Abstract
Producing defensible data for legal proceedings requires strict monitoring of sample integrity. In fire debris analysis, various approved packaging and storage solutions are designed to achieve this by preventing cross-contamination. This study examines the efficiency of current practices at preventing cross-contamination in the presence of a sample matrix (charred wood) via analysis by comprehensive multidimensional gas chromatography coupled with time-of-flight mass spectrometry (GC×GC-ToF MS). The transfer of ignitable liquid residue (ILR) was assessed by comparing percentages of the target ILR area relative to the total chromatogram area and applying chemometric tools developed to detect cross-contamination. All practices reduced cross-contamination in comparison to faulty packaging. Individual practices varied in their performance. Nylon-based packaging performed best, whereas commercial polyethylene-based packaging performed worst due to interfering compounds emitted from the material and sealing mechanism. Heat-sealing was the best sealing mechanism when applied correctly, followed by press-fit connections, and lastly, adhesive sealing. Refrigerated storage offered several advantages, with elevated impact for polyethylene-based packaging and adhesive sealing mechanisms. Triple-layer packaging practices did not show significant benefits over double-layers. The recommended packaging approach based on these findings is mixed-material packaging (metal quart can in a heat-sealed nylon bag), offering advanced prevention of cross-contamination and practical advantages with continued refrigeration during transport.
1. Introduction
Forensic analysis of fire debris plays a crucial role in determining whether ignitable liquids were used to initiate or accelerate a fire, which can indicate arson in conjunction with other forms of evidence. Presenting reliable and defensible data in court requires confidence in the integrity of each sample from the investigation—from collection to analysis to disposal. Cross-contamination can occur during the transport or storage of legal samples and is defined as an unwanted transfer between materials originating from several sources of evidence [1]. The volatile nature of ignitable liquid residue (ILR) evidence causes cross-contamination to occur as compound exchange in the gaseous phase, i.e., volatile organic compound (VOC) transfer. VOC transfer is influenced by factors that either promote or prevent the affinity of VOCs to move into headspace, including time, temperature, and pressure. In practice, cross-contamination is commonly prevented by using clean, non-contaminated, chemical-resistant, and vapor-tight containers for storage until extraction [2,3].
Studies have been conducted to test the performance of containers for fire debris analysis, including metal cans [2], glass mason jars [2,4], polyethylene containers [5], and commercially available sampling bags [2,5,6,7]. Metal and glass containers are known for their imperfect seal [2,4] but remain popular choices for their puncture resistance and easy availability [3]. In contrast, soft materials, including nylon and polyethylene bags, are not puncture-resistant and are susceptible to compound loss caused by adsorption onto packaging material due to its inherent porosity [7]. Research has shown that nylon bags do not retain polar compounds well, are not entirely vapor-proof, and exhibit high leak rates for certain brands [7,8]. Polyethylene materials have exhibited interfering VOC signatures depending on their manufacturing process and can degrade as a result of exposure to chemical cleaning products or higher temperatures [5,6,9].
As imperfect seals and resulting cross-contamination are well documented, practical measures have been adjusted to include recommendations for double-packaging [8] and the use of reliable sealing methods, such as heat sealing, where possible [7,10]. However, in field operations, heat-sealing may not be possible due to additional functionality requirements (e.g., equipment, power, space, sample size) [7]. To compensate for the increased risk of compound transfer or compound loss, multiple layers of packaging (3+) have been employed by fire investigators despite presenting challenges to sampling time, storage space, and general practicality. This practice is based on the principle that additional layers create more VOC boundaries to prevent the unwanted transfer of compounds. However, it has not been sufficiently investigated in the literature and does not consider the potential impact on sample integrity from VOC deposition originating at packaging materials and their seals (e.g., adhesives, rubber), as the analysis via gas chromatography coupled with mass spectrometry (GC-MS) may not provide the selectivity and/or sensitivity to consider the impact of these compounds on potential profile distortion. The focus of this packaging practice remains solely on preventing cross-contamination with other samples.
The process involving cross-contamination with other samples primarily depends on time and temperature. Storage time from the investigation site to receipt by the laboratory should be kept to a minimum. However, storage time may be outside the control of analysts and investigators on account of prolonged transport from remote sites, instrument availability, extended periods of time required for investigations, etc. Storage temperature, however, can be controlled for transport and storage. Elevated temperatures are known to degrade ignitable liquids and promote gaseous compound transfer. Frozen storage is generally recommended for long-term storage as it impedes gaseous transfer the most, thereby reducing the risk of cross-contamination and preserving sample integrity by reducing the potential degradation of the ILR on the fire debris sample. For transport and intermediate storage, which cover the period between receipt and processing of forensic samples in the analytical lab, frozen storage is considered impractical due to additional space and facility requirements, leaving a choice between room temperature and cool (refrigerated) storage. Cool storage is considered advantageous due to reduced reaction rates and microbial activity and is put into practice by using sample coolers and ice packs during transport, where possible. This can present challenges due to space requirements and uneven temperature distributions between samples. Changes in temperature can also affect the sealing ability of containers (material expansion and contraction, glue or rubber corrosion, localized freezing of water content) and the corresponding integrity of samples. Limited research exists on the effect of storage temperature on packaging materials and their sealing ability. Instead, storage temperature comparisons concentrate purely on the charcoal strip and often involve re-extraction of the same sample [11,12,13].
While existing literature does evaluate the suitability of packaging materials for ILR analysis, these evaluations are usually undertaken by GC-MS analysis without a background matrix present [2,4,6,7,9]. Although real-world fire debris samples involve complex matrix effects [13,14,15,16], cross-contamination studies are conducted on non-competing materials, e.g., wipes or cloth [8,11], or pure materials of interest without other interferants present [10]. Several of these studies use thermal desorption for the investigation [6,7], which circumvents the potential for ILR profile distortion caused by extraction saturation and subsequent displacement from adsorbate-adsorbate interactions [2,17] encountered in passive headspace extraction with activated charcoal.
Research on cross-contamination to date does not evaluate packaging using matrix interaction effects, despite their known occurrence. In wildfire debris analysis, the complexity of the matrix and the presence of combustion and pyrolysis compounds frequently lead to co-elutions with target ILR compounds. Comprehensive multidimensional gas chromatography (GC×GC) allows better separation between ILR compounds and complex sample matrix due to its increased separation potential and has been applied successfully in ILR analysis [13,18], and specifically wildfire investigations [13,15,19]. Cross-contamination research also does not evaluate the ability to retain sample integrity with different packaging layers of potentially mixed materials under various storage temperatures, despite this being an employed practice in arson investigations.
