Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
2.2. Cosmetic Samples
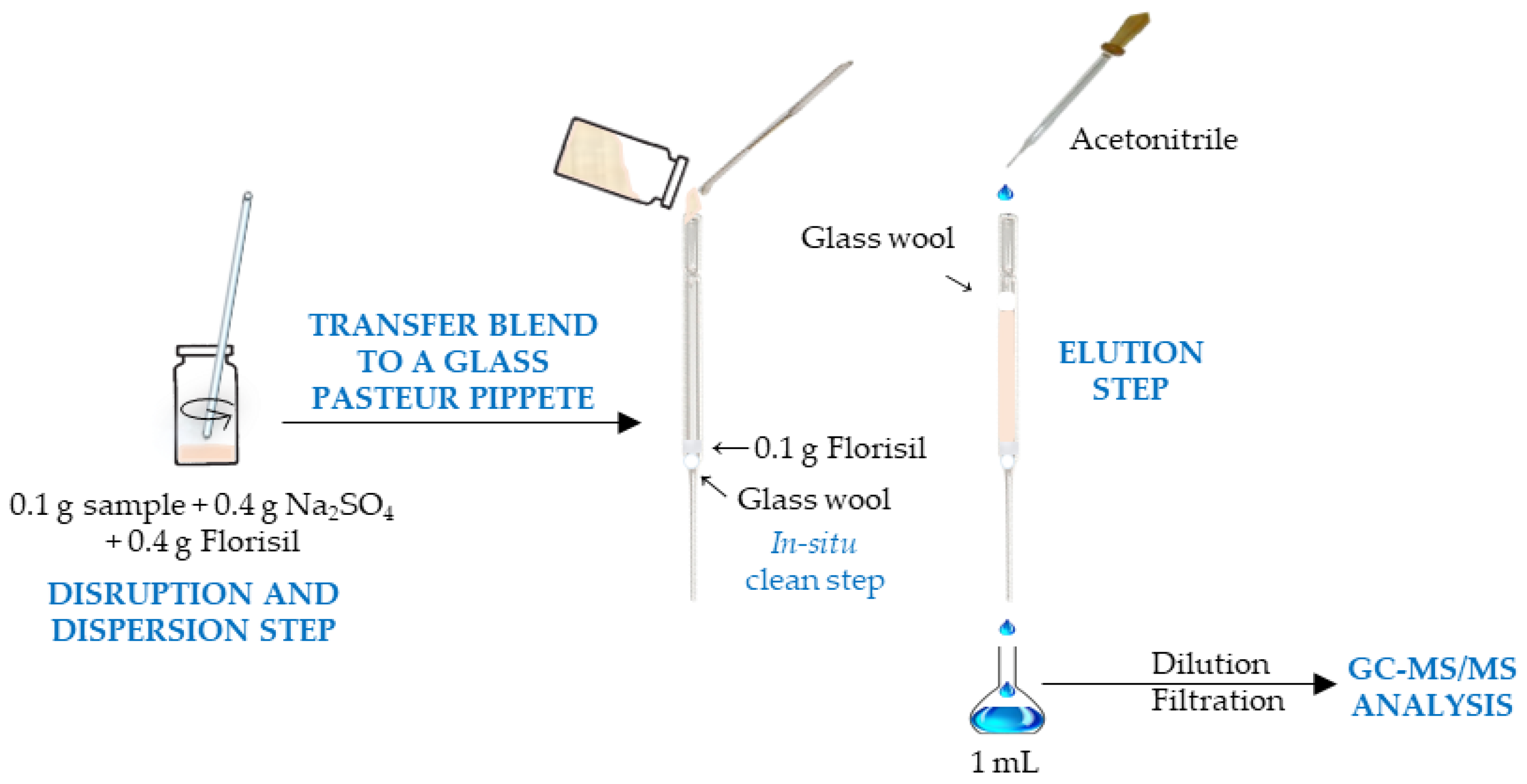
2.3. μ-MSPD Procedure
2.4. GC–MS/MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chromatographic Analysis
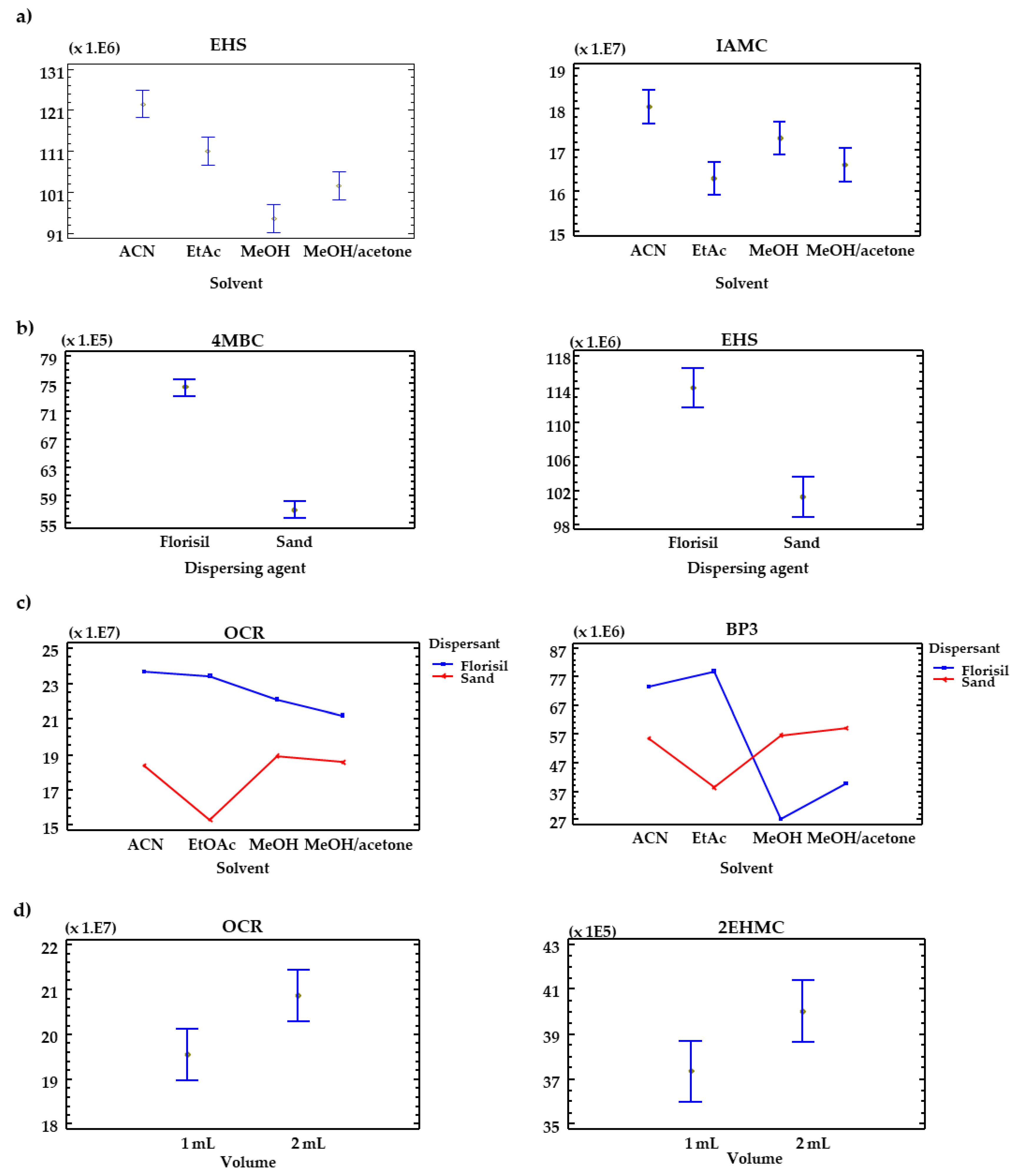
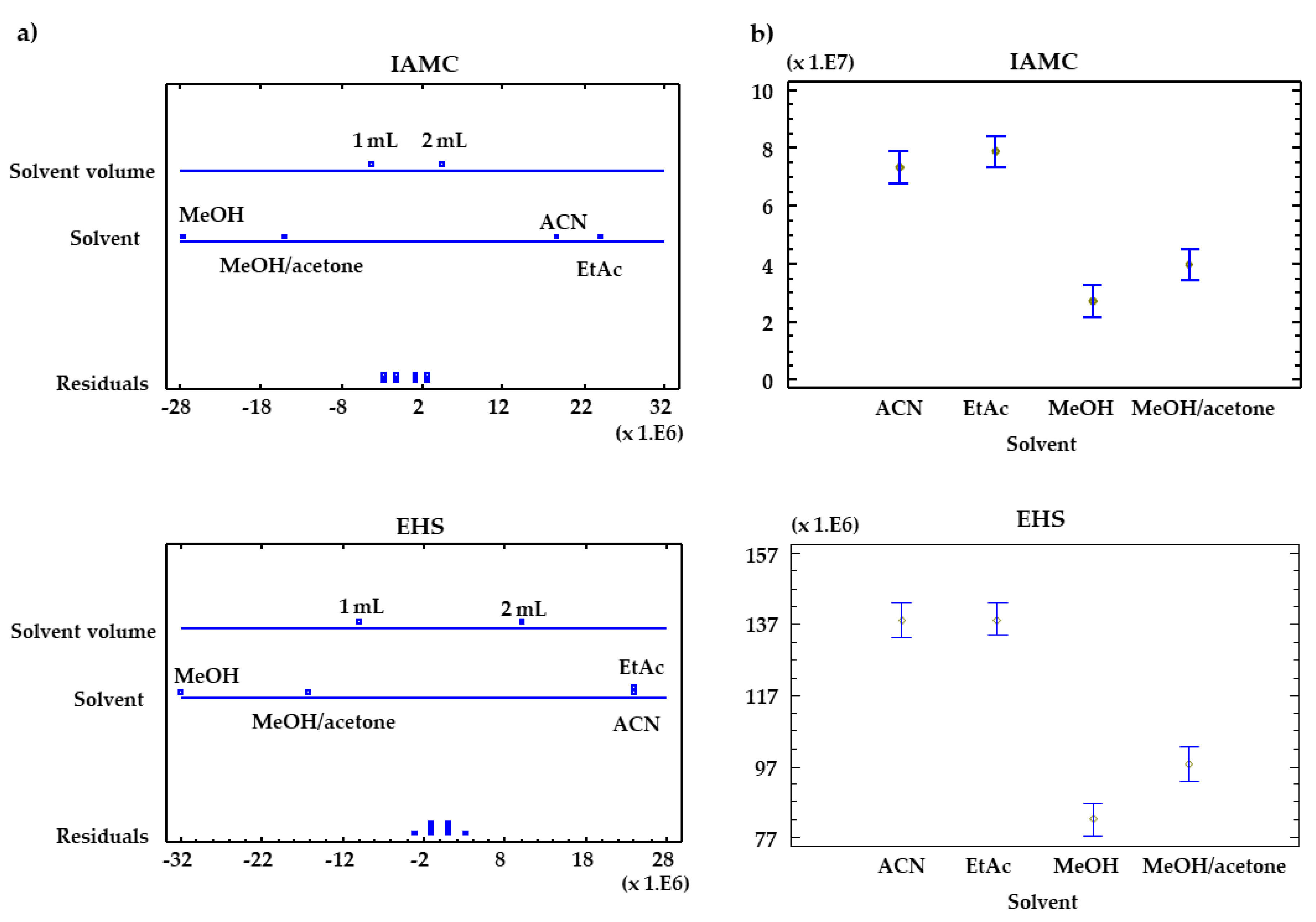
3.2. Optimization of the μ-MSPD Procedure
3.3. Method Performance
3.4. Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union. 2009, 342, 59–209. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223 (accessed on 19 April 2019).
- Salvador, A.; Chisvert, A. Sunscreen analysis: A critical survey on UV filters determination. Anal. Chim. Acta 2005, 537, 1–14. [Google Scholar] [CrossRef]
- Lores, M.; Llompart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls-A. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, G. Current trends in sample preparation for cosmetic analysis. J. Sep. Sci. 2017, 40, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 127, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Lamas, J.P.; Alvarez-Rivera, G.; Lores, M.; Garcia-Jares, C.; Llompart, M. Determination of suspected fragrance allergens in cosmetics by matrix solid-phase dispersion gas chromatography-mass spectrometry analysis. J. Chromatogr. A 2011, 1218, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bai, H.; Zhai, J.; Meng, X.; Guo, X.; Wang, C.; Wang, P.; Lei, H.; Niu, Z.; Ma, Q. Comprehensive screening of 63 coloring agents in cosmetics using matrix solid-phase dispersion and ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2019, 1590, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. TrAC-Trend. Anal. Chem. 2015, 66, 176–192. [Google Scholar] [CrossRef]
- Kamarei, F.; Ebrahimzadeh, H.; Yamini, Y. Optimization of ultrasound-assisted emulsification microextraction with solidification of floating organic droplet followed by high performance liquid chromatography for the analysis of phthalate esters in cosmetic and environmental water samples. Microchem. J. 2011, 99, 26–33. [Google Scholar] [CrossRef]
