Experimental and Numerical Study of Ignition and Flame Propagation for Methane–Air Mixtures in Small Vessels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Influence of Initial Pressure and Initial Composition on Normal Burning Velocity
3.2. Influence of Initial Pressure, Initial Composition and Radioactive Source on Minimum Ignition Energy
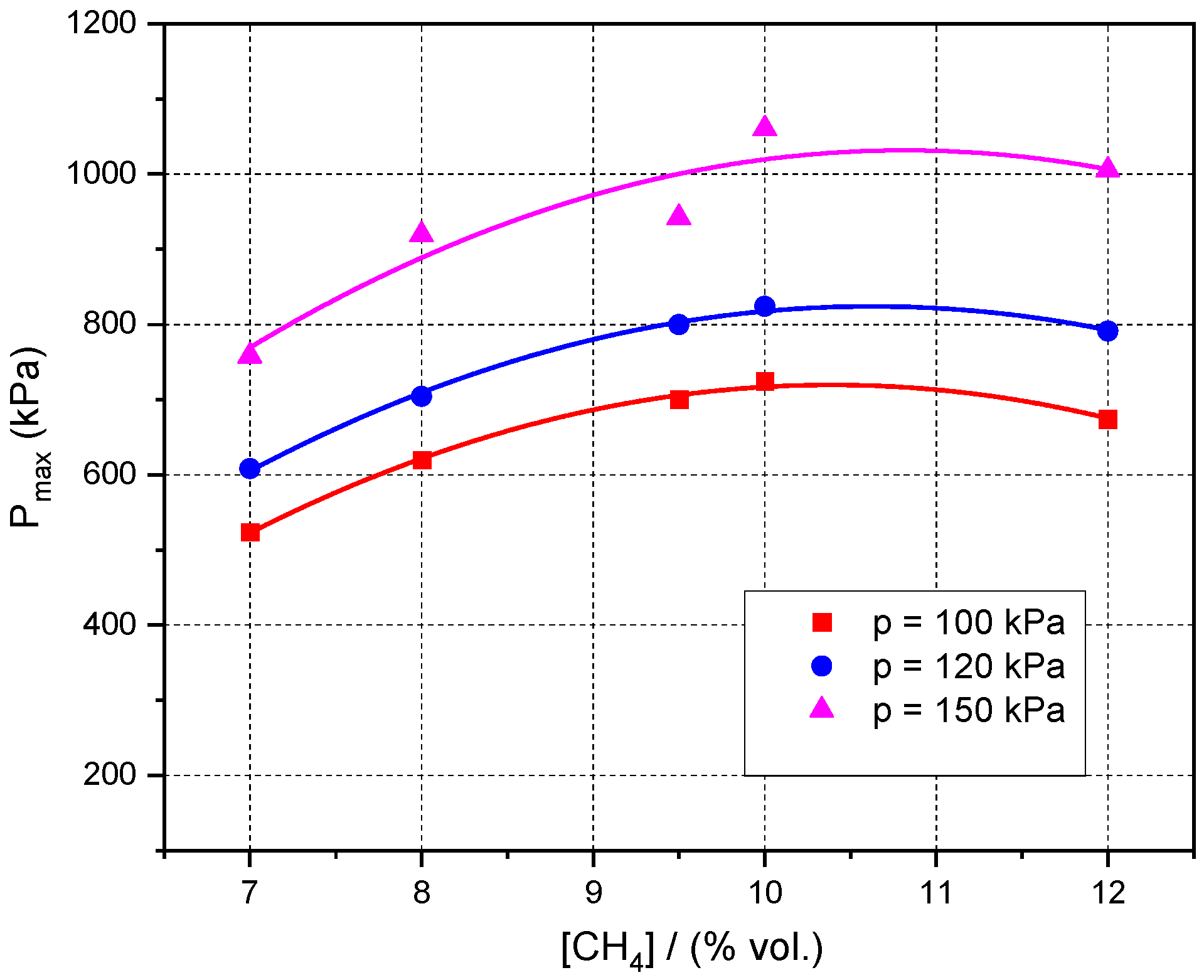
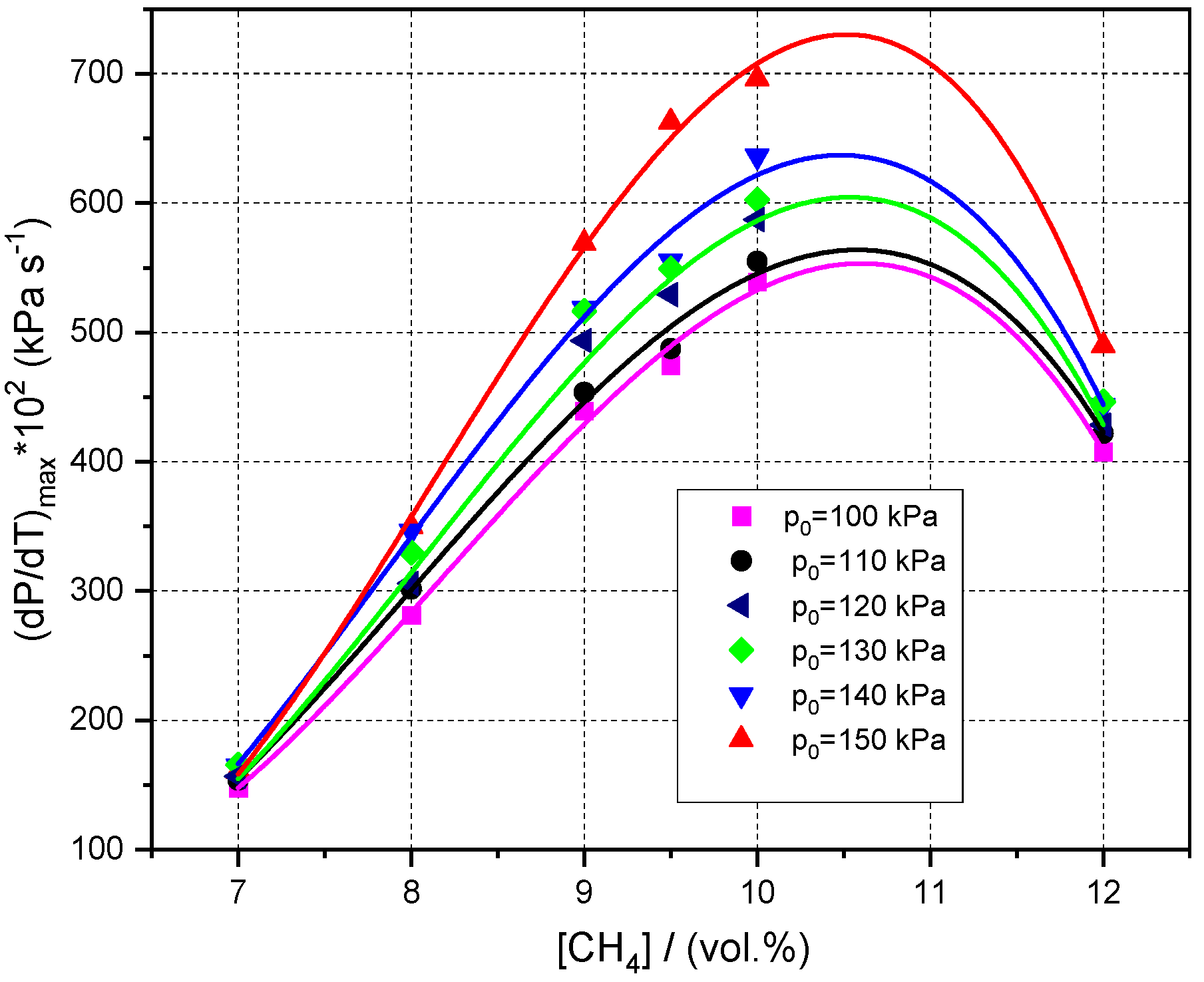
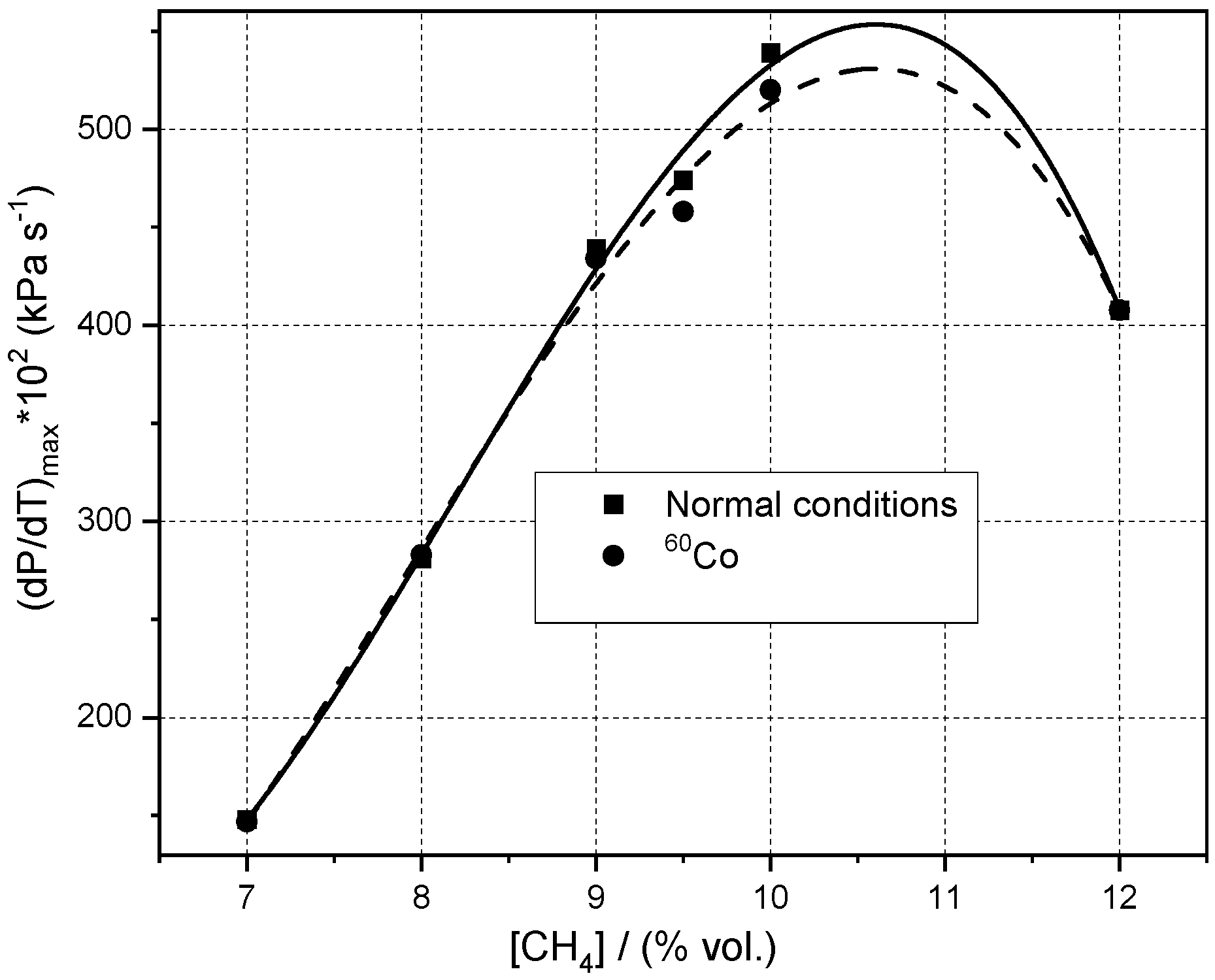
3.3. Influence of Initial Pressure and Initial Composition on Peak Explosion Pressures and Maximum rate of Pressure Rise in Normal and Radioactive Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitu, M.; Giurcan, V.; Razus, D.; Prodan, M.; Oancea, D. Propagation Indices of Methane-Air Explosions in Closed Vessels. J. Loss Prev. Process. Ind. 2017, 47, 110–119. [Google Scholar] [CrossRef]
- Bartknecht, W.; Zwahlen, G. Explosionsschutz: Grundlagen Und Anwendung; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Cashdollar, K.; Zlochower, I.; Green, G.; Thomas, R.; Hertzberg, M. Flammability of Methane, Propane, and Hydrogen Gases. J. Loss Prev. Process. Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Gant, S.E.; Pursell, M.R.; Lea, C.J.; Fletcher, J.; Rattigan, W.; Thyer, A.M.; Connolly, S. Flammability of Hydrocarbon and Carbon Dioxide Mixtures. Process. Saf. Environ. Prot. 2011, 89, 472–481. [Google Scholar] [CrossRef]
