Characterization, Antioxidant and Cytotoxic Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin from Curcuma longa Cultivated in Costa Rica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Solvents
2.2. Extraction of Curcuminoids
2.3. Identification of Curcuminoids by UHPLC-MS
2.4. Isolation of BDM and DMC
2.5. NMR Characterization of Purified BDM and DMC
2.6. Solid-State Characterization of DMC and BDM
2.6.1. Powder X-ray Diffraction
2.6.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6.3. Differential Scanning Calorimetry (DSC)
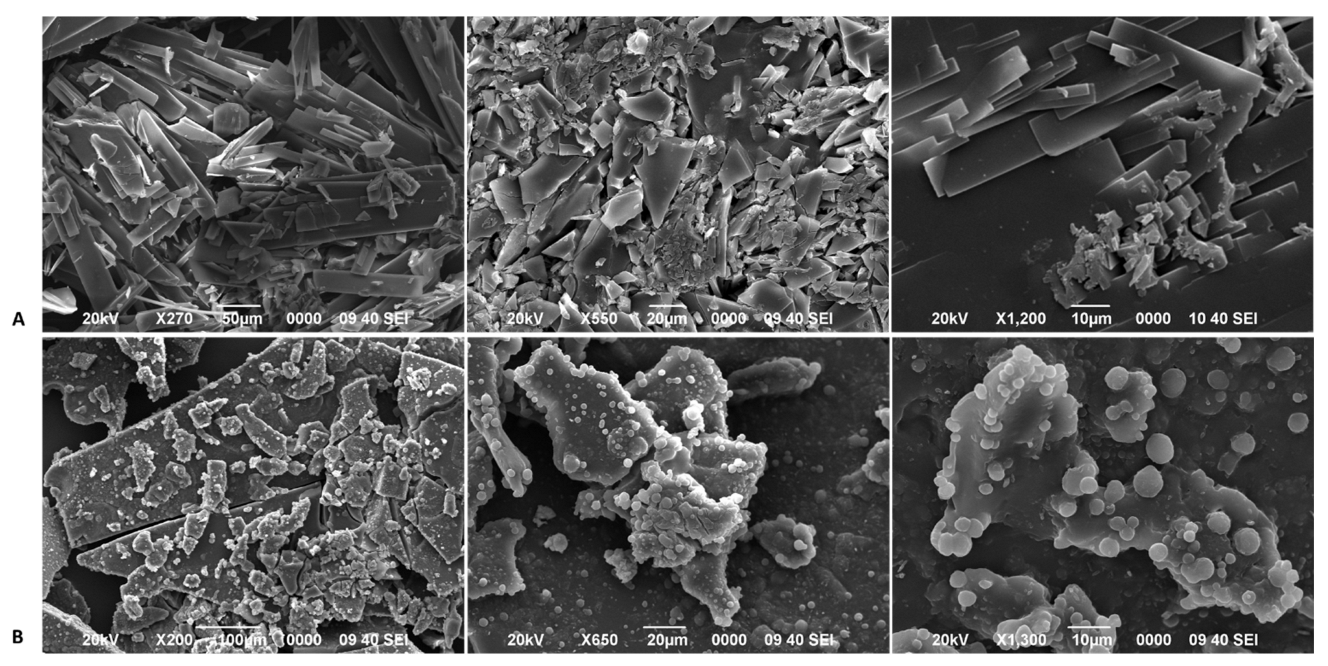
2.6.4. Scanning Electron Microscopy
2.7. In Vitro Studies
2.7.1. Powder Dissolution Test
2.7.2. DPPH Radical-Scavenging Activity
2.7.3. Cytotoxicity assessment on tumoral cells
Cell Culture
Cytotoxicity Evaluation through MTT Assay
3. Results and Discussion
3.1. Isolation and Purification of BDM and DMC
3.2. Solid-State Characterization of BDM and DMC
3.3. In Vitro Studies
3.3.1. Powder Dissolution Test
3.3.2. DPPH Radical-Scavenging Activity
3.3.3. Cytotoxic Activity Evaluation on Tumoral Cells of DMC and BDM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias, J.; Medina, I.; Pazos, M. Galloylation and Polymerization: Role of Structure to Antioxidant Activity of Polyphenols in Lipid Systems. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; p. 1488. [Google Scholar]
- Heffernan, C.; Ukrainczyk, M.; Gamidi, R.K.; Hodnett, B.K.; Rasmuson, Å.C. Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Org. Process Res. Dev. 2017, 21, 821–826. [Google Scholar] [CrossRef]