- Saraji, M.; Mirmahdieh, S. Single-drop microextraction followed by in-syringe derivatization and GC-MS detection for the determination of parabens in water and cosmetic products. J. Sep. Sci. 2009, 32, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Guerra, E.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of a multianalyte method based on micro-matrix-solid-phase dispersion for the analysis of fragrance allergens and preservatives in personal care products. J. Chromatogr. A 2014, 1344, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.; Llompart, M.; Garcia-Jares, C. In-vial micro-matrix-solid phase dispersion for the analysis of fragrance allergens, preservatives, plasticizers, and musks in cosmetics. Cosmetics 2014, 1, 171–201. [Google Scholar] [CrossRef]
- Guerra, E.; Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1415, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Lamas, J.P.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C. Analysis of plasticizers and synthetic musks in cosmetic and personal care products by matrix solid-phase dispersion gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1293, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Celeiro, M.; Lamas, J.P.; Dagnac, T.; Llompart, M.; Garcia-Jares, C. Determination of fourteen UV filters in bathing water by headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry. Anal. Methods 2016, 8, 7069–7079. [Google Scholar] [CrossRef]
- Vila, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Optimization of an analytical methodology for the simultaneous determination of different classes of ultraviolet filters in cosmetics by pressurized liquid extraction-gas chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1405, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Ultrasound-assisted emulsification microextraction followed by gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry for the analysis of UV filters in water. Microchem. J. 2016, 124, 530–539. [Google Scholar] [CrossRef]
| UV Filter | Acronym | CAS | Retention Time (min) | MS/MS Transition (CE a, eV) | ||
|---|---|---|---|---|---|---|
| Ethylhexylsalicylate | EHS | 118-60-5 | 12.85 | 120.0 | → | 92.0 (10) |
| 138.0 | → | 120.0 (10) | ||||
| 250.1 | → | 120.0 (15) | ||||
| Benzyl salicylate | BS | 118-58-1 | 13.73 | 91.0 | → | 39.0 (30) |
| 91.0 | → | 65.0 (15) | ||||
| 228.1 | → | 91.1 (10) | ||||
| Homosalate | HMS | 118-56-9 | 13.88 | 120.0 | → | 92.0 (10) |
| 138.0 | → | 120.0 (10) | ||||
