tong, Y.; Tang, H.; Ma, S.; Wang, X.; et al. Combination of in situ metathesis reaction with a novel “magnetic effervescent tablet-assisted ionic liquid dispersive microextraction” for the determination of endogenous steroids in human fluids. Anal. Bioanal. Chem. 2018, 410, 2921–2935. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, K.; Gao, M.; Qu, J.; Zhang, Z.; Dahlgren, R.A.; Li, Y.; Liu, W.; Huang, H.; Wang, X. Magnetic effervescent tablets containing ionic liquids as a non-conventional extraction and dispersive agent for determination of pyrethroids in milk. Food Chem. 2018, 268, 468–475. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Chen, Y.; Bao, X.; Tang, H.; Ma, S.; Zhou, S.; Xu, M.; Tao, J.; Wang, W.; et al. Preconcentration/Extraction of Phthalate Esters in Milk Samples Using MFe2O4-Based Magnetic Ionic Liquid Effervescent Tablets Consisting of Accessory Functional Fillers. Food Anal. Methods 2019, 12, 2106–2119. [Google Scholar] [CrossRef]
- Fan, C.; Yang, J.; Liang, Y.; Dong, H.; Zhang, W.; Tang, G.; Cao, Y. Effective tuning guanidinium ionic liquid as greener solvent for fast and sensitive determination of auxin herbicides. Microchem. J. 2019, 144, 73–82. [Google Scholar] [CrossRef]
- Du, W.; Yao, L.; Bian, J.; Liu, Y.; Wang, X.; Zhang, J.; Pang, L. Ionic liquid-based air-assisted liquid-liquid microextraction combined with dispersive micro-solid phase extraction for the preconcentration of copper in water samples. Anal. Methods 2018, 10, 3032–3038. [Google Scholar] [CrossRef]
- Yao, L.; Wang, X.; Liu, H.; Lin, C.; Pang, L.; Yang, J.; Zeng, Q. Optimization of ultrasound-assisted magnetic retrieval-linked ionic liquid dispersive liquid–liquid microextraction for the determination of cadmium and lead in water samples by graphite furnace atomic absorption spectrometry. J. Ind. Eng. Chem. 2017, 56, 321–326. [Google Scholar] [CrossRef]
- Fan, C.; Liang, Y.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Wang, D.; Cao, Y. In-situ ionic liquid dispersive liquid-liquid microextraction using a new anion-exchange reagent combined Fe3O4 magnetic nanoparticles for determination of pyrethroid pesticides in water samples. Anal. Chim. Acta 2017, 975, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, J.; Zhou, W.; Lu, R.; Gao, H.; Zhang, S. In situ solvent formation microextraction combined with magnetic dispersive micro-solid-phase extraction for the determination of benzoylurea insecticides in water samples. J. Sep. Sci. 2017, 40, 442–448. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Liu, L.; Cheng, C.; Yang, Y. Ionic Liquid-Based Surfactant Extraction Coupled with Magnetic Dispersive μ-Solid Phase Extraction for the Determination of Phthalate Esters in Packaging Milk Samples by HPLC. Food Anal. Methods 2017, 10, 1745–1754. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, B.; Chen, X.; Wang, B.; Zhang, H.; Zhang, X. Long-chain ionic liquid based mixed hemimicelles and magnetic dispersed solid-phase extraction for the extraction of fluorescent whitening agents in paper materials. J. Sep. Sci. 2017, 40, 2459–2466. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, K.; Liu, F.; Wu, X.; Yang, M.; Li, J.; Gao, H.; Zhang, S.; zhou, W.; Lu, R. Magnetic mixed hemimicelles dispersive solid-phase extraction based on ionic liquid-coated attapulgite/polyaniline-polypyrrole/Fe3O4 nanocomposites for determination of acaricides in fruit juice prior to high-performance liquid chromatography-diode array. Talanta 2017, 166, 93–100. [Google Scholar] [CrossRef]
- Yao, L.; Liu, H.; Wang, X.; Xu, W.; Zhu, Y.; Wang, H.; Pang, L.; Lin, C. Ultrasound-assisted surfactant-enhanced emulsification microextraction using a magnetic ionic liquid coupled with micro-solid phase extraction for the determination of cadmium and lead in edible vegetable oils. Food Chem. 2018, 256, 212–218. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesssis, Properties and Applicationss. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Aladaghlo, Z.; Fakhari, A.R.; Alavioon, S.I.; Dabiri, M. Ultrasound assisted dispersive solid phase extraction of triazole fungicides by using an N-heterocyclic carbene copper complex supported on ionic liquid-modified graphene oxide as a sorbent. Microchim. Acta 2019, 186, 209. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, Z.; Mousavi, H.Z.; Maryam Sajjadi, S. Magnetic carbon nanotubes modified with 1,4-diazabicyclo[2.2.2] octane are a viable sorbent for extraction of selective serotonin reuptake inhibitors. Microchim. Acta 2017, 184, 1427–1436. [Google Scholar] [CrossRef]