Chemometric analysis has been applied to ILR research—most frequently regarding detection and classification [13,20]—but is not commonly utilized in the context of sample integrity. GC×GC coupled with chemometric analysis showed promise in the detection and characterization of cross-contamination under matrix interference [21], with evaluation of false positive and false negative errors, indicating the presence of cross-contamination with confidence. In addition to matrix interaction, the increased layer of complexity that different packaging types and storage methods represent for ILR interpretation with advanced chromatographic separation is yet to be investigated.
This study continues to apply the established analytical workflow [19,22] and data analysis approach [21,22] described previously to evaluate packaging methods currently employed for fire debris. Charred wood chips allow for an investigation of a single competing matrix commonly encountered in wildfire debris [15,19], while excluding the additional complications from microbiome changes in soil [16,21,23,24]. This study aims to evaluate common packaging practices currently employed in wildfire investigations to advise on best practices combining practicality and preservation of sample integrity. Packaging and storage methods are emulated to test efficiency for retaining sample integrity between single- and multi-layer packaging using combinations of different materials, sealing mechanisms, and storage conditions.
2. Materials and Methods
2.1. Standards and Reagents
Standards and reagents were prepared as described by Boegelsack et al. [21]. All solvents [benzene (99.9%), carbon disulfide (99.9%), dichloromethane (99.9%), methanol (99.9%), and toluene (99.9%)], as well as individual standards for naphthalene-d8 (AC174960010) and ethylbenzene-d10 (AC321360010), were obtained from Fisher Scientific (Ottawa, ON, Canada). Additionally, standards for 1,3,5-trimethylbenzene-d12 (372374-1G) and 1,2,4,5-tetramethylbenzene-d14 (D-0269) were purchased from Sigma Aldrich (Supelco, Bellefonte, PA, USA) and CDN isotopes (Pointe-Claire, QC, Canada), respectively. Deuterated Kovats-Lee retention index mix (KLI mix) was procured from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA).
A recovery standard was prepared to monitor and correct for variance in extraction efficiency between individual activated charcoal strips (A-1503, Arrowhead Forensics, Lenexa, KS, USA). The recovery standard combined naphthalene-d8, ethylbenzene-d10, and 1,2,4,5-tetramethylbenzene-d14 in methanol. The internal standard mixture contained KLI mix and 1,3,5-trimethylbenzene-d12 in carbon disulfide. The compounds had a concentration of 500 ng/mL each. The gasoline sample (87% octane) is the same sample as used in Boegelsack et al. [21], purchased locally in Calgary, AB, Canada.
Three reference samples were used in this study: a matrix sample, a contaminated matrix sample, and a spiked matrix sample. The matrix sample is 50% charred wood chips; the contaminated matrix sample is the charred wood matrix exposed to cross-contamination with a gasoline source for 120 h; and the spiked background is the charred matrix spiked with 10 µL of gasoline.
2.2. Storage Experiment
The experimental design was completed in two phases, using a similar approach to an earlier study by Boegelsack et al. [21]. Phase one was conducted with a two-piece laboratory glovebox (Model 818-GBB—Plas-Labs, Lansing, MI, USA), sealed with stainless steel clamps to form an air- and water-tight barrier, at room temperature to compare the effectiveness of packaging types to mitigate uptake from an atmosphere impacted by an ignitable liquid. Packaging types included rigid and soft materials as primary containers in the form of metal quart cans and nylon or low-density polyethylene (LDPE) bags. Rigid containers are a popular choice as they are impervious to puncture, but they are restrictive in size and known to form imperfect seals. Soft materials are flexible for sizing and thereby better suited for transport and storage, but they are prone to puncture and can deposit unwanted VOC contributions. Since double- and triple packaging is common practice with soft materials, nylon, high-density polyethylene (HDPE), and LDPE materials were employed as secondary and tertiary packaging (see Figure 1). Various sealing mechanisms were tested on each packaging layer. Table 1 provides an overview of each packaging type and the respective sealing mechanisms under investigation.

Figure 1.
Packaging methods under investigation: (A) NBH (nylon bag, heat-sealed), (B) NBZ (nylon bag, goose-necked and ziptied), (C) RC (can with commercially available ring seal), (D) NBC (can in nylon bag, heat-sealed), (E) SB (can in commercial evidence bag), (F) DB (can in double-layer commercial evidence bag). Not pictured: NBSB (can in a commercial evidence bag in a nylon bag, heat-sealed) [refer to Table 1 for information on correlating materials and sealing mechanisms].

Table 1.
Packaging codes and their corresponding material types and sealing mechanisms.
Ten grams (10 g) of 50% charred wood chips as sample matrix were added to the primary packaging and sealed in accordance with their respective sealing types (see Table 1). For primary containment in metal quart cans (Uline), each can had a 1/16-inch hole drilled into the lid to mimic consistent leaks from a bad seal, as imperfect metal-on-metal connections are known to occur from field observations and published literature [2]. The hole was consistent with the reference method [21] and allowed a direct comparison between reference samples and packaging materials to evaluate the effectiveness of secondary and tertiary packaging in the event of a leak. Cans sealed with commercially available ring seals (RC) were exempt from the hole in the lid to have comparable results for their testing conditions.
Three replicates were prepared for each packaging type and labeled accordingly before being evenly distributed in the open glovebox with space left to allow neat gasoline to be added. The chamber was sealed, followed by 5 cycles of purging and filling with high-purity nitrogen (99%). Using the total headspace volume of the glovebox (1157 L—provided by the manufacturer), the volume of gasoline required to achieve approximately 2 ppm concentration in the headspace was calculated using the following equation:
where Vsolute is the volume of gasoline needed, Ctarget is the desired concentration, and Vheadspace is the volume of headspace in the glovebox. The calculated volume of gasoline was divided between the three vials and these were added to their designated locations through a transfer chamber before their lids were removed to allow VOC transfer to occur.
Vsolute = Ctarget × (Vheadspace/106)
After 120 h (5 days), samples for phase one (1-GB) were removed from the glovebox and immediately extracted in accordance with previously employed protocols [19] alongside a lab blank. Samples within a soft material primary container were transferred to a clean quart can for extraction. Recovery standard was added to each sample, and an activated charcoal strip (A-1503, Arrowhead Forensics, Lenexa, KS, USA) was attached to the lid. Any rigid primary sampling container with a drilled hole had its lid exchanged for a clean one without a hole. All samples were extracted in a temperature-controlled oven at 90 °C for 16 h. After extraction, all charcoal strips were transferred into pre-labeled GC vials and 1 mL of carbon disulfide with an internal standard added.