| 262.2 | → | 120.0 (15) | ||||
| Benzophenone-3 | BP3 | 131-57-7 | 16.22 | 151.0 | → | 95.0 (10) |
| 227.1 | → | 127.9 (35) | ||||
| 227.1 | → | 184.0 (20) | ||||
| Isoamyl-4-methoxycinnamate | IAMC | 71617-10-2 | 16.38 | 161.0 | → | 133.0 (10) |
| 178.1 | → | 161.1 (10) | ||||
| 248.1 | → | 178.0 (10) | ||||
| 4-methylbenzylidene camphor | 4MBC | 36861-47-9 | 16.63 | 127.9 | → | 102.0 (20) |
| 170.6 | → | 128.1 (15) | ||||
| 254.1 | → | 239.2 (10) | ||||
| Methyl anthranilate | MA | 134-20-3 | 17.66 | 119.0 | → | 91.8 (10) |
| 137.0 | → | 119.0 (10) | ||||
| 275.2 | → | 137.0 (10) | ||||
| Etocrylene | ETO | 5232-99-5 | 18.22 | 231.9 | → | 176.5 (20) |
| 248.0 | → | 164.9 (25) | ||||
| 276.9 | → | 248.1 (10) | ||||
| Ethylhexyl-p-aminobenzoic acid | EHPABA | 21245-02-3 | 19.33 | 148.0 | → | 104.2 (25) |
| 165.1 | → | 148.6 (15) | ||||
| 277.2 | → | 164.9 (10) | ||||
| 2-ethylhexyl 4-methoxycinnamate | 2EHMC | 5466-77-3 | 19.69 | 161.0 | → | 133.1 (10) |
| 177.9 | → | 133.1 (20) | ||||
| 290.2 | → | 178.1 (10) | ||||
| Octocrylene | OCR | 6197-30-4 | 21.48 | 232.0 | → | 203.0 (20) |
| 248.0 | → | 165.0 (30) | ||||
| 360.2 | → | 276.1 (20) | ||||
| Avobenzone | BMDM | 70356-09-1 | 22.44 | 161.1 | → | 118.0 (15) |
| 295.1 | → | 135.1 (15) | ||||
| 309.2 | → | 279.1 (20) | ||||
| Diethylamino hydroxybenzoyl hexyl benzoate | DHHB | 302776-68-7 | 23.10 | 382.2 | → | 280.2 (10) |
| 382.2 | → | 298.1 (10 | ||||
| 397.2 | → | 382.2 (10 | ||||
| Drometrizole trisiloxane | DRT | 155633-54-8 | 25.50 | 221.1 | → | 73.1 (15) |
| 369.1 | → | 250.2 (10) | ||||
| 444.1 | → | 296.1 (25 | ||||
| Factor | Code | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|---|
| Solvent | A | ACN | EtAc | MeOH | MeOH/acetone (1:1, v/v) |
| Dispersant | B | Florisil | Sand | ||
| Volume of solvent (mL) | C | 1 | 2 |
| Compound | Solvent (A) | Dispersant (B) | Volume (C) | AB | AC | BC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | P | F | p | |
| EHS | 63 | 0.0032 | 75 | 0.0032 | 47 | 0.0063 | 150 | 0.0009 | 1.3 | 0.4114 | 0.82 | 0.4313 |
| BP3 | 157 | 0.0008 | 8.1 | 0.0647 | 49 | 0.0059 | 422 | 0.0002 | 4.5 | 0.1238 | 0.64 | 0.4817 |
| IAMC | 18 | 0.0200 | 663 | 0.0001 | 43 | 0.0072 | 65 | 0.0031 | 6.4 | 0.0802 | 0.01 | 0.9361 |
