Abstract
Phthalates are the synthetic chemical plasticizers with the most varied uses and are a source of concern due to their toxicity and ubiquity, so much so that even plasticizer-free polymers can contain them as non-intentionally added substances (NIAS). Food packaging is among the materials with the greatest impact. In this study, a simple protocol is proposed for the location and identification of dimethyl phthalate, diethyl phthalate, dipropyl phthalate, and dibutyl phthalate which is applicable to compliance studies of food packaging materials and for the associated risk assessment. Solid phase microextraction gas chromatography/mass spectrometry was used to evaluate the migration of four NIAS from food packaging to release media simulating food substrates. Three plasticizer-free polymers were used: two that were lab-made and based on sodium alginate and a commercial polyethylene film. Linearity ranged from the LOQ to 10 µg/mL; within-day and between-day precision values were between 12.3–25.7% and 21.9–35.8%, respectively; the LOD and LOQ were in the range 0.029–0.073 µg/mL and 0.122–0.970 µg/mL. Migration tests were conducted for different periods of time at room temperature and at 8 °C. Exposure to microwaves (MW) was also evaluated. All packaging materials tested had global migration limits lower than 10 mg/dm2 of material surface.
1. Introduction
The term plastic includes a wide range of synthetic or semi-synthetic polymeric materials which can be easily modeled, extruded, or pressed into solid objects of various shapes. These properties, together with other characteristics such as lightness, flexibility, durability, and low production costs, have led to the widespread use of plastic in different forms for various applications including food packaging [1,2,3,4,5,6].
Although the use of plastic seems to have positively influenced our daily life, the resulting environmental pollution has far greater economic and social repercussions than the benefits [5,6].
Packaging is the biggest contributor to global plastic waste, being responsible for almost 50% of the total weight [5]. The sometimes poor or non-existent plastic waste management system results in up to 90% of plastic waste being inadequately disposed [5,7].
Moreover, even if pure plastic is characterized by a relatively low toxicity, many additives are used during its production for various reasons, such as phthalic acid esters (phthalates, PAEs), which have been employed to soften plastic during polymerization and are widely recognized as endocrine disruptors and are potentially carcinogenic, teratogenic, and mutagenic [8,9]. Phthalates have a common core structure, but the ester groups can be different, giving the molecules various properties. High-molecular-weight phthalates are more stable and are progressively replacing low-molecular-weight phthalates, which are easily released in the environment since they are only physically linked to the plastic/polymer matrix. Unfortunately, the massive use low-molecular-weight phthalates for decades makes human exposure through direct contact, ingestion, or inhalation inevitable [10].
The EU is adopting strategies on plastic to limit the risk of human and environmental exposure [6,11] by promoting and monitoring compliance controls [12]. In this context, today, great attention is being paid by the scientific community to the development of alternative materials with low environmental impacts which are not dangerous for human health and which can, if not replace, at least reduce the indiscriminate use of plastics. Polymers produced from natural sources represent a valuable alternative for producing these materials [13]. Alginate is a polysaccharide derived from brown seaweed that has found numerous applications in biomedical science and engineering due to its favorable properties including biocompatibility, ease of gelation, and malleability even without the addition of additives [14].
However, PAEs can also be found as unintentionally added substances (NIAS) in plasticizer-free plastics due to contamination through contact with other materials that do contain PAEs, such as inks and/or adhesive labels, places on wrappers, and packaging containing food, for which there are no restrictions of any kind. Phthalates are in fact in the NIAS list, i.e., the list of all chemicals present in a food contact material or article without having been intentionally added during the manufacturing process [10]. They can pose a serious risk to food safety, as their presence is unknown to the consumer or even to the manufacturer. In fact, all food packaging, even packaging declared by the manufacturer to be plasticizer-free, can be an important source of human exposure, as PAEs can reach food by migrating through the different materials in contact with each other,
Rule (EU) no. 10/2011 contains “Plastic Implementation Measures” (PIM), i.e., the guidelines for assessing the risks deriving from the migration of substances from packaging to food [15], while, according to EU Regulation no. 1907/2006 [11], good manufacturing practices allow a release of PAEs of up to 10 mg of substance per 1 dm2 of the surface of the material [15]. Although high-molecular-weight PAEs do not represent a risk to public health, they cannot exceed 0.1% by weight (alone or in combination).
This work targeted four representative PAEs, i.e., dimethyl phthalate (DMP), diethyl phthalate (DEP), dipropyl phthalate (DPP), and dibutyl phthalate (DBP), which have been commonly used as additives by the plastic industry and tend to migrate from packaging to food, having a high polarity and low molecular weight [16]. In fact, the European Commission on endocrine disruption and all current regulations have listed DBP as a priority substance [11], and its use is forbidden in cosmetics and personal care products [17,18] even if (FCM No 157) it is one of the five phthalates allowed in plastic food contact materials [19]. EU Regulation no. 10/2011 [15] indicates maximum levels in the range 0.05–0.1% (w/w) in final products for these analytes in food packaging [19], while different limits have been set for migration levels from packaging to food, e.g., ≤0.3 mg/kg for DBP. The limit for the sum of PAEs is set to ≤60 mg/kg of food product (10 mg/dm2 of material surface).
Usually, PAE determination requires a liquid–liquid extraction (LLE) step followed by liquid or gas chromatography (LC, GC) [17,20,21,22], even if the official methods specifically suggest the use of GC [21]. These procedures are generally time-consuming, labor-intensive, and use a large amount of solvent [6].