- Gieras, M.; Klemens, R.; Rarata, G.; Wolanski, P. Determination of explosion parameters of methane-air mixtures in the chamber of 40 dm3 at normal and elevated temperature. J. Loss Prev. Process. Ind. 2006, 19, 263–270. [Google Scholar] [CrossRef]
- Gieras, M.; Klemens, R. Experimental studies of explosions of methane-air mixtures in a constant chamber. Combust. Sci. Technol. 2010, 181, 641–653. [Google Scholar] [CrossRef]
- Holtappels, K. Report on the Experimentally Determined Explosion Limits, Explosion Pressures and Rates of Explosion Pressure Rise; SAFEKINEX project Part I: Methane, hydrogen and propylene; Deliverable 8; Federal Institute for Materials Research and Testing (BAM): Berglin, Germany, 2006. [Google Scholar]
- Holtappels, K.; Passman, H. Interpretation of Gas. Explosion Tests; Extremes in Explosion Severity; SAFEKINEX project; Deliverable 10; Federal Institute for Materials Research and Testing (BAM): Berglin, Germany, 2007. [Google Scholar]
- Ma, Q.; Zhang, Q.; Chen, J.; Huang, Y.; Shi, Y. Effects of hydrogen on combustion characteristics of methane in air. Int. J. Hydrogen Energy 2014, 39, 11291–11298. [Google Scholar] [CrossRef]
- Mashuga, C.; Crowl, D. Application of the flammability diagram for evaluation of fire and explosion hazards of flammable vapors. Proc. Saf. Progr. 1998, 17, 176–183. [Google Scholar] [CrossRef]