| 4MBC | 13 | 0.0288 | 545 | 0.0002 | 48 | 0.0060 | 45 | 0.0054 | 6.3 | 0.0815 | 0.75 | 0.4490 |
| 2EHMC | 2.6 | 0.2264 | 163 | 0.0010 | 9.7 | 0.0525 | 17 | 0.0202 | 2.2 | 0.2667 | 0.03 | 0.8792 |
| OCR | 4.0 | 0.1425 | 172 | 0.0010 | 13 | 0.0360 | 11 | 0.0374 | 2.3 | 0.2560 | 0.48 | 0.5392 |
| UV Filters | Linearity | Precision a | Recoveries | ||||
|---|---|---|---|---|---|---|---|
| Range (mg L−1) | R2 | RSD, % | Mean Values | 100 μg g−1 | 10 μg g−1 | 1 μg g−1 | |
| EHS | 0.001–10 | 0.9999 | 10 | 109 ± 11 | 106 ± 2 | 111 ± 4 | 110 ± 5 |
| BS | 0.002–10 | 0.9999 | 4.8 | 110 ± 3 | 111 ± 2 | 116 ± 6 | 103 ± 1 |
| HMS | 0.002–10 | 0.9999 | 10 | 109 ± 6 | 110 ± 2 | 109 ± 7 | 108 ± 10 |
| BP3 | 0.002–10 | 0.9980 | 3.9 | 106 ± 6 | 103 ± 3 | 117 ± 10 | 98.7 ± 5.3 |
| IAMC | 0.001–10 | 0.9992 | 14 | 98.4 ± 5.8 | 100 ± 2 | 102 ± 6 | 93.3 ± 9.5 |
| 4MBC | 0.002–10 | 0.9997 | 8.0 | 97.9 ± 6.7 | 97.9 ± 2.8 | 99.4 ± 7.2 | 96.6 ± 10.0 |
| MA | 0.001–10 | 0.9994 | 5.8 | 106 ± 5 | 104 ± 2 | 99.4 ± 8.2 | 114 ± 4 |
| ETO | 0.001–10 | 0.9998 | 5.2 | 97.9 ± 7.3 | 97.4 ± 7.2 | 93.3 ± 9.7 | 103 ± 5 |
| EHPABA | 0.002–10 | 0.9997 | 8.6 | 99.0 ± 4.3 | 101 ± 2 | 95.2 ± 6.8 | 101 ± 4 |
| 2EHMC | 0.002–10 | 0.9992 | 10 | 99.5 ± 4.1 | 99.4 ± 1.5 | 99.1 ± 8.5 | 100 ± 3 |
| OCR | 0.002–10 | 0.9999 | 9.8 | 104 ± 4 | 104 ± 4 | n.c. b | n.c. b |
| BMDM | 1–1000 | 0.9966 | 6.1 | 111 ± 2 | 111 ± 2 | n.c. c | n.c. c |
| DHHB | 1–50 | 0.9922 | 10 | 108 ± 3 | 108 ± 3 | n.c. c | n.c. c |
| DRT | 0.1–100 | 0.9915 | 5.6 | 98.7 ± 2.3 | 98.2 ± 1.2 | 97.4 ± 3.5 | n.c c |
| Compounds | Linearity | Precision a | Recoveries | |||
|---|---|---|---|---|---|---|
| Range (mg L−1) | R2 | RSD, % | Mean Values | 100 μg g−1 | 10 μg g−1 | |
| Fragrance allergens | ||||||
| Pinene | 0.001–10 | 0.9994 | 3.5 | 70.2 ± 6.0 | 77.8 ± 7.8 | 62.6 ± 4.2 |
| Limonene | 0.001–10 | 0.9985 | 7.2 | 85.1 ± 3.7 | 97.1 ± 3.6 | 73.1 ± 3.8 |
| Benzyl alcohol | 0.001–10 | 0.9982 | 9.7 | 109 ± 6 | 107 ± 2 | 111 ± 9 |
| Linalool | 0.005–10 | 0.9994 | 6.7 | 98.6 ± 6.7 | 104 ± 2 | 93. 2 ± 11.4 |
| Methyl-2-octynoate | 0.1–10 | 0.9999 | 6.0 | 106 ± 5 | 105 ± 2 | 107 ± 8 |
| Citronellol | 0.05–10 | 0.9999 | 8.8 | 107 ± 6 | 107 ± 2 | 107 ± 10 |
| Citral | 0.002–10 | 0.9994 | 7.1 | 99.5 ± 4 | 112 ± 1 | 86.9 ± 7.0 |
| Geraniol | 0.02–10 | 0.9998 | 6.9 | 106 ± 3 | 96.4 ± 0.4 | 116 ± 6 |
