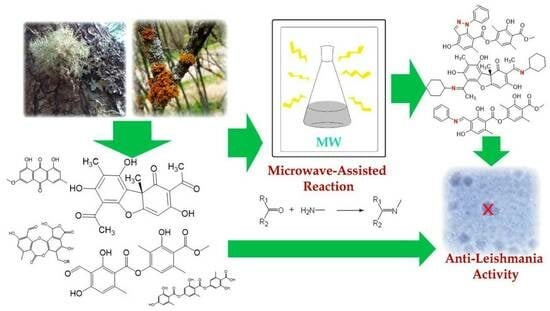
Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.1.1. HPLC Parameters
2.1.2. MS Parameters
2.2. Lichen Material
2.3. Isolation of Lichen Substances
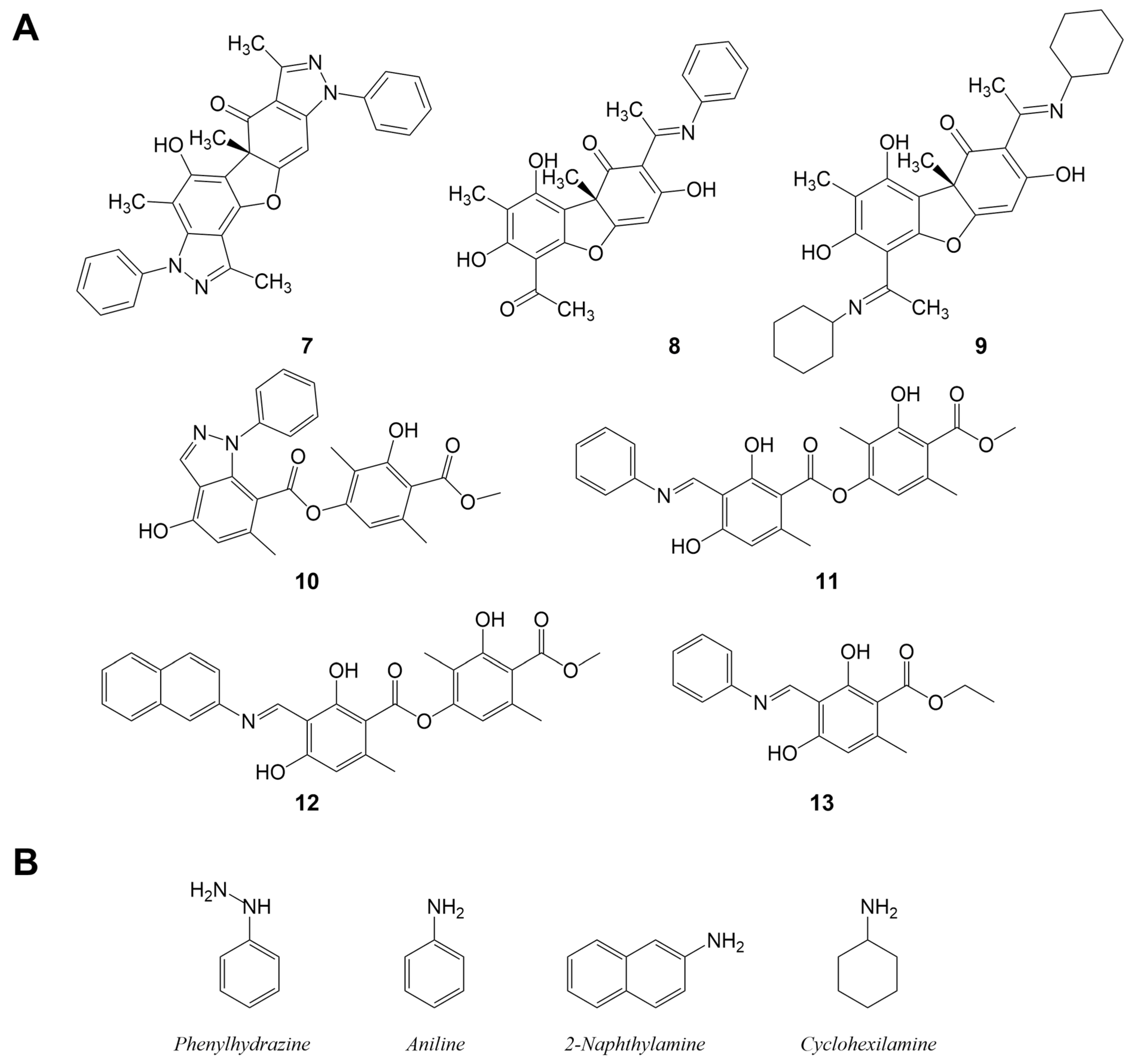
2.4. Semi-Synthesis of Derivatives