- Pekalski, A.A.; Schildberg, H.P.; Smallegange, P.S.D.; Lemkowitz, S.M.; Zevenbergen, J.F.; Braithwaite, M.; Pasman, H.J. Determination of the explosion behaviour of methane and propene in air or oxygen at standard and elevated conditions. Process. Saf. Environ. Prot. 2005, 83, 421–429. [Google Scholar] [CrossRef]
- Prodan, M.; Ghicioi, E.; Oancea, D. Correlation of explosion parameters and explosion type events for preventing environmental pollution. Environ. Eng. Manag. J. 2014, 13, 1409–1414. [Google Scholar] [CrossRef]
- Sapko, M.J.; Furno, A.L.; Kuchta, J.M. Flame and pressure development of large-scale CH4–air–N2 explosions. US Bur. Mines Rep. Investig. 1976, 8, 176. [Google Scholar]
- Senecal, J.; Beaulieu, P.K.G. New data and analysis. Process. Saf. Progr. 1998, 17, 9–15. [Google Scholar] [CrossRef]
- Wang, Z.R.; Ni, L.; Liu, X.; Jiang, J.C.; Wang, R. Effects of N2/CO2 on explosion characteristics of methane and air mixture. J. Loss Prev. Process. Ind. 2014, 31, 10–15. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, N.K.; Shu, C.M. Effects of flammability characteristics of methane with three inert gases. Proc. Saf. Progr. 2010, 29, 349–352. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Lin, D.C.; Duan, Y.; Liang, H.M. Experimental study of gas deflagration temperature distribution and its measurement. Exp. Therm. Fluid Sci. 2011, 35, 503–508. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Zhang, S. Effects of spark duration on minimum ignition energy for methane/air mixture. Proc. Saf. Progr. 2011, 30, 154–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W. Ignition characteristics for methane-air mixtures at various initial temperatures. Process. Saf. Progr. 2013, 32, 37–41. [Google Scholar] [CrossRef]
- Zhang, B.; Xiu, G.; Bai, C. Explosion characteristics of argon/nitrogen diluted natural gas–air mixtures. Fuel 2014, 124, 125–132. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Cammarota, F.; Sarli, V.; Salzano, E.; Russo, G. Anomalous behavior during explosions of CH4 in oxygen-enriched air. Combust. Flame 2011, 158, 2214–2219. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Cammarota, F.; Sarli, V.; Salzano, E.; Russo, G. Reconsidering the flammability diagram for CH4/O2/N2 and CH4/O2/CO2 mixtures in light of combustion-induced Rapid Phase Transition. Chem. Eng. Sci. 2012, 8, 142–147. [Google Scholar] [CrossRef]
- Zhou, B.; Deng, C.; Hao, J.; An, B.; Wu, R. Experimental study on the mechanism of radon exhalation during coal spontaneous combustion in goaf. Tunn. Undergr. Space Technol. 2021, 113, 103776. [Google Scholar] [CrossRef]
