Abstract
Andean blackberry is cultivated in Colombia due to its national and international commercial importance, in addition to its flavour and high nutritional value. Due to its physicochemical, morphological, and physiological characteristics, it constitutes one of the most unstable fruits in the Colombian fruit and vegetable supply chain, thereby generating economic losses. In this study, a polymer-based coating of Aloe vera and essential oil was designed, and its influence on the shelf life of Andean blackberry stored at 4 °C was studied. Once the appropriate composition was established according to the experimental design, Andean blackberries’ physicochemical parameters, the content of total phenols, and antioxidant activity were monitored over 19 days. The total soluble solids present a change between 5.2 and 5.6° Brix and 5.2 and 7.2° Brix for coated and uncoated fruits between 1 and 19 days, respectively. The coated fruits presented a lower loss compared to the uncoated fruits and the total phenol content presented a higher concentration on day 19 in the coated fruits (412.71 ± 37.5 mg Gallic Acid Equivalents L−1 sample). The coating enabled an increase in the shelf life of the blackberries, from 4 to 19 days, while preserving the physicochemical properties of the fruits. Therefore, the application of coating as a method for the post-harvest preservation of Andean blackberries represents a low-cost, easily available strategy.
1. Introduction
Species of the Rubus family are considered an important source of phytochemicals with high nutraceutical value and are beneficial to health [1]. Their fruits are consumed fresh or frozen, or they are processed commercially in a variety of foods and products such as jellies, wine, tea, dyes, and dietary supplements [1]. These fruits are rich in carbohydrates, dietary fiber, vitamins, and minerals [2], and bioactive compounds such as anthocyanins, flavanols or catechins, flavones, flavanones, and isoflavones, to which various biological activities are attributed [3]. These properties have driven the rapid growth of blackberry production for the fresh, processed, and nutraceutical markets.
In Colombia, the species Rubus glaucus Benth., commonly known as Andean blackberry (mora de Castilla), is nationally produced continuously. It is considered a competitive fruit because of its commercial importance at the national and international level due to its flavor and its contribution to the diet, given that it is low in calories and rich in vitamin C and contains potassium, fiber, iron, calcium, tannins, various organic acids, and natural pigments with antioxidant potential [4]. However, these berries are highly perishable fruit, with a shelf life of only 3 to 5 days under refrigeration [5] due to their physicochemical, morphological, and physiological characteristics. These characteristics make them susceptible to various physiopathologies and diseases throughout the harvest, transport, and storage processes, such as dehydration, weight loss, texture changes, cold damage, rot, fermentation, and mechanical damage that ultimately translate into losses for farmers, sellers, and consumers [6].
Currently, the different methods used for blackberry preservation range from basic technologies such as cold treatment via refrigeration and traditional freezing or individual fast freezing to high-end technologies such as modified atmosphere and irradiation. These technologies have been extensively studied [7] but have disadvantages in terms of high energy consumption and altering the physical characteristics of the fruit and causing it to lose suppleness due to water loss [8]. On the other hand, there is growing consumer demand for high-quality and minimally processed fresh products, which has increased the search for new preservation methods and technologies [9].
Edible coatings are effective and environmentally friendly alternatives that act as a semipermeable barrier to reduce respiration and weight loss, maintaining the firmness of fresh fruits and providing shine to the coated products. These coatings can be produced from a wide variety of functional ingredients, such as antimicrobials, antioxidants, nutrients, flavorings, and colouring compounds [10] that contribute to food stability, quality, and safety.
Their main components are polysaccharides of natural origin, including starch, cellulose, pectin, alginates, and chitosan that form films with a high oxygen barrier capacity; plasticizers and emulsifiers that improve flexibility, extensibility, and/or stability of the polymer matrix structure; and essential oils that limit the hygroscopicity of polysaccharide-based materials [9].
In this sense, several studies have been reported on the possibility of implementing edible coatings that help to increase the shelf life of fruits and vegetables that are economical, easily accessible, and that do not modify the nutritional properties of the food. Some of the most used components for developing these edible coatings are polysaccharides, Aloe vera, and essential oils of different species to reduce the loss of water and colour in fruits. In particular, cassava starch and cinnamon oil in coatings are used for strawberry and guava [11,12], chitosan and lemon essential oil are used for strawberries [13], starch and essential oils of oregano, cinnamon, and lime are used in coatings applied to green peppers [14], starch, and Aloe vera are used for tomatoes [15], and coatings for grapes incorporate lemon essential oil [16].
The objective of this study was to examine shelf life and antioxidant proprieties preservation of Andean blackberry (Rubus glaucus Benth) cultivated in Risaralda, Colombia using a coating based on aloe vera, starch, and essential oil.
