Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”
Abstract
1. Introduction
- Physical destruction: through successive cycles of freezing and thawing of fresh Spirulina as the cell membrane is ruptured due to an increase in the volume of the ice crystals and subsequent purification. Another way to release intracellular substances is through the use of micro- or nano-sized spheres [16,17];
- Supercritical fluid extraction, using supercritical carbon dioxide as a solvent [18];
- Methods using alternating electric fields in an aqueous environment [19];
- Speed and efficiency: Due to the presence of cavitation in the sample, mechanical waves arise that quickly destroy the cell walls;
- Ensuring high concentrations of biologically active substances that are heat sensitive: This process avoids a significant increase in temperature and reduces the temperature degradation of the specified components;
- No contamination of the extracts: This specified method uses a minimal amount of chemicals and solvents;
- Ability to easily optimize the process to improve the quality of the extracts in real time;
- Reduction of adverse influences such as oxidation, for example, etc.;
- Multi-functionality: This method can be successfully combined with enzymatic and hydrothermal methods to achieve sustainability and efficiency in the extraction of a larger spectrum of biologically active substances;
- It is suitable for the extraction of substances on a large scale.
2. Materials and Methods
2.1. Investigated Sample
2.2. Determination of Chlorophyll and Carotenoids
2.3. Determination of the Antioxidant Activity of Water and Ethanol Extracts of Spirulina (Arthrosphira platensis)
2.3.1. FRAP Method
2.3.2. DPPH Method
2.4. Green Methods of Extraction
2.4.1. Microwave Extraction
2.4.2. Ultrasonic Extraction
- (1)
- Ultrasonic bath SIEL (Bulgaria) with frequency 36 kHz, power 300 W-(UAE 36 kHz);
- (2)
- Ultrasonic bath ISOLAB (Germany) with frequency 40 kHz, power 60 W-(UAE 40 kHz);
- (3)
- Ultrasonic bath VWR (Malaysia) with frequency 45 kHz, power 30 W-(UAE 45 kHz).
2.5. Spectrophotometric Method for Phycocyanin Analysis
- PC—concentration of phycocyanin in mg mL−1;
- V—volume of extract in mL;
- DB—dry biomass of Spirulina in grams.
2.6. Data Analysis
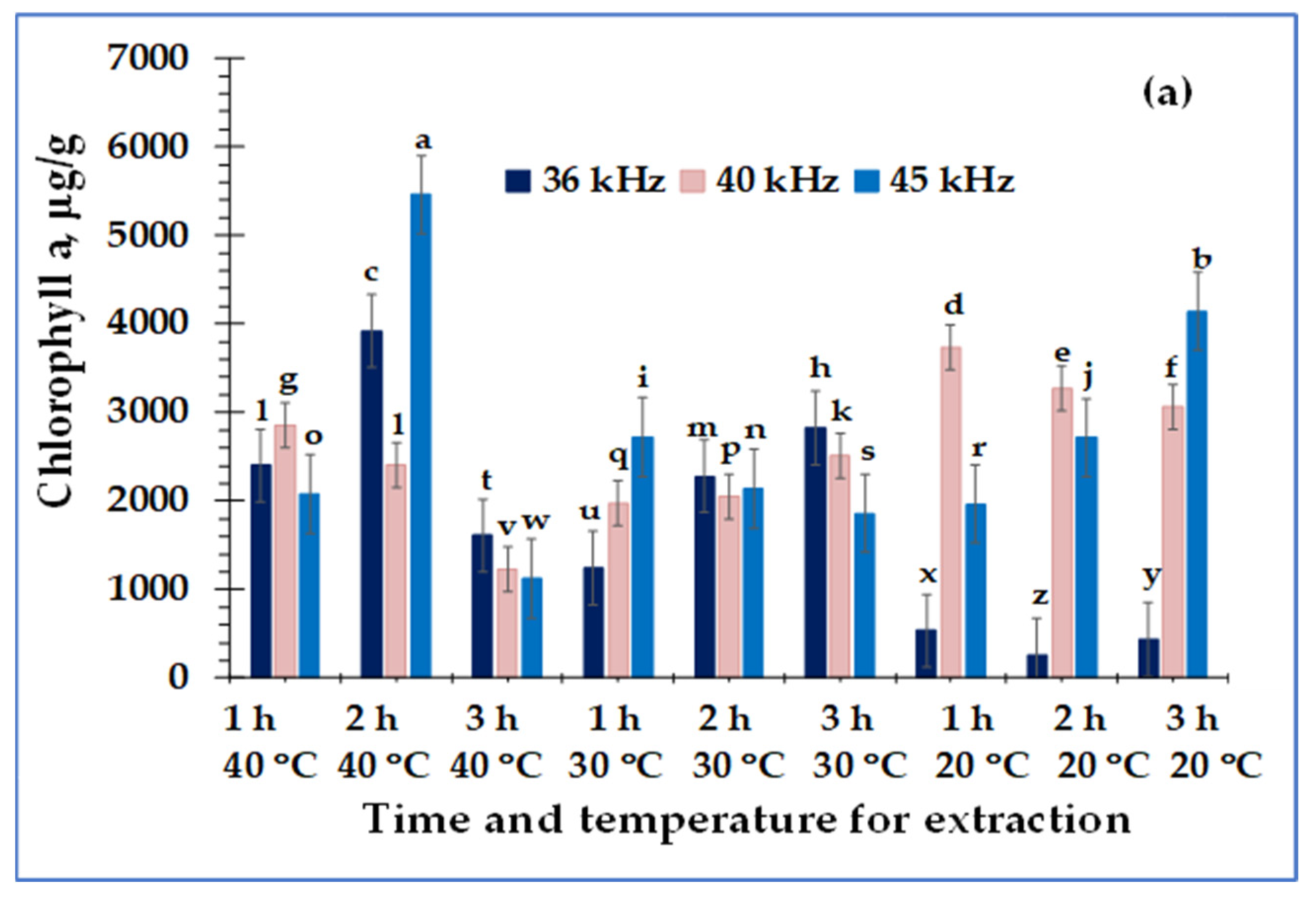
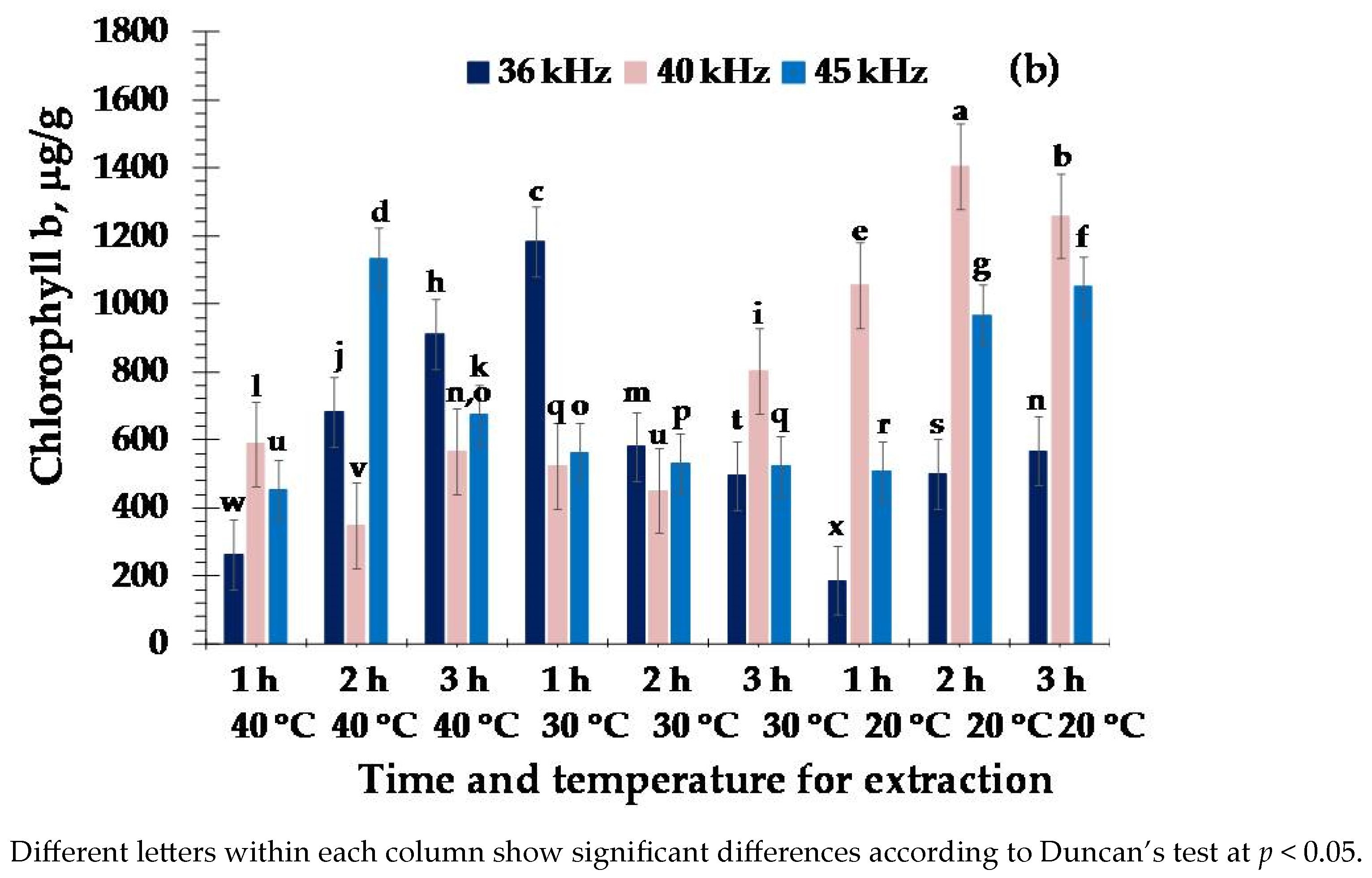
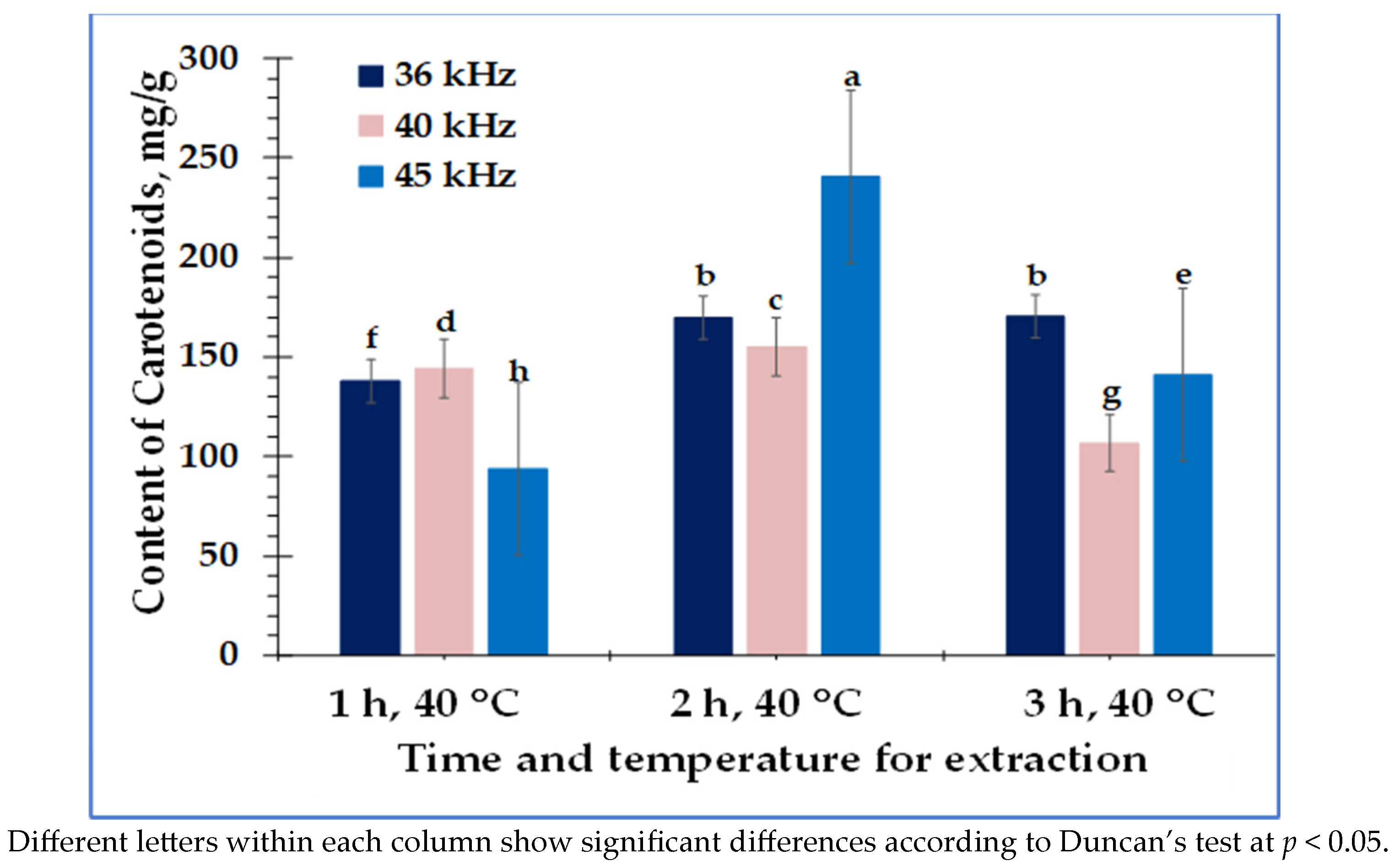
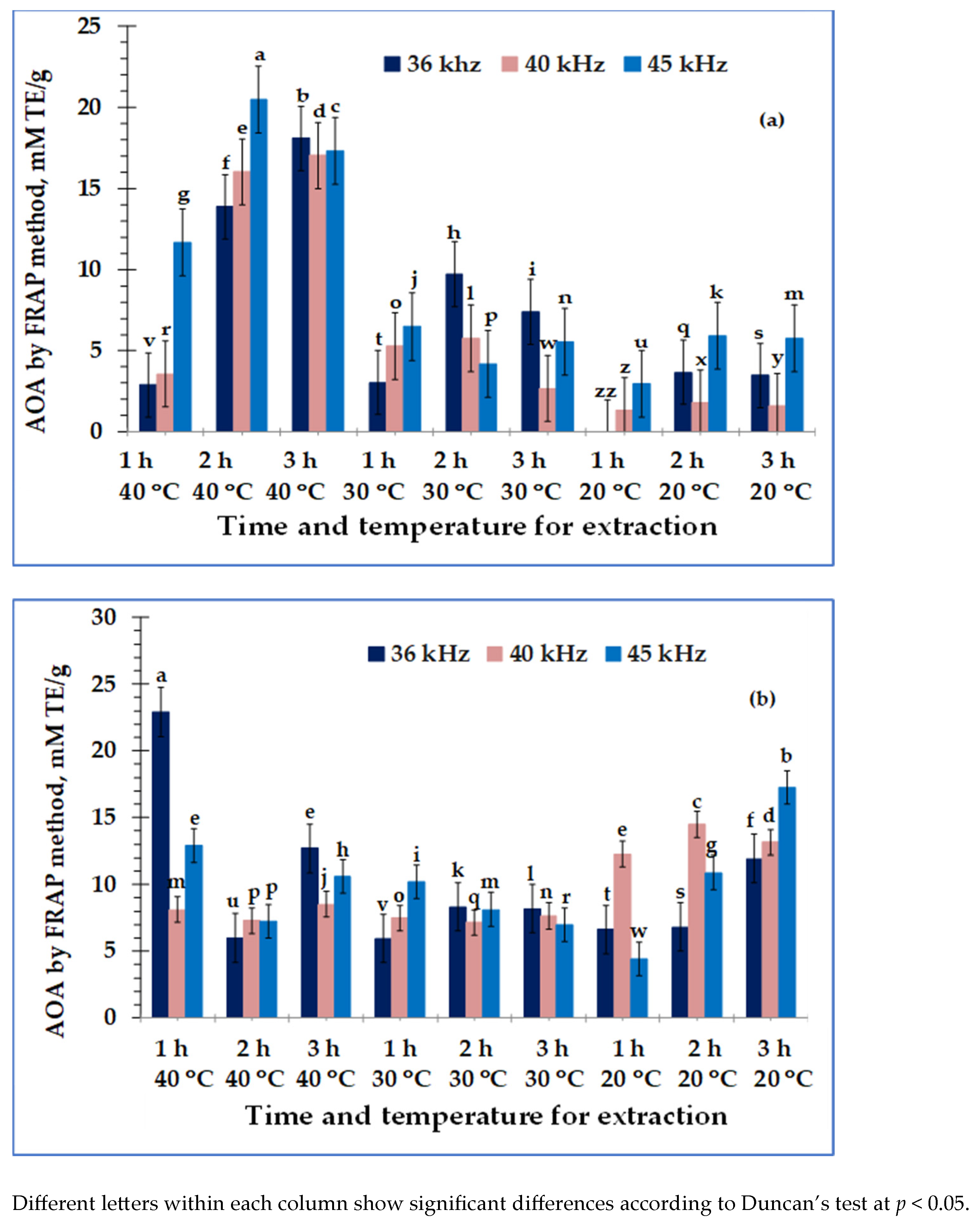
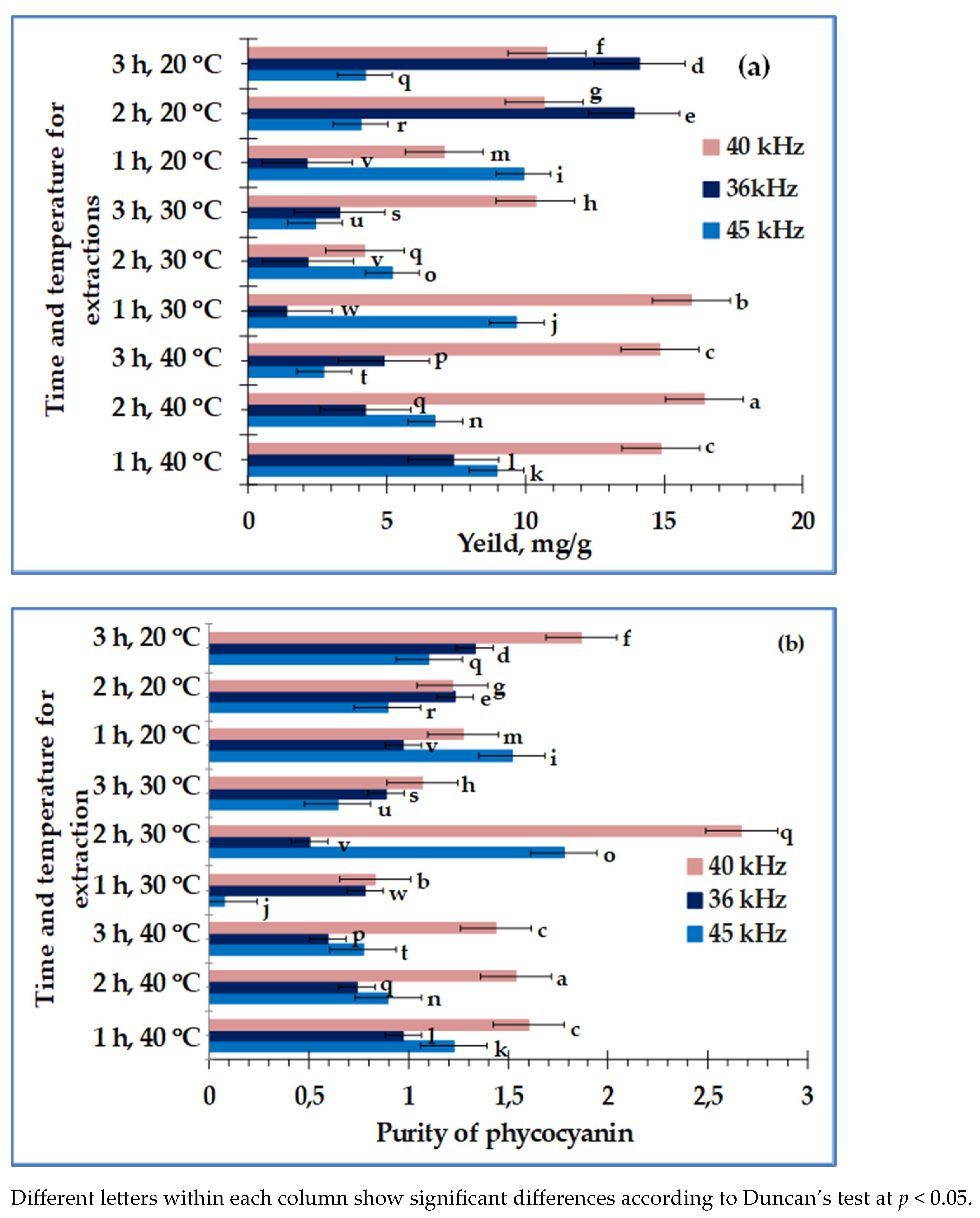
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, J.A.V.; Freitas, B.C.B.; Moraes, L.; Zaparoli, M.; Morais, M.G. Progress in the physicochemical treatment of microalgae biomass for value-added product recovery. Bioresour. Technol. 2020, 301, 122727. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): An overview. Grasas Aceites 2019, 70, e292. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Enhancement of neuroprotective effects of Spirulina platensis extract from a high-pressure homogenization process. Appl. Sci. 2018, 8, 634. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant Activities of Phycocyanin: A bioactive compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Abdelkhalek, N.K.M.; Ghazy, E.W.; Abdel-Daim, M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: Impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. 2015, 22, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímov’a, B.; Wan, D.; Kŭca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.L.; Wang, G.H.H.; Xiang, W.Z.Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Rame, R.; Nilawati, D.S.; Novarina, I.H.; Agus, P.; Harjanto, G.R.D. Cell-wall disruption and characterization of phycocyanin from microalgae: Spirulina platensis using Catalytic ozonation. In Proceedings of the 3rd International Conference on Energy, Environmental and Information System (ICENIS 2018), Semarang, Indonesia, 14–15 August 2018; 2018; Volume 73, pp. 1–4. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; Rangel-Yagui, C.O.; Carvalho, J.C.M.; Sato, S. Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenergy 2004, 26, 329–335. [Google Scholar] [CrossRef]
- Pawar, S.T.; Puranik, P.R. C-phycocyanin production by halotolerant cyanobacteria. PHYKOS-Off. J. Phycol. Soc. India 2014, 44, 25–32. [Google Scholar]
- Puzorjov, A.; McCormick, A.J. Phycobiliproteins from extreme environments and their potential applications. J. Exp. Bot. 2020, 71, 3827–3842. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid green extractions of C-phycocyanin from Arthrospira maxima for functional applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Kalavadia, B.; Trivedi, U.; Madamwar, D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata-Isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006, 41, 2017–2023. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Aftari, R.V.; Rezaei, K.; Bandani, A.R.; Mortazavi, A. Antioxidant activity optimisation of Spirulina platensis C-phycocyanin obtained by freeze-thaw, microwave-assisted and ultrasound-assisted extraction methods. Qual. Assur. Saf. Crops Foods 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Jain, T.; Jain, V.; Pandey, R.; Shukila, S.S. Microwave assisted extraction for phytoconstituents-an overview. Asian J. Res. Chem. 2009, 2, 19–25. [Google Scholar]
- Aftari, R.V.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. The optimized concentration and purity index of Spirulina platensis C-phycocyanin: A comparative study on microwave-assisted and ultrasound-assisted extraction methods. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- Viskari, P.J.; Colyer, C.L. Rapid extraction of phycobiliproteins from cultured cyanobacteria samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Pisanova, E. Chemical characterization of selected algae and cyanobacteria from Bulgaria as sources of compounds with antioxidant activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, I.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Abalde, J. Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef]
- Silveira, S.T.; Quines, L.K.D.M.; Burkert, C.A.V.; Kalil, S.J. Separation of phycocyanin from Spirulina platensis using ion exchange chromatography. Bioprocess Biosyst. Eng. 2008, 31, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Su, C.H.; Liu, C.S.; Yang, P.C.; Syu, K.S.; Chiuh, C.C. Solid-liquid extraction of phycocyanin from Spirulina platensis: Kinetic modeling of influential factors. Sep. Purif. Technol. 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.D.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Irawati, D.; Abdillah, A.A.; Pramono, H.; Sulmartiwi, L. The effect of different polar solvents on the stability of thermal extraction phycocyanin from Spirulina platensis. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012050. [Google Scholar] [CrossRef]
- Kamble, S.P.; Gaikar, R.B.; Padilla, R.B.; Shinde, K.D. Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluation of its antioxidant, anticoagulation, and prevention of DNA damage activity. J. Appl. Pharm. Sci. 2013, 3, 149–153. [Google Scholar] [CrossRef]
- Dejsungkranont, M.; Chisti, Y.; Sirisansaneeyakul, S. Simultaneous production of C-phycocyanin and extracellular polymeric substances by photoautotrophic cultures of Arthrospira platensis. J. Chem. Technol. Biotechnol. 2017, 92, 2709–2718. [Google Scholar] [CrossRef]
- Puglisi, R.; Biazzi, E.; Gesmundo, D.; Vanni, R.; Tava, A.; Cenadelli, S. The Antioxidant Activity of a Commercial and a Fractionated Phycocyanin on Human Skin Cells in Vitro. Molecules 2022, 27, 5276. [Google Scholar] [CrossRef] [PubMed]