The second phase compared how different storage conditions affect the progression of cross-contamination for each packaging type (see Table 1). Cross-contamination processes primarily depend on time and temperature, according to gas transfer processes. Storage time frequently lies outside the control of analysts and investigators on account of prolonged transport from remote sites, instrument availability, extended periods of time required for investigations, etc. Temperature, however, can be controlled during storage and presents a choice between elevated temperature, room temperature, refrigerated, and frozen. Elevated temperatures are known to degrade ignitable liquids and promote gaseous compound transfer, and thus, should be avoided. Frozen storage is generally recommended for long-term storage as it impedes gaseous transfer the most, thereby reducing the risk of cross-contamination and preserving sample integrity by reducing chemical reaction rates. In this study, the focus lay on short- to intermediate-term storage conditions and how they impact cross-contamination rates during transport, covering the period between receipt and processing of forensic samples in the analytical lab. As such, only room temperature and refrigeration were under consideration in the study design. Temperatures were monitored with mercury thermometers.
For room temperature storage (2-GB), the glovebox from phase one was cleaned in accordance with manufacturing instructions. For refrigerated storage (2-FG), a laboratory fridge was prepared by monitoring that the operating temperature remained stable. Six replicates for each packaging type were prepared and labeled according to their packaging and storage method. All replicates were evenly spaced in the open glovebox, with space left for gasoline vials. After sealing the chamber, 5 cycles of purging and filling with high-purity nitrogen (99%) were conducted before fresh gasoline vials were moved to their designated locations through a transfer chamber. An adjusted volume of neat gasoline was also added to the laboratory fridge, ensuring that both storage methods equated to approximately 2 ppm concentration in their total headspace.
For the first 120 h, all samples remained in the glovebox to simulate initial packaging and transport conditions encountered in the field, as well as allow direct comparison to reference samples. After 120 h, samples were split into continued room temperature storage (2-GB) and refrigerated storage (2-FG), where they remained for an additional 168 h (7 days). At the conclusion of 288 h (12 days), all samples were removed from their storage and extracted as outlined for phase one of this study.
2.3. Analysis by GC×GC-TOFMS
Sample analysis follows previously established parameters from the method developed in Boegelsack et al. [19]. An Agilent 7890A GC (Palo Alto, CA, USA), retrofitted with an Insight flow modulator (Sepsolve, Peterborough, UK) and coupled to a Markes BenchTOF-Select mass spectrometer (Llantrisant, UK), was operated with helium (5.0 grade) as carrier gas at an average linear velocity of 4.0 cm s−1. The injector was kept at 250 °C and operated with a 5:1 split ratio. Injections were performed via an Agilent G4567A (Palo Alto, CA, USA) autosampler at 1 μL volumes. Operating at a flow ratio of 40, the column set included a non-polar, 5% diphenyl column (Restek, Bellefonte, PA, USA; 25 m × 0.18 mm i.d. 0.18 µm film thickness) at 0.5 mL/min flow rate in the first dimension and a semi-polar, 50% diphenyl column (Restek, Bellefonte, PA, USA; 5 m × 0.25 mm i.d. 0.18 µm film thickness) at 20 mL/min flow in the second dimension. The modulator set up consisted of a 25 cm × 0.53 mm sample loop and a 2 m length × 0.1 mm i.d. bleed line, operating at a 4 s modulation period PM. The transfer line to the TOF MS was 0.8 m × 0.25 mm, with a bleed line of 2 m × 0.25 mm going to the FID outlet. The oven ramp started at an initial temperature of 40 °C held for 5 min, followed by a 4 °C/min hold to 280 °C and a 3-min hold at the final temperature. The MS transfer line and ion source were operated at 250 °C. The electron energy applied was 70 eV, and the scanned mass range was 50–400 m/z in electron ionization mode at a 50 Hz acquisition rate.
2.4. Data Acquisition, Processing, and Analysis
Data was acquired and processed using ChromCompare+ (V2.1.7, Sepsolve, Peterborough, UK). Deconvolution was applied after alignment and baseline correction. Integration parameters included a minimum ion count of 300, a minimum absolute area and height of 1000, and peak merging at 10% overlap. For comparable analysis with the reference samples [21], a total of 128 target compounds were analyzed in each sample, including internal and recovery standards (n = 10). Table 2 shows native target compounds in their respective compound groups as used in Boegelsack et al. [21], with “Other” compound groups denoting additional compounds found statistically significant to differentiate cross-contamination in that study. In the context of ILR analysis, compound groups can further be differentiated as light, medium, or heavy with regard to their volatility. Light compounds (most volatile) refer to an equivalent n-alkane range up to C9; heavy compounds (least volatile) refer to larger compounds with an equivalent n-alkane range starting at C9 and exceeding C20; and medium compounds are considered within the equivalent n-alkane range of C8 to C13. The detailed strategy for analyte identification can be found in the Supplementary Materials (Figure S1).

Table 2.
Target compounds in affiliation with their respective compound groups. (Other references: non-traditional compounds of interest identified in Boegelsack et al. [21]).
Internal and recovery standard corrections were applied to minimize the effects of variance caused by instrumental or extraction performance. Data tabulation, graphing, and analysis of variance (ANOVA) were completed in Microsoft Excel 365 (Microsoft, Redmond, WA, USA).
The chemometric analysis was accomplished using ChromCompare+ and followed the approach developed in Boegelsack et al. [21]. Using the model developed therein, the packaged samples were compared to the applicable matrix (wood). Each sample was aligned with the automated alignment feature, which deploys a localized compression and decompression algorithm across chromatograms. Both targeted and untargeted analyses had built an unsupervised principal component analysis (PCA), either with its respective list of target compounds (targeted) or using the tile-based fisher ratio approach (untargeted) across chromatograms. The data for each approach was normalized using probabilistic quotient normalization (PQN) to the mean and compared to the established predictive models. Each model employed a random forest design and used the nearest neighbor algorithm for class prediction.
3. Results and Discussion
3.1. Total Chromatogram Analysis
An initial assessment of the effectiveness of different packaging types was conducted by comparing the relative percentages of the target ILR compound areas to the total chromatogram areas (Table 3).

Table 3.
Average target compound areas expressed as a percentage of the total chromatogram area for reference samples and packaging types, with their respective standard deviations in brackets. * Outlier removed.
Using a single-factor ANOVA with α = 95%, the three different reference samples were found to be significantly different (F value >> Fcrit and p << 0.05). Samples NBH (nylon bag, heat-sealed), NBZ (nylon bag, goose-necked and zip-tied), and NBC (metal can packaged in a heat-sealed nylon bag) were statistically insignificant with the matrix background, while SBNB (metal can in commercial evidence bag in a heat-sealed nylon bag) and RC (metal can with a commercially available ring seal) were statistically insignificant with the contaminated matrix. Samples SB (metal can in commercial evidence bag) and DB (can in double-layer commercial evidence bag) are considered significantly different from all reference matrix samples, falling between contaminated and spiked reference samples. Approximating the effectiveness of packaging methods at inhibiting cross-contamination by evaluating relative target compound areas suggests the following order of effectiveness: NBH > NBC > NBZ > SBNB > RC > SB > DB.