2.5. Evaluation against Leishmania Parasites
2.6. Cytotoxicity
2.7. 96-Well Plate Preparation
2.8. IC50 by Fluorometric Method (Rezasurin)
2.9. Selective Index
3. Results and Discussion
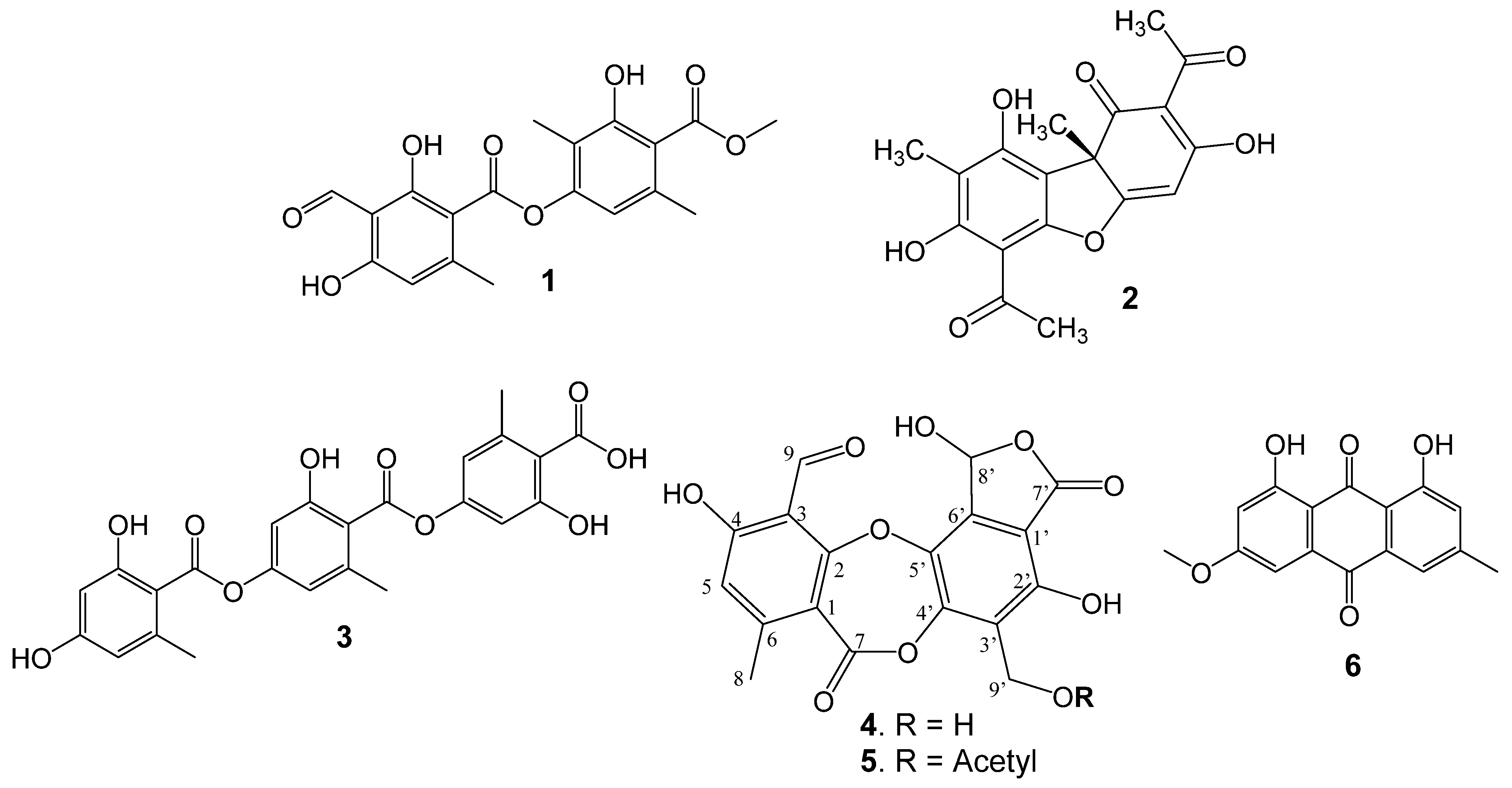
3.1. Lichen Compounds