- Dahoe, A.E.; de Goey, L.P.H. On the determination of the laminar burning velocity of closed vessel explosions. J. Loss Prevent. Proc. Ind. 2003, 16, 457–478. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Lipatnikov, A.N.; Shy, S.S.; Li, W. Experimental assessment of various methods of determination of laminar flame speed in experiments with expanding spherical flames with positive Markstein lengths. Combust. Flame 2015, 16, 22840–22854. [Google Scholar] [CrossRef]
- Hassan, M.I.; Aung, K.T.; Faeth, G.M. Measured and predicted properties of laminar premixed methane/air flames at various pressures. Combust. Flame 1998, 11, 539–550. [Google Scholar] [CrossRef]
- Gu, X.J.; Haq, M.Z.; Lawes, M.; Woolley, R. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combust. Flame 2000, 121, 41–58. [Google Scholar] [CrossRef]
- Duva, C.B.; Wang, C.Y.; Chance, L.E.; Toulson, E. Correlations for the laminar burning velocity and burned gas Markstein length of methane-air mixtures diluted with flue gases at high temperatures and pressures. Fuel 2020, 281, 118721. [Google Scholar] [CrossRef]
- Varghese, R.J.; Kolekar, H.; Kishore, R.; Kumar, S. Measurement of laminar burning velocities of methane-air mixtures simultaneously at elevated pressures and elevated temperatures. Fuel 2019, 25, 116120. [Google Scholar] [CrossRef]
- Salzano, E.; Pioa, G.; Ricca, A.; Palma, V. The effect of a hydrogen addition to the premixed flame structure of light alkanes. Fuel 2018, 234, 1064–1070. [Google Scholar] [CrossRef]