- Ukrainczyk, M.; Hodnett, B.K.; Rasmuson, Å.C. Process Parameters in the Purification of Curcumin by Cooling Crystallization. Org. Process Res. Dev. 2016, 20, 1593–1602. [Google Scholar] [CrossRef]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef]
- Jin, C.Y.; Lee, J.D.; Park, C.; Choi, Y.H.; Kim, G.Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef]
- Rahardjo, B.; Widjajanto, E.; Sujuti, H.; Keman, K. Curcumin decreased level of proinflammatory cytokines in monocyte cultures exposed to preeclamptic plasma by affecting the transcription factors NF-κB and PPAR-γ. Biomark. Genom. Med. 2014, 6, 105–115. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Omari-Siaw, E.; Adu-Frimpong, M.; Liu, J.; Xu, X.; Yu, J. Enhanced oral bioavailability of Bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: Formulation design, in vitro and in vivo evaluation. Int. J. Pharm. 2020, 590, 119887. [Google Scholar] [CrossRef]
- Guo, L.Y.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Shin, E.M.; Zhou, H.Y.; Jung, J.W.; Kim, Y.S. Comparison of suppressive effects of demethoxycurcumin and bisdemethoxycurcumin on expressions of inflammatory mediators In Vitro and In Vivo. Arch. Pharm. Res. 2008, 31, 490–496. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Natarajan, S.R.; Thangaraj, K.; Manoharan, R. Therapeutic aspects of AMPK in breast cancer: Progress, challenges, and future directions. Biochim. Biophys. Acta-Rev. Cancer 2020, 1874, 188379. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Chaiwangyen, W.; Garbisa, S.; Limtrakul, P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J. Nutr. Biochem. 2009, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xian, J.; Wang, J.; Huang, J.; Xu, Z. Pharmacokinetic Characteristics of Bisdemethoxycurcumin and Its Mechanism of Reversing Cardiomyocyte Apoptosis. Indian J. Pharm. Sci. 2023, 85, 64–73. [Google Scholar] [CrossRef]
- Huang, C.; Lu, H.-F.; Chen, Y.-H.; Chen, J.-C.; Chou, W.-H.; Huang, H.-C. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase-dependent and –independent apoptosis via Smad or Akt signaling pathways in HOS cells. BMC Complement. Med. Ther. 2020, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Fallas, M.I.; Vargas-Huertas, F.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A.; Navarro-Hoyos, M. Polyphenolic HRMS Characterization, Contents and Antioxidant Activity of Curcuma longa Rhizomes from Costa Rica. Antioxidants 2022, 11, 620. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Nagana Gowda, G.A.; Marquez, S.; Patil, B.S. Rapid separation and quantitation of curcuminoids combining pseudo two-dimensional liquid flash chromatography and NMR spectroscopy. J. Chromatogr. B 2013, 937, 25–32. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR Study of the Solution Structure of Curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef]
- Prasad, D.; Praveen, A.; Mahapatra, S.; Mogurampelly, S.; Chaudhari, S.R. Existence of β-diketone form of curcuminoids revealed by NMR spectroscopy. Food Chem. 2021, 360, 130000. [Google Scholar] [CrossRef]