- Hosseini, F.; Shirkhanloo, H.; Kazemi, N.M. Nano Analysis in Biochemistry: In Vitro Separation and Determination of Aluminium in Blood of Dialysis Patients Based on Graphene Oxide Nanoparticles Dispersed to Ionic Liquid. J. Nanoanal. 2017, 4, 99–109. [Google Scholar] [CrossRef]
- Hamidi, S.; Azami, A.; Mehdizadeh Aghdam, E. A novel mixed hemimicelles dispersive micro-solid phase extraction using ionic liquid functionalized magnetic graphene oxide/polypyrrole for extraction and pre-concentration of methotrexate from urine samples followed by the spectrophotometric method. Clin. Chim. Acta 2019, 488, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, S.; Rofouei, M.K.; Seidi, S.; Emmer, Å. Applicability of a magnetic bucky gel for microextraction of mercury from complicated matrices followed by cold vapor atomic absorption spectroscopy. Sep. Sci. Technol. 2020, 55, 1505–1514. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Li, X.; Wang, H.; Ji, X.; Zhang, Z. Determination of microcystins in environmental water samples with ionic liquid magnetic graphene. Ecotoxicol. Environ. Saf. 2019, 176, 20–26. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Liu, H.; Liu, G.; Xu, X.; Li, L.; Lv, J.; Liu, Z.; Zhou, W.; Xu, D. Magnetic Solid-Phase Extraction of Dichlorodiphenyltrichloroethane and Its Metabolites from Environmental Water Samples Using Ionic Liquid Modified Magnetic Multiwalled Carbon Nanotube/Zeolitic Imidazolate Framework-8 as Sorbent. Molecules 2019, 24, 2758. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Y.; Zhang, L.; Xi, C.; Li, X.; Tang, B.; Wang, G.; Chen, Z. Designed multifunctional ionic liquids-magnetic graphene nanocomposites as the adsorbent of MSPE for the determination of preservatives. Anal. Methods 2018, 10, 1420–1430. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Jamshidi, S.; Seidi, S.; Saleh, A. A bucky gel consisting of Fe3O4 nanoparticles, graphene oxide and ionic liquid as an efficient sorbent for extraction of heavy metal ions from water prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 3425–3432. [Google Scholar] [CrossRef]
- Nasrollahpour, A.; Moradi, S.M.J.; Moradi, S.E. Dispersive solid phase micro-extraction of mercury(II) from environmental water and vegetable samples with ionic liquid modified graphene oxide nanoparticles. J. Serb. Chem. Soc. 2017, 82, 551–565. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X. Nanoparticles of type Fe3O4-SiO2-graphene oxide and coated with an amino acid-derived ionic liquid for extraction of Al(III), Cr(III), Cu(II), Pb(II) prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 4279–4286. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Zhang, Z.H.; Wu, X.L.; Chen, W.G.; Zhu, Y.; Fang, C.F.; Zhao, Y.G. Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils. J. Chromatogr. A 2017, 1489, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Nie, H.; Yin, J.; Han, Y.; Lv, Y.; Yan, H. Selective extraction and detection of β-agonists in swine urine for monitoring illegal use in livestock breeding. Food Chem. 2020, 313, 126155. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Row, K.H. Simultaneous determination of levofloxacin and ciprofloxacin in human urine by ionic-liquid-based, dual-template molecularly imprinted coated graphene oxide monolithic solid-phase extraction. J. Sep. Sci. 2019, 42, 642–649. [Google Scholar] [CrossRef]
- Erdem, P.; Tashakkori, P.; Merdivan, M.; Seyhan Bozkurt, S. Ionic liquid-based graphene oxide-coated fiber for headspace-solid phase microextraction of polycyclic aromatic hydrocarbons in water samples. Turk. J. Chem. 2019, 43, 1383–1397. [Google Scholar] [CrossRef]
- Tashakkori, P.; Erdem, P.; Merdivan, M.; Bozkurt, S.S. Determination of Phthalate Esters in Water and Coffee by Solid-Phase Microextraction Using Vinyl Terminated Imidazolium Based Ionic Liquid Grafted on Graphene Oxide Coatings. ChemistrySelect 2019, 4, 2307–2313. [Google Scholar] [CrossRef]