- Pagliaro, J.L.; Linteris, G.T.; Sunderland, P.B.; Baker, P.T. Combustion inhibition and enhancement of premixed methane–air flames by halon replacements. Combust. Flame 2015, 162, 41–49. [Google Scholar] [CrossRef]
- Chan, Y.L.; Zhu, M.M.; Zhang, Z.Z.; Liu, P.F.; Zhang, D.K. The Effect of CO2 dilution on the laminar burning velocity of premixed methane/air flames. Energy Procedia 2015, 75, 3048–3053. [Google Scholar] [CrossRef]
- Park, O.; Veloo, P.S.; Liu, N.; Egolfopoulos, F.N. Combustion characteristics of alternative gaseous fuels. Proc. Combust. Inst. 2011, 33, 887–894. [Google Scholar] [CrossRef]
- Akram, M.; Saxena, P.; Kumar, S. Laminar burning velocity of methane–air mixtures at elevated temperatures. Energy Fuels 2013, 27, 3460–3466. [Google Scholar] [CrossRef]
- Konnov, A.; Akram, M.; Kishore, V.R.; Kim, N.; Pratha, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+airmixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Razus, D.; Oancea, D.; Movileanu, C. Burning velocity evaluation from pressure evolution during the early stage of closed-vessel explosions. J. Loss Prevent. Proc. Ind. 2006, 19, 334–342. [Google Scholar] [CrossRef]
- Razus, D.; Oancea, D.; Brinzea, V.; Mitu, M.; Movileanu, C. Experimental and computed burning velocities of propane–air mixtures. Energy Convers. Manag. 2010, 51, 2979–2984. [Google Scholar] [CrossRef]