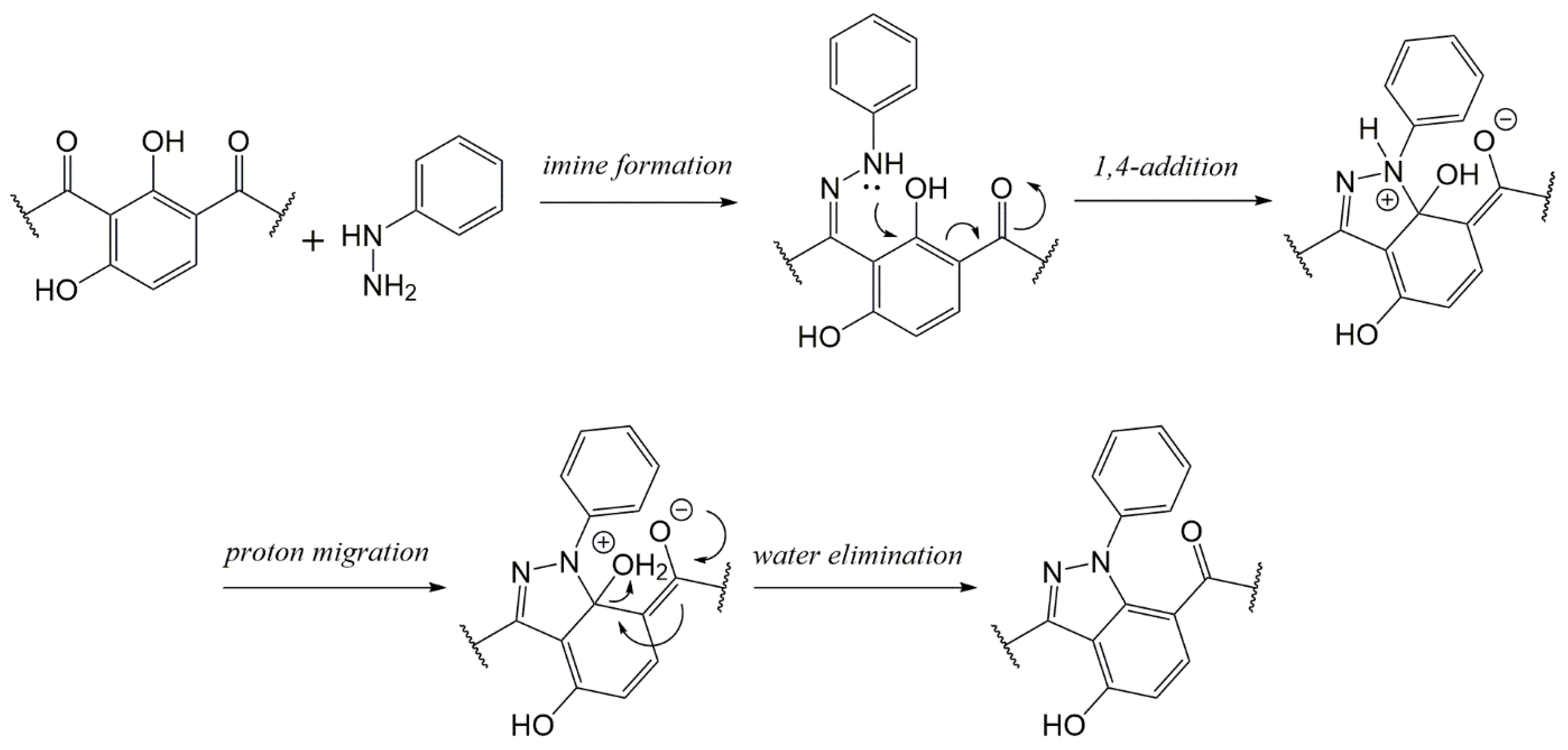
3.2. Microwave Semisynthesis
3.3. Leishmania Activity and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Commitee on the Control of Leishmaniases; Report No. 9789241209496; World Health Organization: Geneva, Switzerland, 2010.