These results were supplemented by chemometric analysis, which showed a classification of negative for NBH and NBC, a mixture of negative (1-GB, 2-FG) and contaminated (2-GB) for NBZ, contaminated for RC and SBNB, and positive for SB and DB. (Select chromatograms to illustrate the visual difference, which are attached in Figure S2).
Williams and Sigman [2] observed ILR profile distortion effects caused by various leak rates between container types, which do not apply to relative target area analysis. Distortion effects impact both background and ILR compounds in the same manner, by displacing very volatile compounds with less volatile compounds. This does not affect the relative area of target compounds as all volatility ranges are summed, thereby circumventing their ability to differentiate between packaging types. Outliers from faulty seals can likewise be recognized using either absolute areas (as described in [2]) or relative target compound areas as used herein (see NBH 2-FG).
Under current packaging practice, a metal quart can is commonly double- or triple-bagged in polyethylene bags with a self-adhesive sealing mechanism. Concurrent with other findings [7,10], heat-sealing (NBH, NBC, SBNB) is the most effective sealing mechanism when compared with zip-tied goose neck closure (NBZ), press fit (RC), or self-adhesive sealing (SB, DB, SBNB). In addition, double-bagging a quart can showed no advantage over single-bagging, with both methods statistically considered a single grouping. The exception to this was presented when a mixture of materials was employed with better performance of SBNB compared to both SB and DB. While existing literature stresses the importance of double-packaging in cases of imperfect seals on the primary container [8], additional layers are not commonly studied, especially when including mixtures of packaging materials or sealing mechanisms. Based on the results in Table 3, there are no benefits to including more than two layers of packaging. The benefit SBNB provides over SB can be attributed to the packaging material. The single heat-sealed nylon layer ranked better than any of the polyethylene packaging methods, including SBNB.
Nylon-based packaging materials outperform other materials at preventing cross-contamination by displaying the lowest relative target compound area. This currently does not take into account the potential contribution of interfering VOC signatures from polyethylene-based materials, as shown in the literature [2,4,5,6,7,9,10], which was investigated by examining compound class distributions between packaging methods.
3.2. Compound Class Analysis
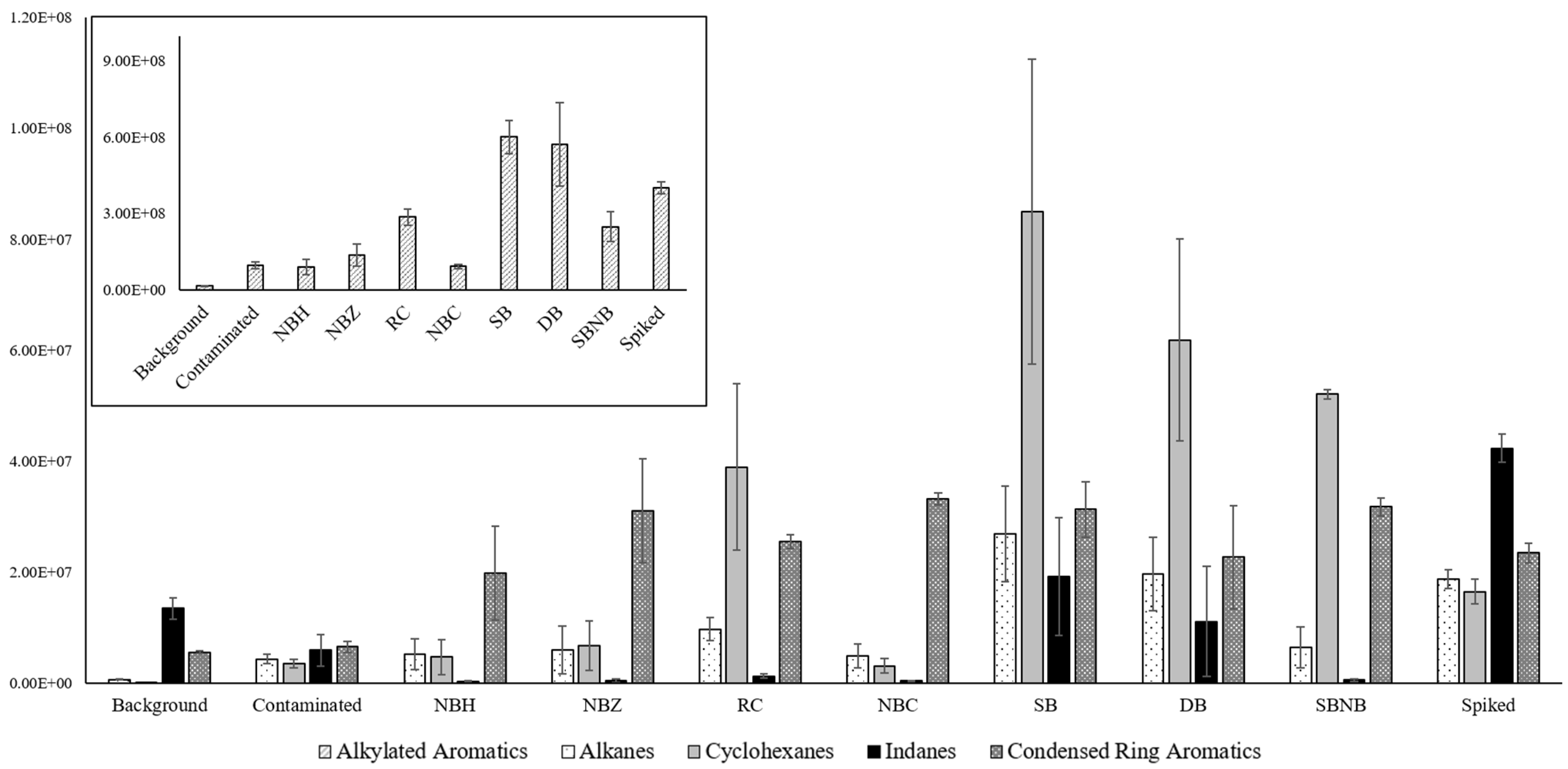
A relative area comparison approach as described above was deemed unreliable to analyze compound classes, as minor changes in one compound group would affect subsequent results of other groups as an effect of normalization and potentially misrepresent direct comparisons. Instead, a direct comparison of absolute abundances was chosen with the understanding that total areas between chromatograms would differ, but all results adhered to the same order of magnitude for signal (×109). Relevant abundances for various compound classes of interest in ILR analysis are shown in Figure 2.
