Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices
Abstract
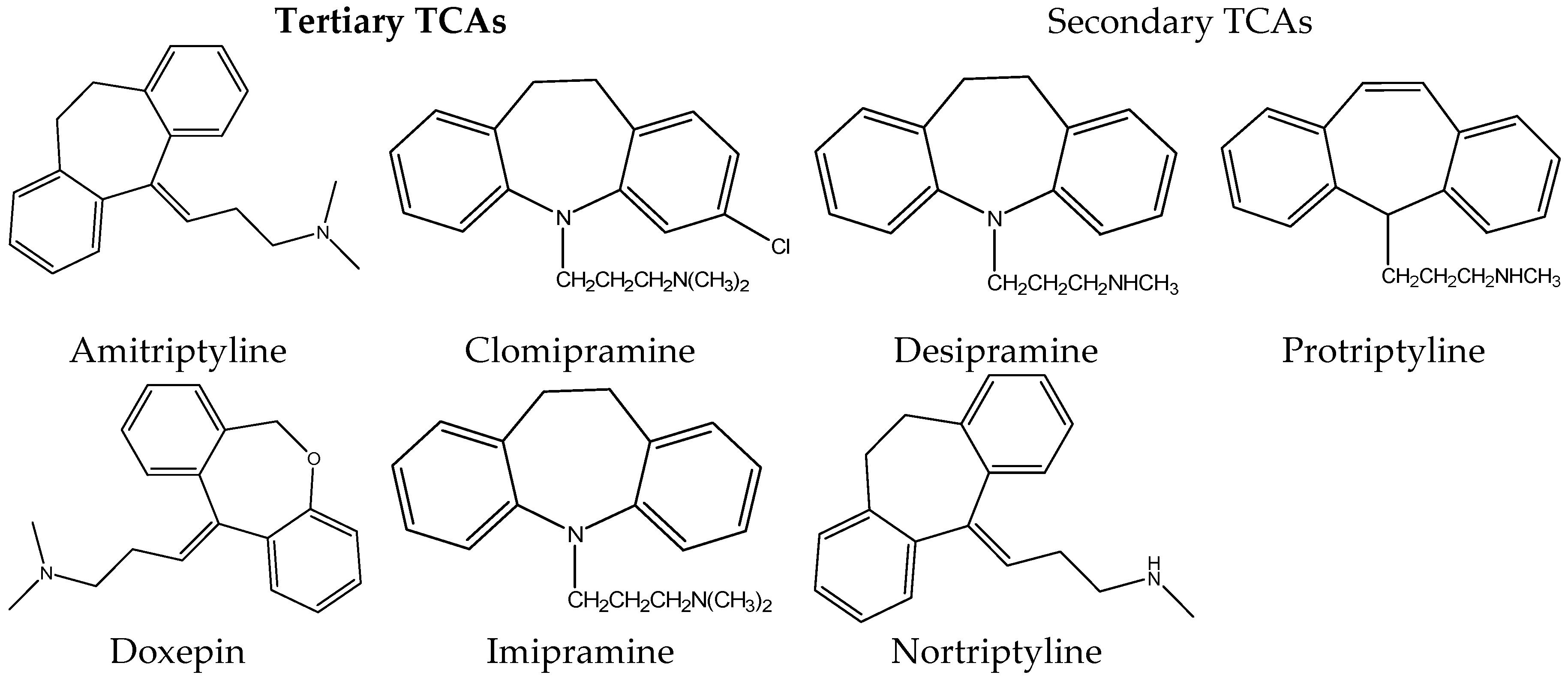
1. Introduction
2. Early Use of Gas Chromatography
3. Recent Advances in the Use of Gas Chromatography
3.1. Gas Chromatography-Mass Spectrometry Methods
3.2. Gas Chromatography with Various Detector Systems
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Samanidou, V.; Nika, M.; Papadoyannis, I. HPLC as a Tool in Medicinal Chemistry for the Monitoring of Tricyclic Antidepressants in Biofluids. Mini Rev. Med. Chem. 2008, 7, 256–275. [Google Scholar] [CrossRef]
- Brown, W.A.; Rosdolsky, M. The clinical discovery of imipramine. Am. J. Psychiatry 2015, 172, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Samanidou, V.F.; Papadoyannis, I.N. Bio-sample preparation and analytical methods for the determination of tricyclic antidepressants. Bioanalysis 2011, 3, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Jain, A.; Verma, K.K. Determination of amoxapine and nortriptyline in blood plasma and serum by salt-assisted liquid–liquid microextraction and high-performance liquid chromatography. J. Sep. Sci. 2010, 33, 3774–3780. [Google Scholar] [CrossRef]
- Melanson, S.E.F.; Tewandrowski, E.L.; Griggs, D.A.; Flood, J.G. Interpreting Tricyclic Antidepressant Measurements in Urine in an Emergency Department Setting: Comparison of Two Qualitative Point-of-Care Urine Tricyclic Antidepressant Drug Immunoassays with Quantitative Serum Chromatographic Analysis. J. Anal. Toxicol. 2007, 31, 270–275. [Google Scholar] [CrossRef]
- Risch, S.C.; Huey, L.Y.; Janowsky, D.S. Plasma levels of tricyclic antidepressants and clinical efficacy: Review of the literature—part II. J. Clin. Psychiatry 1979, 40, 58–69. [Google Scholar] [PubMed]
- Mohebbi, A.; Farajzadeh, M.A.; Yaripour, S.; Mogaddam, M.R.A. Determination of tricyclic antidepressants in human urine samples by the three-step sample pretreatment followed by HPLC-UV analysis: An efficient analytical method for further pharmacokinetic and forensic studies. EXCLI J. 2008, 17, 952–963. [Google Scholar] [CrossRef]
- Uddin, M.N.; Samanidou, V.F.; Papadoyannis, I.N. Simultaneous Determination of 1,4-Benzodiazepines and Tricyclic Antidepressants in Saliva after Sequential SPE Elution by the Same HPLC Conditions. J. Chin. Chem. Soc. 2011, 58, 142–154. [Google Scholar] [CrossRef]
- Coulter, C.; Taruc, M.; Tuyay, J.; Moore, C. Antidepressant Drugs in Oral Fluid Using Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Toxicol. 2010, 34, 65–72. [Google Scholar] [CrossRef][Green Version]
- Sempio, C.; Morini, L.; Vignali, C.; Groppi, A. Simple and sensitive screening and quantitative determination of 88 psychoactive drugs and their metabolites in blood through LC-MS/MS: Application on postmortem samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 970, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, M.; Morini, L.; Sempio, C.; Groppi, A. Validation of a multi-analyte LC-MS/MS method for screening and quantification of 87 psychoactive drugs and their metabolites in hair. Anal. Bioanal. Chem. 2014, 406, 3497–3506. [Google Scholar] [CrossRef]
- Acedoo-Valenzuela, M.; Mora-Diez, N.; Galeano-Diaz, D.; Silva-Rodriguez, A. Determination of Tricyclic Antidepressants in Human Breast Milk by Capillary Electrophoresis. Anal. Sci. 2010, 26, 26699–26702. [Google Scholar] [CrossRef]
- Wang, J.; Golden, T.; Ozsoz, M.; Lu, Z. Sensitive and selective voltammetric measurements of tricyclic antidepressants using lipid-coated electrodes. Bioelectrochem. Bioenerg. 1990, 23, 217–226. [Google Scholar] [CrossRef]