- Da Luz, J.; de Oliveira, E.; Martins, M.; da Silva, N.; Alves, L.; dos Santos, F.; da Silva, L.; Silva, E.; de Medeiros, P. Ultrastructural Analysis of Leishmania infantum chagasi Promastigotes Forms Treated In Vitro with Usnic Acid. Sci. World J. 2015, 2015, 617401–617408. [Google Scholar] [CrossRef]
- Cheuka, P.; Mayoka, G.; Mutai, P.; Chibale, K. The Role of Natural Products in Drug Discovery and Development against Neglected Tropical Diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef]
- Kappe, C.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef]
- Leadbeater, N. Microwave Heating as a Tool for Sustainable Chemistry; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429075339. [Google Scholar]
- Sahu, N.; Sharma, R.; Suhas, K.; Joshi, J.; Prakash, K.; Sharma, R.; Pratap, R.; Hu, X.; Kaur, S.; Jain, M.; et al. Natural-Product-Inspired Microwave-Assisted Synthesis of Novel Spirooxindoles as Antileishmanial Agents: Synthesis, Stereochemical Assignment, Bioevaluation, SAR, and Molecular Docking Studies. Molecules 2023, 28, 4817–4836. [Google Scholar] [CrossRef]
- Pinatto-Botelho, M.; Crotti, A.; Souza, J.; Magalhães, L.; Donate, P. Microwave-assisted synthesis and antileishmanial activity of 3-methoxycarbonyl-γ-butyrolactone derivatives. J. Braz. Chem. Soc. 2014, 25, 1331–1337. [Google Scholar] [CrossRef]
- Jellali, H.; Amri, N.; Mukhrish, Y.; Al Nasr, I.; Koko, W.; Khan, T.; Deniau, E.; Sauthier, M.; Ghalla, H.; Hamdi, N. Copper-Catalyzed Asymmetric Hydroboration Reaction of Novel Methylene Isoindolinone Compounds through Microwave Irradiation and Their Antileishmanial and Antitoxoplasma Activities. ACS Omega 2023, 8, 23067–23077. [Google Scholar] [CrossRef]
- Patil, S.; Bollikonda, S.; Patil, R.; Sangshetti, J.; Bobade, A.; Asrondkar, A.; Reddy, P.; Shinde, D. Microwave-assisted synthesis of novel 5-substituted benzylidene amino-2-butyl benzofuran-3-yl-4-methoxyphenyl methanones as antileishmanial and antioxidant agents. Bioorg. Med. Chem. 2018, 28, 482–487. [Google Scholar] [CrossRef]
- Chakraborty, M.; Baweja, S.; Bhagat, S.; Chundawat, T. Microwave Assisted Synthesis of Schiff Bases: A Green Approach. Int. J. Chem. React. Eng. 2012, 10, 1–4. [Google Scholar] [CrossRef]
- Luzina, O.; Polovinka, M.; Salakhutdinov, N.; Tolstikov, G. Chemical modification of usnic acid: III.* Reaction of (+)-usnic acid with substituted phenylhydrazines. Russ. J. Org. Chem. 2009, 45, 1783–1789. [Google Scholar] [CrossRef]