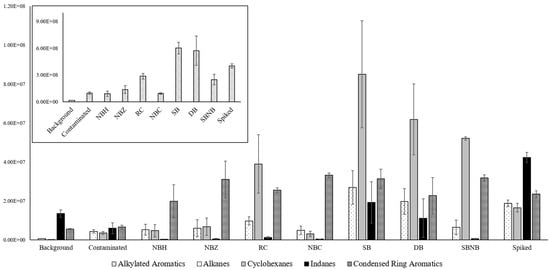
Figure 2.
Absolute compound class distribution for references and packaging types comparing averages across replicates with standard deviation error bars for compound classes of interest in ILR analysis. Due to the vast difference in abundance, n-alkanes, cycloalkanes, indanes, and condensed ring aromatics are displayed on the y-axis at the ×107 scale (foreground), and alkylated aromatics are displayed on the y-axis at the ×108 scale (top left).
Figure 2 shows that alkylated aromatics are the most abundant compound class across all samples. Alkanes, cyclohexanes, and alkylated aromatics follow the same distribution pattern, with lower abundances in nylon-based packaging, roughly equivalent to contaminated reference samples, and very high contributions in polyethylene-based packaging, which compare to or exceed those of spiked reference samples. Indanes are largely absent in nylon packaging and RC, but do have a higher presence than background reference levels and lower than spiked reference levels in SB and DB. Lastly, condensed ring aromatics are elevated at spiked background levels (NBH, DB) or higher in all packaging types.
There is broad agreement in the literature that nylon does not contribute significant background signal [7,8] or reduces signal when blended with polyethylene [10], whereas polyethylene materials can contribute a wide range of compounds, including alkanes, alkenes, aldehydes, ketones, and other carbohydrates, including alkylated aromatics [2,5,6,7]. In some cases, background contributions can render polyethylene containers unusable for fire debris storage [9], which led to adding the condition of only using approved containers for collection and storage. Beyond the material of the containers in use, sealing mechanisms can also contribute compounds when rubbers or adhesives are used [10]. Where the material additions explain the vast difference in compound group abundance between nylon- and polyethylene-based packaging types, the sealing mechanism likely adds a significant contribution to the alkylated aromatics apparent between RC and SB/DB.
In addition to the background contributions of polyethylene materials, nylon is also known to have higher leak rates and partial absorbance onto the material for alkylated aromatics in particular [7]. The comparative increase in condensed ring aromatics of NBC to NBH could be an indicator that this effect is diminished when using nylon as secondary containment as opposed to primary containment. This confirms that authentic sample composition is preserved in the metal container, which also provides additional benefits of rigidity and heat resistance for extraction. As a result, the ranking for most effective packaging method changes slightly from the relative comparison, putting NBC as the best option.
The comparative increase in uptake for NBZ when compared to NBH further confirms that heat-sealing is the best method of closure by avoiding both compound loss from imperfect seals and compound deposition sealing materials (e.g., rubber, adhesives). These findings concur with available literature [7,10] and observations in field application.
Figure 2 shows a slight advantage to triple packaging, as DB has lower average absolute areas for compound classes of interest compared to SB. Regardless, interpretation would be unaffected as it is based on relative comparisons, and Table 3 did not yield a significant difference between the two packaging groups.
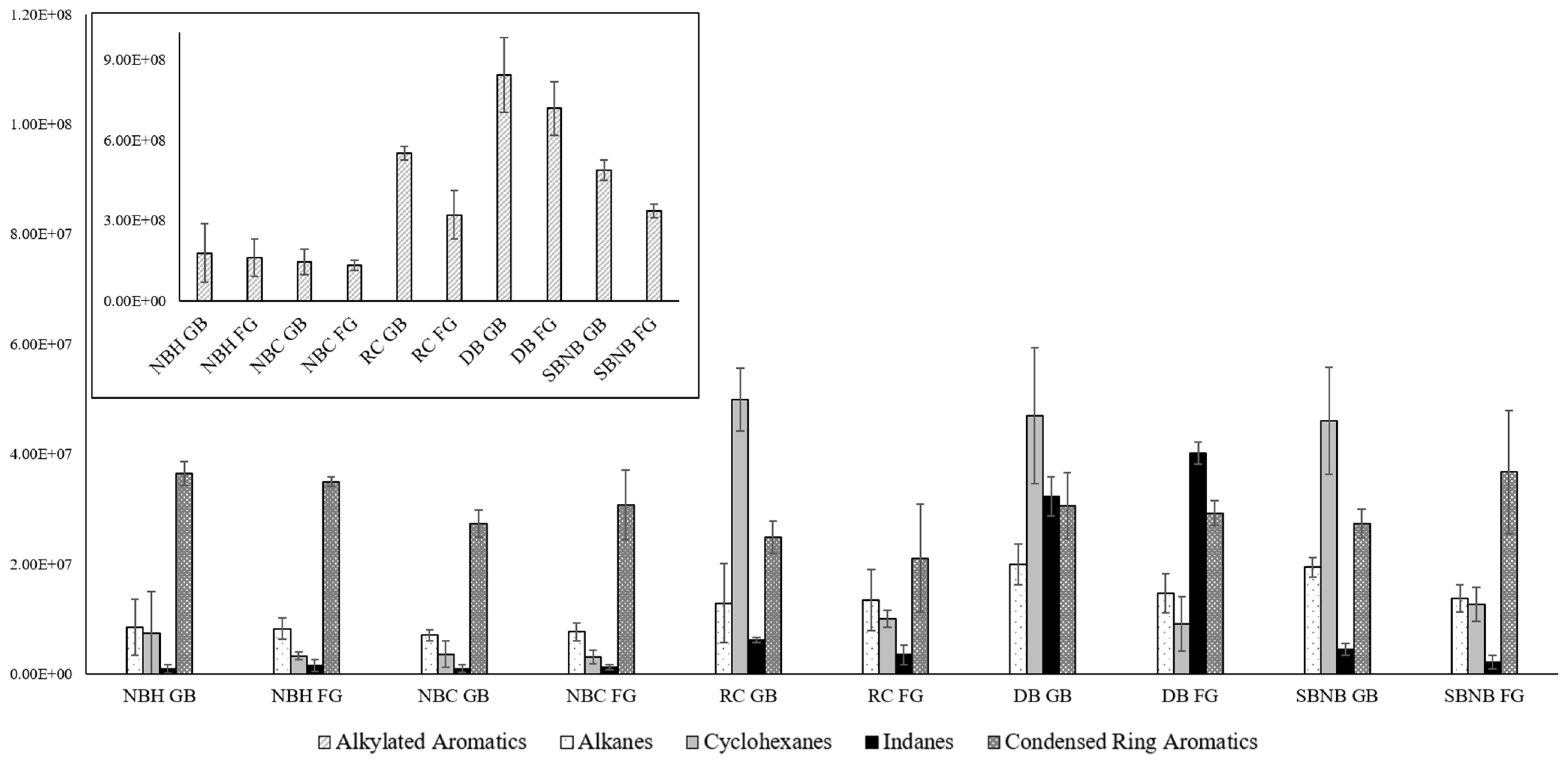
Confirmation of the background contribution in polyethylene-based materials versus potential increased leakage/absorption onto nylon-based materials was cross-checked when investigating the ideal storage temperature for intermediate storage length. Using the best performing methods for each representative packaging type, NBH for single packaging, NBC and RC for double packaging, as well as DB and SBNB for triple packaging, Figure 3 shows the absolute abundances of compound classes of interest in ILR analysis for each packaging type at room temperature and refrigerated storage for time point 2.