- Yuan, L.; Horosanskaia, E.; Engelhardt, F.; Edelmann, F.T.; Couvrat, N.; Sanselme, M.; Cartigny, Y.; Coquerel, G.; Seidel-Morgenstern, A.; Lorenz, H. Solvate Formation of Bis(demethoxy)curcumin: Crystal Structure Analyses and Stability Investigations. Cryst. Growth Des. 2019, 19, 854–867. [Google Scholar] [CrossRef]
- Wünsche, S.; Yuan, L.; Seidel-Morgenstern, A.; Lorenz, H. A Contribution to the Solid State Forms of Bis(demethoxy)curcumin: Co-Crystal Screening and Characterization. Molecules 2021, 26, 720. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Natarajan, R.K.; Natarajan, S.; Rathikha, R. Structural investigation on curcumin. Asian J. Chem. 2008, 20, 2903–2913. [Google Scholar]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Ge, Y.; Della Porta, G.; Pederson, C.L.; Lokier, S.W.; Hoffmann, R.; Immenhauser, A. Botryoidal and Spherulitic Aragonite in Carbonates Associated with Microbial Mats: Precipitation or Diagenetic Replacement Product? Front. Earth Sci. 2021, 9, 698952. [Google Scholar] [CrossRef]
- Lateh, L.; Kaewnopparat, N.; Yuenyongsawad, S.; Panichayupakaranant, P. Enhancing the water-solubility of curcuminoids-rich extract using a ternary inclusion complex system: Preparation, characterization, and anti-cancer activity. Food Chem. 2022, 368, 130827. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A. Investigation by response surface methodology of the combined effect of pH and composition of water-methanol mixtures on the stability of curcuminoids. Food Chem. 2017, 219, 414–418. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Prajakta, D.; Ratnesh, J.; Chandan, K.; Suresh, S.; Grace, S.; Meera, V.; Vandana, P. Curcumin Loaded pH-Sensitive Nanoparticles for the Treatment of Colon Cancer. J. Biomed. Nanotechnol. 2009, 5, 445–455. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant Capacity: Which Capacity and How to Assess It? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Foti, M.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH_ Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Mahavorasirikul, W.; Viyanant, V.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement. Altern. Med. 2010, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, S.; Qiu, X.; Cong, J.; Zhou, J.; Miu, W. Curcumin Inhibits Cell Viability and Increases Apoptosis of SW620 Human Colon Adenocarcinoma Cells via the Caudal Type Homeobox-2 (CDX2)/Wnt/β-Catenin Pathway. Med. Sci. Monit. 2019, 25, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, L.; Jiang, X.; Wang, Y.; Wang, Z.; Li, D. Curcumin Inhibits Cell Viability by Inducing Apoptosis and Autophagy in Human Colon Cancer Cells. Proc. Anticancer Res. 2019, 3, 133180. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sahu, P.K.; Sahu, P.L.; Agarwal, D.D. Structure activity relationship, cytotoxicity and evaluation of antioxidant activity of curcumin derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 1342–1347. [Google Scholar] [CrossRef]
- Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of curcuminoids and evaluation of their cytotoxic and antioxidant properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef]