| Cinnamaldehyde | 0.005–10 | 0.9999 | 6.8 | 101 ± 7 | 106 ± 2 | 95.7 ± 11.3 |
| Hydroxycitronellal | 0.005–10 | 0.9995 | 6.0 | 108 ± 2 | 100 ± 2 | 116 ± 3 |
| Anise alcohol | 0.01–10 | 0.9998 | 8.3 | 102 ± 5 | 101 ± 2 | 103 ± 9 |
| Cinnamyl alcohol | 0.001–10 | 0.9996 | 8.7 | 105 ± 6 | 105 ± 4 | 105 ± 7 |
| Eugenol | 0.005–10 | 0.9965 | 6.4 | 108 ± 4 | 105 ± 3 | 111 ± 5 |
| Methyleugenol | 0.005–10 | 0.9981 | 6.8 | 95.6 ± 3.7 | 102 ± 2 | 89.2 ± 5.4 |
| Isoeugenol | 0.02–10 | 0.9992 | 8.4 | 100 ± 4 | 103 ± 3 | 97.0 ± 5.3 |
| Coumarin | 0.02–10 | 0.9980 | 6.6 | 102 ± 8 | 104 ± 3 | 100 ± 13 |
| α-isomethylionone | 0.005–10 | 0.9975 | 8.6 | 99.5 ± 5.6 | 101 ± 2 | 98.1 ± 9.3 |
| Lilial® | 0.005–10 | 0.9995 | 6.6 | 100 ± 7 | 106 ± 2 | 94.2 ± 12.1 |
| Amylcinnamaldehyde | 0.005–10 | 0.9991 | 8.1 | 106 ± 4 | 106 ± 1 | 106 ± 7 |
| Lyral® | 0.002–2 | 0.9971 | 7.2 | 107 ± 4 | 108 ± 1 | 105 ± 7 |
| Amylcinnamyl alcohol | 0.005–10 | 0.9992 | 8.1 | 110 ± 7 | 107 ± 1 | 112 ± 12 |
| Farnesol | 0.02–10 | 0.9994 | 12 | 107 ± 7 | 106 ± 4 | 107 ± 10 |
| Hexylcinnamaldehyde | 0.01–10 | 0.9922 | 8.1 | 107 ± 5 | 107 ± 2 | 106 ± 7 |
| Benzyl benzoate | 0.002–10 | 0.9992 | 6.7 | 102 ± 6 | 104 ± 2 | 99.3 ± 10.2 |
| Benzyl cinnamate | 0.001–10 | 0.9999 | 5.4 | 103 ± 4 | 103 ± 3 | 102 ± 5 |
| Preservatives | ||||||
| Bronidox | 0.002–10 | 0.9999 | 4.8 | 103 ± 6 | 110 ± 1 | 95.6 ± 11.1 |
| Phenoxyethanol (PhEtOH) | 0.001–10 | 0.9999 | 7.4 | 110 ± 7 | 101 ± 1 | 120 ± 14 |
| Methyl paraben (MeP) | 0.001–10 | 0.9997 | 10 | 110 ± 6 | 102 ± 2 | 117 ± 11 |
| Butylhydroxyanisole (BHA) | 0.0001–10 | 0.9990 | 5.5 | 95.6 ± 4.5 | 103 ± 3 | 88.1 ± 5.9 |
| Butylhydroxytoluene (BHT) | 0.005–10 | 0.9996 | 4.9 | 95.1 ± 3.3 | 103 ± 2 | 87.2 ± 4.7 |
| Ethyl paraben (EtP) | 0.02–10 | 0.9999 | 11 | 102 ± 8 | 100 ± 2 | 103 ± 14 |
| Isopropyl paraben (iPrP) | 0.05–10 | 0.9995 | 10 | 103 ± 4 | 105 ± 3 | 99.9 ± 4.6 |
| Propyl paraben (PrP) | 0.01–10 | 0.9998 | 11 | 98.5 ± 4.2 | 102 ± 2 | 94.9 ± 6.4 |
| Iodopropynylbutyl carbamate (IPBC) | 0.002–10 | 0.9997 | 3.5 | 104 ± 9 | 103 ± 2 | 105 ± 15 |
| Isobutyl paraben (iBuP) | 0.005–10 | 0.9999 | 8.1 | 103 ± 2 | 102 ± 2 | 104 ± 2 |
| Butyl paraben (BuP) | 0.005–10 | 0.9999 | 7.6 | 99.6 ± 1.9 | 101 ± 1 | 98.3 ± 2.8 |