- Calcott, M.; Ackerley, D.; Knight, A.; Keyzers, R.; Owen, J. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Jha, B.; Shrestha, M.; Pandey, D.; Bhattarai, T.; Bhattarai, H.; Paudel, B. Investigation of antioxidant, antimicrobial and toxicity activities of lichens from high altitude regions of Nepal. BMC Complement. Altern. Med. 2017, 17, 282. [Google Scholar] [CrossRef] [PubMed]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Deep, P.; Singh, S.; Nayak, B. Lichen Secondary Metabolites and Its Biological Activity. Am. J. PharmTech Res. 2016, 6, 28–44. [Google Scholar]
- Vila, J.; Mollinedo, P.; Flores, Y.; Sterner, O. 1,3,7-Trimethylguanine from the lichen Stereocaulon ramulosum. Rev. Bol. Quim. 2008, 25, 1–3. [Google Scholar]
- Edwards, H.G.M.; Newton, E.M.; Wynn-Williams, D.D. Molecular structural studies of lichen substances II: Atranorin, gyrophoric acid, fumarprotocetraric acid, rhizocarpic acid, calycin, pulvinic dilactone and usnic acid. J. Mol. Struct. 2003, 651–653, 27–37. [Google Scholar] [CrossRef]
- Rashid, M.; Majid, M.; Quader, M. Complete NMR assignments of (+)-usnic acid. Fitoterapia 1999, 70, 113–115. [Google Scholar] [CrossRef]
- Narui, T.; Sawada, K.; Takatsuki, S.; Okuyama, T.; Culberson, C.F.; Culberson, W.L.; Shibata, S. NMR assignments of depsides and tridepsides of the lichen family umbilicariaceae. Phytochemistry 1998, 48, 815–822. [Google Scholar] [CrossRef]
- Eifler-Lima, V.L.; Sperry, A.; Sinbandhit, S.; Boustie, J.; Tomasi, S.; Schenkel, E. NMR spectral data of salazinic acid isolated from some species of Parmotrema. Magn. Reson. Chem. 2000, 38, 472–474. [Google Scholar] [CrossRef]
- Vila, J.; Mollinedo, P.; Sterner, O. Spectroscopic studies of lichen depsides and depsidones. Rev. Bol. Quim. 2011, 28, 28–34. [Google Scholar]
- Castañeta, G.; Sepulveda, B.; Vargas, R.; Garcia-Beltran, O.; Simirgiotis, M.; Areche, C. A sustainable application for the extraction of lichen metabolites from Usnea cornuta: Nontargeted metabolomics and antioxidant activity. Nat. Prod. Res. 2022, 37, 2076–2082. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Newton, E.M.; Wynn-Williams, D.D.; Coombes, S.R. Molecular spectroscopic studies of lichen substances 1: Parietin and emodin. J. Mol. Struct. 2003, 648, 49–59. [Google Scholar] [CrossRef]
- Canaviri, P.; Bergquist, K.-E.; Vila, J. Estudio fitoquímico del liquen Teloschistes flavicans. Rev. Bol. Quim. 2006, 23, 9–12. [Google Scholar]