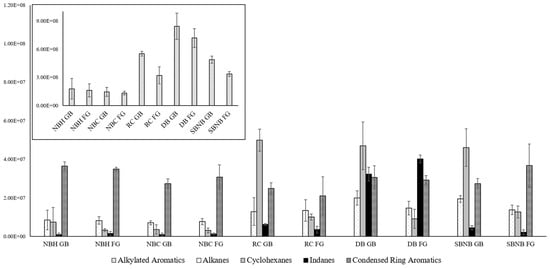
Figure 3.
Absolute compound class distribution between storage temperatures (GB—room temperature, FG—refrigerated) for select packaging types comparing averages across replicates with standard deviation error bars for compound classes of interest in ILR analysis. Due to the vast difference in abundance, n-alkanes, cycloalkanes, indanes, and condensed ring aromatics are displayed on the y-axis at the ×107 scale (foreground), and alkylated aromatics are displayed on the y-axis at the ×108 scale (top left).
Figure 3 shows that nylon-based packaging (NBH, NBC) has an approximately even distribution of compound classes and signal strength between storage temperatures, whereas polyethylene-based packaging (RC, DB, SBNB) has lower signal abundance for refrigerated storage for alkylated aromatics and cyclohexanes, with alkanes following the same trend only for LDPE (DB, SBNB). This confirms that these compounds are contributed by packaging materials while simultaneously confirming that refrigerated storage is advantageous by reducing transfer and reaction rates from packaging materials. An increase in abundance for indanes and condensed ring aromatics at refrigerated storage can be observed for DB and SBNB, respectively. As this increase likewise correlates to a decrease in alkylated aromatics, it is possible that they are caused by carbon strip saturation leading to ILR profile distortion, as described in Williams et al. [17]. The signal abundance for each compound group of interest approximately doubled in comparison to time point 1. Total chromatogram areas did not always double, particularly for polyethylene-based samples, suggesting that carbon strip saturation occurred for select time point 2 extractions. This effect is a known possibility when using carbon strip extractions and is a common occurrence when dealing with real samples of unknown concentrations. While the distribution across volatility ranges (i.e., light, medium, heavy) is skewed, compounds contributed from the background or ILR are affected in the same manner, and conclusions about the presence or absence of ILR profiles can still be drawn.
NBH FG did contain a single outlier where the seal had failed, which showed a considerable increase in alkylated aromatic and indane signals contributed from the fridge background at much higher concentrations than the original gasoline source. These imperfect seals cannot be detected by visual inspection, similar to imperfect metal-on-metal press-fit connections. Uptake from the fridge environment as a result of potential failed seals cannot be excluded for DB and SBNB, although it is considered highly unlikely that all seals failed.
In general, refrigerated storage has shown distinct advantages for polyethylene-based packaging materials, with limited impact on nylon-based packaging. Regardless of packaging type, reaction rates are inhibited by lower temperatures. As a result, cool storage is recommended as a supplementary preventative measure for inhibiting cross-contamination processes and matrix interaction effects for potential degradation or distortion of ILR profiles.
3.3. Individual Compound Analysis
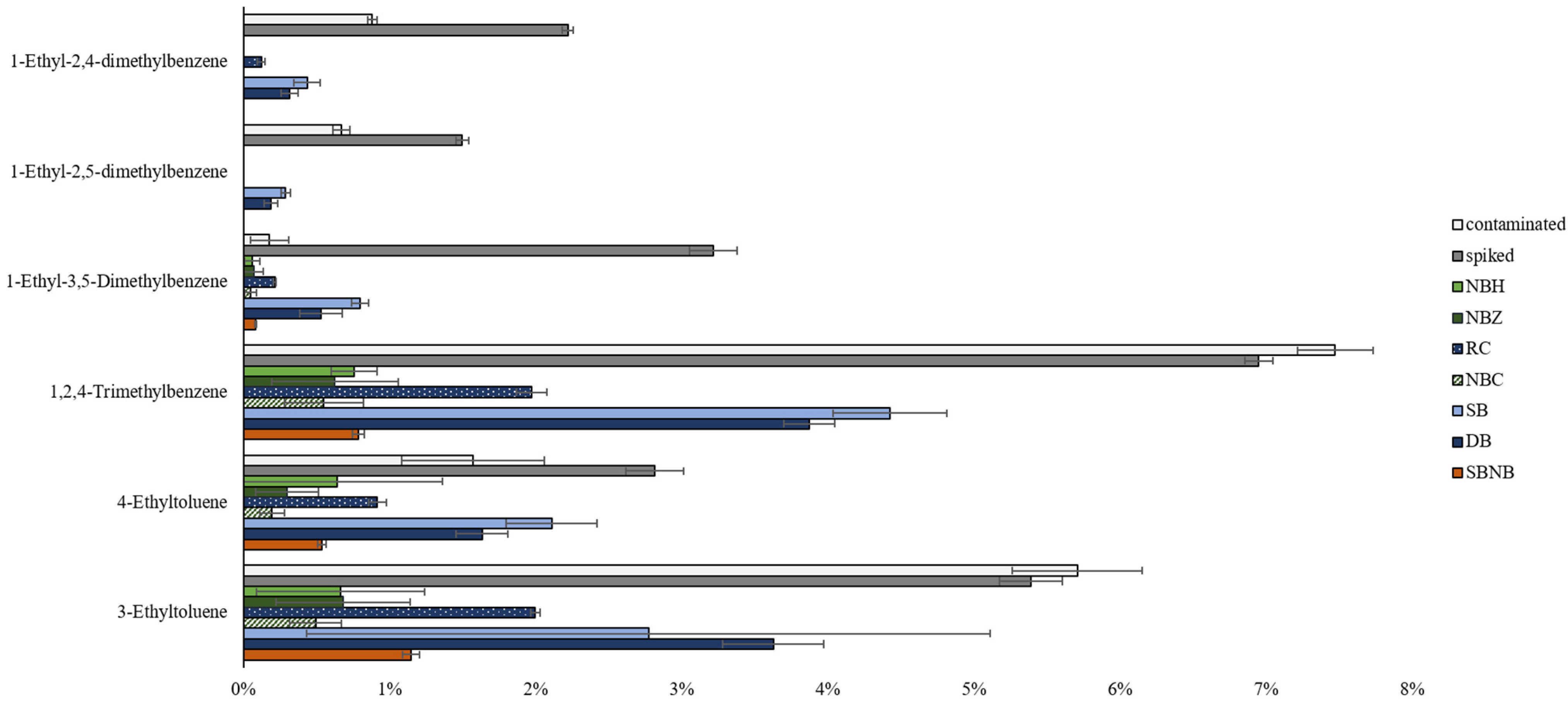
Increased leakage or adsorption onto packaging materials for nylon-based packaging could not be sufficiently investigated via group-type analysis. Thus, individual compound comparisons were investigated. Mid-range alkylated aromatics are common marker compounds used to differentiate cross-contamination from positive ILR samples, especially when used in relative abundance in lighter and/or heavier compound ranges [21].