| Triclosan (TCS) | 0.002–10 | 0.9983 | 2.3 | 115 ± 9 | 113 ± 3 | 117 ± 14 |
| Benzyl paraben (BzP) | 0.05–10 | 0.9995 | 4.2 | 101 ± 6 | 99.2 ± 3.3 | 103 ± 8 |
| Plasticizers | ||||||
| Dimethyl adipate (DMA) | 0.01–10 | 0.9998 | 5.3 | 116 ± 5 | 104 ± 1 | 118 ± 8 |
| Diethyl adipate (DEA) | 0.001–10 | 0.9989 | 6.5 | 98.5 ± 7.7 | 103 ± 2 | 93.9 ± 13.4 |
| Diethyl phthalate (DEP) | 0.005–10 | 0.9984 | 6.2 | 99.9 ± 4.0 | 102 ± 1 | 97.8 ± 7.1 |
| Diisobutyl phthalate (DIBP) | 0.001–10 | 0.9992 | 4.7 | 98.9 ± 4.9 | 101 ± 2 | 96.8 ± 7.8 |
| Dibutyl phtahalte (DBP) | 0.001–10 | 0.9997 | 9.1 | 100 ± 5 | 102 ± 2 | 98.4 ± 8.7 |
| Dimethoxyethyl phthalate (DMEP) | 0.005–10 | 0.9999 | 6.3 | 105 ± 6 | 105 ± 2 | 105 ± 9 |
| Diisopentyl phthalate (DIPP) | 0.002–10 | 0.9998 | 8.2 | 99.3 ± 5.5 | 100 ± 3 | 98.7 ± 7.9 |
| Dipentyl phthalate (DPP) | 0.001–10 | 0.9997 | 11 | 101 ± 3 | 101 ± 2 | 101 ± 3 |
| Benzylbutyl phthalate (BBP) | 0.002–10 | 0.9997 | 13 | 99.7 ± 5.5 | 100 ± 3 | 99.5 ± 8.0 |
| Diethylhexyl adipate (DEHA) | 0.005–10 | 0.9997 | 14 | 95.4 ± 5.4 | 96.2 ± 2.8 | 94.6 ± 8.0 |
| Diisoheptyl phthalate (DIHP) | 0.002–10 | 0.9998 | 3.9 | 97.6 ± 5.3 | 100 ± 3 | 95.3 ± 7.6 |
| Dicyclohexyl phthalate (DCHP) | 0.005-10 | 0.9996 | 9.5 | 101 ± 4 | 101 ± 3 | 101 ± 4 |
| Diethylhexyl phthalate (DEHP) | 0.01–10 | 0.9997 | 9.2 | 100 ± 3 | 100 ± 4 | 100 ± 1 |
| Diphenyl phthalate (DPhP) | 0.001–10 | 0.9999 | 11 | 101 ± 5 | 98.7 ± 4.5 | 103 ± 5 |
| Di-n-octyl phthalate (DnOP) | 0.005–10 | 0.9998 | 8.0 | 105 ± 3 | 107 ± 5 | 102 ± 1 |
| Synthetic musks | ||||||
| Cashmeran | 0.001–10 | 0.9976 | 7.1 | 100 ± 4 | 103 ± 2 | 97.0 ± 5.0 |
| Celestolide | 0.002–10 | 0.9979 | 5.4 | 100 ± 8 | 106 ± 2 | 94.2 ± 13.8 |
| Phantolide | 0.005–10 | 0.9977 | 5.9 | 99.8 ± 7.3 | 104 ± 2 | 95.6 ± 12.6 |
| Ambrette | 0.005–10 | 0.9998 | 10 | 97.7 ± 7 | 104 ± 1 | 91.4 ± 13.0 |
| Trasolide | 0.1–10 | 0.9996 | 9.6 | 102 ± 9 | 98.3 ± 9.9 | 105 ± 9 |
| Galaxolide | 0.001–10 | 0.9995 | 6.7 | 97.3 ± 5.1 | 101 ± 3 | 97.3 ± 8.2 |
| Tonalide | 0.005–10 | 0.9988 | 7.2 | 96.1 ± 7.2 | 101 ± 1 | 91.3 ± 13.5 |
| Musk Moskene | 0.002–10 | 0.9999 | 9.4 | 108 ± 5 | 112 ± 2 | 103 ± 8 |
| Musk Tibetene | 0.005–10 | 0.9995 | 7.1 | 99.1 ± 4.9 | 104 ± 1 | 94.3 ± 8.8 |
| Ambrettolide | 0.002–10 | 0.9988 | 7.0 | 105 ± 3 | 104 ± 3 | 106 ± 2 |