- Arévalo-Lopéz, D.; Nina, N.; Ticona, J.C.; Limachi, I.; Salamanca, E.; Udaeta, E.; Paredes, C.; Espinoza, B.; Serato, A.; Garnica, D.; et al. Leishmanicidal and cytotoxic activity from plants used in Tacana traditional medicine (Bolivia). J. Ethnopharmacol. 2018, 216, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Campos-Buzzi, F.; Fracasso, M.; Clasen, B.K.; Ticona, J.C.; Gimenez, A.; Cechinel-Filho, V. Evaluation of antinociceptive effects of Galipea longiflora alkaloid extract and major alkaloid 2-phenylquinoline. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 707–711. [Google Scholar] [CrossRef]
- Calla-Magarinos, J.; Gimenez, A.; Troye-Blomberg, M.; Fernandez, C. An Alkaloid Extract of Evanta, Traditionally Used as Anti-leishmania Agent in Bolivia, Inhibits Cellular Proliferation and Interferon-γ Production in Polyclonally Activated Cells. Scan. J. Immunol. A 2009, 69, 251–258. [Google Scholar] [CrossRef]
- Bonny, S.; Hitti, E.; Boustie, J.; Bernard, A.; Tomasi, S. Optimization of a microwave-assisted extraction of secondary metabolites from crustose lichens with quantitative spectrophotodensitometry analysis. J. Chromatogr. A 2009, 1216, 7651–7656. [Google Scholar] [CrossRef]
- Calla-Quispe, E.; Robles, J.; Areche, C.; Sepulveda, B. Are Ionic Liquids Better Extracting Agents Than Toxic Volatile Organic Solvents? A Combination of Ionic Liquids, Microwave and LC/MS/MS, Applied to the Lichen Stereocaulon glareosum. Front. Chem. 2020, 8, 450. [Google Scholar] [CrossRef]
- Komaty, S.; Sauvager, A.; Bazureau, J.; Tomasi, S.; Paquin, L. Efficiency and selectivity of ionic liquids in microwave-assisted extraction of major lichen phenolic compounds: A scalable process with recycling of ionic liquids. Phytochem. Anal. 2021, 32, 592–600. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801–131823. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Kutney, J.; Sanchez, I. Studies in the usnic acid series. I. The condensation of (+)-usnic acid with aliphatic and aromatic amines. Can. J. Chem. 1976, 54, 2795–2803. [Google Scholar] [CrossRef]
- Bazin, M.; Lamer, A.; Delcros, J.; Rouaud, I.; Uriac, P.; Boustie, J.; Corbel, J.; Tomasi, S. Synthesis and cytotoxic activities of usnic acid derivatives. Biorg. Med. Chem. 2008, 16, 6860–6866. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.; Sokolov, D.; Pokrovskii, M.; Pokrovskii, A.; Bekker, O.; Danilenko, V.; Salakhutdinov, N. Synthesis and Biological Activity of Usnic Acid Enamine Derivatives. Chem. Nat. Compd. 2015, 51, 646–651. [Google Scholar] [CrossRef]
- Victor, K.; Boris, L.; Athina, G.; Anthi, P.; Marija, S.; Marina, K.; Oliver, R.; Marina, S. Design, synthesis and antimicrobial activity of usnic acid derivatives. MedChemComm 2018, 9, 870–882. [Google Scholar] [CrossRef]
- Samuelsen, L.; Hansen, P.; Vang, O. Derivatives of usnic acid cause cytostatic effect in Caco-2 cells. Nat. Prod. Res. 2021, 35, 4953–4959. [Google Scholar] [CrossRef]