- Rao, M.L.; Staberock, U.; Baumann, P.; Hiemke, C.; Deister, A.; Cuendet, C.; Amey, M.; Hartter, S.; Kraemer, M. Monitoring tricyclic antidepressant concentrations in serum by fluorescence polarization immunoassay compared with gas chromatography and HPLC. Clin. Chem. 1994, 40, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kataky, R.; Palmer, S.; Parker, D.; Spurling, D. Alkylated cyclodextrin-based potentiometric and amperometric electrodes applied to the measurement of tricyclic antidepressants. Electroanalysis 1997, 9, 1267–1272. [Google Scholar] [CrossRef]
- Acedo-Valenzuela, M.I.; Galeano-Diaz, T.; Mora-Diez, N.; Silva-Rondriguez, A. Response surface methodology for the optimisation of flow-injection analysis with in situ solvent extraction and fluorimetric assay of tricyclic antidepressants. Talanta 2005, 66, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Knihnicki, P.; Wieczorek, M.; Moos, A.; Koscielniak, P.; Wietecha-Posłuszny, R.; Wozniakiewicz, M. Electrochemical sensor for determination of desipramine in biological material. Sens. Actuators B 2013, 189, 37–42. [Google Scholar] [CrossRef]
- Santos, M.G.; Tavares, I.M.C.; Barbosa, A.F.; Bettini, J.; Figueiredo, E.C. Analysis of tricyclic antidepressants in human plasma using online-restricted access molecularly imprinted solid phase extraction followed by direct mass spectrometry identification/quantification. Talanta 2017, 163, 8–16. [Google Scholar] [CrossRef]
- Aladaghlo, Z.; Fakhari, A.R.; Hasheminasab, K.S. Application of electromembrane extraction followed by corona discharge ion mobility spectrometry analysis as a fast and sensitive technique for determination of tricyclic antidepressants in urine samples. Microchem. J. 2016, 129, 41–48. [Google Scholar] [CrossRef]
- Jafari, M.T.; Saraji, M.; Sherafatm, H. Electrospray ionization-ion mobility spectrometry as a detection system for three-phase hollow fiber microextraction technique and simultaneous determination of trimipramine and desipramine in urine and plasma samples. Anal. Bioanal. Chem. 2011, 399, 3555–3564. [Google Scholar] [CrossRef]
- Breaud, A.R.; Harlan, R.; Di Bussolo, J.M.; McMillin, G.A.; Clark, W. A rapid and fully-automated method for the quantitation of tricyclic antidepressants in serum using turbulent-flow liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 2010, 411, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Berm, E.J.J.; Paardekooper, J.; Brummel-Mulder, E.; Hak, E.; Wilffert, B.; Maring, J.G. A simple dried blood spot method for therapeutic drug monitoring of the tricyclic antidepressants amitriptyline, nortriptyline, imipramine, clomipramine, and their active metabolites using LC-MS/MS. Talanta 2015, 134, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Alidoust, M.; Seidi, S.; Rouhollahi, A.; Shanehsaz, M. In-tube electrochemically controlled solid phase microextraction of amitriptyline, imipramine and chlorpromazine from human plasma by using an indole-thiophene copolymer nanocomposite. Microchim. Acta 2017, 184, 2473–2481. [Google Scholar] [CrossRef]
- Alves, V.; Conceicao, C.; Goncalves, J.; Teixeira, H.M.; Camara, J.S. Improved Analytical Approach Based on QuECHERS/UHPLC-PDA for Quantification of Fluoxetine, Clomipramine and their Active Metabolites in Human Urine Samples. J. Anal. Toxicol. 2016, 41, 45–53. [Google Scholar] [CrossRef]
- Safari, M.; Shahlaei, M.; Yamini, Y.; Shakorian, M.; Arkan, E. Magnetic framework composite as sorbent for magnetic solid phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of tricyclic antidepressants. Anal. Chim. Acta 2018, 1034, 204–213. [Google Scholar] [CrossRef]
- Hamidi, F.; Hadjmohammadi, M.R.; Aghaie, A.B.G. Ultrasound-assisted dispersive magnetic solid phase extraction based on amino-functionalized Fe3O4 adsorbent for recovery of clomipramine from human plasma and its determination by high performance liquid chromatography: Optimization by experimental design. J. Chromatogr. B 2017, 1063, 18–24. [Google Scholar] [CrossRef]