- Rito-Palomares, M.; Nũnez, L.; Amador, D. Practical application of aqueous two-phase systems for developing a prototype process for c-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Figueira, F.S.; Moraes, C.C.; Kalil, S.J. C-phycocyanin purification: Multiple processes for different applications. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Fernandez-Rojas, B.; Hernandez-Juarez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Purohit, A.; Kumar, V.; Chownk, M.; Yadav, S.K. Processing-independent extracellular production of high purity index C-Phycocyanin from Spirulina platensis. ACS Biomater. Sci. Eng. 2019, 5, 3237–3245. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M. Two Classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Sivasankari, S.; Naganandhini, N.; Ravindran, D. Comparison of Different Extraction methods for Phycocyanin Extraction and Yield, from Spirulina platensis. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 904–909. [Google Scholar]
- Minchev, I.; Petkova, N.; Milkova-Tomova, I. Ultrasound-assisted extraction of chlorophylls and phycocyanin from Spirulina platensis. Biointerface Res. Appl. Chem. 2020, 11, 9296–9304. [Google Scholar]
- Larrosa, A.P.Q.; Camara, A.S.; Moura, J.M.; Pinto, L.A.A. Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Sci. Biotechnol. 2018, 27, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Stromdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Hernadez, A.C.; Nieves, I.; Meckes, M.; Chamorro, G.; Barron, B.L. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antivir. Res. 2002, 56, 279–285. [Google Scholar]