- Duong, T.-H.; Paramita Devi, A.; Tran, N.-M.-A.; Phan, H.-V.-T.; Huynh, N.-V.; Sichaem, J.; Tran, H.-D.; Alam, M.; Nguyen, T.-P.; Nguyen, H.-H.; et al. Synthesis, α-glucosidase inhibition, and molecular docking studies of novel N-substituted hydrazide derivatives of atranorin as antidiabetic agents. Bioorg. Med. Chem. Lett. 2020, 30, 127359. [Google Scholar] [CrossRef]
- Nguyen, G.; Luu, K.; Nguyen, P. Preparation of some derivatives of (+)-usnic acid with aromatic amines under microwave irradiation condition. STDJ 2015, 18, 113–120. [Google Scholar] [CrossRef]
- Luzina, O.; Polovinka, M.; Salakhutdinov, N.; Tolstikov, G. Chemical modification of usnic acid—2. Reactions of (+)-usnic acid with amino acids. Russ. Chem. Bull. 2007, 56, 1249–1251. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Fallah-Mehrjardi, M.; Torabi, V.; Munawar, K.; Ashfaq, M.; Tahir, M. Ultrasound-based synthesis, SC-XRD, NMR, DFT, HSA of new Schiff bases derived from 2-aminopyridine: Experimental and theoretical studies. J. Mol. Struct. 2021, 1233, 130105. [Google Scholar] [CrossRef]
- Armenta, R.; Vinatoru, M.; Burja, A.; Kralovec, J.; Barrow, C. Transesterification of Fish Oil to Produce Fatty Acid Ethyl Esters Using Ultrasonic Energy. JAOCS 2007, 84, 1045–1052. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Tapia, A.; Lima, B.; Pertino, M.; Sortino, M.; Zacchino, S.; Rojas de Arias, A.; Feresin, G. A new antifungal and antiprotozoal depside from the andean lichen Protousnea poeppigii. Phytother. Res. 2008, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Fournet, A.; Ferreira, M.; de Arias, A.; de Ortiz, S.; Inchausti, A.; Yalaff, G.; Quilhot, W.; Fernandez, E.; Hidalgo, M. Activity of Compounds Isolated from Chilean Lichens Against Experimental Cutaneous Leishmaniasis. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 1997, 116, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Derici, M.; Cansaran-Duman, D.; Taylan-Özkan, A. Usnic acid causes apoptotic-like death in Leishmania major, L. infantum and L. tropica. 3 Biotech 2018, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.; Wang, C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ. Toxicol. Pharmacol. 2020, 80, 103493. [Google Scholar] [CrossRef]
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef]
- Iranpour, S.; Hosseinzadeh, A.; Alipour, A. Efficacy of miltefosine compared with glucantime for the treatment of cutaneous leishmaniasis: A systematic review and meta-analysis. Epidemiol. Health 2019, 41, e2019011. [Google Scholar] [CrossRef]
