Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DES
2.3. Extraction of Flavonoids by the UAE-DES Method
2.4. Determination of the Total Flavonoids Content
2.5. Recovery of Flavonoids from the DES Extract
2.6. Evaluation of Antioxidant Activities
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.6.3. Fe3+ Reducing Assay
2.6.4. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.6.5. Fe2+ Chelating Assay
2.7. Antibacterial Activity
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Selection of DES
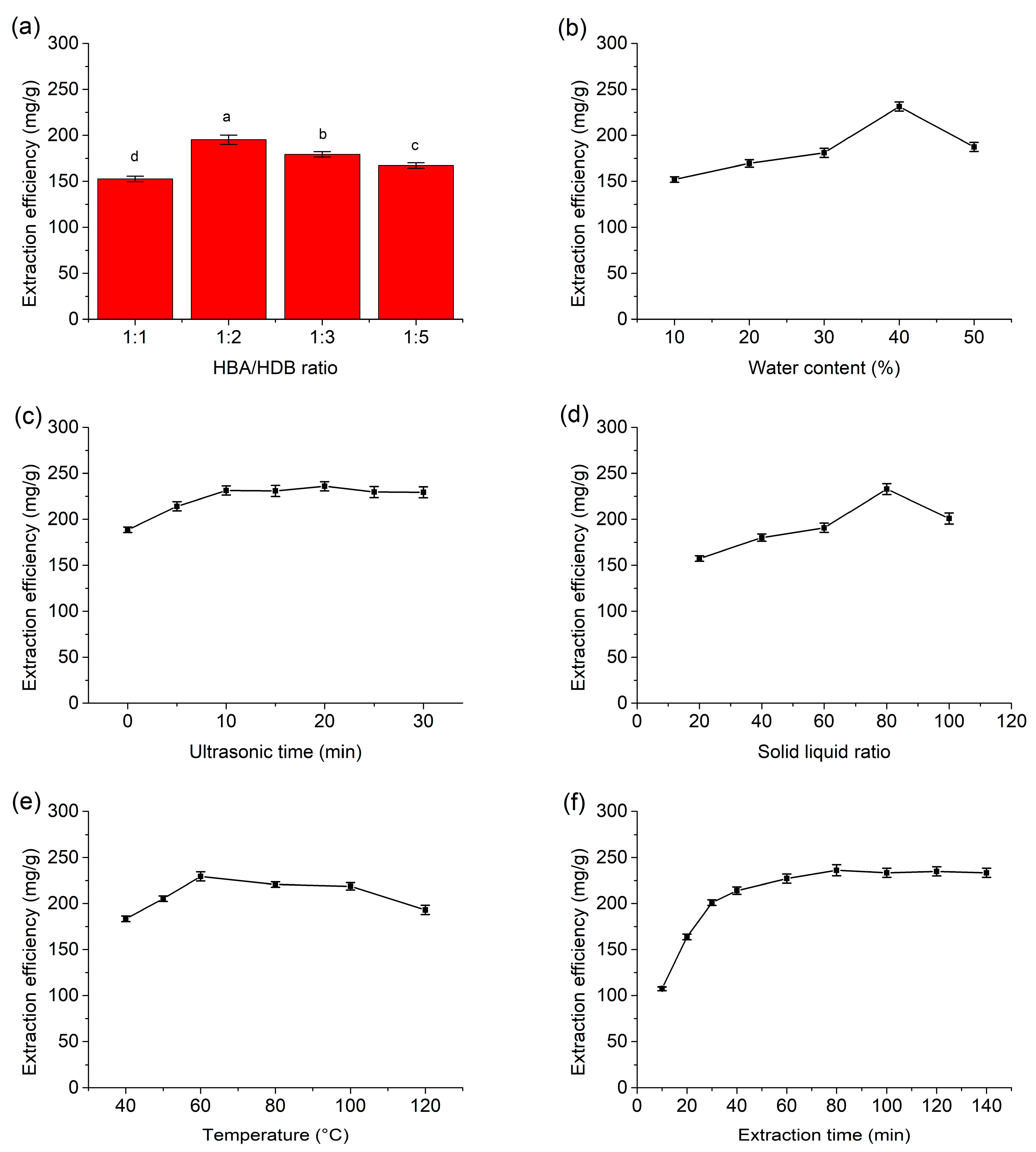
3.2. Optimization of Extraction Parameters
3.2.1. Effect of the HBA/HDB Ratio
3.2.2. Effect of Water Content in DES
3.2.3. Effect of Ultrasonic Time
3.2.4. Effect of the Solid–Liquid Ratio
3.2.5. Effect of Temperature
3.2.6. Effect of Extraction Time
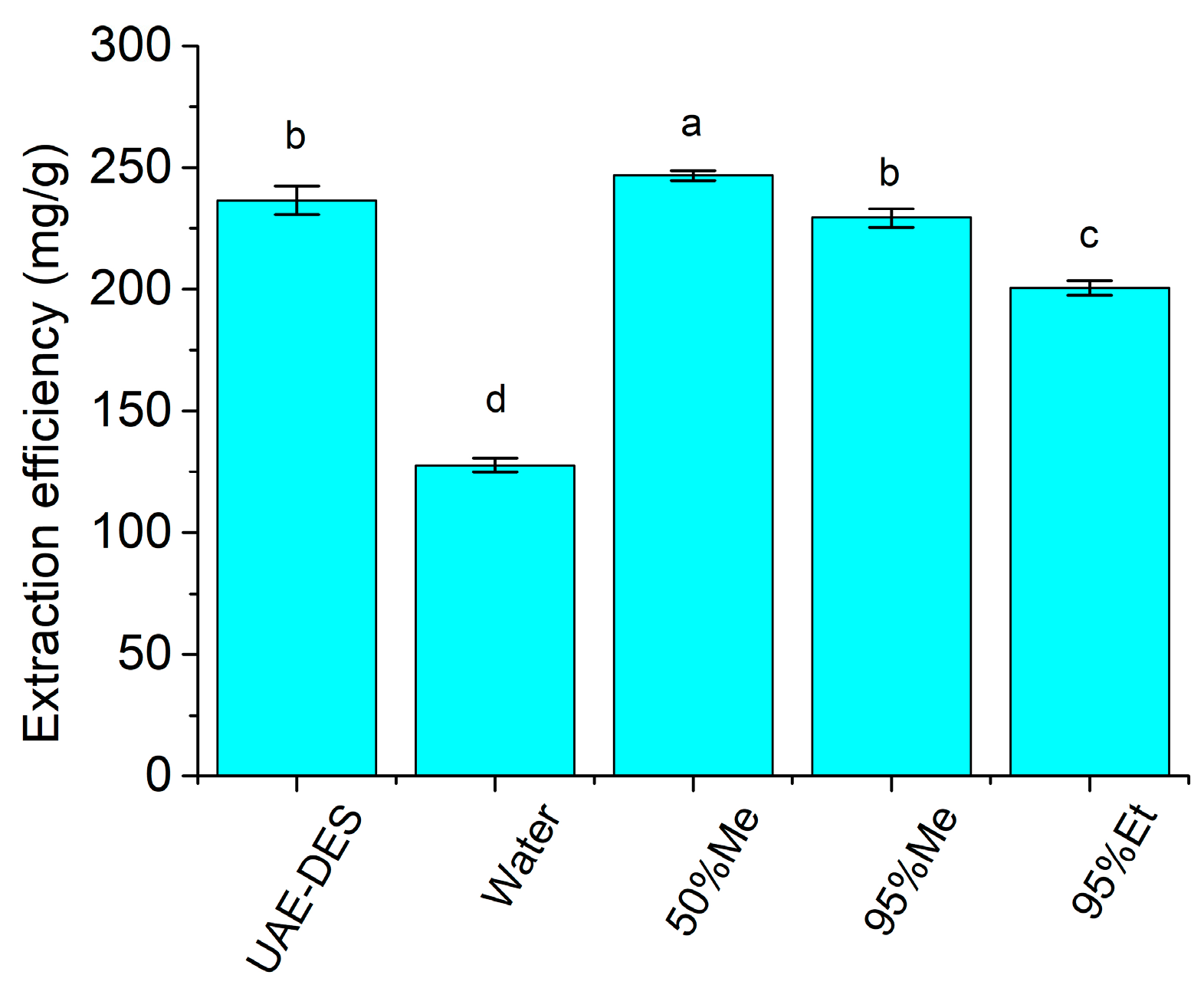
3.2.7. Comparison to Conventional Solvents
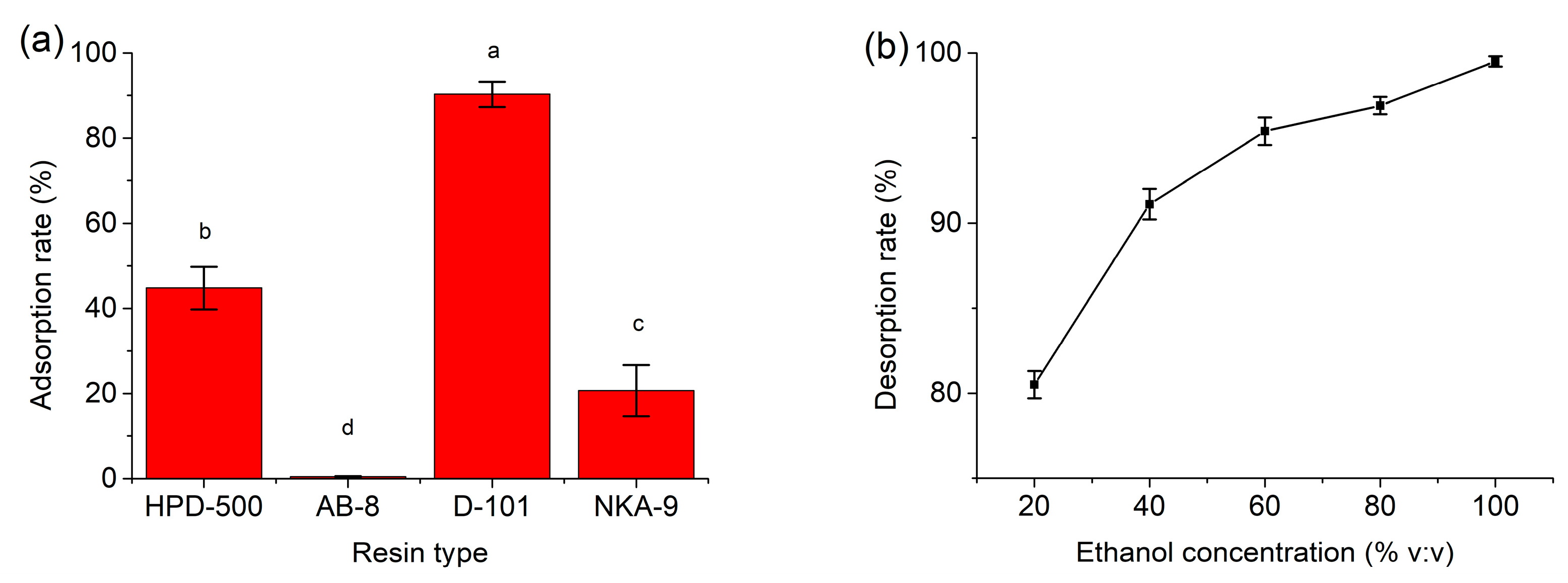
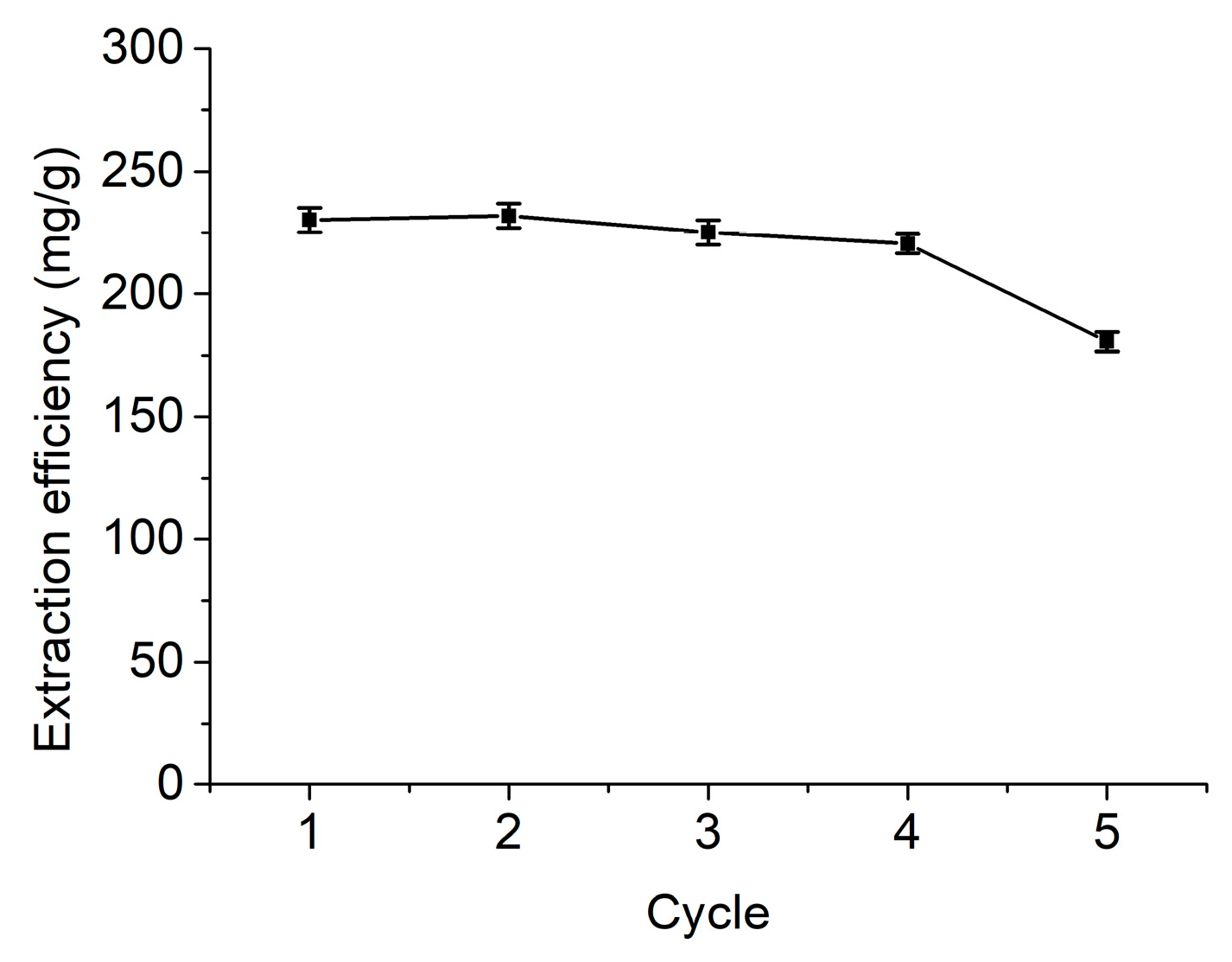
3.3. Recovery of Flavonoids and Reusability of DES
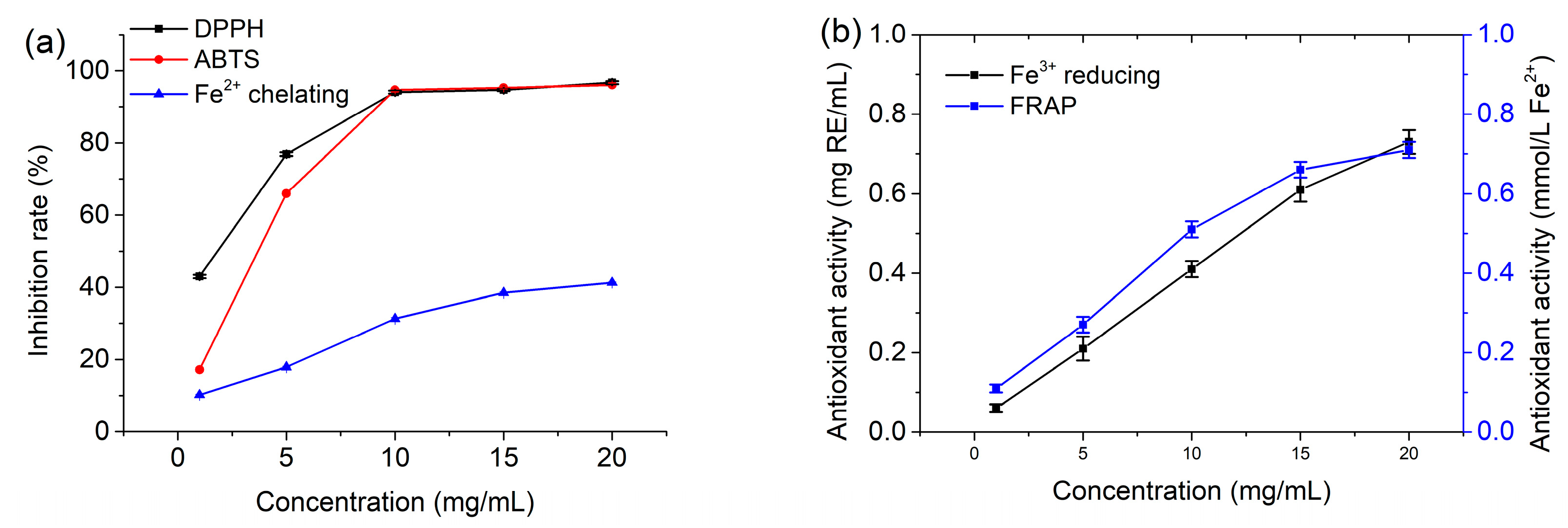
3.4. Antioxidant Activities of the DES Extract
3.5. Antibacterial Activity of the DES Extract
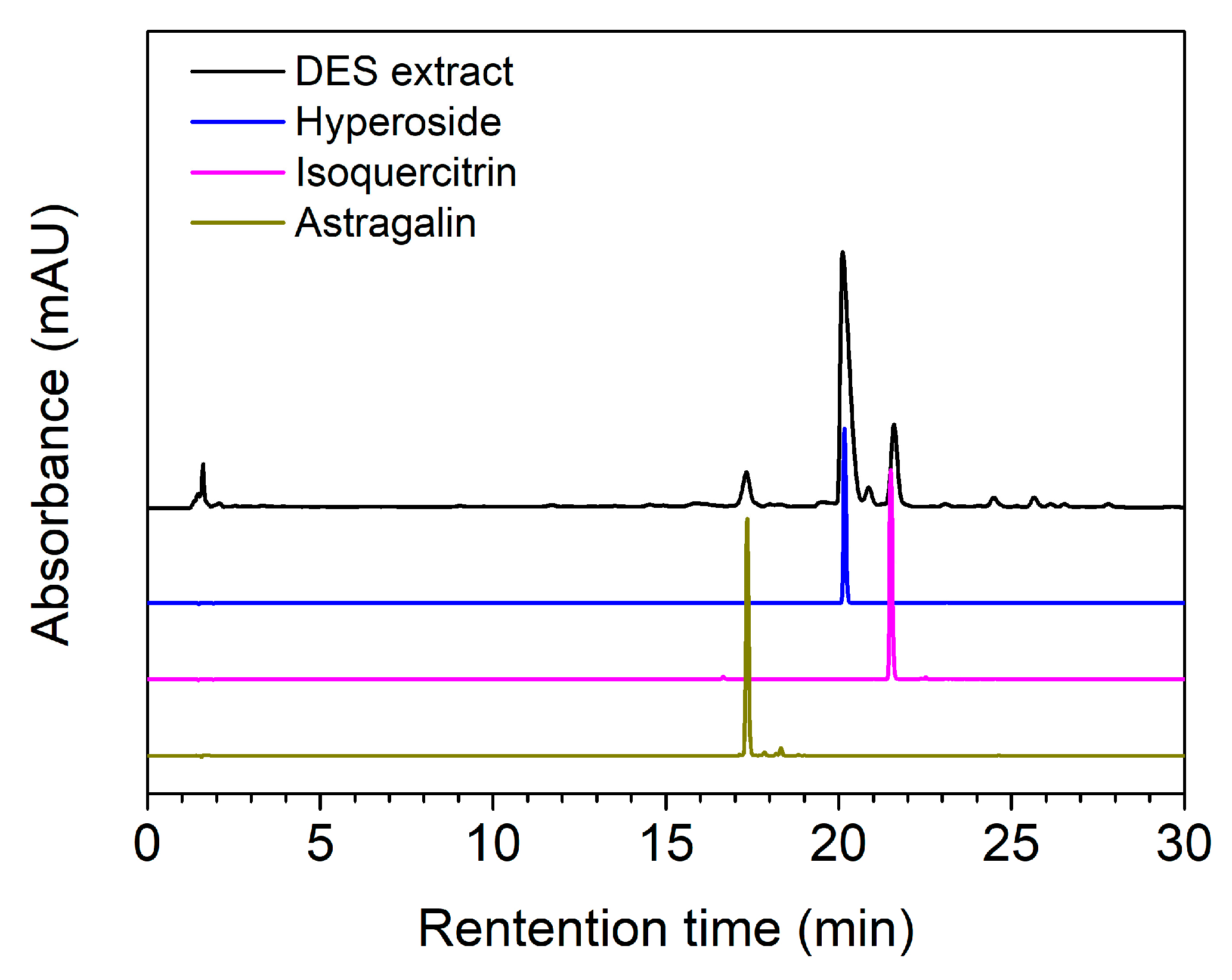
3.6. HPLC Analysis of the DES Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Shi, K.; Shi, J.; Feng, Y.; Hao, C.; Peng, J.; Chen, S. A simple strategy to monitor the temporal and spatial distribution of alkaloids in sacred lotus leaves. Biosci. Biotechnol. Biochem. 2021, 85, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, Y.; Fang, J.-B.; Liu, Y.-L.; Li, S.-H. Flavonoids in lotus (Nelumbo) leaves evaluated by HPLC–MSn at the germplasm level. Food Res. Int. 2013, 54, 796–803. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Simeonov, E.; Rusenova, N.; Ivanova, D.; Nikolova, G.; Karamalakova, Y.; Chilev, C.; Beev, G. Flavonoids Extraction Kinetics, Antimicrobial Activity and Radical Scavenging Potential of Bulgarian Woundwort (Solidago virgaurea L.). Separations 2022, 9, 27. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Kanaan, M.Q.; Hussein, N.A.; AlOmar, M.K.; Sabran, S.F.; Abu Bakar, M.F. Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents. Separations 2022, 9, 271. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Muhamad Hanafi, A.F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Welton, T.; Labidi, J. Extraction of flavonoid compounds from bark using sustainable deep eutectic solvents. Sustain. Chem. Pharm. 2021, 24, 100544. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Hao, H.; Lin, L.; Liu, S.; Kang, Y.; Wang, Y.; Huang, J.; Weng, W. Deep Eutectic Solvent-Based Microwave-Assisted Extraction for the Chromatographic Analysis of Bioactive Flavonoids in Spirodela polyrrhiza. J. Chromatogr. Sci. 2022, 60, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, F.; Busuioc, A.C.; Botezatu, A.V.; Gosav, S.; Avramescu, S.M.; Furdui, B.; Dinica, R.M. Comparative Study of Natural Antioxidants from Glycine max, Anethum graveolensand Pimpinella anisum Seed and Sprout Extracts Obtained by Ultrasound-Assisted Extraction. Separations 2022, 9, 152. [Google Scholar] [CrossRef]
- Boli, E.; Prinos, N.; Louli, V.; Pappa, G.; Stamatis, H.; Magoulas, K.; Voutsas, E. Recovery of Bioactive Extracts from Olive Leaves Using Conventional and Microwave-Assisted Extraction with Classical and Deep Eutectic Solvents. Separations 2022, 9, 255. [Google Scholar] [CrossRef]