Untargeted chemometric analysis from the detection and characterization of cross-contamination study [21] identified statistically significant alkylated aromatics via PCA and volcano plot interrogations to differentiate between negative, contaminated, and positive reference clusters. The displayed compounds in Figure 4 are largely responsible for the classification of each sample in the respective targeted and untargeted chemometric models. The negative reference sample, non-impacted wood background, did not contain any of these compounds, whereas concentrations expressed as percentages of the total chromatogram area are depicted in Figure 4 for reference samples and packaging types under investigation at time point 1.
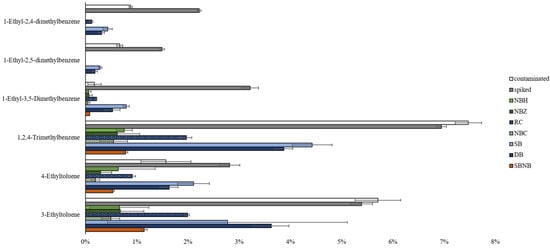
Figure 4.
Statistically significant compounds (sorted along the y-axis in order of volatility, with the most volatile at the bottom) determined in untargeted PCA and volcano plot interrogations by Boegelsack et al. [21] expressed as a percentage of the total chromatogram area (x-axis) with standard deviation error bars by packaging type as compared to a contaminated and spiked reference sample. (Note: the background matrix does not contain any of these compounds).
The trend between contaminated and spiked references shows higher concentrations of more volatile compounds in contaminated samples and higher concentrations of less volatile compounds in spiked samples, as follows: the overall expectations for cross-contamination transfer based on vapor pressures. The exceptions are 4-ethyltoluene and 1-ethyl-3,5-dimethylbenzene, which could mean a higher degree of matrix interaction with wood, resulting in a lower relative abundance than expected for the contaminated reference.
As Figure 4 shows, all packaging types display a lower percentage of relative abundance, signifying that all packaging types reduced the rate of compound transfer. Nylon-based packaging shows lower concentrations for all marker compounds than polyethylene-based packaging materials. The observed trend of compound concentration decreasing with increasing volatility suggests that this is due to successfully inhibiting the occurrence of cross-contamination. If this trend was related to selective compound loss as observed in the literature [7], it would be expected to occur more prominently where nylon is the primary material (NBH, NBZ) but not where it constitutes the secondary layer (NBC, SBNB). As this is not the case, results from Figure 4 suggest nylon-based material is more effective at inhibiting cross-contamination in comparison to polyethylene-based packaging. In addition, Figure 4 confirms that NBC is the most effective choice of packaging, which further provides the practical benefit of having a rigid, puncture-resistant container as primary packaging.
4. Conclusions
Analysis of forensic packaging effectiveness and related prevention of cross-contamination commonly assumes that potential VOC uptake will be strictly based on the vapor pressure of relevant compounds, occurring as early uptake of more volatile compounds and later uptake of less volatile compounds. As a result, it is usually studied under non-competing or no matrix conditions. In a recent study [21], we confirmed interaction effects and matrix-dependent displacement to occur during cross-contamination processes, affecting both the detection and characterization of cross-contamination.
Initial packaging comparisons showed that all packaging materials were effective in reducing cross-contamination but varied in their performance. Nylon-based packaging performed best with the lowest relative percentage of ILR target compounds present. This could be partially attributed to polyethylene-based packaging emitting VOC signatures in the compound groups of interest, which was further impacted by a self-adhesive sealing mechanism. In combination, the sealing mechanism and LDPE bags largely contributed alkanes, cyclohexanes, and alkylated aromatics. Nylon-based packaging, on the other hand, is suspected to have higher adsorption and selective leak rates, leading to increased loss of indanes and mid-range alkylated aromatics. No controls for this interaction were included in the study design, but the comparative performances of NBH and NBC suggest this to be a minor effect if present.
Chemometric analysis showed a high degree of sensitivity in the detection of cross-contamination gained from GC×GC analysis. It further delivered proof-of-concept confirmation under simulated fire debris conditions for potential application in packaging evaluations. The results obtained herein confirm the potential for further development of chemometric tools as ILR interpretation aids, despite the complex sample matrix composition and low ILR concentration.
The results for packaging evaluation in this study suggest that triple packaging with the same material type does not offer significant benefits. Although this practice assumes that additional layers of packaging will preserve sample integrity better, this could not be confirmed. In fact, it may create the potential for more VOC deposition, depending on the composition of the packaging material and the sealing mechanism. The advantages of refrigerated storage were evident in the reduction of chemical transmission and reaction rates, particularly where polyethylene bags are concerned. Heat-sealing was shown to be the most effective sealing mechanism, as evident by the reduced loss of compounds of interest. Lastly, the importance of clean storage conditions was highlighted when compounds from the impacted fridge could be detected in samples with compromised seals despite preferred storage conditions.
A combined method of metal quart cans, packaged in a heat-sealed nylon bag showed the best performance at preventing cross-contamination and retaining sample integrity while simultaneously offering the practical advantages of a puncture- and heat-resistant primary container that can be used for extractions without further manual handling of the sample.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations11020058/s1, Figure S1. Analyte identification strategy and corresponding identification confidence for target compounds in this study. Figure S2. Example chromatograms of wood matrix blank (A), spiked wood matrix (B), NBC-2GB (C), and DB-2GB (D), highlighting the chromatographic location of statistically significant target compounds from chemometric analysis: 3-Ethyltoluene (1), 4-Ethyltoluene (2), 1,2,4-Trimethylbenzene (3), 1-Ethyl-3,5-Dimethylbenzene (4), 1-Ethyl-2,5-Dimethylbenzene (5), and 1-Ethyl-2,4-Dimethylbenzene (6). With the exception of the matrix blank, where none of the target compounds were present, each chromatogram also displays the mass spectral comparison for 1,2,4-Trimethylbenzene (3) between the obtained spectrum (red, top) and the library spectrum (blue, bottom).