- Ahmadi, F.; Mahmoudi-Yamchi, T.; Azizian, H. Super paramagnetic core-shells anchored onto silica grafted with C8/NH2 nano-particles for ultrasound-assisted magnetic solid phase extraction of imipramine and desipramine from plasma. J. Chrom. B 2018, 1077–1078, 52–59. [Google Scholar] [CrossRef]
- Scoggins, B.A.; Maguire, K.P.; Norman, T.R.; Burrows, G.D. Measurement of tricyclic antidepressants. Part, I. A review of methodology. Clin. Chem. 1980, 26, 5–17. [Google Scholar]
- Gupta, R.N.; Stefanec, M.; Eng, F. Determination of tricyclic antidepressant drugs by gas chromatography with the use of a capillary column. Clin. Biochem. 1983, 2, 94–97. [Google Scholar] [CrossRef]
- Van Brunt, N. Application of new technology for the measurement of tricyclic antidepressants using capillary gas chromatography with a fused silica DB5 column and nitrogen phosphorus detection. Ther. Drug Monit. 1983, 5, 11–37. [Google Scholar] [PubMed]
- Norman, T.R.; Maguire, K.P. Analysis of tricyclic antidepressant drugs in plasma and serum by chromatographic techniques. J. Chromatogr. 1985, 340, 173–197. [Google Scholar] [CrossRef]
- Smyth, W.F.J. Recent studies on the electrospray ionisation mass spectrometric behavior of selected nitrogen-containing drug molecules and its application to drug analysis using liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogr. B 2005, 824, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.H. Multi-analyte procedures for screening for and quantification of drugs in blood, plasma, or serum by liquid chromatography-single stage or tandem mass spectrometry (LC-MS or LC-MS/MS) relevant to clinical and forensic toxicology. Clin. Biochem. 2005, 38, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.H. Determination of Nanogram Quantities of Chlorpromazine and Some of Its metabolites in Plasma Using Gas-Liquid Chromatography with an Electron Capture Detector. Anal. Chem. 1968, 40, 1251–1255. [Google Scholar] [CrossRef]
- Gifford, A.; Turner, P.; Pare, C.M.B. Sensitive method for the routine determination of tricyclic antidepressants in plasma using a specific nitrogen detector. J. Chromatogr. 1975, 105, 107–113. [Google Scholar] [CrossRef]
- Jorgensen, A. Gas Chromatographic Method for the Determination of Amitriptyline and Nortriptyline in Human Serum. Acta Pharmacol. Toxicol. 1975, 36, 79–90. [Google Scholar] [CrossRef]
- Vasiliades, J.; Buch, K.C. Gas Liquid Chromatographic Determination of Therapeutic and Toxic Levels of Amitriptyline in Human Serum with a Nitrogen-Sensitive Detector. Anal. Chem. 1976, 48, 1708–1711. [Google Scholar] [CrossRef]
- Bailey, D.N.; Jatlow, P.I. Gas-Chromatographic Analysis for Therapeutic Concentrations of lmiprarnine and Desipramine in Plasma, with Use of a Nitrogen Detector. Clin. Chem. 1976, 22, 1697–1701. [Google Scholar]
- Claeys, M.; Muscettola, G.; Markey, S.P. Simultaneous measurement of imipramine and desipramine by selected ion recording with deuterated internal standards. Biomed. Mass Spectrom. 1976, 3, 110–116. [Google Scholar] [CrossRef]
- Dorrity, F.; Linnolla, M.; Habig, R.L. Therapeutic Monitoringo f Tricyclic Antidepressantsin Plasma by Gas Chromatography. Clin. Chem. 1977, 23, 1326–1328. [Google Scholar] [PubMed]
- Wilson, J.M.; Williamson, L.J.; Raisys, V.A. Simultaneous Measurement of Secondary and Tertiary Tricyclic Antidepressants by GC/MS Chemical Ionization Mass Fragmentography. Clin. Chem. 1977, 23, 1012–1017. [Google Scholar]
- Garland, W.A. Quantitative determination of amitriptyline and its principal metabolite, nortriptyline, by GLC-chemical ionization mass spectrometry. J. Pharm. Sci. 1977, 1, 77–81. [Google Scholar] [CrossRef]