- García, J.L.; Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Clark, J.G.; Kostal, K.M.; Marino, B.A. Modulation of collagen production following bleomycin-induced pulmonary fibrosis in hamsters. The presence of a factor in the lung that increases fibroblast prostaglandin E2 and cAMP and suppresses fibroblast proliferation and collagen production. J. Biol. Chem. 1982, 257, 8098–8105. [Google Scholar] [CrossRef]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from Spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. J. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadeniz, F.; Koca, N.; Soyer, Y. Effect of heat treatment on chlorophyll degradation and color loss in green peas. Gida 2008, 33, 225–233. [Google Scholar]
- Wu, L.C.; Ho, J.A.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.D.; Prietto, L.; de Souza, M.M.; Furlong, E.B. Profile, antioxidant potential, and applicability of phenolic compounds extracted from Spirulina platensis. Afr. J. Biotechnol. 2015, 14, 2903–2909. [Google Scholar]
- Ali, H.E.A.; Shanab, S.M.M.; Abo-State, M.A.M.; Shalaby, E.A.A.; El Demerdash, U.M.N.; Abdullah, M.A. Evaluation of antioxidants, pigments and secondary metabolites contents in Spirulina platensis. Appl. Mech. Mater. 2014, 625, 160–163. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Safari, R.; Amiri, Z.R.; Kenari, R.E. Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2020, 19, 1911–1927. [Google Scholar] [CrossRef]