- Adeyeni, T.A.; Khatwani, N.; San, K.; Ezekiel, U.R. BMI1 is downregulated by the natural compound curcumin, but not by bisdemethoxycurcumin and dimethoxycurcumin. Physiol. Rep. 2016, 4, e12906. [Google Scholar] [CrossRef][Green Version]
- Cao, A.; Li, Q.; Yin, P.; Dong, Y.; Shi, H.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis 2013, 18, 1391–1402. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Hsu, C.-W.; Chang, W.-C.; Wang, M.-Y.; Wu, J.-F.; Hsu, Y.-C. Demethoxycurcumin Retards Cell Growth and Induces Apoptosis in Human Brain Malignant Glioma GBM 8401 Cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 396573. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Woo, S.; Jee, J.-G.; Sung, S.; Kim, H. Bisdemethoxycurcumin Induces Apoptosis in Activated Hepatic Stellate Cells via Cannabinoid Receptor 2. Molecules 2015, 20, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Ampasavate, C.; Sung, B.; Aggarwal, B.B.; Limtrakul, P. Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur. J. Pharmacol. 2010, 627, 8–15. [Google Scholar] [CrossRef]
- Strzyga-Łach, P.; Chrzanowska, A.; Podsadni, K.; Bielenica, A. Investigation of the Mechanisms of Cytotoxic Activity of 1,3-Disubstituted Thiourea Derivatives. Pharmaceuticals 2021, 14, 1097. [Google Scholar] [CrossRef]
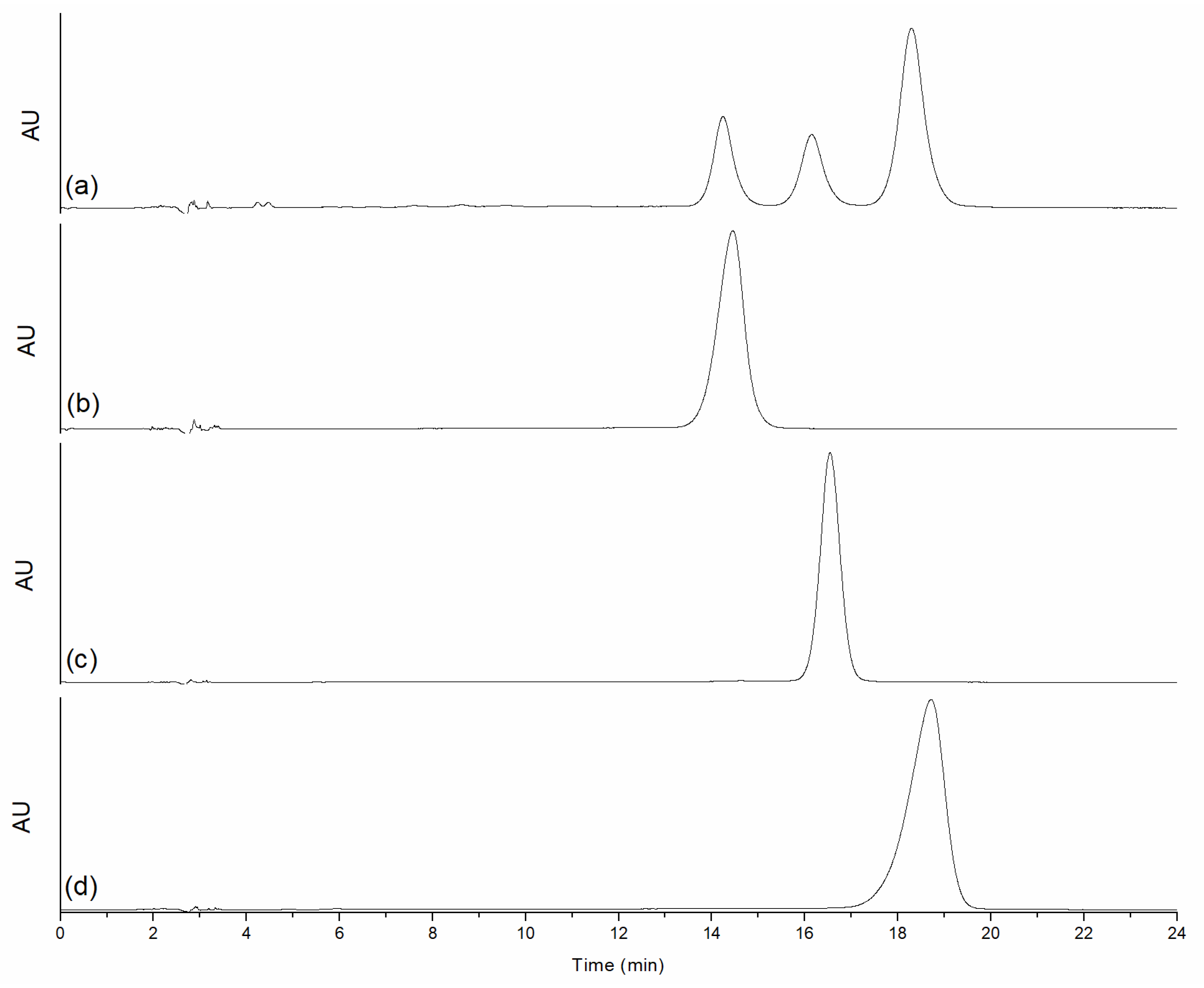
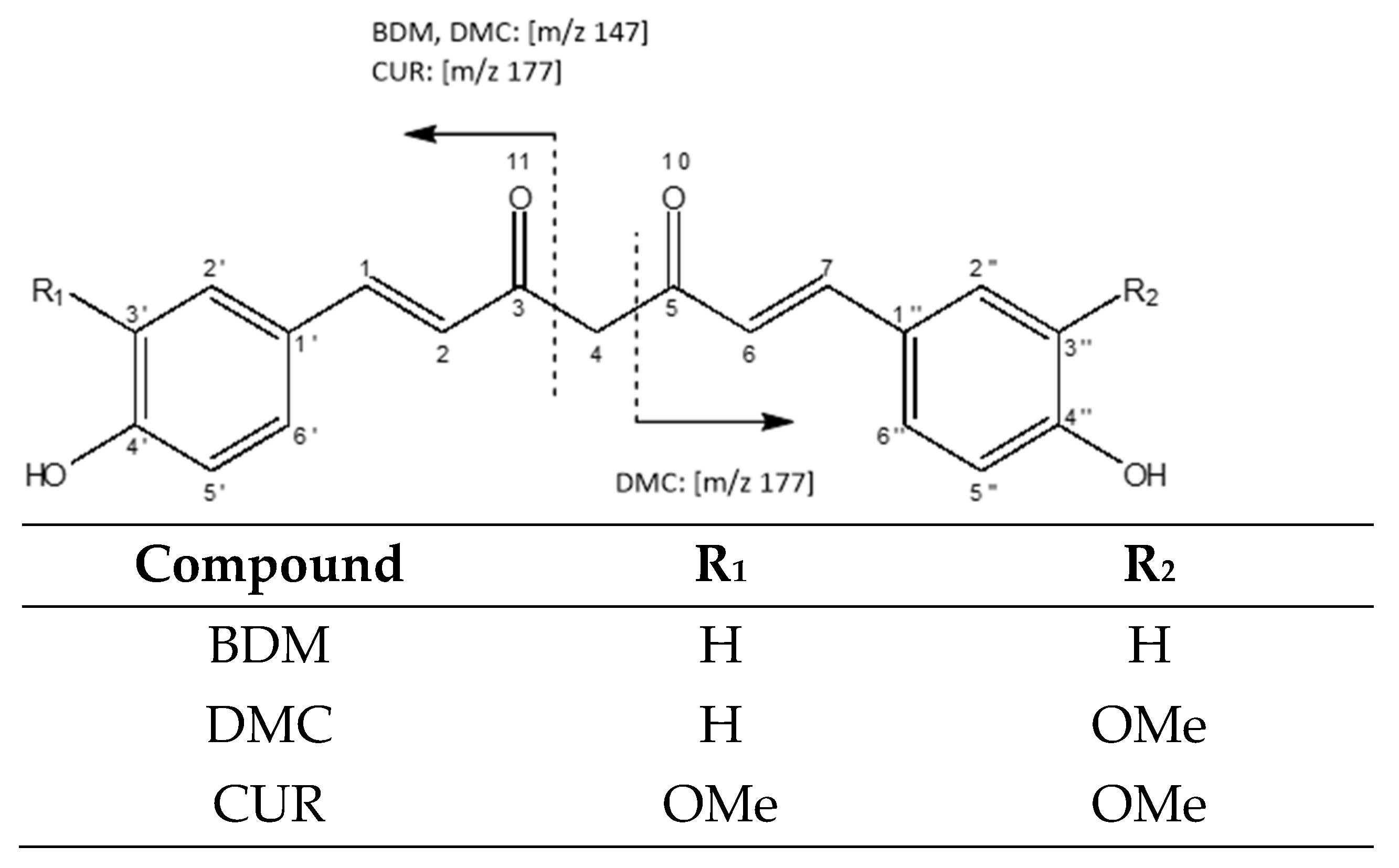
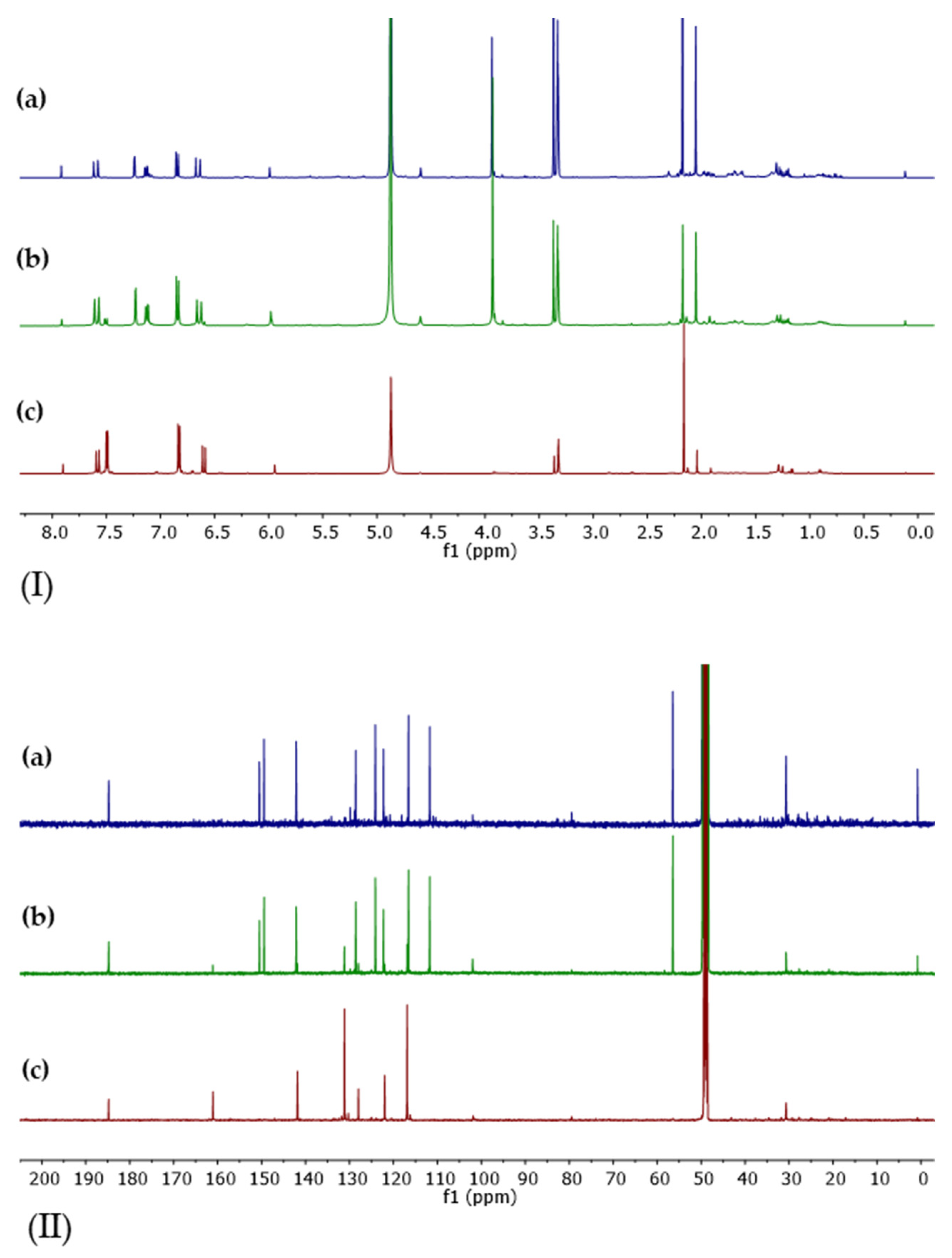

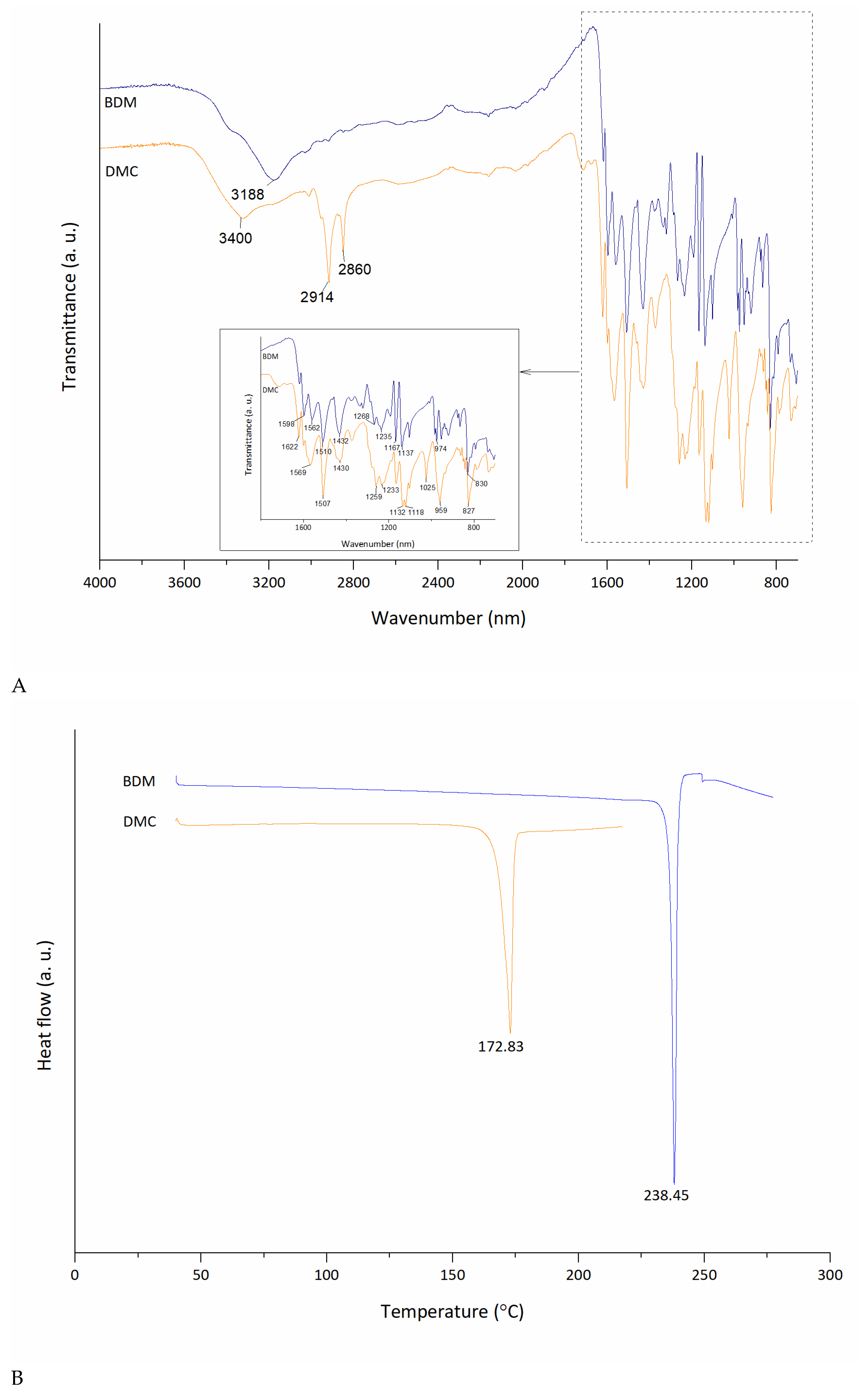

| # C | 13C (ppm) | 1H (ppm) |
|---|---|---|
| 3, 5 | 184.76 | |
| 4′, 4″ | 150.49 | |