- Garland, W.A.; Muccino, R.R.; Min, B.H.; Cupano, J.; Fann, W.E. A method for the determination of amitriptyline and its metabolites nortriptyline, 10-hydroxyamitriptyline, and 10-hydroxynortriptyline in human plasma using stable isotope dilution and gas chromatography-chemical ionization mass spectrometry (GC-CIMS). Clin Pharmacol. Ther. 1979, 6, 844–856. [Google Scholar] [CrossRef]
- Dhar, A.K.; Kutt, H. An improved gas-liquid chromatographic procedure for the determination of amitriptyline and nortriptyline levels in plasma using nitrogen-sensitive detectors. Ther. Drug Monit. 1979, 31, 209–216. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Greenblatt, D.J.; Shader, R.I. Tricyclic Antidepressants Determination in Human Plasma by Gas-Liquid Chromatgraphy Using Nitrogen-Phosporous Detection: Application to Single-Dose Pharmacokinetic Studies. Pharmacology 1981, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Narasimhachari, N.; Saady, J.; Friedel, R.O. Quantitative mapping of metabolites of imipramine and desipramine in plasma samples by gas chromatographic-mass spectrometry. Biol. Psychiatry 1981, 10, 937–944. [Google Scholar]
- Hals, P.A.; Lundgren, T.I.; Aarbakke, J. A sensitive gas chromatographic assay for amitriptyline and nortriptyline in plasma. Ther. Drug Monit. 1982, 4, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Lukey, B.J.; Hurst, H.E. Quantification of amitriptyline, nortriptyline, and 10-hydroxy metabolite isomers in plasma by capillary gas chromatography with nitrogen-sensitive detection. J. Chromatogr. 1983, 278, 291–299. [Google Scholar] [CrossRef]
- Ishida, R.; Ozaki, T.; Uchida, H.; Irikura, T. Gas chromatographic--mass spectrometric determination of amitriptyline and its major metabolites in human serum. J. Chromatogr. 1984, 305, 73–82. [Google Scholar] [CrossRef]
- Hattori, H.; Takashima, E.; Yamada, T. Detection of tricyclic antidepressants in body fluids by gas chromatography with a surface ionization detector. J. Chromatogr. 1990, 529, 189–193. [Google Scholar] [CrossRef]
- Ulrich, S.; Isensee, T.; Pester, U. Simultaneous determination of amitriptyline, nortriptyline and four hydroxylated metabolites in serum by capillary gas-liquid chromatography with nitrogen-phosphorus-selective detection. J. Chromatogr. B Biomed. Appl. 1996, 685, 81–89. [Google Scholar] [CrossRef]
- Pommier, F.; Sioufli, A.; Godbillon, J. Simultaneous determination of imipramine and its metabolite desipramine in human plasma by capillary gas chromatography with mass-selective detection. J. Chromatogr. B 1997, 703, 147–158. [Google Scholar] [CrossRef]
- Lee, X.; Kumazawa, T.; Sato, K. Detection of Tricyclic Antidepressants in Whole Blood by Headspace Solid-Phase Microextraction and Capillary Gas Chromatography. J. Chromatogr. Sci. 1997, 37, 302–308. [Google Scholar] [CrossRef]
- De la Torre, R.; Ortuño, J.; Pascual, J.A.; González, S.; Ballesta, J. Quantitative Determination of Tricyclic Antidepressants and Their Metabolites in Plasma by Solid-Phase Extraction (Bond- Elut TCA) and Separation by Capillary Gas Chromatography with Nitrogen- Phosphorous Detection. Ther. Drug Monit. 1998, 20, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Way, B.A.; Stickle, D.; Mitchell, M.E.; Koenig, J.W.; Turk, J. Isotope Dilution Gas Chromatographic- Mass Spectrometric Measurement of Tricyclic Antidepressant Drugs. Utility of the 4-Carbethoxyhexafluorobutyryl Derivatives of Secondary Amines. J. Anal. Toxicol. 1998, 22, 374–382. [Google Scholar] [CrossRef]
- MeIIstrom, B.; Eksborg, S. Determination of chlorimipramine and desmethylchlorimipramine in human plasma by ion-pair partition chromatography. J. Chromatogr. 1976, 116, 475–479. [Google Scholar] [CrossRef]