2. Materials and Methods
2.1. Plant Material
The fruits of Andean blackberry (Rubus glaucus Benth) used in the study were collected in the municipality of Santa Rosa de Cabal, Risaralda, Colombia, in maturity states 4 and 5, as established in NTC 4106 [17], with the following coordinates N: 04°53′24.1″; W: 075°33′44.0″ and 2085 ± 3 m. The fruits were washed and disinfected with 1% Tego 51 soap (Merck, Ref. CO15005149050) and 5% commercial sodium hypochlorite.
The leaves of Aloe vera (Aloe barbadensis Miller) cultivated in the municipality of Pereira, Risaralda were washed to remove dirt; the edges were cut and left to rest for 24 h. The pulp or gel to be used in the coating is in the central part of the leaf and represents 65 to 80% of the total weight of this. For the gel extraction, the rind was removed using a plastic knife, the internal material was washed with water for 2 min, after washing, the pulp was homogenised and filtered to eliminate residues. The obtained solution was stabilised by ascorbic acid (Supelco, Ref. 1004681000) and citric acid (Supelco, Ref. 1002441000) [18]. For the coating, the aloe vera content was 20% of the total extract. Banana starch and mandarin essential oil were provided by the Food Science and Technology Research Group (CYTA) of the University of Quindío, Armenia, Quindío.
2.2. Determination of the Optimal Coating Composition
Experimental Design
To determine the optimal concentration of the coating, a 22 factorial design was proposed. The test factors were the concentration of starch and mandarin essential oil, with a total of four coatings evaluated with the concentrations specified in Table 1, as established by Sánchez et al., (2012) [19], Guancha et al., (2016) [20] and Acevedo-Guevara & Nieto-Suaza, (2017) [18].

Table 1.
Concentrations of starch and essential oil used in the coating.
2.3. Preparation of Coatings and Films
The coatings were prepared using the concentrations of Aloe vera (20%), glycerol (1.5%), starch, and mandarin essential oil set out in Table 1, the remaining percentage was completed with distilled water. In a container, the corresponding percentages of Aloe vera, starch and glycerol were added for each of the coatings, the mixtures were subjected to heating (80 °C) with constant stirring until complete gelling; they were then allowed to cool to 25 °C. The mandarin essential oil was added, and the volume was completed with distilled water. Finally, 25 mL per mixture were spread in Petri dishes and dried at 35 °C for 24 h [19].
2.4. Characterisation of the Films
2.4.1. Physical Properties
- Thickness: The thickness was determined by averaging the measurements made with a micrometer (Newton, MA, USA, Fowler) in six different locations of ten films for each coating [18].
- Transparency: Absorbance readings were taken for each film at 600 nm in a UV-Vis spectrophotometer (ColorQuest XE, Reston, Virginia, USA, HunterLab), and the transparency was subsequently calculated according to Equation (1). The absorbance was measured on three films per coating, each in triplicateTransparency = Absorbance/Thickness
- Water solubility index (WSI): This index was determined using weight differences. The films were dispersed in 80 mL of water with constant stirring for one hour and then dried at 60 °C. The index was calculated according to Equation (2) [21]. The WSI was measured for three films per coating, each in triplicate.WSI = (Dry weight initial-dry weight final)/(dry weight initial)
2.4.2. Barrier Properties
- Water Vapour Permeability (WVP): To determine the WVP, the ASTM E96-05 standard was followed according to Equation (3), with modifications proposed by García, et al., (2004) [22] for hydrophilic films. Films were placed in permeation cells and maintained in a controlled humidity cabinet at 65% RH and 25 °C for 48 h. The permeation cells were weighted at 1 h intervals for 8 h. The WVP was calculated using the thickness of each film studied and was measured on three films per coating, each in triplicatewhere WVP: water vapour permeability [g (Pa × s × m) −1]; m: slope of the weight loss curve; L: average thickness of the film (m); A: exposed area of the film (m2); (hr1 − hr2): difference in relative humidity; Pw: partial pressure of water vapour at the test temperature (Pa).WVP = [(m/A) L)/(Pw (hr1 − hr2)],
2.4.3. Mechanical Properties
- Tensile stress (TS) and elongation percentage (% E): These parameters were determined according to the ASTM method D882-09. The equipment used was a TA-XT Plus Texture Analyzer (texture technologies), the biofilm was placed in the equipment holder and stretched until it ruptured. The tensile strength (TS [MPa]) was calculated as the quotient between the force to rupture and the cross-sectional area, while the elongation percentage (% E) was determined as the percentage ratio between the elongation and the initial length of the film.