A faster and green extraction is represented by solid phase microextraction (SPME) combined with LC [4] or GC coupled with mass spectrometry (GC/MS) [22], which enables PAE determination in water at sub-ppb levels [22].
The extraction of analytes from aqueous samples can be performed either through direct immersion (DI) of the fiber into the liquid phase or through headspace (HS) sampling. Adsorbed analytes are then thermally desorbed in the injection port of a gas chromatograph and analyzed using an appropriate column and detector [23,24].
In this work, direct immersion (DI)-SPME coupled with GC/MS was used to study the migration of four NIAS, i.e., DMP, DEP, DPP, and DBP, from different films to various liquids simulating food with different properties, i.e., hydrophilic, alcoholic, and fatty food, in accordance with the Commission Regulation (EU) No 10/2011 [15]. The innovative lab-made SA-based film [25] with and without GPE and a commercial polyethylene (PE) film for food use were used as test substrates.
2. Materials and Methods
2.1. SA Films
2.1.1. Reagents
Alginic acid sodium salt from brown algae (medium viscosity), anhydrous CaCl2 (granular, ≤7.0 mm, purity ≥ 93.0%), and glycerol (anhydrous, reagent grade, purity ≥ 99.5%) were purchased from Sigma Aldrich (Milan, Italy).
Grape pomace waste was received from a local supplier (L’Archetipo, Contrada Tafuri sp21, km 7, Castellaneta, Taranto/Puglia 74011 (Italy)) and stored at −19 °C in glass bottles before the use.
Distilled water was used to prepare the SA hydrogel and obtain the polyphenolic extract.
2.1.2. GPE from Grape Pomace By-Products
GPE was obtained by adding 50 g of grape waste (seeds, skin, and stems) into 1500 mL of distilled water that was boiled for 30 min. Subsequently, vacuum filtration was conducted to remove the residual coarse solids. Then, the derived aqueous extract was centrifuged with a Heraeus Multifuge X3R centrifuge (Thermo Scientific, Waltham, MA, USA) and stored at −19 °C in glass bottles until use [25].
2.1.3. Preparation
Three food packaging materials were used: two were lab-made and based on SA with and without GPE, respectively, and one was a commercial PE film.
The SA hydrogels (1% w/v) were prepared by solubilizing the alginic acid sodium salt powder in distilled water. CaCl2 (2.5% w/v) and glycerol (1 mL/100 mL) were added as plasticizers. The mixture was stirred for 24 h at room temperature to ensure complete alginate dissolution before being transferred to Petri plates and left at 60 °C for 24 h until the solvent dried. Eventually, after soaking for 10 min in a CaCl2 5% (w/v) solution, free-standing, water-resistant solid films were obtained. The same procedure was applied to obtain SA/GPE (60:40, v/v) composite films [26].
2.2. Determination of the PAE Release
2.2.1. Chemicals
PAE standards (purity > 99%) were purchased from Sigma-Aldrich (Milan, Italy). Stock solutions (1 mg/mL) were prepared in sterile filtered ultrapure water (SFUW, Sigma-Aldrich) with 20% (w/v) NaCl (Sigma Aldrich) and stored in glass vials at 8 °C [22]. Working solutions were prepared daily by diluting stock solutions with SFUW and were stored at 8 °C until use.
2.2.2. Experimental Design of Food PAE Release Simulation
To study the release of PAEs from the packaging, amber vials equipped with caps with pre-drilled silicon septa were filled with 2 mL of sunflower oil (purchased from a local market), SFUW solutions, ethanol (Sigma Aldrich) in different percentages (10, 20 and 50%), or 3% (v/v) acetic acid (Sigma Aldrich), which were used as food simulants (FS) with different properties, as shown in Table 1 [15]. Then, each film was cut into 2 × 2 cm pieces which were immersed in the vials for different periods of time (1 h, 1 d, 7 d, and 15 d) at room temperature and at 8 °C.

Table 1.
Food simulants (FS) used for the determination of PAEs released from packaging materials [11].