- Paterson, S.; Cordero, R.; Burlinson, S. Screening and semi-quantitative analysis of post mortem blood for basic drugs using gas chromatography/ion trap mass spectrometry. J. Chromatogr. B 2004, 813, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Crifasi, J.A.; Bruder, M.F.; Long, C.W.; Janssen, K. Performance Evaluation of Thermal Desorption System (TDS) for Detection of Basic Drugs in Forensic Samples by GC-MS. J. Anal. Toxicol. 2006, 30, 582–592. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Yazdinejad, S.R.; Es’haghi, Z. Directly Suspended Droplet Microextraction and Analysis of Amitriptyline and Nortriptyline by GC. Chromatographia 2007, 66, 613–617. [Google Scholar] [CrossRef]
- Rana, S.; Uralets, V.P.; Ross, W. A New Method for Simultaneous Determination of Cyclic Antidepressants and their Metabolites in Urine Using Enzymatic Hydrolysis and Fast GC-MS. J. Anal. Toxicol. 2008, 32, 355–363. [Google Scholar] [CrossRef]
- Lee, X.; Hasegawa, C.; Kumazawa, T.; Shinmen, N.; Shoji, Y.; Seno, H.; Sato, K. Determination of tricyclic antidepressants in human plasma using pipette tip solid-phase extraction and gas chromatography–mass spectrometry. J. Sep. Sci. 2008, 31, 2265–2271. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Ushiro, M.; Takahashi, Y.; Saito, K.; Ookubo, T.; Iwasaki, Y.; Nakazawa, H. Improvement and validation the method using dispersive liquid–liquid microextraction with in situ derivatization followed by gas chromatography–mass spectrometry for determination of tricyclic antidepressants in human urine samples. J. Chromatogr. B 2011, 879, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumar, A.; Malik, A.K.; Singh, B. Quantification of Tricyclic and Nontricyclic Antidepressants in Spiked Plasma and Urine Samples Using Microextraction in Packed Syringe and Analysis by LC and GC-MS. Chromatographia 2011, 74, 235–242. [Google Scholar] [CrossRef]
- Papoutsis, Ι.; Khraiwesh, A.; Nikolaou, P.; Pistos, C.; Spiliopoulou, C.; Athanaselis, S. A fully validated method for the simultaneous determination of 11 antidepressant drugs in whole blood by gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2012, 70, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Darwish, M.Z.; Hammad, H.A.; Fathy, W.M. Validated method for the simultaneous determination of some tricyclic antidepressants in human urine samples by gas chromatography–mass spectrometry. Int. J. Anal. Bioanal. Chem. 2013, 3, 59–63. [Google Scholar]
- Dos Santos, M.F.; Ferri, C.C.; Seulin, S.C.; Leyton, V.; Pasqualucci, C.A.G.; Munoz, D.R.; Yonamine, M. Determination of antidepressants in whole blood using hollow-fiber liquid-phase microextraction and gas chromatography–mass spectrometry. Forensic Toxicol. 2014, 32, 214–224. [Google Scholar] [CrossRef]
- Banitaba, M.H.; Davarani, S.S.H.; Ahmar, H.; Movahed, S.K. Application of a new fiber coating based on electrochemically reduced graphene oxide for the cold-fiber headspace solid-phase microextraction of tricyclic antidepressants. J. Sep. Sci. 2014, 37, 1162–1169. [Google Scholar] [CrossRef]
- Mohebbi, A.; Yaripour, S.; Farajzadeha, M.A.; Mogaddamd, M.R.A. Combination of dispersive solid phase extraction and deep eutectic solvent–based air–assisted liquid–liquid microextraction followed by gas chromatography–mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids. J. Chromatogr. A 2018, 1571, 84–93. [Google Scholar] [CrossRef]
- Jagtap, P.K.; Tapadia, K. Pharmacokinetic determination and analysis of nortriptyline based on GC–MS coupled with hollow-fiber drop-to-drop solvent microextraction technique. Bioanalysis 2018, 10, 143–152. [Google Scholar] [CrossRef]