| Extraction Time, s | Phycocyanin Yield ± SD, mg g−1 Extract | Phycocyanin Purity Index ± SD | AOA by DPPH, mM TE g−1 Extract | AOA by FRAP, mM TE g−1 Extract |
|---|---|---|---|---|
| 60 | 4.58 a ± 0.23 | 1.17 a ± 0.07 | 13.31 b ± 0.56 | 1869.6 b ± 5.34 |
| 120 | 3.34 b ± 0.35 | 0.92 b ± 0.04 | 13.35 b ± 1.34 | 1595.6 c± 7.23 |
| 180 | 3.40 b ± 0.30 | 0.88 b ± 0.08 | 16.13 a ± 1.67 | 1925.4 a ± 11.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, K.; Petkova, N.; Mihaylova, D.; Gentscheva, G.; Gavrailov, G.; Pehlivanov, I.; Andonova, V. Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”. Separations 2024, 11, 57. https://doi.org/10.3390/separations11020057
Nikolova K, Petkova N, Mihaylova D, Gentscheva G, Gavrailov G, Pehlivanov I, Andonova V. Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”. Separations. 2024; 11(2):57. https://doi.org/10.3390/separations11020057
Chicago/Turabian StyleNikolova, Krastena, Nadezhda Petkova, Dasha Mihaylova, Galia Gentscheva, Georgi Gavrailov, Ivaylo Pehlivanov, and Velichka Andonova. 2024. "Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”" Separations 11, no. 2: 57. https://doi.org/10.3390/separations11020057
APA StyleNikolova, K., Petkova, N., Mihaylova, D., Gentscheva, G., Gavrailov, G., Pehlivanov, I., & Andonova, V. (2024). Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”. Separations, 11(2), 57. https://doi.org/10.3390/separations11020057