2.5. Evaluation of the Influence of the Coating on the Shelf Life of the Blackberries
The physicochemical and antioxidant properties of the blackberry fruits three days after coating were monitored in triplicate for 19 days using uncoated blackberry fruits as a control.
2.5.1. Application of the Coating
Each Andean blackberry was submerged in the selected coating according to the best physical, barrier, and mechanical properties. The berries rested in a sieve for 10 min to remove the excess coating and were then dried for two hours with cold air (27 °C) [23].
2.5.2. Parameters Evaluated
- pH: The pH was determined by the potentiometric method according to the Colombian technical norm NTC 4592 [24], with digital pH meter Orion 3 Star (North America, Thermo Scientific).
- Acidity: The titratable acidity expressed as malic acid was determined by potentiometric titration with sodium hydroxide and phenolphthalein as an indicator according to the Colombian technical norm NTC 4106 [17].
- Total soluble solids (TSS): The total soluble solids were measured in °Brix by the refractometric method according to the Colombian technical standard NTC 4106 [17].
- Weight loss: The weight loss was evaluated by the gravimetric method using Equation (4) [25].where X is the weight of the fruit on each day of analysis.Weight loss = Weight day 1 − Weight day X
- Total phenols: The Folin–Ciocalteu method was used. In a 5 mL flask, 50 µL of the extract, 250 µL of Folin-Ciocalteu reagent (1: 1), 750 µL of Na2CO3 (20%) were mixed and made up with distilled water. After incubating this mixture for 30 min, the absorbance was measured at 760 nm wavelength (GEN10S UV-Vis, Deutschland, Germany, Thermo Scientific). A calibration curve was used using gallic acid as a reference standard (R2: 0.9992). The results were expressed as milligrams of gallic acid equivalents per gram of sample (mg GAE g−1 sample).
- Antioxidant activity: Two spectrophotometric methods were used. The first was the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method, in which 30 μL of the extract were mixed with 2 mL of the ethanolic DPPH solution at 50.7 μM (20 mg L−1). After incubating this mixture for 30 min, the absorbance was measured at 517 nm wavelength (GEN10S UV-Vis, Deutschland, Germany, Thermo Scientific). Then a calibration curve was made with Trolox as the reference standard (R2: 0.9950). The results were expressed as micromolar of the Trolox Equivalents (μM TE)
The second was the Ferric Reduction Activity Potential (FRAP) method, The FRAP reagent was prepared by mixing a 300 mM acetate buffer solution (pH 3.6) with 10 mM TPTZ, 40 mM HCl, and 20 mM FeCl3 solution (10:1:1). In which 1.8 mL of the FRAP were mixed with 60 μL of extract. After incubating this mixture for 30 min, the absorbance was measured at 593 nm (GEN10S UV-Vis, Deutschland, Germany, Thermo Scientific). Then, a calibration curve was used with Trolox as the reference standard (R2: 0.9880). The results were expressed as micromolar of the Trolox Equivalents (μM TE).
2.6. Statistical Analysis
All data were expressed as mean ± standard deviation. For the determination of the coating, optimum concentrations were applied in a 22 factorial arrangement, the test factors being the concentration of starch and essential oil, and factorial analysis. Each treatment was replicated twice. An analysis of variance (ANOVA) was conducted followed by Tukey’s test. p-values of <0.05 were considered to indicate statistically significant differences in the evaluated parameters, antioxidant activity, total phenolic content, and the time of application. The Infostat program, version 2008i, was used for all statistical analyses.
3. Results
3.1. Determination of the Coating Composition
The results obtained for the physical, barrier, and mechanical properties evaluated for the coatings are shown in Table 2.

Table 2.
Results of film characterisation.
The factor analysis shows in Figure 1. Figure 1A shows that factor 1 is composed of permeability, tension, and elongation with a higher percentage than 84%, while factor 2 is composed of the thickness variable with a higher percentage than 90%. According to the estimated factors, the dispersion graph (Figure 1B) shows that the R4 coating presents the best conditions in relation to factor 1.
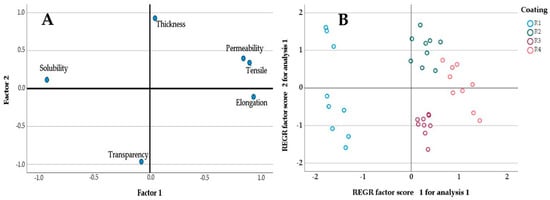
Figure 1.
Factor analysis results. (A) Component plot in rotated space (B) Scatter plot of points.