| Lichen | Providence (Site) | Jurisdiction |
|---|---|---|
| Usnea patagonica * | Sud Yungas (Pongo) | La Paz |
| Stereocaulon ramulosum * | Sud Yungas (Unduavi) | La Paz |
| Teloschistes chrysophthalmus * | Murillo (Cota-Cota) | La Paz |
| Xanthoparmelia lineola * | Omasuyos (Apuvillque) | La Paz |
| Usnea Cornuta ⁑ | Chillan | VIII region |
| Parmotrema paramoreliensis ⁑ | Longavi | VII region |
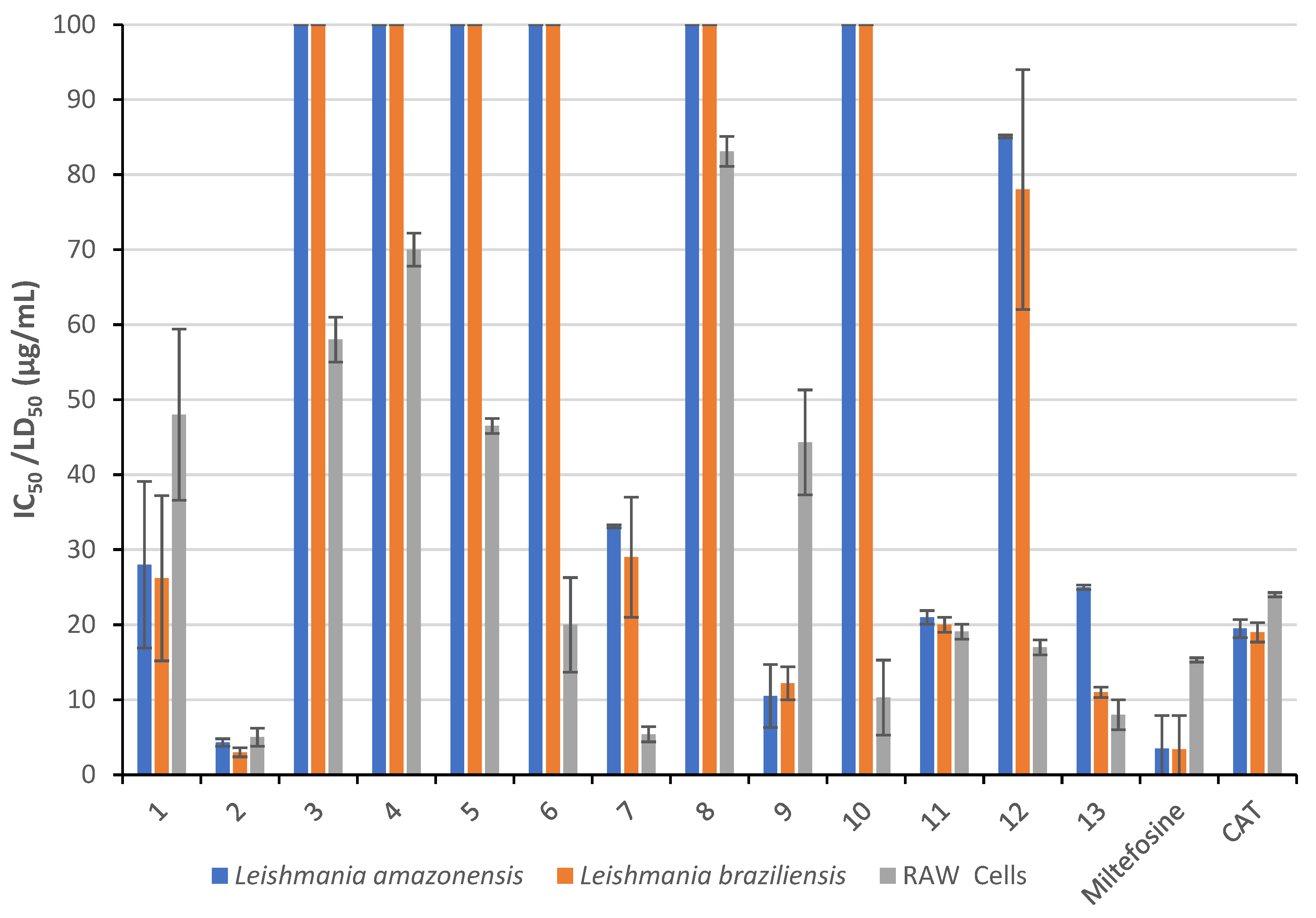
| Compound | Leishmania amazonensis IC50 (µg/mL) | SI | Leishmania braziliensis IC50 (µg/mL) | SI | RAW Cells LD50 (µg/mL) |
|---|---|---|---|---|---|
| 1 | 28.0 ± 11.1 | 1.7 | 26.2 ± 11 | 1.8 | 48.0 ± 11.4 |
| 2 | 4.3 ± 0.5 | 1.2 | 3.0 ± 0.6 | 1.7 | 5.0 ± 1.2 |
| 3 | >100 | - | >100 | - | 58.0 ± 3.0 |
| 4 | >100 | - | >100 | - | 70.0 ± 2.2 |
| 5 | >100 | - | >100 | - | 46.5 ± 1.0 |
| 6 | >100 | - | >100 | - | 20.0 ± 6.3 |
| 7 | 33.1 ± 6.5 | 0.2 | 29.0 ± 8.0 | 0.2 | 5.4 ± 1.0 |
| 8 | >100 | - | >100 | - | 83.1 ± 2.0 |
| 9 | 10.5 ± 0.3 | 4.2 | 12.2 ± 2.2 | 3.6 | 44.3 ± 7.0 |
| 10 | >100 | - | >100 | - | 10.3 ± 5.0 |
| 11 | 21.0 ± 2.0 | 0.9 | 20.0 ± 1.0 | 1.0 | 19.1 ± 1.0 |
| 12 | 85.1 ± 13 | 0.2 | 78.0 ± 16 | 0.2 | 17.0 ± 1.0 |
| 13 | 25 ± 2.0 | 0.3 | 11.0 ± 0.2 | 0.7 | 8.0 ± 2.0 |
| Miltefosine | 3.5 ± 0.6 | 4.4 | 3.4 ± 1.0 | 4.5 | 15.3 ± 0.3 |
| CAT | 19.5 ± 1.4 | 1.2 | 19.0 ± 2.4 | 1.3 | 24.0 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeta, G.; Villagomez, R.; Salamanca, E.; Canaviri-Paz, P.; Bravo, J.A.; Vila, J.L.; Bárcenas-Pérez, D.; Cheel, J.; Sepúlveda, B.; Giménez, A.; et al. Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens. Separations 2023, 10, 524. https://doi.org/10.3390/separations10100524
Castañeta G, Villagomez R, Salamanca E, Canaviri-Paz P, Bravo JA, Vila JL, Bárcenas-Pérez D, Cheel J, Sepúlveda B, Giménez A, et al. Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens. Separations. 2023; 10(10):524. https://doi.org/10.3390/separations10100524
Chicago/Turabian StyleCastañeta, Grover, Rodrigo Villagomez, Efrain Salamanca, Pamela Canaviri-Paz, José A. Bravo, José L. Vila, Daniela Bárcenas-Pérez, José Cheel, Beatriz Sepúlveda, Alberto Giménez, and et al. 2023. "Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens" Separations 10, no. 10: 524. https://doi.org/10.3390/separations10100524
APA StyleCastañeta, G., Villagomez, R., Salamanca, E., Canaviri-Paz, P., Bravo, J. A., Vila, J. L., Bárcenas-Pérez, D., Cheel, J., Sepúlveda, B., Giménez, A., & Areche, C. (2023). Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens. Separations, 10(10), 524. https://doi.org/10.3390/separations10100524