- Hou, X.; Lu, X.; Tang, S.; Wang, L.; Guo, Y. Graphene oxide reinforced ionic liquid-functionalized adsorbent for solid-phase extraction of phenolic acids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1072, 123–129. [Google Scholar] [CrossRef]
- Sotolongo, A.C.; Martinis, E.M.; Wuilloud, R.G. An easily prepared graphene oxide-ionic liquid hybrid nanomaterial for micro-solid phase extraction and preconcentration of Hg in water samples. Anal. Methods 2018, 10, 338–346. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Row, K.H. Graphene and Graphene Oxide Modified by Deep Eutectic Solvents and Ionic Liquids Supported on Silica as Adsorbents for Solid-Phase Extraction. Bull. Korean Chem. Soc. 2017, 38, 251–257. [Google Scholar] [CrossRef]
- Chu, F.; Gao, M.; Wang, H.; Li, J.; Zheng, Z.; Wang, X.; Zhang, Z. Development of an effervescent reaction-enhanced microextraction method for preconcentration/extraction of trace estrogens in milk using a reduced graphene oxide-assisted ionic liquid-based nanofluid. Anal. Methods 2019, 11, 3608–3618. [Google Scholar] [CrossRef]
- Braun, D.; Cherdron, H.; Rehahn, M.; Ritter, H.; Voit, B. Polymer Synthesis: Theory and Paractice, 5th ed.; Springer: Heidelberg, Germnay, 2013. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Ulbricht, M. Design and synthesis of organic polymers for molecular separation membranes. Curr. Opin. Chem. Eng. 2020, 28, 60–65. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated polymers as materials of membranes for gas and vapor separation. J. Memb. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Zhang, S.; Yin, R.; Liu, X.; He, Y.; Dai, K.; Shan, C.; Guo, J.; Liu, C.; et al. Electrically conductive polymer composites for smart flexible strain sensors: A critical review. J. Mater. Chem. C 2018, 6, 12121–12141. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Fadillah, G.; Saputra, O.A.; Saleh, T.A. Trends in polymers functionalized nanostructures for analysis of environmental pollutants. Trends Environ. Anal. Chem. 2020, 26, e00084. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Marć, M.; Szczepańska, N.; Namieśnik, J. New Polymeric Materials for Solid Phase Extraction. Crit. Rev. Anal. Chem. 2017, 47, 373–383. [Google Scholar] [CrossRef]
- Santos, H.; Martins, R.O.; Soares, D.A.; Chaves, A.R. Molecularly imprinted polymers for miniaturized sample preparation techniques: Strategies for chromatographic and mass spectrometry methods. Anal. Methods 2020, 12, 894–911. [Google Scholar] [CrossRef]
- Zhu, G.; Li, W.; Wang, P.; Cheng, G.; Chen, L.; Zhang, K.; Li, X. One-step polymerization of hydrophilic ionic liquid imprinted polymer in water for selective separation and detection of levofloxacin from environmental matrices. J. Sep. Sci. 2020, 43, 639–647. [Google Scholar] [CrossRef]
- Jia, M.; Yang, J.; Sun, Y.K.; Bai, X.; Wu, T.; Liu, Z.S.; Aisa, H.A. Improvement of imprinting effect of ionic liquid molecularly imprinted polymers by use of a molecular crowding agent. Anal. Bioanal. Chem. 2018, 410, 595–604. [Google Scholar] [CrossRef]
- Han, Y.; Yang, C.; Zhou, Y.; Han, D.; Yan, H. Ionic Liquid-Hybrid Molecularly Imprinted Material-Filter Solid-Phase Extraction Coupled with HPLC for Determination of 6-Benzyladenine and 4-Chlorophenoxyacetic Acid in Bean Sprouts. J. Agric. Food Chem. 2017, 65, 1750–1757. [Google Scholar] [CrossRef]
- Sun, Y.K.; Sun, G.Y.; Jia, M.; Yang, J.; Liu, Z.S.; Huang, Y.P.; Aisa, H.A. Cost-effective imprinting to minimize consumption of template in room-temperature ionic liquid for fast purification of chlorogenic acid from the extract of E. ulmoides leaves. Anal. Bioanal. Chem. 2019, 411, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Jia, M.; Yang, J.; Huang, Y.P.; Liu, Z.S.; Aisa, H.A. A strategy of utilizing Zn(II) as metallic pivot in room temperature ionic liquid to prepare molecularly imprinted polymers for compound with intramolecular hydrogen bonds. Anal. Bioanal. Chem. 2018, 410, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhang, L.S.; Song, W.F.; Huang, Y.P.; Liu, Z.S. A polymer monolith incorporating stellate mesoporous silica nanospheres for use in capillary electrochromatography and solid phase microextraction of polycyclic aromatic hydrocarbons and organic small molecules. Microchim. Acta 2018, 185, 444. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.J.; Yang, J.; Zhao, Y.X.; Liu, Z.S.; Aisa, H.A. Preparation of ionic liquid-mediated imprinted monolith for selective capture and purification of corilagin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1041–1042, 98–103. [Google Scholar] [CrossRef]