Through the statistical analysis, it was possible to determine that the best coating was R4, where statistically significant differences (p < 0.005) were observed in the parameters of solubility, permeability, TS, and thickness with respect to the other evaluated coatings. It had a thickness of 0.115 ± 0.006 mm, a transparency of 14.050 ± 0.781 AU mm−1, a solubility of 50.520 ± 1.637 mg mL−1, a permeability of 5.847 ± 0.052 g (Pa s m)−1, and a TS of 3.853 ± 0.349 MPa.
3.2. Evaluation of the Influence of the Coating on the Shelf Life of the Blackberry
The coating used to evaluate its influence on the Rubus glaucus Benth fruit shelf life was R4. Blackberries appearance (Rubus glaucus Benth) for the day 1 and 19 shows in Figure 2. Figure 3 shows the results of monitoring the pH, acidity, TSS (°Brix), and weight loss. There were statistically significant differences (p < 0.005) between the evaluated parameters, such as pH, acidity, TSS, and weight loss, among the coated and uncoated Andean blackberry fruits.
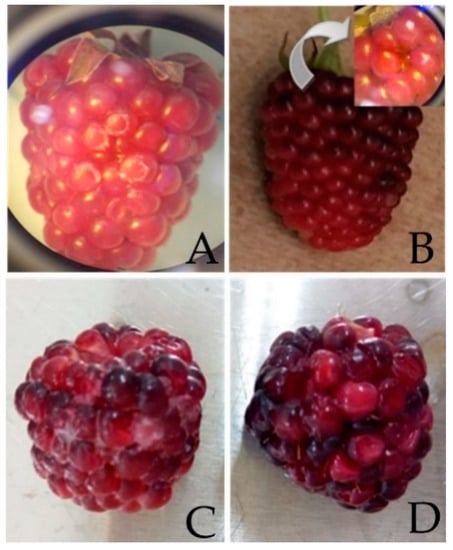
Figure 2.
Blackberries appearance. (A,B) Coated and uncoated fruits on day 1. (C,D) Coated and uncoated fruits on day 19.
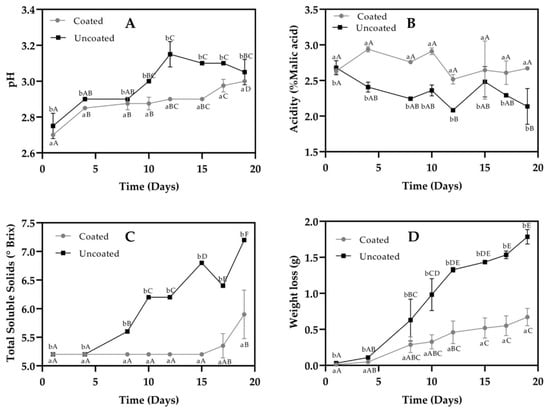
Figure 3.
Monitoring the physicochemical properties of coated and uncoated fruits. (A) pH (B) Acidity (C) Total Soluble Solids (D) Weight loss. The averages with a common letter are not significantly different according to the Tukey test (p ≥ 0.05). Uppercase letters differences between days and lowercase letters differences between material coated and uncoated fruits.
Regarding pH, values from 2.7 ± 0.0 to 3.0 ± 0.0 and 2.8 ± 0.1 to 3.1 ± 0.1 were observed for the coated and uncoated fruits, respectively. For titratable acidity, the values were 2.5 ± 0.06 to 2.9 ± 0.047% malic acid for coated fruits; these values were similar on all days of the measurement, while for uncoated fruits, the values obtained for this parameter were between 2.1 ± 0.3 and 2.7 ± 0.1% malic acid.
The coated fruits had a lower TSS content, ranging from 5.2 ± 0.0 to 5.9 ± 0.4 °Brix, and the uncoated fruits ranged from 5.2 ± 0.0 to 7.2 ± 0.0 °Brix. A lower weight loss was observed for coated fruits compared to uncoated fruits, with respective values between 0.012 ± 0.00 g to 0.671 ± 0.120 g and 0.03 ± 0.002 g to 1.8 ± 0.100 g.
The results obtained for the total phenol contents evaluated by the Folin–Ciocalteu method are presented in Figure 4. According to the analysis of variance, significant differences were found between for the fruits on the different days evaluated (p < 0.05), that is, the fruits coated and uncoated presented a higher content of total phenols on day 19 (450.00 ± 3.9 and 470.00 ± 3.93 mg GAE L−1 sample) compared to day one (316.2.75 ± and 329.44 ± 6.28 mg GAE L−1 sample), which was confirmed by Tukey′s test.