- Tsvetov, N.; Sereda, L.; Korovkina, A.; Artemkina, N.; Kozerozhets, I.; Samarov, A. Ultrasound-assisted extraction of phytochemicals from Empetrum hermafroditum Hager. using acid-based deep eutectic solvent: Kinetics and optimization. Biomass Convers. Biorefinery 2022, 12, 145–156. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.; Wang, G.; Zuo, W.; Zeng, Y.; Liu, L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents. Molecules 2022, 27, 5794. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Guo, Z.; Liu, Y.; Wu, Q.; Xiao, J. Optimization of ultrasound-assisted extraction of phenolics from Asparagopsis taxiformis with deep eutectic solvent and their characterization by ultra-high-performance liquid chromatography-mass spectrometry. Front. Nutr. 2022, 9, 1036436. [Google Scholar] [CrossRef]
- Qin, G.; Lei, J.; Li, S.; Jiang, Y.; Qiao, L.; Ren, M.; Gao, Q.; Song, C.; Fu, S.; Zhou, J.; et al. Efficient, green extraction of two biflavonoids from Selaginella uncinata with deep eutectic solvents. Microchem. J. 2022, 183, 108085. [Google Scholar] [CrossRef]
- Aouam, I.; El Atki, Y.; Taleb, M.; Taroq, A.; El Kamari, F.; Lyoussi, B.; Abdellaoui, A. Antioxidant Capacities and Total Phenolic Contents of Thymus riatarum. Mater. Today Proc. 2019, 13, 579–586. [Google Scholar] [CrossRef]
- Sang, J.; Liu, K.; Ma, Q.; Li, B.; Li, C.-Q. Combination of a deep eutectic solvent and macroporous resin for green recovery of anthocyanins from Nitraria tangutorun Bobr. fruit. Sep. Sci. Technol. 2019, 54, 3082–3090. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Xiao, A.; Huang, S.; Li, D. Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells. Open Chem. 2020, 18, 1481–1494. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Xue, D. Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem. 2017, 226, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Buravlev, E.V.; Shevehenko, O.G. 2-Hydroxy-3-isobornyl-5-methylbenzaldehyde derivatives: Synthesis and antioxidant activity in vitro. Russ. Chem. Bull. 2019, 68, 79–85. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial Activity of Polyphenols: Structure-Activity Relationship and Influence of Hyperglycemic Condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.K.; Alqasmi, M.H.; Alrouji, M.; Kuriri, F.A.; Almuhanna, Y.; Joseph, B.; Asad, M. Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 8551. [Google Scholar] [CrossRef]
- Naim, N.; Bouymajane, A.; Oulad El Majdoub, Y.; Ezrari, S.; Lahlali, R.; Tahiri, A.; Ennahli, S.; Laganà Vinci, R.; Cacciola, F.; Mondello, L.; et al. Flavonoid Composition and Antibacterial Properties of Crocus sativus L. Petal Extracts. Molecules 2023, 28, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cui, Q.; Yin, L.-J.; Zheng, X.; Gao, M.-Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2019, 170, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Ind. Crops Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- Sui, M.; Feng, S.; Liu, G.; Chen, B.; Li, Z.; Shao, P. Deep eutectic solvent on extraction of flavonoid glycosides from Dendrobium officinale and rapid identification with UPLC-triple-TOF/MS. Food Chem. 2023, 401, 134054. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Dou, Y.; Yan, N.; Li, N.; Zhang, H.; Tan, J.-N. Optimizing Ultrasound-Assisted Deep Eutectic Solvent Extraction of Bioactive Compounds from Chinese Wild Rice. Molecules 2019, 24, 2718. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Vladić, J.; Vidović, S.; Pastor, K.; Jokić, S.; Molnar, M.; Jerković, I. Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants 2020, 9, 153. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Ma, P.; Geng, S.; Kong, Y.; Li, X.; Fan, Z.; Zhang, Y.; Dong, A.; Zhou, Q. Optimization of the extraction process of flavonoids from Trollius ledebouri with natural deep eutectic solvents. J. Sep. Sci. 2022, 45, 717–727. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crops Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, F.; Yao, Z.; Zhu, J.; Jing, X.; Wang, X. Efficient extraction of flavonoids from Polygonatum sibiricum using a deep eutectic solvent as a green extraction solvent. Microchem. J. 2022, 175, 107168. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, D.; Zhu, X.; Su, A.; Zhang, H. Extraction of Illegal Dyes from Red Chili Peppers with Cholinium-Based Deep Eutectic Solvents. J. Anal. Methods Chem. 2017, 2017, 2753752. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Wang, J.-P. Effective extraction with deep eutectic solvents and enrichment by macroporous adsorption resin of flavonoids from Carthamus tinctorius L. J. Pharm. Biomed. Anal. 2019, 176, 112804. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, H.; Tong, M.; Gong, Y. Green and enhanced extraction of coumarins from Cortex Fraxini by ultrasound-assisted deep eutectic solvent extraction. J. Sep. Sci. 2020, 43, 3441–3448. [Google Scholar] [CrossRef]
- Xia, G.-H.; Li, X.-H.; Jiang, Y.-H. Deep eutectic solvents as green media for flavonoids extraction from the rhizomes of Polygonatum odoratum. Alex. Eng. J. 2021, 60, 1991–2000. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Chu, X.; Wang, X. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax senticosus Using Deep Eutectic Solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef]
- Gao, M.; Wang, D.; Deng, L.; Liu, S.; Zhang, K.; Quan, T.; Yang, L.; Kang, X.; Xia, Z.; Gao, D. High-Crystallinity Covalent Organic Framework Synthesized in Deep Eutectic Solvent: Potentially Effective Adsorbents Alternative to Macroporous Resin for Flavonoids. Chem. Mater. 2021, 33, 8036–8051. [Google Scholar] [CrossRef]