- Ma, W.; Row, K.H. Solid-phase extraction of chlorophenols in seawater using a magnetic ionic liquid molecularly imprinted polymer with incorporated silicon dioxide as a sorbent. J. Chromatogr. A 2018, 1559, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Row, K.H. Magnetic solid-phase extraction with Fe3O4/molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids for the rapid purification of alkaloid isomers (Theobromine and Theophylline) from green tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dai, Q.; Wang, Y.; Hu, X.; Xu, P.; Ni, R.; Meng, J. Creating magnetic ionic liquid-molecularly imprinted polymers for selective extraction of lysozyme. RSC Adv. 2018, 8, 21850–21856. [Google Scholar] [CrossRef]
- Md Yusoff, M.; Raoov, M.; Yahaya, N.; Md Salleh, N. An ionic liquid loaded magnetically confined polymeric mesoporous adsorbent for extraction of parabens from environmental and cosmetic samples. RSC Adv. 2017, 7, 35832–35844. [Google Scholar] [CrossRef]
- Yih Hui, B.; Mohamad Zain, N.N.; Mohamad, S.; Varanusupakul, P.; Osman, H.; Raoov, M. Poly(cyclodextrin-ionic liquid) based ferrofluid: A new class of magnetic colloid for dispersive liquid phase microextraction of polycyclic aromatic hydrocarbons from food samples prior to GC-FID analysis. Food Chem. 2020, 314, 126214. [Google Scholar] [CrossRef]
- Tarannum, N.; Suhani; Kumar, D. Synthesis, characterization and applications of copolymer of β–cyclodextrin: A review. J. Polym. Res. 2020, 27, 89. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110267. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; Del Rio, A.M.; Sánchez-García, D. Functionalized ordered mesoporous silicas (MCM-41): Synthesis and applications in catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef]
- Bagheri, E.; Ansari, L.; Sameiyan, E.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Sensors design based on hybrid gold-silica nanostructures. Biosens. Bioelectron. 2020, 153, 112054. [Google Scholar] [CrossRef] [PubMed]
- Diagboya, P.N.E.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Bapat, G.; Labade, C.; Chaudhari, A.; Zinjarde, S. Silica nanoparticle based techniques for extraction, detection, and degradation of pesticides. Adv. Colloid Interface Sci. 2016, 237, 1–14. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ. Chem. Lett. 2019, 17, 683–696. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, D.; Li, X.; Yu, Y.; Luo, P.; Chen, J.; Dai, C.; Wu, Y. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. Trends Anal. Chem. 2019, 121, 115669. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Qi, J.; Hou, Y.; Wei, X. Graphene-supported ordered mesoporous composites used for environmental remediation: A review. Sep. Purif. Technol. 2020, 239, 116511. [Google Scholar] [CrossRef]
- Ma, M.; Lu, L.; Li, H.; Xiong, Y.; Dong, F. Functional metal organic framework/SiO2 nanocomposites: From versatile synthesis to advanced applications. Polymers 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Zajickova, Z. Advances in the development and applications of organic–silica hybrid monoliths. J. Sep. Sci. 2017, 40, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.; MacHoke, A.; Schwieger, W. Supported ionic liquids on non-porous and porous inorganic materials - A topical review. Appl. Catal. A Gen. 2012, 445–446, 92–101. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, X.; Cui, B. Synthesis of a polymeric imidazolium-embedded octadecyl ionic liquid-grafted silica sorbent for extraction of flavonoids. J. Chromatogr. A 2019, 1606, 460376. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.C.; de Toffoli, A.L.; Maciel, E.V.S.; Lanças, F.M. Online fully automated SPE-HPLC-MS/MS determination of ceftiofur in bovine milk samples employing a silica-anchored ionic liquid as sorbent. Electrophoresis 2018, 39, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Han, Y.; Wu, D.; Qiao, F.; Bai, L.; Wang, Z.; Yan, H. Ionic liquid-organic-functionalized ordered mesoporous silica-integrated dispersive solid-phase extraction for determination of plant growth regulators in fresh Panax ginseng. Talanta 2020, 207, 120247. [Google Scholar] [CrossRef]