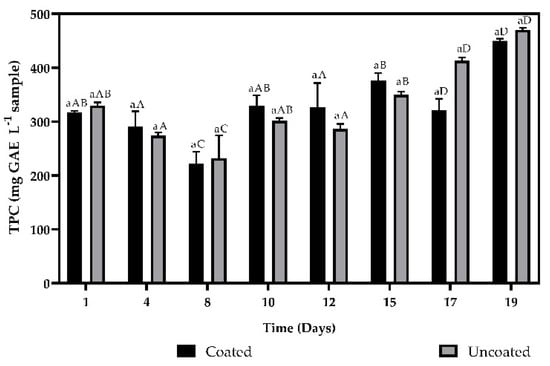
Figure 4.
Total phenolic content of coated and uncoated fruits. The averages with a common letter are not significantly different according to the Tukey test (p ≥ 0.05). Uppercase letters differences between days and lowercase letters differences between material coated and uncoated fruits.
The results obtained antioxidant activity are shown in Figure 5 and Figure 6. In the antioxidant activity determined by the DPPH method (Figure 5), statistical analysis showed significant differences (p < 0.05) between coated and uncoated fruits, with values between 1220.653 ± 15.74 μM and 1498.596 ± 22.562 μM and between 1247.95 ± 10.018 μM and 1531.87 ± 71.819 μM, respectively.
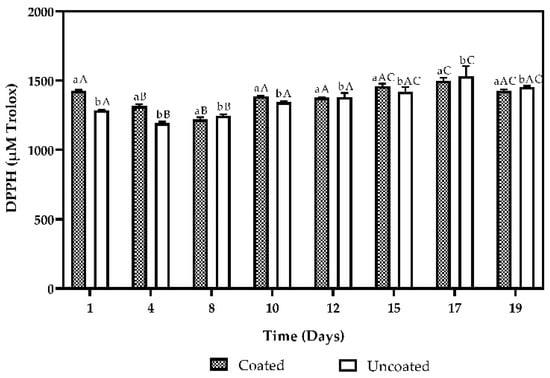
Figure 5.
Antioxidant activity by the DPPH method and total phenol content of coated and uncoated fruits. The averages with a common letter are not significantly different according to the Tukey test (p ≥ 0.05). Uppercase letters differences between days and lowercase letters differences between material coated and uncoated fruits.
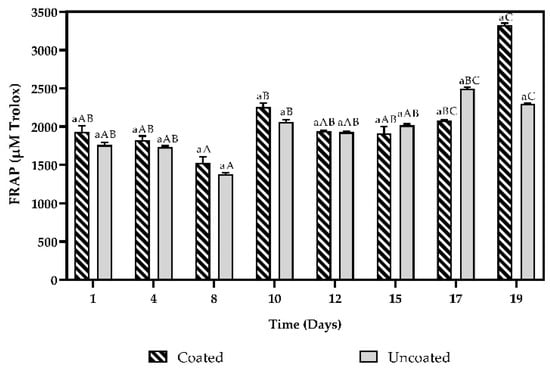
Figure 6.
Antioxidant activity by the FRAP method and total phenol content of coated and uncoated fruits. The averages with a common letter are not significantly different according to the Tukey test (p ≥ 0.05). Uppercase letters differences between days and lowercase letters differences between material coated and uncoated fruits.
Using the FRAP method (Figure 6), the antioxidant activity presented a similar behaviour for the two plant materials, with values between 1526.037 ± 80.680 μM up to 3325.681 ± 26.078 μM and 1380.821 ± 18.744 μM up to 2495.490 ± 20.102 μM for coated and uncoated fruits, respectively.
4. Discussion
When investigating the physical, barrier, and mechanical properties of the coating, it was observed that the thickness of the films increased 0.018 mm and the transparency decreased with increasing concentrations of starch and with decreasing concentrations of essential oil. The decrease in transparency is because the banana starch used lends a brownish colour to the films due to chemical modifications, such as enzymatic oxidation [26], and due to the increase in solids in the coating, which also produces an increase in film thickness [18]. The thickness of edible films and coatings is usually less than 0.3 mm, so those developed in this research are within this value [27,28].
The WSI decreased and the permeability increased when increasing the concentration of starch and essential oil, because these components allow a greater hydrophobicity, preventing the interaction of water with the components of the coating and the exchange of water vapor molecules between the fruit and surrounding air.
According to the physical, mechanical, and barrier properties evaluated, it was established that coating four (R4) presented the appropriate composition because of its thickness and the best observed mechanical properties, which provided greater rigidity to the fruit, improving its resistance to possible damage. That is corroborated with the factor analysis; however, the amount of essential oil could possibly be reduced (R2) while maintaining the good properties of the coating. By having low water solubility and a higher permeability, it will be less likely that the coating will be susceptible to deterioration during fruit storage. The results obtained in this study demonstrated the ability of the Aloe vera coating to extend the shelf life of Andean blackberry fruit, similar results were obtained by [23] who evaluated the preservation of the polyphenolic content and antioxidant properties of Rubus glaucus Benth.