- Al-Azzawi, A.; Al Dibsawi, A.; Talath, S.; Wali, A.F.; Sarheed, O. Method Development: The Antioxidant and Antifungal Activity of the Isolated Component from the Ethanolic Extract of Tecoma stans Leaves Using Flash Chromatography. Separations 2022, 9, 317. [Google Scholar] [CrossRef]
- Oliveira, G.; Marques, C.; de Oliveira, A.; de Almeida dos Santos, A.; do Amaral, W.; Ineu, R.P.; Leimann, F.V.; Peron, A.P.; Igarashi-Mafra, L.; Mafra, M.R. Extraction of bioactive compounds from Curcuma longa L. using deep eutectic solvents: In vitro and in vivo biological activities. Innov. Food Sci. Emerg. Technol. 2021, 70, 102697. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Wu, W.; Jiao, L.-L.; Yang, P.-F.; Guo, M.-Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, N.; Chen, M.; Yuan, Y.; He, S.; Wang, Y.; Wu, Q.; Li, L.; Yang, H.; Zeng, Q. Effects of in vitro digestion on the composition of flavonoids and antioxidant activities of the lotus leaf at different growth stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. [Google Scholar] [CrossRef]
- An, J.-Y.; Wang, L.-T.; Lv, M.-J.; Wang, J.-D.; Cai, Z.-H.; Wang, Y.-Q.; Zhang, S.; Yang, Q.; Fu, Y.-J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Naseem, Z.; Zahid, M.; Hanif, M.A.; Shahid, M. Green extraction of ethnomedicinal compounds from Cymbopogon citratus Stapf using hydrogen-bonded supramolecular network. Sep. Sci. Technol. 2021, 56, 1520–1533. [Google Scholar] [CrossRef]
- Benslama, A.; Harrar, A.; Gul, F.; Demirtas, I. Phenolic Compounds, Antioxidant and Antibacterial Activities of Zizyphus lotus L. Leaves Extracts. Nat. Prod. J. 2017, 7, 316–322. [Google Scholar] [CrossRef]