| 3′, 3″ | 149.43 | |
| 1, 7 | 142.14 | 7.60 (d, J = 15.8 Hz, 2 H) |
| 1′, 1″ | 128.58 | |
| 6′, 6″ | 124.13 | 7.13 (dd, J = 8.2, 1.9 Hz, 2 H) |
| 2, 6 | 122.26 | 6.65 (d, J = 15.8 Hz, 2 H) |
| 5′, 5″ | 116.57 | 6.85 (d, J = 8.2 Hz, 2 H) |
| 2′, 2″ | 111.72 | 7.24 (d, J = 1.9 Hz, 2 H) |
| 4 | 101.98 | 5.99 (s, 2 H) |
| (C3′,C3″)-O-CH 3 | 56.46 | 3.94 (s, 6 Hh) |
| # C | 13C (ppm) | 1H (ppm) |
|---|---|---|
| 3, 5 | 184.79 | |
| 4′, 4″ | 161.06 | |
| 1, 7 | 141.84 | 7.58 (d, J = 15.8 Hz, 2 H) |
| 2′, 2″, 6′, 6″ | 131.14 | 7.49 (d, J = 8.6 Hz, 4 H) |
| 1′, 1″ | 127.99 | |
| 2, 6 | 121.97 | 6.60 (d, J = 15.8 Hz, 2 H) |
| 3′, 3″, 5′, 5″ | 116.88 | 6.83 (d, J = 8.6 Hz, 4 H) |
| 4 | 101.88 | 5.94 (s, 2 H) |
| # C | 13C (ppm) | 1H (ppm) |
|---|---|---|
| 5 | 184.81 | |
| 3 | 184.76 | |
| 4′ | 161.09 | |
| 4″ | 150.47 | |
| 3″ | 149.42 | |
| 1, 7 | 142.13 | 7.59 (d, J = 15.8 Hz) |
| 2′, 6′ | 131.5 | 7.51 (d, 8.7 Hz) |
| 1″ | 128.58 | |
| 1′ | 127.99 | |
| 6″ | 124.12 | 7.12 (dd, J = 8.2, 1.9 Hz) |
| 6 | 122.29 | 6.64 (d, J = 15.8 Hz) |
| 2 | 122.25 | 6.61 (d, J = 15.6 Hz) |
| 5″ | 116.89 | 6.84 (d, J = 8.2 Hz) |
| 3′, 5′ | 116.57 | 6.84 (d, J = 8.2 Hz) |
| 2″ | 111.73 | 7.23 (d, J = 1.9 Hz) |
| 4 | 102.00 | 5.98 (s) |
| (C3″)-O-CH3 | 56.45 | 3.93 (s) |
| DMC | BDM | |
|---|---|---|
| Cell Line | IC50 μM (μg/mL) | IC50 μM (μg/mL) |
| AGS | 52.2 ± 1.4 a (17.6 ± 0.5) | 57.2 ± 2.5 a (17.6 ± 0.8) |
| SW-620 | 42.9 ± 2.4 a (14.5 ± 0.8) | 52.5 ± 0.1 a (16.2 ± 0.1) |
| HepG2 | 115.6 ± 8.0 a (39.1 ± 2.7) | 64.7 ± 0.8 b (19.9 ± 0.2) |