- Martinez, M.A.; de la Torre, C.S.; Almarza, E. Simultaneous Determination of Viloxazine, Venlafaxine, Imipramine, Desipramine, Sertraline, and Amoxapine in Whole Blood: Comparison of Two Extraction/Cleanup Procedures for Capillary Gas Chromatography with Nitrogen-Phosphorus Detection. J. Anal. Toxicol. 2002, 26, 296–302. [Google Scholar] [CrossRef]
- Martinez, M.A.; de la Torre, C.S.; Almarza, E. A Comparative Solid-Phase Extraction Study for the Simultaneous Determination of Fiuoxetine, Amitriptyline, Nortriptyline, Trimipramine, Maprotiline, Clomipramine and Trazodone in Whole Blood by Capillary Gas-Liquid Chromatography with Nitrogen-Phosphorus Detection. J. Anal. Toxicol. 2003, 27, 353–358. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Razavi, N.; Yazdinejad, S.R. Separation and determination of amitriptyline and nortriptyline by dispersive liquid–liquid microextraction combined with gas chromatography flame ionization detection. Talanta 2008, 75, 1293–1299. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Yamini, Y.; Seidi, S.; Ebrahimpour, B. Electromembrane surrounded solid phase microextraction: A novel approach for efficient extraction from complicated matrices. J. Chromatogr. A 2013, 1280, 16–22. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Razavi, N. Separation and Determination of Amitriptyline and Nortriptyline in Biological Samples Using Single-Drop Microextraction with GC. Chromatographia 2011, 73, 549–557. [Google Scholar] [CrossRef]
- Jeannot, M.A.; Cantwell, F.F. Solvent microextraction into a single drop. Anal. Chem. 1996, 68, 2236–2240. [Google Scholar] [CrossRef]
- Davarani, S.S.H.; Najarian, A.M.; Nojavan, S.; Tabatabaei, M. Electromembrane extraction combined with gas chromatography for quantification of tricyclic antidepressants in human body fluid. Anal. Chim. Acta 2012, 725, 51–56. [Google Scholar] [CrossRef]
- Saraji, M.; Mehrafza, N.; Hajialiakbari, A.A.; Mohammad, B.; Jafari, T. Determination of desipramine in biological samples using liquid–liquid–liquid microextraction combined with in-syringe derivatization, gas chromatography, and nitrogen/phosphorus detection. J. Sep. Sci. 2012, 35, 2637–2644. [Google Scholar] [CrossRef]
- Davarani, S.S.H.; Nojavan, S.; Asadi, R.; Banitaba, H.M. Electro-assisted solid-phase microextraction based on poly(3,4-etylenedioxythiophen) combined with GC for the quantification of tricyclic antidepressants. J. Sep. Sci. 2013, 36, 2315–2322. [Google Scholar] [CrossRef]
- Seidi, S.; Yamini, Y.; Rezazadeh, M. Combination of electromembrane extraction with dispersive liquid–liquid microextraction followed by gas chromatographic analysis as a fast and sensitive technique for determination of tricyclic antidepressants. J. Chromatogr. B 2013, 913–914, 138–146. [Google Scholar] [CrossRef]
- Asghari, A.; Saffarzadeh, Z.; Bazregar, M.; Rajabi, M.; Boutorabi, L. Low-toxic air-agitated liquid-liquid microextraction using a solidifiable organic solvent followed by gas chromatography for analysis of amitriptyline and imipramine in human plasma and wastewater samples. Microchem. J. 2017, 130, 122–128. [Google Scholar] [CrossRef]
- Karageorgou, E.; Manousi, N.; Samanidou, V.F.; Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extraction for the Fast Isolation of Sulfonamides Residues from Raw Milk Followed by High Performance Liquid Chromatography with Ultraviolet Detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Georgiadis, D.; Kabir, A.; Furton, K.G. Capsule Phase Microextraction: The Total and Ultimate Sample Preparation Approach. J. Chromogr. Sep. Tech 2018, 9, 1–4. [Google Scholar] [CrossRef]
| TCAs | Matrix | Sample Preparation | GC Parameters | Detector | LOQs (ng/mL) | Recoveries | Ref. |
|---|---|---|---|---|---|---|---|
| Imipramine, desipramine | Plasma | MSPE | Column: CP-Sil 8 CB column (30 m × 0.25 mm i.d., 0.25 μm) Carrier gas: He (pressure of 10 psi). Injection mode: splitless Injector: 270 °C and Detector: 270 °C Oven: 80 °C (1 min), 30 °C/min to 280 °C (5 min). | FID | 10–4000 | 94% | [28] |