| Musk Ketone | 0.001–10 | 0.9998 | 10 | 98.4 ± 7.1 | 90.8 ± 3.4 | 98.0 ± 10.7 |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | |
| UV filters | |||||||||||||
| EHS | 26923 ± 2851 | 6 ± 1 | 39706 ± 1131 | 28372 ± 698 | 17 ± 3 | 23925 ± 3115 | 12 ± 1 | 1.1 ± 0.1 | |||||
| BS | 8.8 ± 0.3 | 17 ± 2 | 1.7 ± 0.5 | 0.5 ± 0.1 | |||||||||
| HMS | 0.5 ± 0.1 | 1.2 ± 0.3 | 52597 ± 2980 | 1.4 ± 0.2 | 8.4 ± 0.4 | 6.5 ± 0.1 | |||||||
| BP3 | 1.0 ± 0.2 | 46 ± 1 | 3 ± 1 | 18 ± 1 | 4693 ± 1727 | ||||||||
| IAMC | 1.8 ± 0.1 | 6 ± 2 | 24 ± 1 | ||||||||||
| 4MBC | 27061 ± 3013 | ||||||||||||
| 2-EHMC | 4927 ± 272 | 46364 ± 3939 | 350 ± 77 | 12 ± 3 | 17230 ± 3233 | 158 ± 4 | 46154 ± 3290 | 3 ± 1 | 0.9 ± 0.07 | 4 ± 1 | 1 ± 0.07 | ||
| OCR | 49327 ± 4146 | 7722 ± 1063 | 28 ± 10 | 29378 ± 1118 | 14065 ± 2442 | 42633 ± 2059 | 3 ± 0.1 | ||||||
| BMDM | 2970 ± 116 | 66444 ± 20047 | 3260 ± 763 | 86318 ± 35293 | 53437 ± 4486 | 19397 ± 7542 | 19490 ± 3001 | ||||||
| DHHB | 99111 ± 17536 | ||||||||||||
| DRT | 13300 ± 820 | ||||||||||||
| Fragrance allergens | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 |
| Limonene | 61 ± 5 | 2.1 ± 0.4 | 281 ± 35 | 0.4 ± 0.01 | 17 ± 1 | 0.3 ± 0.04 | 0.6 ± 0.01 | 4.3 ± 0.3 | 0.5 ± 0.02 | 18 ± 2 | 0.3 ± 0.01 | 2132 ± 120 | |
| Benzyl alcohol | 3.6 ± 0.2 | 0.7 ± 0.1 | 2.5 ± 0.4 | 4.9 ± 0.1 | 1.8 ± 0.5 | 1.2 ± 0.1 | 0.7 ± 0.04 | 1.2 ± 0.1 | 0.4 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 113 ± 40 | |
| Linalool | 120 ± 7 | 4.6 ± 0.6 | 234 ± 22 | 0.7 ± 0.01 | 2.0 ± 0.1 | 127 ± 50 | |||||||
| Citronellol | 34 ± 4 | ||||||||||||
| Citral | 12 ± 2 | 34 ± 11 | |||||||||||
| Hydroxycitronellal | 31 ± 2 | ||||||||||||
| Cinnamyl alcohol | 2.0 ± 0.2 | ||||||||||||
| Eugenol | 12 ± 1 | 1.1 ± 0.1 | 0.9 ± 0.02 | ||||||||||
| Coumarin | 5.7 ± 0.5 | 22 ± 3 | |||||||||||
| α-isomethylionone | 4.9 ± 0.4 | 270 ± 32 | 55 ± 7 | ||||||||||
| Lilial® | 6.6 ± 0.4 | 80 ± 1 | 1.1 ± 0.3 | ||||||||||
| Lyral® | 87 ± 12 | ||||||||||||
| Farnesol | 3.9 ± 0.2 | 20 ± 2 | 6.7 ± 0.02 | ||||||||||
| Hexylcinnamaldehyde | 63 ± 4 | 0.8 ± 0.1 | 1.7 ± 0.5 | 5.5 ± 0.6 | |||||||||
| Benzyl benzoate | 2.2 ± 0.2 | 1.0 ± 0.1 | 11 ± 1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | |||||||