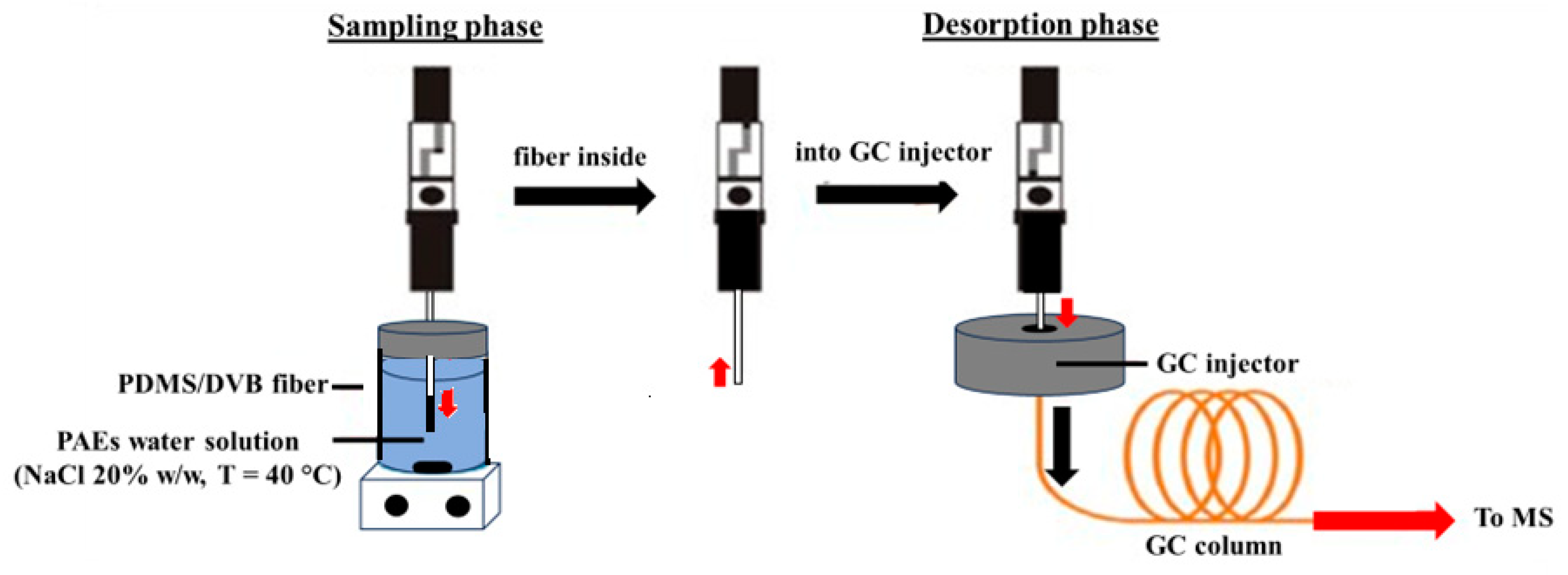
Then, 150 µL of each solution was transferred into 1.5 mL glass vials equipped with pierceable silicone septa caps containing 1.35 mL of 20% saline solution and a magnetic stir bar [22]. Then, each vial was subjected to the SPME procedure schematized in Figure 1.
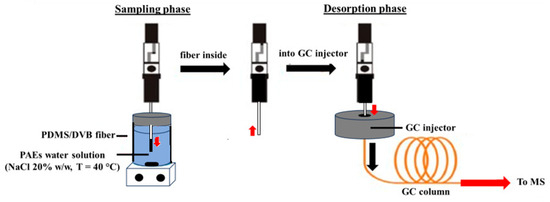
Figure 1.
Scheme of the SPME–GC/MS procedure.
The effect of microwaves (600 W) on the packaging immersed in the liquids of Table 1 was tested for 20 and 120 s [27] with the same procedure.
All samples were prepared in triplicate and analyzed three times. During all experimental procedures, the use of plastic objects of any type (tips, containers, etc.) was always avoided.
2.2.3. SPME–GC/MS Experimental Conditions
The SPME device, consisting of a manual holder, a polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber with a film thickness diameter of 65 mm, was obtained from Supelco (Sigma-Aldrich).
DI-SPME PAE extraction was conducted under constant stirring for 20 min at 40 °C. To avoid a possible “memory effect”, after the desorption step and before performing the subsequent extraction, the fiber was kept at 200 °C for 30 min in the GC injector.
The GC/MS system was a Finnigan TRACE GC ultra gas chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a split/splitless injector interfaced to an ion trap MS (Finnigan Polaris Q, Thermo Fisher Scientific). The capillary column was a Supelco SPB-5 (30 m, 0.25 μm i.d., 0.25 μm film thickness), with helium (purity > 99.999%, Rivoira, Bari, Italy) as a carrier gas (flow rate 1 mL/min). The temperature of the transfer line was 220 °C, while the injector (splitless mode for 2 min) was kept at 270 °C. The oven temperature was 50 °C initially, increasing by 10 °C/ min from 50 °C to 260 °C, where it was held for 3 min. The ion source temperature of the electron impact MS was set at 250 °C. The electron energy was 70 eV and the filament current was 150 μA. Total ion current (TIC, m/z range 40–300) acquisition mode was used [27]. Analytes were detected using extracted ion chromatograms (XICs) obtained in TIC mode.
2.2.4. Method Validation
Calibration curves were estimated in the concentration range of 0.001–10 μg/mL. The limits of detection (LOD) were determined as (3·sda)/b and the limit of quantification (LOQ) was (10·sda)/b, where sda is the standard deviation of the y-intercept and b is the slope of the regression line.
The within-day (n = 3) and between-day (n = 3 over 5 days) percentages of relative standard deviations (RSD%) were calculated at the concentration levels of 0.5, 2.5, and 5 μg/mL.
The stability of the samples was analyzed every 2 h over 24 h at room temperature. Variations were expressed as RSD %.
Since NaCl solution could damage the SPME fibers, their robustness was evaluated as the number of extractions which could be performed while obtaining the same analytical performances.
Recovery was assessed using the standard addition method. Known amounts of each standard analyte solution (0.5 μg/mL) were added to the film samples (2 × 2 cm) that, after 1 h, were subsequently subjected to the developed SPME–GC/MS procedure. Recovery was calculated according to the following formula:
Recovery% = amount found − original amount/amount spiked × 100% [28].