As shown in Figure 3A, the pH showed statistically significant differences (p < 0.05) between coated and uncoated fruits, i.e., this parameter was higher for uncoated fruits. This increase in pH during storage is possibly related to fruit senescence, as reported by González, (2010) [29]. Possibly, it can occur due to anabolic biochemical processes that give way to catabolic processes producing aging and death during maturation, and after this, organic acids decrease due to sugar formation, which is why the pH could increase [30]. Another aspect to highlight is that, possibly, the pH increases because the acidity goes down, where the acids of the fruit would be used as a substrate for respiration [31]. Concerning acidity (Figure 3B), a decrease was observed for uncoated fruits (p < 0.05)—an inverse behavior to pH. When comparing the results obtained with the guidelines of the Colombian Technical Standard NTC 4106, it was determined that coated fruits (2.71 ± 0.040% malic acid) comply with the maximum acidity determined for fruits in maturity classes 5 and 6 (2.8 and 2.5% malic acid, respectively), making the fruits better suited for fresh consumption or as a raw material for processing. Meanwhile, the uncoated fruits from day 4 did not meet the requirements according to NTC 4106, because their acidity was less than 2.5% malic acid. Regarding the TSS content (Figure 3C), the uncoated fruits were characterised by a marked increase, from 5.2 ± 0.000 on day one to 7.2 ± 0.000 °Brix on day 19, with higher values in comparison with coated fruits. The increase in TSS in uncoated fruits is possibly influenced by the transformation of organic acids into sugars (gluconeogenesis), which translates into a decrease in titratable acidity [32].
Regarding weight loss (Figure 3D), the uncoated fruits lost 1.754 g of initial weight during the 19 days of analysis, while coated fruits lost 0.659 g, indicating that the coating decreased fruit transpiration, conserving its texture. The initial weights of coated and uncoated fruits were: 3.7979 g and 4.6713 g respectively. It has been reported that edible coatings can control water vapor and other gases, such as O2 and CO2 permeability from the fruit to the exterior. Those gases are mainly the product of the degradation of complex sugars during storage, and using edible coatings can deaccelerate the degradation process leading to a smaller increase in TSS values [33]. Furthermore, it has been reported that the inclusion of Aloe vera in banana starch films leads to a reduction of water vapor permeability, due to a crosslinking effect between Aloe vera components and starch molecules [34]. It seems that the higher concentrations of both polymers, also lead to lower CO2 and O2 permeability, reducing blackberries degradation during storage and lesser weight loss.
According to the study carried out by Velasquez-Castro, et al., (2019) [23], the total phenols content did not show differences between coated and uncoated fruits (p > 0.05), while during storage the differences became statistically significant, a similar behavior found in this study. On the first day, the total phenol content was 316.94 ± 2.75 mg GAE L−1 sample and 329.44 ± 6.285 mg GAE L−1 sample and increased significantly to 450 ± 3.93 mg GAE L−1 sample and 470 ± 3.93 mg GAE L−1 sample after storage 19 days for the coated and uncoated fruits, respectively. It has been shown that the accumulation of phenolic compounds in fruits during storage can be promoted by the activity of Phenylalanine Ammonia Lyase (PAL) [35]. In addition, it depends on the species, crop, temperature, climatic, and environmental conditions during the growing period [34].
According to the antioxidant activity by the DPPH method (Figure 5), the coated and uncoated fruits present significant differences, as well as the days evaluated (p < 0.05), where the coated fruits have higher antioxidant activity, similar results found for blackberry (Rubus glaucus Benth) for ten days [23], raspberry (Rubus spp) for eight days [36], raspberry (Rubus ulmifolius subsp sanctus) for nine days [37], and strawberry (Fragaria ananassa cv Hongyan) for six days [38].
Regarding the antioxidant activity by the FRAP method (Figure 6), significant differences were only observed between the evaluated storage days (p < 0.05), both coated and uncoated fruits present a higher activity on day 19 compared to day 1.