| Preservatives | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 |
| PhEtOH | d | 8461 ± 1164 | 2384 ± 275 | 6029 ± 178 | 6181 ± 1673 | 3663 ± 526 | 47 ± 1 | 88 ± 3 | 6.1 ± 0.2 | 6660 ± 1323 | 1608 ± 52 | 3650 ± 153 | |
| MeP | 3094± 244 | 0.4 ± 0.1 | 5.4 ± 0.1 | 2778 ± 615 | 1.4 ± 0.4 | 1382 ± 46 | 0.3 ± 0.001 | 978 ± 356 | |||||
| BHA | |||||||||||||
| BHT | 3.1 ± 0.4 | 52 ± 7 | 31 ± 3 | 1.0 ± 0.2 | 0.9 ± 0.1 | 2.0 ± 0.5 | 1.2 ± 0.1 | 0.9 ± 0.0004 | 20 ± 1 | 1 ± 0.2 | 69 ± 2 | 80 ± 31 | |
| EtP | 895 ± 73 | 644 ± 131 | 6.9 ± 0.1 | 226 ± 81 | |||||||||
| PrP | 793 ± 57 | 318 ± 71 | 545 ± 18 | 100 ± 36 | |||||||||
| iBuP | 436 ± 13 | 110 ± 31 | |||||||||||
| BuP | 947 ± 54 | 763 ± 199 | 3.9 ± 0.7 | 209 ± 81 | |||||||||
| Plasticizers | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 |
| DEP | 13 ± 2 | 396 ± 43 | 3.4 ± 1.0 | 26 ± 1 | 0.7 ± 0.1 | ||||||||
| DBP | 1.7 ± 0.2 | 3.8 ± 0.9 | 15 ± 1 | ||||||||||
| DEHA | 3.2 ± 0.1 | 52 ± 4 | 26305 ± 2379 | 2.6 ± 0.1 | 2.6 ± 0.2 | 45 ± 22 | 2.4 ± 0.2 | 2.9 ± 0.04 | 24 ± 1 | ||||
| DEHP | 9 ± 3 | 6.8 ± 0.4 | 9 ± 1 | 5.0 ± 0.3 | 5 ± 2 | 54 ± 16 | 2.8 ± 0.5 | 5.7 ± 0.2 | 51 ± 2 | ||||
| Synthetic musks | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 |
| Celestolide | 27 ± 1 | ||||||||||||
| Galaxolide | 534 ± 57 | 1.8 ± 0.2 | 2.0 ± 0.04 | ||||||||||
| Ambrettolide | 12.6 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celeiro, M.; Vazquez, L.; Lamas, J.P.; Vila, M.; Garcia-Jares, C.; Llompart, M. Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products. Separations 2019, 6, 30. https://doi.org/10.3390/separations6020030
Celeiro M, Vazquez L, Lamas JP, Vila M, Garcia-Jares C, Llompart M. Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products. Separations. 2019; 6(2):30. https://doi.org/10.3390/separations6020030
Chicago/Turabian StyleCeleiro, Maria, Lua Vazquez, J. Pablo Lamas, Marlene Vila, Carmen Garcia-Jares, and Maria Llompart. 2019. "Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products" Separations 6, no. 2: 30. https://doi.org/10.3390/separations6020030
APA StyleCeleiro, M., Vazquez, L., Lamas, J. P., Vila, M., Garcia-Jares, C., & Llompart, M. (2019). Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products. Separations, 6(2), 30. https://doi.org/10.3390/separations6020030