3. Results
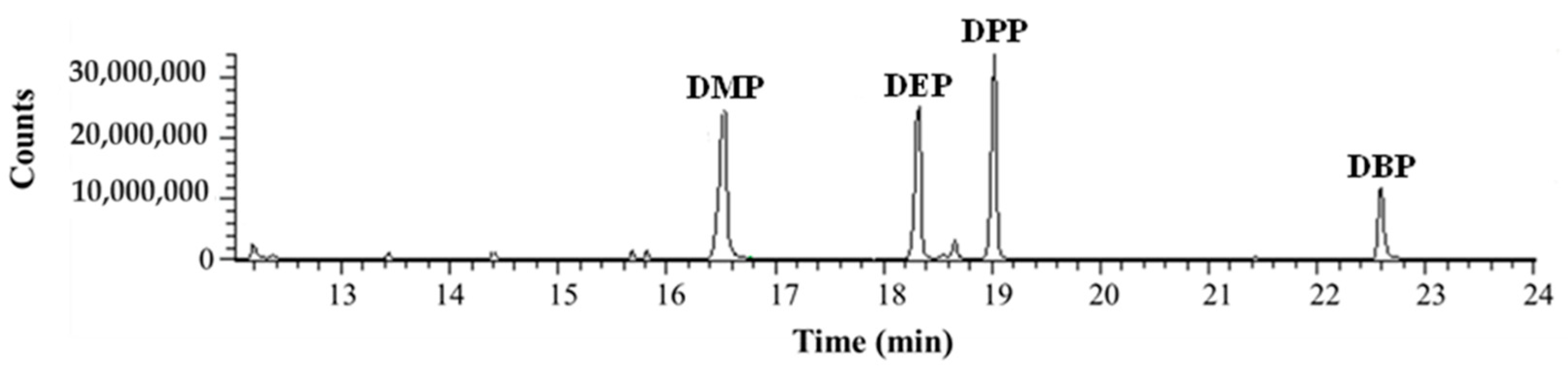
Figure 2 shows a DI-SPME–GC/MS chromatogram obtained in total ion current (TIC) acquisition mode from the analysis of a standard solution (1 µg/mL) of DMP, DEP, DPP, and DBP, while Table 2 reports their retention time (RT), the m/z ions used for quantification, the percentage concentrations in the standard solution, and the percentage area extracted from the chromatogram.
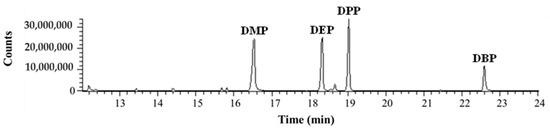
Figure 2.
DI-SPME–GC/MS chromatogram (TIC acquisition mode) obtained from the analysis of a standard solution (1 µg/mL) of DMP, DEP, DPP, and DBP.

Table 2.
Main GC/MS data relevant to the selected PAEs.
The method was validated in terms of linearity, limits of detection (LOD) and quantification (LOQ), and precision. The results showed good linearity for all the analytes in the range 0.001–10 µg/mL with correlation coefficients always better than 0.9867. The LOD and LOQ ranged from 0.029 to 0.073 µg/mL and from 0.122–0.970 µg/mL, respectively. The intermediate precision of the method (expressed in % RSD) for the same replicate sample ranged from 12.9 (DMP) to 25.7 (DBP) and from 21.9 (DPP) to 35.8 (DBP) for within-day and between-day reliability, respectively. All of the validation parameters are reported in Table 3.

Table 3.
Analytical method validation parameters.
Stability tests showed that the solutions were stable within 24 h at room temperature, finding values of RSD % comparable to those reported in Table 3 for within-day measurements.
The robustness of the SPME fibers was established as reaching up to 43 extraction–desorption cycles. It can be improved to more than 100 cycles by rinsing the fiber after each cycle and storing it overnight in fresh water.
Table 4 reports the total PAEs released by the three considered coatings in each food simulant (Table 1) at the different exposure times tested, which was estimated using the developed SPME–GC/MS protocol.

Table 4.
Total PAEs released by CF, SA, and SA + GPE coatings in each FS at different exposure times.
It is worth noting that the extraction procedure showed good results even when applied to the extraction of the analytes in food simulants D and E, which contain amounts of organic solvent that are usually not compatible with SPME, since samples were diluted 1:10 with NaCl water solutions leading to a final ethanol concentration of 5% and an oil–water emulsion, respectively, which, as previously reported [29,30], can be easily subjected to SPME.