The coating developed based on Aloe vera and starch for the fruits of Andean blackberry showed low solubility, high permeability, a high elongation percentage, and a high TS, enabling a delay in fruit transpiration and decreasing the weight loss during storage, which also increased the shelf life of the blackberries by 15 days compared to the control and by 19 days compared to that reported by Ramírez et al., (2013) [39]. These authors managed to conserve blackberries for up to 10 days using coatings based on Aloe vera, carnauba wax, and glycerol [23,39]
5. Conclusions
The objective of this investigation was to examine shelf life and antioxidant proprieties preservation of Andean blackberry (Rubus glaucus Benth) cultivated in Risaralda, Colombia using a coating based on aloe vera, starch, and mandarin essential oil. In this study, a low-cost and highly available edible coating with a content of 20% Aloe vera, 3% plantain starch, 1.5% glycerol, and 0.1% mandarin essential oil was obtained, which shows great potential for preserving Andean blackberries for approximately 19 days. The thickness of edible films and coatings is usually less than 0.3 mm, so those developed in this research are within this value. This coating provides a barrier to reduce weight loss, preserve pH, and reduce TSS and titratable acidity, in addition to preserving the total phenol content and antioxidant fruit activity in storage time. In later studies, a sensory analysis will continue to establish the potential of this coating that could provide commercial value for producers.
Author Contributions
Conceptualization J.P.A.V., G.E.G.Á., M.I.P., and C.C.V.; methodology, M.C.V.S. and N.C.H.; validation, M.C.V.S. and N.C.H.; formal analysis, M.C.V.S.; investigation, J.P.A.V., G.E.G.Á., M.I.P., and C.C.V.; resources, J.P.A.V., G.E.G.Á., M.I.P., and C.C.V.; data curation, N.C.H. and G.E.G.Á.; writing—origi.nal draft preparation, N.C.H., G.E.G.Á., and J.P.A.V.; writing—review and editing, N.C.H., G.E.G.Á. and J.P.A.V.; supervision G.E.G.Á. and J.P.A.V.; project administration, G.E.G.Á. and J.P.A.V.; funding acquisition, J.P.A.V., G.E.G.Á., M.I.P., and C.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fund of the General Royalty System grant number BPIN 201200010050 and Universidad del Quindío (code: Call N°4- 28-01-2021, support for the financing of publication in indexed journals) for financing the project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data obtained are published in this article with open access to the public according to the journal policy. The participating research groups have the primary information files obtained confirming their originality, and manifest scientific honesty in obtaining and processing the information. There is no conflict of interest.
Acknowledgments
The authors are grateful to Vice-Rector for Research, Innovation and Extension of the Technological University of Pereira and the project Development of Scientific and Technological Capabilities in Biotechnology Applied to the Health and Agro-industry Sectors in the Department of Risaralda (Code: BPIN 201200010050), financed with resources from the CTeI Fund of the General Royalty System for financing the project and Universidad del Quindío (code: Call N°4- 28-01-2021, support for the financing of publication in indexed journals).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milosevic, T.; Mratinic, E.; Milosevic, N.; Glisic, I.; Mladenovic, J. Segregation of Blackberry Cultivars Based on the Fruit Physico- Chemical Attributes. J. Agric. Sci. 2012, 18, 100–109. [Google Scholar]
- Gündoğdu, M.; Kan, T.; Canan, İ. Bioactive and antioxidant characteristics of blackberry cultivars from East Anatolia. Turk. J. Agric. For. 2016, 40, 344–351. [Google Scholar] [CrossRef]
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving Human Health and Healthy Aging and Promoting Quality Life—A Review. Plant. Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Perdomo, E.; Aller, A.; Cruz-Quintana, S.M.; Giampieri, F.; Alvarez-Suarez, J.M.; Riera, M.E. Andean berries from Ecuador: A review on Botany, Agronomy, Chemistry and Health Potential. J. Berry Res. 2015, 5, 49–69. [Google Scholar] [CrossRef]
- Guzmán, T.M.; Cuenca, K.; Tacuri, E. Caracterización de la poscosecha de la mora de castilla (Rubus glaucus) tratada con 1-metilciclopropeno. Cienc. Técnicas Agropecu. 2018, 27, 66–75. [Google Scholar]
- Ayala, L.C.; Valenzuela, C.P.; Bohórquez, Y. Phytochemical characterization of Castilla Blackberry (Rubus glaucus Benth) in six maturity stage. Biotecnol. Sect. Agropecu. Agroind. 2013, 11, 10–18. [Google Scholar]
- Ramírez, J.D.; Aristizábal, I.D.; Restrepo, J.I. Blackberry conservation through the application of edible coating of aloe vera mucilaginous gel. Vitae 2013, 20, 172–183. [Google Scholar]