- Razus, D.; Brinzea, V.; Mitu, M.; Oancea, D. Burning Velocity of Liquefied Petroleum Gas (LPG)−air mixtures in the presence of exhaust gas. Energy Fuel 2010, 24, 1487–1494. [Google Scholar] [CrossRef]
- Movileanu, C.; Razus, D.; Oancea, D. Additive Effects on the Burning Velocity of Ethylene–Air Mixtures. Energy Fuel 2011, 25, 2444–2451. [Google Scholar] [CrossRef]
- Razus, D.; Brinzea, V.; Mitu, M.; Movileanu, C.; Oancea, D. Burning velocity of propane–air mixtures from pressure–time records during explosions in a closed spherical vessel. Energy Fuels 2012, 26, 901–909. [Google Scholar] [CrossRef]
- Mitu, M.; Razus, D.; Giurcan, V.; Oancea, D. Normal burning velocity and propagation speed of ethane–air: Pressure and temperature dependence. Fuel 2015, 147, 27–34. [Google Scholar] [CrossRef]
- Brinzea, V.; Mitu, M.; Movileanu, C.; Musuc, A.; Razus, D. Expansion coefficients and normal burning velocities of propane-air mixtures by the closed vessel technique. Buchar. Univ. Chem. Fac. Ann. 2010, 19, 31–37. [Google Scholar]
- Bradley, D.; Mitcheson, A. Mathematical solutions for explosions in spherical vessels. Combust. Flame 1976, 26, 201–217. [Google Scholar] [CrossRef]
- Metghalchi, M.; Keck, J. Laminar burning velocity of propane-air mixtures at high temperature and pressure. Combust. Flame 1980, 38, 143–154. [Google Scholar] [CrossRef]