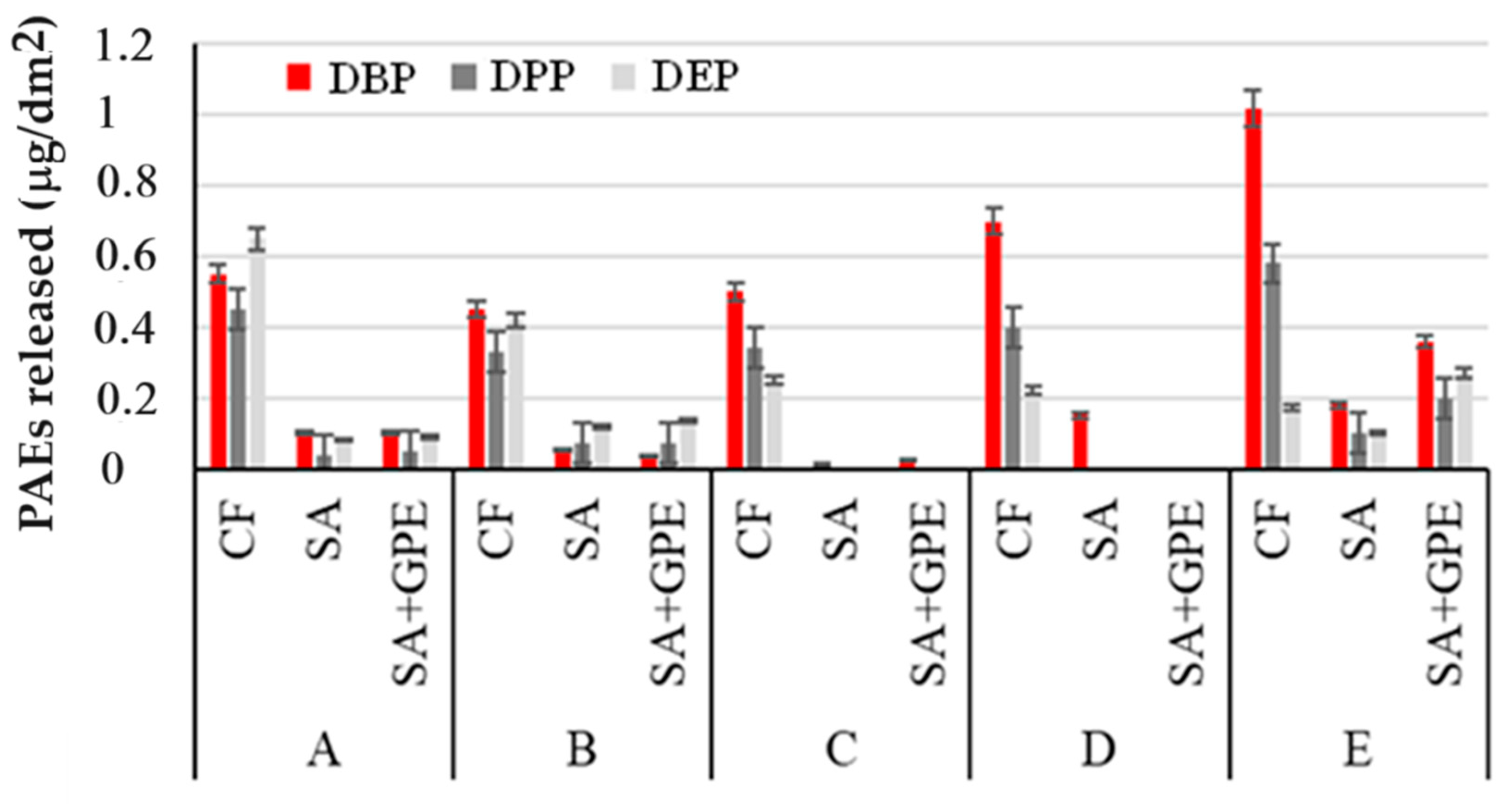
Figure 3 shows the amounts of PAEs released per dm2 from the three packaging polymers in FS after 14 days of immersion at room temperature.
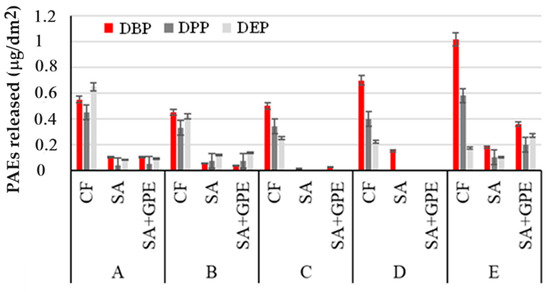
Figure 3.
Amount of PAEs released per dm2 of packaging material (CF, SA, and SA + GPE) in 3% (v/v) acetic acid (A), in 10% (v/v; (B)), 20% (v/v; (C)), and 50% (v/v; (D)) ethanol, and vegetable oil (E), after 14 days of immersion at room temperature.
To simulate the domestic refrigerator effect, CF, SA, and SA + GPE films were immersed in the four test liquids and stored at 8 °C for 1 h, 1 d, 7 d, and 15 d. It was found that the low temperature always hinders the release of the four considered PAEs, as well as in the case of CF. For SA and SA + GPE, a release close to the LOD level for all the three considered compounds was observed, even after 14 days.
The accuracy of the estimates was assessed through recovery studies, finding RSD % values between 88.3 and 98.2.