| Amitriptyline | Blood | LLE | Column: DB-5 (30 m × 0.25 mm, 0.25 μm) Carrier gas: He (flow rate of 1 mL/min) Injection mode: Splitless Injector temperature: 280 °C and Detector: 300 °C Oven: 50 °C (2 min), 30 °C/min to 180 °C, 5 °C/min to 280 °C (19 min) | MS | NA | NA | [58] |
| Amitriptyline, nortriptyline | Urine | directly suspended droplet microextraction | Column: CP-Sil 24CB (30 m × 0.32 mm, 0.25 μm) Carrier gas: He was delivered at a flow rate of 1.11 mL/min. Injection mode: split ratio of 46.04/1 Injector: 280 °C and Detector: 280 °C Oven: 100 °C (1 min), 50 °C/min to 240 °C, 2 °C/min to 260 °C | MS | 132–165 | 76.1–82.6% | [60] |
| Amitriptyline, nortriptyline, imipramine, desipramine | Urine | LLE after enzymic hydrolysis | Column: CP-SIL 5CB (10 m × 0.15 mm, 0.12 μm) Injection mode: Splitless Carrier gas: H2 delivered at a flow rate of 1 mL/min. Injector: 250 °C Oven: two different acquisition programs | MS | 5–100 | NA | [61] |
| amitriptyline, amoxapine, imipramine, trimipramine | Plasma | pipette tip SPE | Column: DB-5MS fused silica capillary column (30 m × 0.32 mm id, 0.25 μm) Carrier gas: He (flow rate of 2.0 mL/min). Injection mode: splitless Injector: 250 °C and Detector: 280 °C Oven: 100 °C (1 min), 20 °C/min to 300 °C. | MS | 0.2–5 | 80.2–92.1% | [62] |
| Imipramine, desipramine, amitriptyline, nortriptyline, clomipramine | Urine | DLLME | Column: DB-5MS (30 m × 0.25 mm i.d., 0.5 μm) Carrier gas: He (flow rate of 1.0 mL/min) Oven temperature program: 60 °C (3 min), 15 °C/min to 300 °C (4 min). | MS | 2–5 | 88.2–104.3% | [63] |
| Amitriptyline, imipramine, clomipramine | Urine, plasma | Microextraction by packed syringe | Column: Rtx-1MS (30 m × 0.25 mm id, 0.25 μm) Carrier gas: He (flow rate of 1 mL/min) Injection mode: split (10:1) Injector: 270 °C and Detector: 300 °C. Oven: 100 °C (1 min), 10 °C/min to 200 °C, 15°C/min to 260 °C, 30 °C/min to 300 °C. | MS | 0.330–0.608 | 77–99% | [64] |
| Amitriptyline, clomipramine, nortriptyline | Whole blood | SPE | Column: HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm) Carrier gas: He (flow rate of 1 mL/min) Injector: 280 °C and Detector: 300 °C. Oven: 100 °C (1 min), 40 °C/min to 300 °C (4 min). | MS | 1–5 | 79.2–102.6% | [65] |
| Amitriptyline, imipramine | Urine | LLE | Column: DB-5MS column (30 m × 0.25 mm i.d, 0.5 μm Carrier gas: Helium was delivered as carrier gas at flow rate of 1 mL/min Oven: 80 °C held for 3 min then raised to 300 °C at a rate of 30 °C and held for 4 min | MS | 100000 | 89.7 | [66] |
| Amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, | Whole blood | HF-LPME | Column: HP-5MS column (30 m × 0.25 mm i.d., 0.25 μm) Carrier gas: He (flow rate of 0.8 mL/min) Injection mode: splitless Injector: 280 °C Oven: 125 °C (1 min), 50 °C/min to 190 °C, 5 °C/min to 225 °C (3 min), 50 °C/min to 230 °C (1 min). | MS | 20 | 36–89%. | [67] |
| Amitriptyline, imipramine clomipramine | Plasma | HS-SPME | Column: CP-Sil 8 CB (25 m × 0.32 mm id, 0.25 μm) Carrier gas: N2 (pressure of 17 psi) Injector port: 280 °C and Detector: 300 °C Oven: 80 °C (3 min), 30 °C/min to 180 °C, 5 °C/min to 210 °C, 30 °C/min to 300 °C (1 min). | FID | 1.0–1.7 | 73–96% | [68] |
| Amitriptyline, nortriptyline, imipramine, desipramine, clomipramine | Plasma, urine | d-SPE and deep eutectic solvent-based air-assisted LPME | Column: HP–5MS column (60 m × 0.25 mm i.d., 0.25 μm). Carrier gas: He (flow rate of 1 mL/min). Detector: 260 °C Column: 100 °C (1 min), 50 °C/min to 190 °C, 5 °C/min to 225 °C (3 min), 20 °C/min to 300 °C (5 min). | MS | 0.027–0.191 | 62–74% | [69] |