- Piryaei, M.; Abolghasemi, M.M.; Karimi, B. Determination and analysis of volatile components from Thymus kotschyanus Boiss with a new solid-phase microextraction fibre and microwave-assisted hydrodistillation by periodic mesoporous organosilica based on alkylimidazolium ionic liquid. Phytochem. Anal. 2019, 30, 193–197. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Osman, M.M.; Yakout, A.A.; Abdelfattah, A.M. Water and soil decontamination of toxic heavy metals using aminosilica-functionalized-ionic liquid nanocomposite. J. Mol. Liq. 2018, 266, 834–845. [Google Scholar] [CrossRef]
- Llaver, M.; Coronado, E.A.; Wuilloud, R.G. High performance preconcentration of inorganic Se species by dispersive micro-solid phase extraction with a nanosilica-ionic liquid hybrid material. Spectrochim. Acta B 2017, 138, 23–30. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Mauro Lanças, F. Evaluation of ionic liquids supported on silica as a sorbent for fully automated online solid-phase extraction with LC–MS determination of sulfonamides in bovine milk samples. J. Sep. Sci. 2018, 41, 2237–2244. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Raza, W.; Kukkar, D.; Saulat, H.; Raza, N.; Azam, M.; Mehmood, A.; Kim, K.H. Metal-organic frameworks as an emerging tool for sensing various targets in aqueous and biological media. Trends Anal. Chem. 2019, 120, 115654. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Guan, J. Applications of metal–organic framework-derived materials in fuel cells and metal-air batteries. Coord. Chem. Rev. 2020, 409, 213214. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Pasán, J.; Ayala, J.H.; Pino, V. The Rise of Metal-Organic Frameworks in Analytical Chemistry. In Handbook of Smart Materials in Analytical Chemistry, 1st ed.; de la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2019; pp. 463–502. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yang, L.; Wang, Z.; Liu, H. Recent advances in applications of metal–organic frameworks for sample preparation in pharmaceutical analysis. Coord. Chem. Rev. 2020, 411, 213235. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Taima-Mancera, I.; Pasán, J.; Ayala, J.H.; Pino, V. Application of MOFs and Their Derived Materials in Solid-Phase Extraction. In Applications of Metal–Organic Frameworks and Their Derived Materials, 1st ed.; Inamuddin Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Scrivener Publising: Beverly, MA, USA, 2020; pp. 219–262. [Google Scholar]
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. Trends Anal. Chem. 2017, 90, 114–134. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Gao, M.; Huang, X.; Xu, D. Recent Advances and Applications of Magnetic Metal-Organic Frameworks in Adsorption and Enrichment Removal of Food and Environmental Pollutants. Crit. Rev. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.; Yao, S. Preconcentration of tropane alkaloids by a metal organic framework (MOF)-immobilized ionic liquid with the same nucleus for their quantitation in Huashanshen tablets. Analyst 2019, 144, 6989–7000. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Tian, M.; Row, K.H.; Yan, X.; Xiao, W. Isolation of aristolochic acid I from herbal plant using molecular imprinted polymer composited ionic liquid-based zeolitic imidazolate framework-67. J. Sep. Sci. 2019, 42, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Ahmed, I.; Jhung, S.H. Adsorptive removal of herbicides from water over nitrogen-doped carbon obtained from ionic liquid@ZIF-8. Chem. Eng. J. 2017, 323, 203–211. [Google Scholar] [CrossRef]
- Nasrollahpour, A.; Moradi, S.E.; Baniamerian, M.J. Vortex-Assisted Dispersive Solid-Phase Microextraction Using Ionic Liquid-Modified Metal-Organic Frameworks of PAHs from Environmental Water, Vegetable, and Fruit Juice Samples. Food Anal. Methods 2017, 10, 2815–2826. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Ionic liquid@MIL-101 prepared via the ship-in-bottle technique: Remarkable adsorbents for the removal of benzothiophene from liquid fuel. Chem. Commun. 2016, 52, 2561–2564. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).