- Tang, X.; Tang, P.; Liu, L. Molecular Structure–Affinity Relationship of Flavonoids in Lotus Leaf (Nelumbo nucifera Gaertn.) on Binding to Human Serum Albumin and Bovine Serum Albumin by Spectroscopic Method. Molecules 2017, 22, 1036. [Google Scholar] [CrossRef]
- Jung, S.Y.; Jung, W.S.; Jung, H.K.; Lee, G.H.; Cho, J.H.; Cho, H.W.; Choi, I.Y. The mixture of different parts of Nelumbo nucifera and two bioactive components inhibited tyrosinase activity and melanogenesis. J. Cosmet. Sci. 2014, 65, 377–388. [Google Scholar]
- Cho, W.-K.; Yang, H.J.; Ma, J.Y. Lotus (Nelumbo nucifera Gaertn.) leaf water extracts suppress influenza a viral infection via inhibition of neuraminidase and hemagglutinin. J. Funct. Foods 2022, 91, 105019. [Google Scholar] [CrossRef]
| No. | HBA | HBD | Molar Ratio | Extraction Efficiency (mg/g) |
|---|---|---|---|---|
| 1 | Choline chloride | Ethylene glycol | 1:2 | 114.9 ± 2.5 d |
| 2 | Triethylene glycol | 1:2 | 91.5 ± 1.2 h | |
| 3 | Glycerol | 1:2 | 49.6 ± 1.2 p | |
| 4 | 2-Chloropropionic acid | 1:2 | 33.7 ± 0.1 rs | |
| 5 | Malonic acid | 1:2 | 36.3 ± 0.4 r | |
| 6 | Lactic acid | 1:2 | 54.1 ± 1.5 o | |
| 7 | Formic acid | 1:2 | 81.4 ± 0.7 l | |
| 8 | Acetic acid | 1:2 | 88.3 ± 0.8 ij | |
| 9 | Urea | 1:2 | 178.9 ± 2.8 a | |
| 10 | Benzyltriethylammonium chloride | Ethylene glycol | 1:2 | 82.1 ± 0.9 kl |
| 11 | Triethylene glycol | 1:2 | 100.9 ± 1.7 g | |
| 12 | Glycerol | 1:2 | 92.2 ± 1.3 h | |
| 13 | 2-Chloropropionic acid | 1:2 | 49.6 ± 0.4 p | |
| 14 | Malonic acid | 1:2 | 49.3 ± 0.5 p | |
| 15 | Lactic acid | 1:2 | 83.4 ± 1.4 kl | |
| 16 | Formic acid | 1:2 | 154.9 ± 2.7 b | |
| 17 | Acetic acid | 1:2 | 48.0 ± 0.9 p | |
| 18 | Oxalic acid | 1:2 | 107.4 ± 0.8 f | |
| 19 | Acetamide | 1:2 | 30.7 ± 0.2 st | |