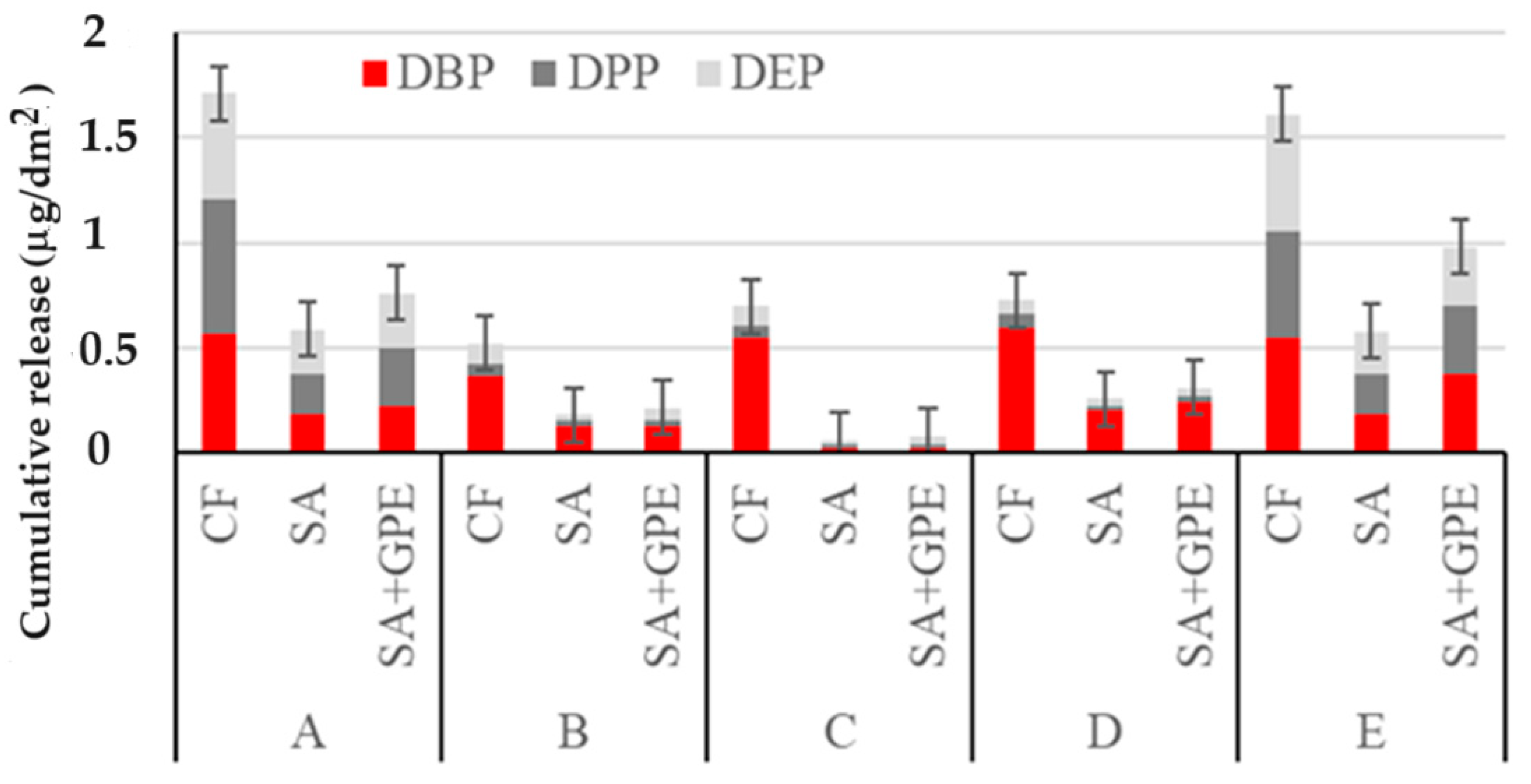
Figure 4 reports the cumulative release of PAEs per dm2 from the three packaging materials considered in selected simulants (Table 2) after 20 s of exposure to microwaves (600 W).
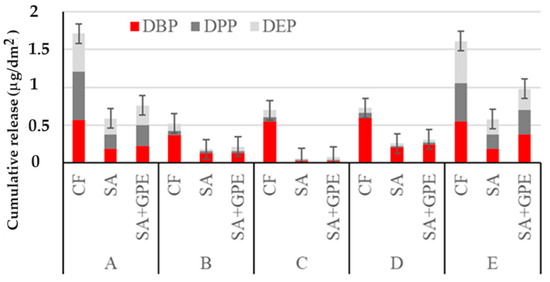
Figure 4.
Cumulative release of PAEs per dm2 from the packaging material (CF, SA, and SA + GPE in 3% (v/v) acetic acid (A), in 10% (v/v, (B)), 20% (v/v, (C)), and 50% (v/v, (D)) ethanol, and in vegetable oil (E), after 20 s of exposure to microwaves (600 W).
4. Discussion
Plasticizers can be intentionally added during manufacturing (IAS) but can also be present as pollutants (NIAS) in food packaging. In the latter case, they cause greater concern because their presence is unknown to the consumer and the producer. In this work, three materials produced without the addition of plasticizers were selected to develop a simple, fast, and easily automatable protocol applicable to conformity studies that are potentially useful for consumer protection. For this purpose, the SPME–GC/MS technique was proposed, as, after optimization, it can combine in a single step the almost selective extraction of the target analytes from different matrices (gas, liquid or solid) with their pre-concentration and directly transfer the results to the chromatographic separation and detection system [24,31].
A 65 μm PDMS/DVB fiber, which was suitable for extracting the target analytes under optimal conditions and thermally desorbing them directly into the injector of the GC/MS system, was selected [4,22]. Traditional sample preparation methods (i.e., LLE, SPE, MW) and more innovative ones (i.e., accelerated solvent extraction or the ASE method) are generally very laborious, not always environmentally friendly, given that they make extensive use of organic solvents, or difficult to automate [32].
To test the conformity of the materials selected through the migration experiments, the food simulants indicated in EU Regulation No. 10/2011 [6] were used. Numerous studies in the literature have reported migration in Tenax® FS when assessing the migration of contaminants in dry foods [33]. Here, for the first time the SPME–GC/MS technique has been proposed for compliance studies.
The results obtained showed that all the films were polluted in traces by three of the four selected PAEs, namely, DEP, DPP, and DBP. No traces of DMP or other PAEs, recognizable by the National Institute of Standards and Technology (NIST) mass spectrometer library, were detectable during careful inspection of the chromatograms.
As can be seen from Table 4, the total migration limit of quantified PAEs never exceeds 10 mg/dm2, as required by current regulations [15]. The largest quantity of PAEs, which increased over time, was always released by the commercial PE film, probably because, being an industrial product, it may be more exposed to contamination by NIAS. Furthermore, DEP, DPP, and DBP are polar compounds and tend to be more easily released into food from non-polar polyethylene than from SA-based films.