Author Contributions
N.B.: Conceptualization, Methodology, Investigation, Formal Analysis, Validation, Writing—Original Draft, Writing—Review and Editing, Visualization. J.W.: Investigation. C.D.S.: Conceptualization, Writing—Review and Editing. J.M.W.: Writing—Review and Editing, Funding Acquisition. D.W.M.: Writing—Review and Editing, Funding Acquisition. G.O.: Conceptualization, Methodology, Investigation, Resources, Funding Acquisition, Writing—Review and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Council of Canada (NSERC), grant number DWM 2020-04014, the Canada Foundation for Innovation (CFI), project number 40177, and the Wildfire Management Science and Technology Fund, Alberta Agriculture and Forestry.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Dr. Court Sandau was employed by the company Chemistry Matters Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- National Institute of Justice. Glossary for Crime Scene Investigation: Guides for Law Enforcement; U.S. Department of Justice: Washington, DC, USA, 2009. Available online: https://nij.ojp.gov/topics/articles/glossary-crime-scene-investigation-guides-law-enforcement (accessed on 10 June 2023).
- Williams, M.R.; Sigman, M. Performance Testing of Commercial Containers for Collection and Storage of Fire Debris Evidence. J. Forensic Sci. 2007, 52, 579–585. [Google Scholar] [CrossRef]
- Stauffer, E.; Dolan, J.A.; Newman, R. Fire Debris Analysis; Academic Press: Burlington, NJ, USA, 2008. [Google Scholar]
- Lang, T. A Study of Contamination in Fire Debris Containers. Can. Soc. Forensic Sci. J. 1999, 32, 75–83. [Google Scholar] [CrossRef]
- Borusiewicz, R.; Kowalski, R. Volatile organic compounds in polyethylene bags—A forensic perspective. Forensic Sci. Int. 2016, 266, 462–468. [Google Scholar] [CrossRef]
- Borusiewicz, R. Comparison of New Ampac Bags and FireDebrisPAK Bags as Packaging for Fire Debris Analysis. J. Forensic Sci. 2012, 57, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Grutters, M.M.P.; Dogger, J.; Hendrikse, J.N. Performance Testing of the New AMPAC Fire Debris Bag Against Three Other Commercial Fire Debris Bags. J. Forensic Sci. 2012, 57, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Belchior, F.; Andrews, S. Evaluation of Cross-contamination of Nylon Bags with Heavy-loaded Gasoline Fire Debris and with Automotive Paint Thinner. J. Forensic Sci. 2016, 61, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Tontarski, R.E.J. Evaluation of polyethylene containers used to collect evidence for accelerant collection. J. Forensic Sci. JFSCA 1983, 28, 440–445. [Google Scholar] [CrossRef]
- Saiz, J.; Ferrando, J.-L.; Atoche, J.-C.; Garcia-Ruiz, C. Study of the suitability of DUO plastic bags for the storage of dynamites. Forensic Sci. Int. 2013, 232, e33–e37. [Google Scholar] [CrossRef] [PubMed]
- Baerncopf, J.; Hutches, K. Evaluation of long term preservation of ignitable liquids adsorbed onto charcoal strips: 0 to 2 years. Forensic Chem. 2020, 18, 100234. [Google Scholar] [CrossRef]
- Sandercock, P. Retention of Gasoline and Diesel Fuel Samples on Charcoal: Evaluation of Long Term Preservation of Petroleum Residues. Can. Soc. Forensic Sci. J. 1997, 30, 219–224. [Google Scholar] [CrossRef]
- Evans, M. Interpol review of fire debris analysis and fire investigation 2019–2022. Forensic Sci. Int. Synerg. 2023, 6, 100310. [Google Scholar] [CrossRef]
- Borusiewicz, R. Substrate interferences in identifying flammable liquids in food, environmental and biological samples: Case studies. Sci. Justice 2015, 55, 176–180. [Google Scholar] [CrossRef]
- Kates, L.N.; Richards, P.I.; Sandau, C.D. The application of comprehensive two-dimensional gas chromatography to the analysis of wildfire debris for ignitable liquid residue. Forensic Sci. Int. 2020, 310, 110256. [Google Scholar] [CrossRef] [PubMed]
- Calle, J.; Ferreiro-González, M.; Aliaño-González, M.; Barbero, G.; Palma, M. Characterization of Biodegraded Ignitable Liquids by Headspace–Ion Mobility Spectrometry. Sensors 2020, 20, 6005. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Fernandes, D.; Bridge, C.; Dorrien, D.; Elliott, S.; Sigman, M. Adsorption saturation and chromatographic distortion effects on passive headspace sampling with activated charcoal in fire debris analysis. J. Forensic Sci. 2005, 50, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sampat, A.; van Daelen, B.; Lopatka, M.; Mol, H.; van der Weg, G.; Vivó-Truyols, G.; Sjerps, M.; Schoenmakers, P.; van Asten, A. Detection and Characterization of Ignitable Liquid Residues in Forensic Fire Debris Samples by Comprehensive Two-Dimensional Gas Chromatography. Separations 2018, 5, 43. [Google Scholar] [CrossRef]
- Boegelsack, N.; Hayes, K.; Sandau, C.; Withey, J.M.; McMartin, D.W.; O’Sullivan, G. Method development for optimizing analysis of ignitable liquid residues using flow-modulated comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2021, 1656, 462495. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, Z.; Avissar, Y.; Ehila, F.; Grafit, A. Automatic detection and classification of ignitable liquids from GC–MS data of casework samples in forensic fire-debris analysis. Forensic Chem. 2022, 29, 100419. [Google Scholar] [CrossRef]
- Boegelsack, N.; Walker, J.; Sandau, C.; Withey, J.; McMartin, D.; O’Sullivan, G. Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes. Separations 2023, 10, 491. [Google Scholar] [CrossRef]
- Boegelsack, N.; Sandau, C.; McMartin, D.; Withey, J.; O’Sullivan, G. Development of retention time indices for comprehensive multidimensional gas chromatography and application to ignitable liquid residue mapping in wildfire investigations. J. Chromatogr. A 2021, 1635, 461717. [Google Scholar] [CrossRef]
- Franchina, F.; Purcaro, G.; Burklund, A.; Beccaria, M.; Hill, J. Evaluation of different adsorbent materials for the untargeted and targeted bacterial VOC analysis. Anal. Chim. Acta 2019, 1066, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.; Yan, S.; Cassista, A.; Hrynchuk, R.; Sandercock, P. Degradation of Gasoline, Barbecue Starter Fluid, and Diesel Fuel by Microbial Action in Soil. Can. Soc. Forensic Sci. J. 2001, 34, 49–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).