| Vero | 126.9 ± 5.3 a (42.9 ± 1.8) | 134.7 ± 5.3 a (41.5 ± 1.6) |
| Cell Line | DMC | BDM |
|---|---|---|
| AGS | 2.4 | 2.4 |
| SW-620 | 3.0 | 2.6 |
| HepG2 | 1.1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya-Sibaja, A.M.; Vargas-Huertas, F.; Quesada, S.; Azofeifa, G.; Vega-Baudrit, J.R.; Navarro-Hoyos, M. Characterization, Antioxidant and Cytotoxic Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin from Curcuma longa Cultivated in Costa Rica. Separations 2024, 11, 23. https://doi.org/10.3390/separations11010023
Araya-Sibaja AM, Vargas-Huertas F, Quesada S, Azofeifa G, Vega-Baudrit JR, Navarro-Hoyos M. Characterization, Antioxidant and Cytotoxic Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin from Curcuma longa Cultivated in Costa Rica. Separations. 2024; 11(1):23. https://doi.org/10.3390/separations11010023
Chicago/Turabian StyleAraya-Sibaja, Andrea Mariela, Felipe Vargas-Huertas, Silvia Quesada, Gabriela Azofeifa, José Roberto Vega-Baudrit, and Mirtha Navarro-Hoyos. 2024. "Characterization, Antioxidant and Cytotoxic Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin from Curcuma longa Cultivated in Costa Rica" Separations 11, no. 1: 23. https://doi.org/10.3390/separations11010023
APA StyleAraya-Sibaja, A. M., Vargas-Huertas, F., Quesada, S., Azofeifa, G., Vega-Baudrit, J. R., & Navarro-Hoyos, M. (2024). Characterization, Antioxidant and Cytotoxic Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin from Curcuma longa Cultivated in Costa Rica. Separations, 11(1), 23. https://doi.org/10.3390/separations11010023