| Notriptyiline | Urine, blood | hollow-fiber drop-to-drop solvent microextraction | Column: DB-5 (30 m × 0.25 mm i.d., 1 μm) Carrier gas: of helium (flow: 1 mL/min) Injection mode: splitless Injector: 250 °C and Detector: 300 °C Oven: 80 °C (3min), 20 °C/min to 250 °C (3 min). | MS | 234 | 97.33–103.66% | [70] |
| Imipramine, desipramine | Whole Blood | SPE | Column: HP-1 (25 m × 0.20 mm i.d., 0.11 μm) Carrier gas: He (constant pressure of 195 kPa) Injection mode: split (split ratio of 1:20) Injector: 280 °C and Detector: 300 °C Oven: 180 °C (1 min), 10 °C/min to 300 °C (3 min) | NPD | 70–222 | 64–86% | [71] |
| Amitriptyline, nortriptyline, trimipramine, clomipramine | Whole Blood | SPE | Column: HP-1 (25 m × 0.20 mm i.d., 0.11 μm) Carrier gas: He (constant pressure of 195 kPa) Injection mode: split (split ratio of 1:20) Injector: 280 °C and Detector: 300 °C Oven: 180 °C (1 min), 10 °C /min to 300 °C (3 min). | NPD | 44–485 | 59–84% | [72] |
| Amitriptyline | Plasma, urine | Electromembrane SPME | Column: HP-5 (30 m × 0.32 mm i.d., 0.25 μm) Carrier gas: He (constant flow rate of 0.6 mL/min) Injector: 280 and Detector: 300 °C Oven: 160 °C (3 min), 30 °C/min to 280 °C (3 min) | FID | 5 | 3.1–11.5% | [74] |
| Amitriptyline, nortriptyline | Plasma, urine | Single-drop microextraction | Column: CP-Sil 24 CB (30 m × 0.32 mm id, 0.25 μm) Carrier gas: He (flow rate: 1.11 mL/min) Injection mode: split (1:46) Injector: 280 °C and Detector: 280 °C Oven: 100 °C (1 min), 20 °C/min to 240 °C, 2 °C /min to 260 °C. | FID | 33–66 | 66.5–97.4% | [75] |
| Imipramine, clomipramine | Plasma, urine | EME | Column: CP-Sil 8 CB column (25 m × 0.32 mm i.d., 0.25 μm) Carrier gas: N2 (constant pressure of 20 psi). Injection mode: splitless Injector: 270 °C and Detector: 300 °C. Oven: 140 °C, 32 °C/min to 280 °C (of 32 °C/min and held at 280 °C (1 min) | FID | 2.3 | 90–95% | [77] |
| Desipramine | Plasma, urine | HF-LPME | Column: DB-35MS (30 m × 0.25 mm, 0.15 μm) Carrier gas: N2 (head pressure of 0.12 MPa) Injection mode: splitless Injector: 260 °C and Detector: 310 °C Oven: 100 °C (1 min), 30 °C/min to 300 °C (9 min). | NPD | 66 | 32% | [78] |
| Imipramine, clomipramine, desipramine | Urine | electro-assisted SPME | Column: CP-Sil 8 CB column (25 m × 0.32 mm i.d., 0.25 μm) Carrier gas: nitrogen as carrier gas, delivered with constant pressure of 16 psi Injector: 270 °C and Detector: 300 °C Oven: 100 °C, 30 °C/min to 300 °C (1 min) | FID | 0.5–1.5 | 16–74.2% | [79] |
| Amitriptyline, trimipramine | Plasma, urine | EME and DLLME | Column: HP-5 (30 m × 0.32 mm i.d., 0.25 μm) Carrier gas: He (constant flow rate of 4 mL/min) Injection mode: split, ratio 1:5 Injector: 280 °C and Detector: 300 °C Oven: 185 °C (12 min), 30 °C/min to 280 °C (3 min). | FID | 10 and 40 | 88.8–92.3% | [80] |
| Amitriptyline, imipramine | Plasma | air-agitated liquid–liquid microextraction | Column: BP-5 capillary column (30 m × 0.32 mm i.d., 0.25 μm). Injection mode: splitless Injector: 280 °C and FID: 280 °C Oven: 100 °C (3 min), 20 °C/min to 280 °C (2 min). | FID | 15–20 | 68–73% | [81] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Samanidou, V.F. Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices. Separations 2019, 6, 24. https://doi.org/10.3390/separations6020024
Manousi N, Samanidou VF. Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices. Separations. 2019; 6(2):24. https://doi.org/10.3390/separations6020024
Chicago/Turabian StyleManousi, Natalia, and Victoria F. Samanidou. 2019. "Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices" Separations 6, no. 2: 24. https://doi.org/10.3390/separations6020024
APA StyleManousi, N., & Samanidou, V. F. (2019). Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices. Separations, 6(2), 24. https://doi.org/10.3390/separations6020024