As far as PAE contamination of SA-based films is concerned, it could be ascribed to the plastic container of pure alginate dispensed by the manufacturing company, to the Petri dish employed to polymerize the film, or the plastic containers used by the manufacturing companies to supply the grape processing wastes (seeds, skin, and stems), which could extract PAEs during the storage time, as confirmed by SPME–GC/MS analyses. However, these causes of contamination could only partially explain the amount of PAEs released by the polymeric films, confirming their ubiquitous nature, which leads to non-intentional contamination [15]. To limit this release of PAEs, the possibility of carrying out the polymerization phase in glass, ceramic, and metal plates was evaluated.
Unfortunately, the SA-based films did not polymerize correctly, always losing elasticity, which is essential packaging applications.
Figure 3 suggests that the three re-elevated PAEs interact in a different way with packaging materials and simulants. The longer the branched chain of the PAE, the lower the interaction of the PAE with the polymer structure and therefore the tendency to migrate easily towards foods [34]. Furthermore, as the branched chain increases, the lipophilic character increases; therefore, DBP was extracted better with a 50% aqueous ethanol solution compared to the 20 and 10% solutions, respectively [35]. For the same reason, DBP tends to be better extracted by vegetable oil.
The amount of PAEs released was found to be dependent on the plasticizer structure and the diffusion conditions such as MW heating. After 20 s of exposure to MWs, the cumulative release of low-molecular-weight plasticizers reached values between 0.5 and 1.7 mg/dm2 (Figure 4), comparable to those obtained after 14 days at room temperature. Therefore, exposure to MWs can be used to rapidly evaluate high levels of PAE pollution for a considered plastic material. Moreover, even when increasing the MW exposure time up to 120 s there is no significant increase (F-test, p < 0.05) in the amount of released NIAS. The WW assistance is likely linked to the temperature increase (approximately 100 °C) [27] which, in accordance with the Arrehenius equation, favors the rise in migration [16], reaching values for PAEs released into the medium comparable to those observed after the longest polymer/simulant contact time at room temperature. In these conditions, a molding of the polymers was also observed.
5. Limitations of the Study and Future Recommendations
The SPME–GC/MS method described has currently been validated only at our laboratory level. In order, therefore, to complete its validation and to be able to possibly propose it as an alternative, simple, fast, cheap, and low-environmental impact method to the official methods for the determination of PAEs in food matrices, in the near future, a comparison with an official method for the determination of the considered substances and the estimation of intra-laboratory reproducibility will be carried out.
6. Conclusions
Although PAEs do not exhibit any acute toxicity evident in toxicology experiments, if ingested they can accumulate in the body’s internal organs and cause long-term damage. Therefore, their use has been legally regulated. However, given their diffusion and persistence in the environment, they can pollute the most disparate materials and reach humans unknown. Food packaging is a viable source of PAEs. Consequently, the possibility of carrying out rigorous checks in a simple manner is desirable.
In this work, an SPME–GC/MS protocol was successfully developed for the determination of four PAEs (i.e., DMP, DEP, DDP, and DBD). The method was used to assess the conformity of selected materials for food packaging: two lab-made polymers based on SA and SA enriched with GPE, respectively, and a commercial PE film for food use that did not contain PVC and plasticizers.
The release of PAEs from the considered plasticizer-free polymers was studied in five liquid food simulants with different properties, in which each film was immersed for different periods of time at room temperature and at 8 °C. The effect of microwaves (600 W) for 20 and 120 s was also tested.
The method was found to be simple, fast, and sensitive, enabling the detection of the target NIAS released from the considered packaging in different selected media. Specifically, a global migration limit lower than 10 mg/dm2 of material surface was registered for all tested polymers, meaning they were legally compliant.
Author Contributions
N.D.V. and A.M.A. conceived the original idea. J.G. and V.R. prepared the SA and SA + GPE films. N.D.V. and A.M.A. carried out the SPME–GC/MS analysis, also validating the indicated analytical methods and processing all the experimental data. The manuscript was written by N.D.V., A.M.A. and C.Z. C.Z. also provided the financial support. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Research data can reasonably be requested from nicoletta.devietro@uniba.it.
Acknowledgments
This work was carried out within the framework of the research activities envisaged by the project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3-Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union–NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods-Research and innovation network on food and nutrition Sustainability, Safety and Security–Working ON Foods”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment. Off. J. Eur. Union 2019, 155, 1–19.
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Fobil, J.; Hogarh, J. The dilemmas of plastic wastes in a developing economy: Proposals for a sustainable management approach for Ghana. West Afr. J. Appl. Ecol. 2006, 10. [Google Scholar] [CrossRef]
- Vimal, K.E.K.; Mathiyazhagan, K.; Agarwal, V.; Luthra, S.; Sivakumar, K. Analysis of barriers that impede the elimination of single-use plastic in developing economy context. J. Clean. Prod. 2020, 272, 122629. [Google Scholar] [CrossRef]
- Golwalaa, H.; Zhanga, X.; Iskandera, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Comm. 2011, 12, 42–130.
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Environ. Contamin. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, G.; Zheng, H.; Liu, N.; Shi, M.; Luo, X.; Chen, L.; Li, F.; Hu, S. Fate of four phthalate esters with presence of Karenia brevis: Uptake and biodegradation. Aquat Toxicol. 2019, 206, 81–90. [Google Scholar] [CrossRef]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2017, 10, S1938–S1954. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410 (accessed on 1 January 2023).