- Antunes, L.E.C.; Duarte Filho, J.; De Souza, C.M. Conservação pós-colheita de frutos de amoreira-preta. Pesqui. Agropecuária Bras. 2003, 38, 413–419. [Google Scholar] [CrossRef][Green Version]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Combinational Edible Antimicrobial Films and Coatings. Antimicrob. Food Packag. 2016, 1, 633–646. [Google Scholar]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant, and antimicrobial properties of chitosan–cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Palou, E.; Jiménez-Munguía, M.T.; Nevárez-Morillón, G.V.; Navarro-Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Kim, I.H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. LWT Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). NTC 4106. Frutas Frescas. Mora de Castilla. Especificaciones; ICONTEC: Bogotá, Colombia, 1997; p. 13. [Google Scholar]
- Acevedo-Guevara, L.; Nieto-Suaza, L. Diseño de nanocompuestos de aloe vera. In Desarrollo y caracterización de nanocompuestos comestibles de Aloe Vera (Aloe Barbadensis Miller) y nanopartículas de almidón plátano (Musa x Paradisiaca l) nativo y modificado; Facultad de Ciencias Básicas y Tecnologías—Universidad del Quindío: Armenia-Quindío, Colombia, 2017. [Google Scholar]
- Sánchez, T.; García, O.; Pinzón, M. Elaboración y caracterización de películas de almidón de yuca (Manihot esculenta) variedad ICA cultivada en el departamento de Quindío. Vitae 2012, 1, S426–S429. [Google Scholar]
- Guancha-Chalapud, M.A.; Caicedo, C.; Ruiz, E.M.; Valencia, M.F. Propiedades de conservación: Recubrimiento a base de quitosano y Aloe vera aplicado en papa criolla (Solanum phureja). Inf. Técnico 2016, 80, 9–19. [Google Scholar] [CrossRef][Green Version]
- García, M.A.; Pinotti, A.; Martino, M.N.; Zaritzky, N.E. Characterization of composite hydrocolloid films. Carbohydr. Polym. 2004, 54, 339–345. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Gooding, C.H. Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. J. Food Eng. 1994, 21, 395–409. [Google Scholar] [CrossRef]
- Velasquez-Castro, J.; Arrubla-Vélez, J.P.; Guerrero-Álvarez, G.E.; Cardona-Hurtado, N.C. Preservation of the polyphenolic content and antioxidant properties of Rubus glaucus Benth. Curr. Res. Nutr. Food Sci. 2019, 7, 886–893. [Google Scholar] [CrossRef]
- Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). NTC 4592. Productos de Frutas y Verduras. Determinación del pH; ICONTEC: Bogotá, Colombia, 1999; p. 4. [Google Scholar]
- García-Mera, G.A.; Salas-Macías, C.A.; Canales-Torres, H.G. Recubrimiento comestible natural con base en Aloe vera como estrategia de conservación de Psidium guajava. Rev. Científica 2017, 3, 1–13. [Google Scholar] [CrossRef]
- García-Tejeda, Y.; Zamudio-Flores, P.; Luis, A.; Romero-Bastida, C.; Solorza-Feria, J. Oxidación del almidón nativo de plátano para su uso potencial en la fabricación de materiales de empaque biodegradables: Caracterización física, química, térmica y morfológica. Rev. Iberoam. Polímeros 2011, 12, 125–135. [Google Scholar]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; Volume 9. [Google Scholar]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and physical characteristics of edible films, based on κ-and ι-carrageenans with the addition of lapacho tea extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef]
- González, M.V. Conservación de Mora, Uvilla y Frutilla Mediante La utilización del Aceite Esencial de Canela (Cinnamomum zeynalicum). Bachelor’s Thesis, Facultad de Ciencias, Escuela de Bioquimica y Farmacia, Escuela Superior Técnica de Chimborazo, Riobamba, Ecuador, 2010. [Google Scholar]
- Hassan, F.A.S.; Mahfouz, S.A. Effect of 1-methylcyclopropene (1-MCP) on the postharvest senescence of coriander leaves during storage and its relation to antioxidant enzyme activity. Sci. Hortic. 2012, 141, 69–75. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Merchán, P.J.; Balaguera-López, H.E. Determinación de los estadios fenológicos del fruto de Vitis vinifera L. bajo condiciones del altiplano tropical en Boyacá. Rev. UDCA Actual. Divulg. Científica 2009, 12, 141–150. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The influence of Aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses, and quality attributes of table grapefruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Rahmanzadeh-Ishkeh, S.; Asghari, M.; Shirzad, H.; Alirezalu, A.; Ghasemi, G. Lemon verbena (Lippia citrodora) essential oil effects on antioxidant capacity and phytochemical content of raspberry (Rubus ulmifolius subsp. sanctus). Sci. Hortic. 2019, 248, 297–304. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Aristizabal, I.D.; Restrepo, J.I. Conservación de mora de castilla mediante la aplicación de un recubrimiento comestible de gel de mucílago de penca de sábila. Vitae 2013, 20, 172–183. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).