| 20 | Betaine | Glycerol | 1:2 | 87.6 ± 0.6 ij |
| 21 | Malonic acid | 1:2 | 73.3 ± 0.4 m | |
| 22 | Lactic acid | 1:2 | 97.7 ± 1.0 g | |
| 23 | Formic acid | 1:2 | 84.4 ± 0.5 g | |
| 24 | Acetic acid | 1:2 | 113.3 ± 1.1 d | |
| 25 | Urea | 1:2 | 59.3 ± 0.4 n | |
| 26 | Oxalic acid | 1:2 | 39.8 ± 0.1 q | |
| 27 | Guanidine hydrochloride | Ethylene glycol | 1:2 | 99.3 ± 0.9 g |
| 28 | Triethylene glycol | 1:2 | 105.2 ± 1.1 f | |
| 29 | Glycerol | 1:2 | 85.3 ± 0.7 jk | |
| 30 | 2-Chloropropionic acid | 1:2 | 83.7 ± 0.4 kl | |
| 31 | Lactic acid | 1:2 | 111.4 ± 3.0 e | |
| 32 | Formic acid | 1:2 | 98.7 ± 1.0 g | |
| 33 | Acetic acid | 1:2 | 29.1 ± 0.8 t | |
| 34 | Acetamide | 1:2 | 138.9 ± 2.5 c |
| Antibacterial Properties | Samples | S. aureus | E. coli |
|---|---|---|---|
| DIZ (mm) | DES extract (50 mg/mL) | 7.29 ± 0.01 | 9.02 ± 0.02 |
| DES extract (100 mg/mL) | 8.49 ± 0.01 | 12.29 ± 0.01 | |
| Rutin (1 mg/mL) | 7.21 ± 0.01 | 7.11 ± 0.01 | |
| MIC (μg/mL) | DES extract | 1666 | 208 |
| Rutin | 100 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Xiao, A.; Zhang, Y.; Duan, S. Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities. Separations 2023, 10, 65. https://doi.org/10.3390/separations10020065
Liu L, Xiao A, Zhang Y, Duan S. Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities. Separations. 2023; 10(2):65. https://doi.org/10.3390/separations10020065
Chicago/Turabian StyleLiu, Liangliang, Aiping Xiao, Yi Zhang, and Shengwen Duan. 2023. "Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities" Separations 10, no. 2: 65. https://doi.org/10.3390/separations10020065
APA StyleLiu, L., Xiao, A., Zhang, Y., & Duan, S. (2023). Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities. Separations, 10(2), 65. https://doi.org/10.3390/separations10020065