- Etxabide, A.; Young, B.; Bremer, P.J.; Kilmartin, P.A. Non-permanent primary food packaging materials assessment: Identification, migration, toxicity, and consumption of substances. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4130–4145. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.B.; Schnorr, C.; Silva, L.F.O.; Morais, M.M.; Moraes, C.C.; da Rosa, G.S.; Dotto, G.L.; Lima, É.C.; Naushad, M. Trends in bioactive multilayer films: Perspectives in the use of polysaccharides, proteins, and carbohydrates with natural additives for application in food packaging. Foods 2023, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/ EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Comm. 2006, 1–516.
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Compr. Rev. Food Sci. Food. Saf. 2023, 2, 1337–1359. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, P.J.; Garcia-Jares, C.; Llompart, M. Pressurized liquid extraction-gas chromatography-mass spectrometry analysis of fragrance allergens, musks, phthalates and preservatives in baby wipes. J. Chrom. A 2015, 1384, 9–21. [Google Scholar] [CrossRef]
- European Commission Recomandation (EU) 2019/794: “On a coordinated control plan with a view to establishing the prevalence of certain substances migrating from materials and articles intended to come into contact with food”. Off. J. Eur. Union 2019, 129, 37–42.
- Rascon, A.J.; Rocío-Bautista, P.; Moreno-Gonzalez, D.; García-Reyes, J.F.; Ballesteros, E. Fiber coating based on a green metal-organic framework to determine phthalates in bottled waters by direct-immersion microsolid-phase extraction. Microchem. J. 2023, 191, 108767–108777. [Google Scholar] [CrossRef]
- Environment Protection Agency. Official EPA METHOD 8061 Phthalate Esters by Gas Chromatography with Electron Capture Detection (GC/ECD); EPA Meths. Rev 1; Environment Protection Agency: Washington, DC, USA, 1996.
- Liu, W.; Lun, Y. Determination of Sub-Ppb Level of Phthalates in Water by Auto-SPME and GC–MS; Application 5989-7726EN; Agilent Technologies: Santa Clara, CA, USA, 2008. [Google Scholar]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12–31. [Google Scholar] [CrossRef]
- Bojko, B.; Cudjoe, E.; German, A.; Ríos, G.; Gorynski, K.; Jiang, R.; Reyes-Garcés, N.; Risticevic, S.; Silva, É.A.S.; Togunde, O.; et al. SPME—Quo vadis? Anal. Chim. Acta 2012, 75, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing eco-friendly water-resistant sodium-alginate-based films blended with a polyphenolic aqueous extract from grape pomace waste for potential food packaging applications. Int. J. Mol. Sci. 2023, 24, 11462–11480. [Google Scholar] [CrossRef] [PubMed]
- Dirpan, A.; Hidayat, S.H.; Djalal, M.; Ainani, A.F.; Kasmira, D.S.Y.; Khosuma, M.; Solon, G.T.; Ismayanti, N. Trends over the last 25 years and future research into smart packaging for food: A review. Future Foods 2023, 8, 100252–100268. [Google Scholar] [CrossRef]
- Farag, R.S.; Hewedi, F.M.; Abu-Raiia, S.H.; El-Baroty, G.S. Comparative study on the deterioration of oils by microwave and conventional heating. J. Food Prot. 1992, 55, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Sun, H.; Tai, Z.; Gao, S.; Xu, W.; Chen, W. A Simple and Sensitive HPLC Method for the Simultaneous Determination of Eight Bioactive Components and Fingerprint Analysis of Schisandra sphenanthera. Anal. Chim. Acta 2010, 662, 97–104. [Google Scholar] [CrossRef]
- Batlle, R.; Sánchez, C.; Nerín, C. A systematic approach to optimize solid-phase microextraction. Determination of pesticides in ethanol/water mixtures used as food simulants. Anal. Chem. 1999, 71, 2417–2422. [Google Scholar] [CrossRef]
- Gionfriddo, A.; Gruszecka, D.; Li, X.; Pawliszyn, J. Direct-immersion SPME in soy milk for pesticide analysis at trace levels by means of a matrix-compatible coating. Talanta 2020, 211, 120746–120752. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Liu, J.M.; Li, C.Y.; Yang, F.; Zhao, N.; Lv, S.-W.; Liu, J.C.; Chen, L.J.; He, Z.; Zhang, Y.; Wang, S. Assessment of migration regularity of phthalates from food packaging materials. Food Addit. Contam. 2020, 31, 546–555. [Google Scholar] [CrossRef]
- Van Den Houwe, K.; Van Loco, J.; Lynen, F.; Van Hoeck, E. The Use of Tenax® as a Simulant for the Migration of Contaminants in Dry Foodstuffs: A Review. Packag. Technol. Sci. 2018, 31, 781–790. [Google Scholar] [CrossRef]
- Agyekum, A.A.; Derick, C.; Dontoh, D. Assessment of phthalate migration in polyethylene food contact materials sold on the Ghanaian market. Cogent Environ. Sci. 2020, 6, 1794242. [Google Scholar]
- Doan, K.; Bronaugh, R.L.; Yourick, J.J. In vivo and in vitro skin absorption of lipophilic compounds, dibutyl phthalate, farnesol and geraniol in the hairless guinea pig. Food Chem. Toxicol. 2010, 48, 18–23. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).