- Huzayyin, A.S.; Moneib, H.A.; Shehatta, M.S.; Attia, A.M.A. Laminar burning velocity and explosion index of LPG–air and propane–air mixtures. Fuel 2008, 87, 39–57. [Google Scholar] [CrossRef]
- Andrews, G.; Bradley, D. The burning velocity of methane-air mixtures. Combust. Flame 1972, 19, 275–288. [Google Scholar] [CrossRef]
- Potter, A.E., Jr.; Berlad, A.L. The effect of fuel type and pressure on flame quenching. Symp. Int. Combust. 1957, 6, 27–36. [Google Scholar] [CrossRef]
- Lewis, B.; von Elbe, G. Combustion Flames and Explosion of Gases; Academic Press: New York, NY, USA, 1987; Chapter 5. [Google Scholar]
- Moorhouse, J.; William, A.; Maddison, T.E. An investigation of the minimum ignition energies of some C1 to C7 hydrocarbons. Combust. Flame 1974, 23, 203–213. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, C.; Xiu, G.; Liu, Q.; Gong, G. Explosion and flame characteristics of methane/air mixtures in a large-scale vessel. Process. Saf. Progr. 2014, 33, 362–368. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Si, Z.; Huang, Z.; Zhang, K.; Jin, Z. High methane natural gas/air explosion characteristics in confined vessel. J. Hazard. Mater. 2014, 278, 520–528. [Google Scholar] [CrossRef]
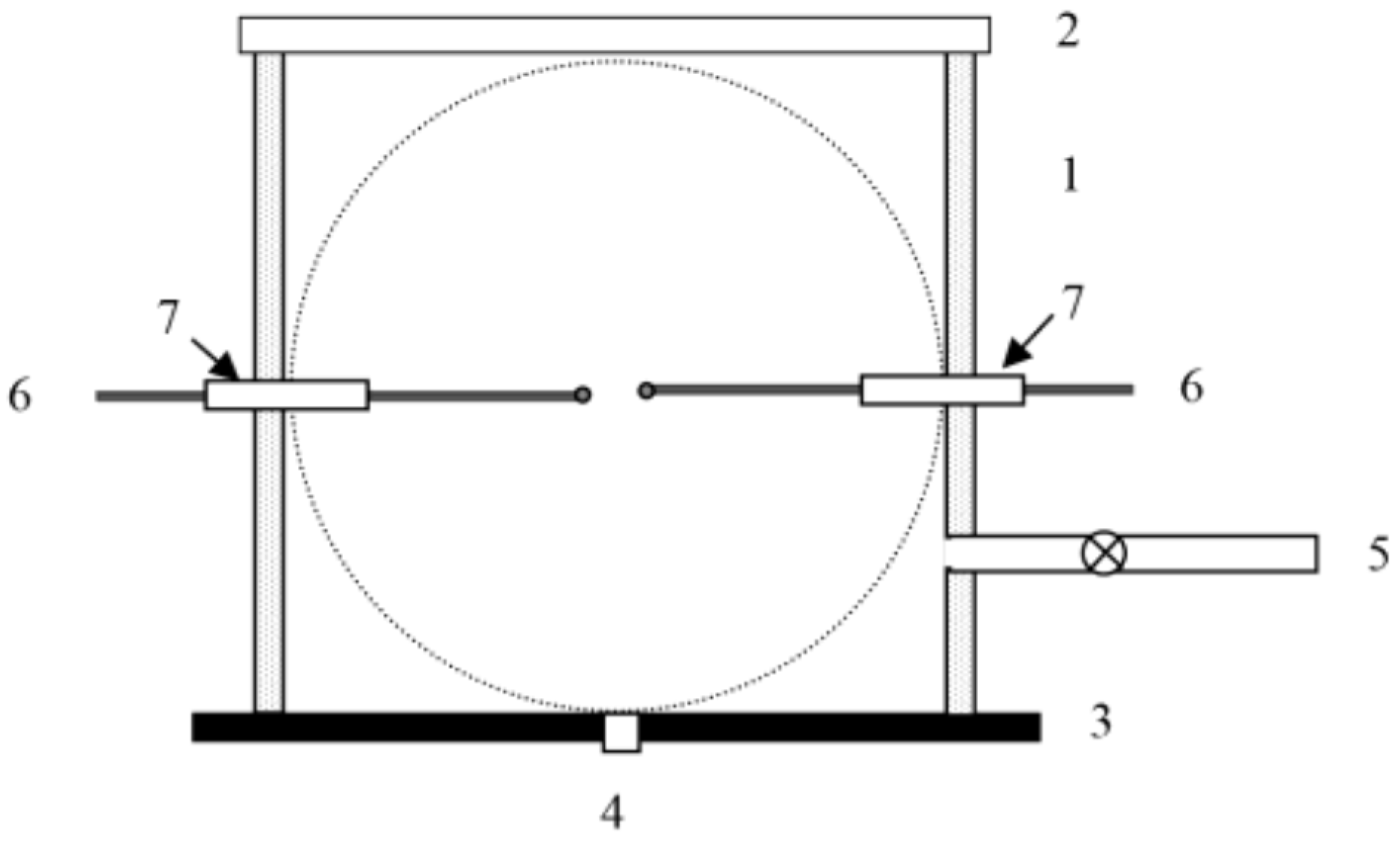
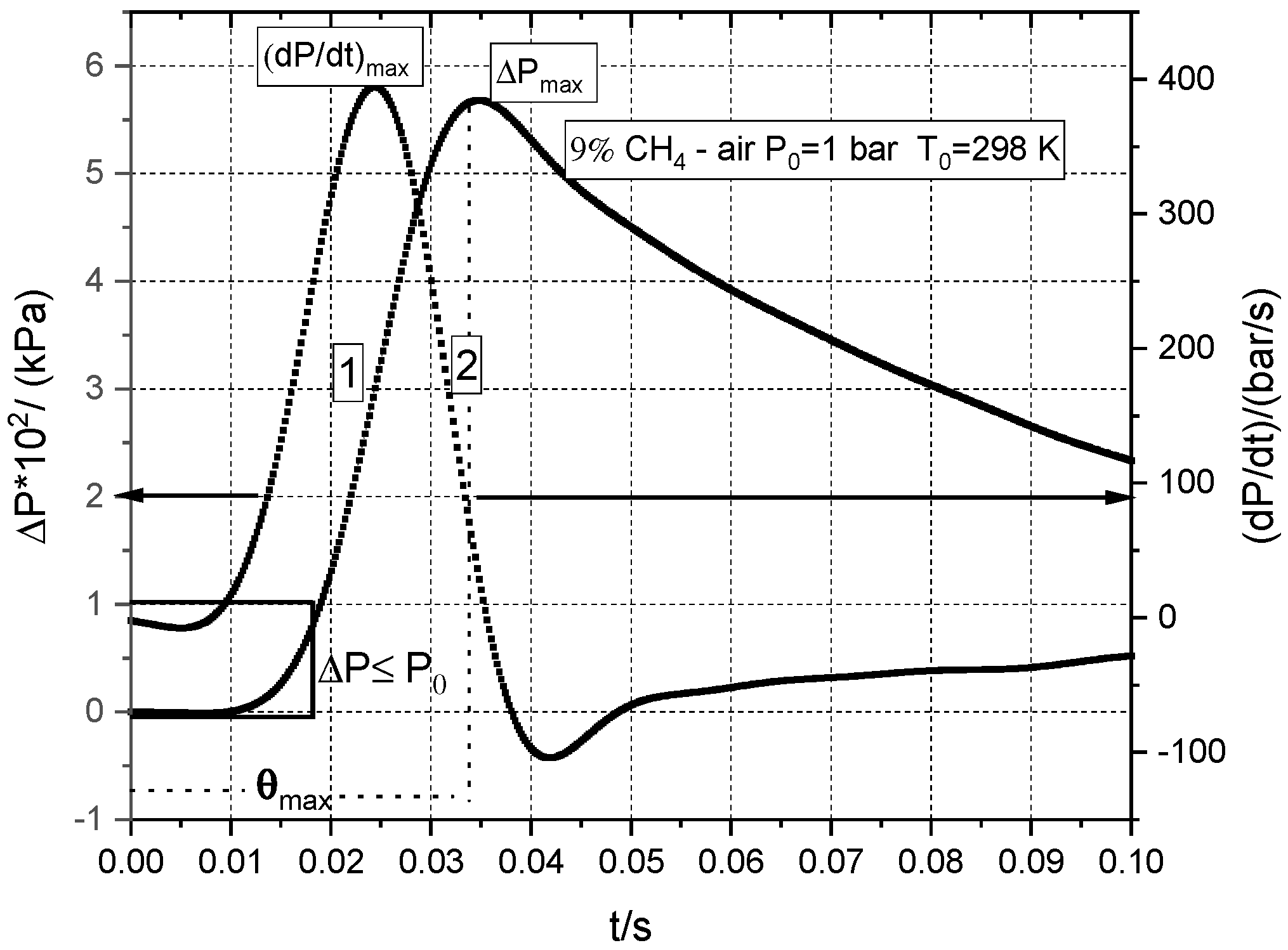


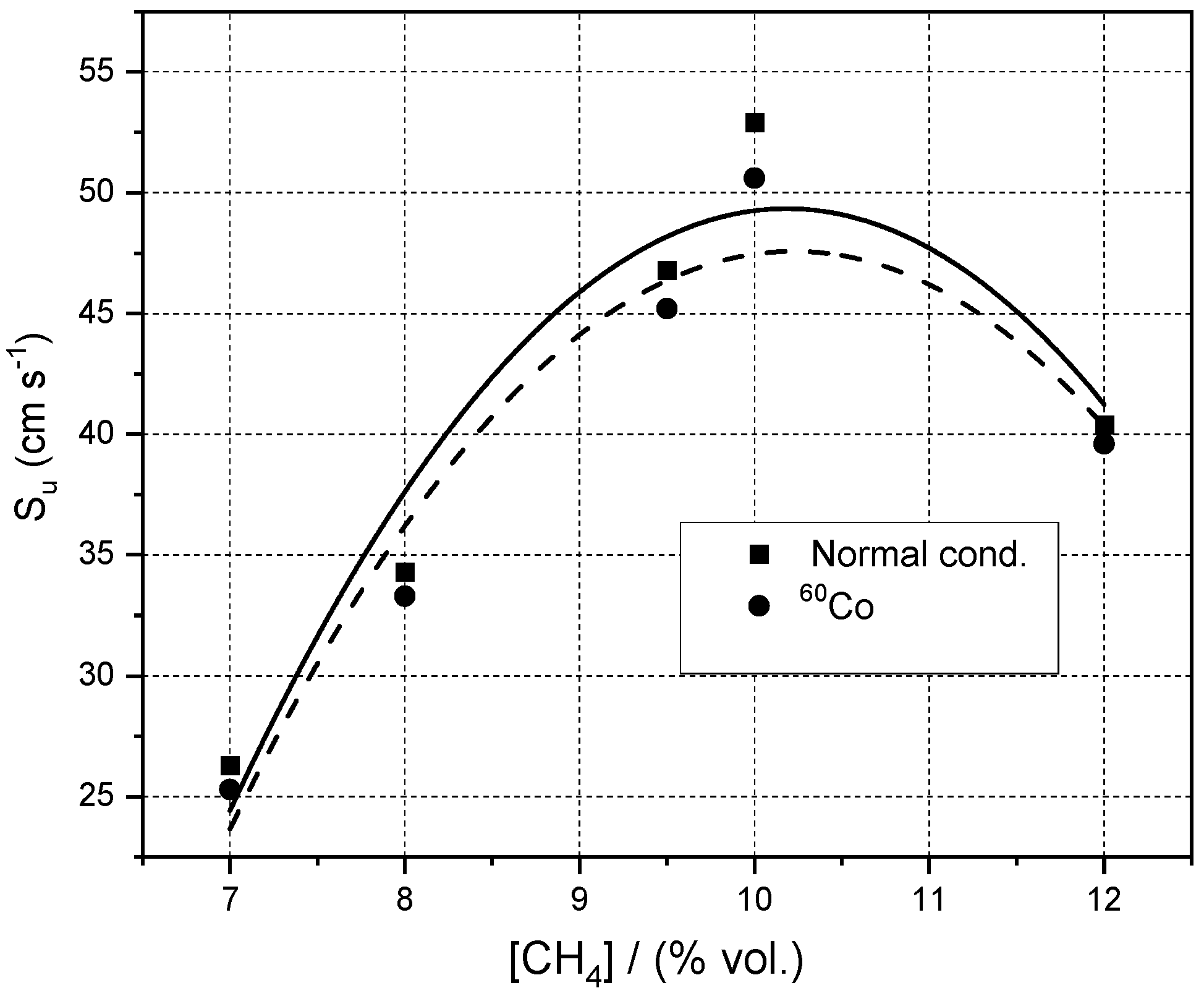

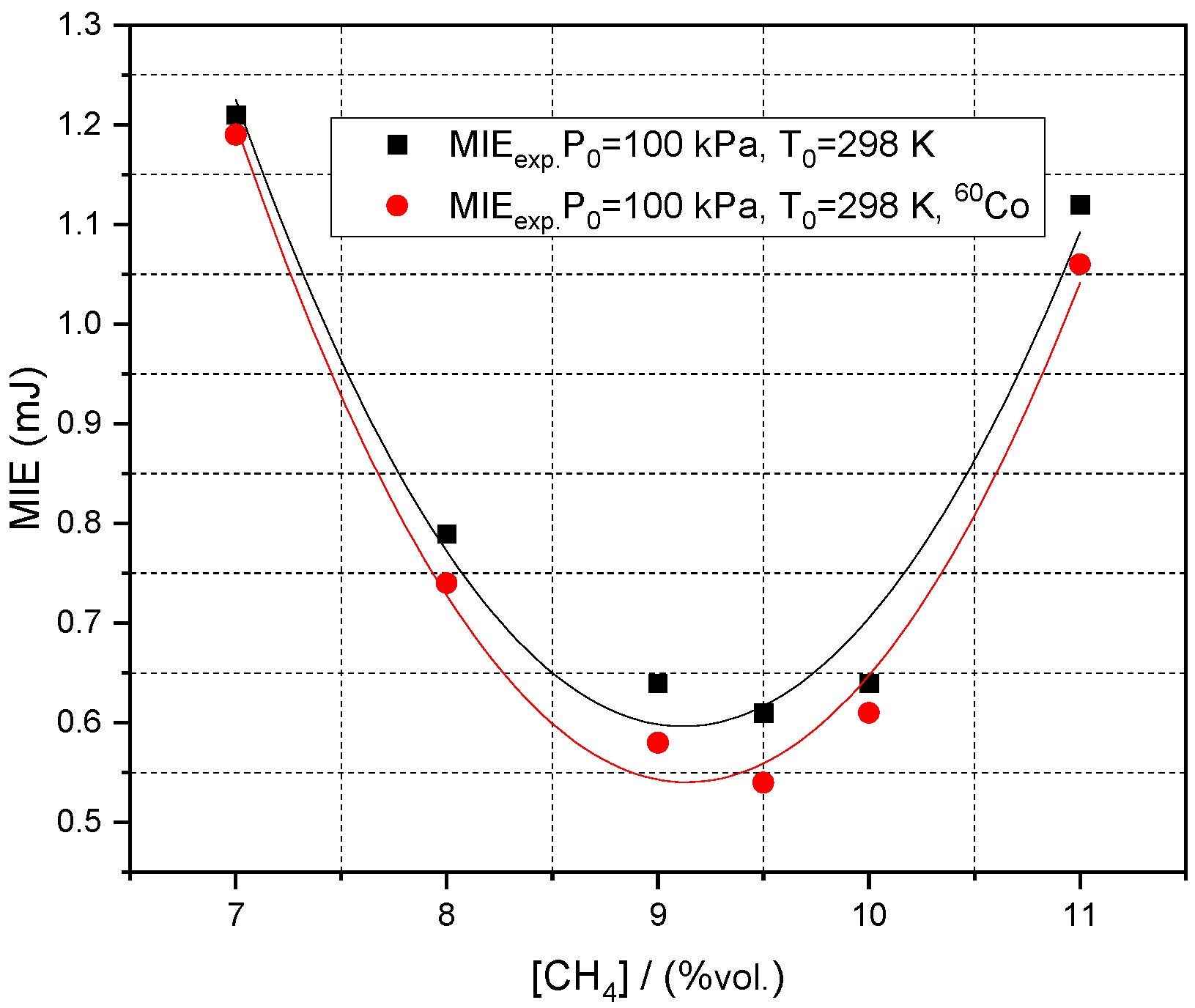

| % CH4 | φ | Su/(m/s) | −ν | nr (from ν) |
|---|---|---|---|---|
| 8.0 | 0.83 | 0.343 | 0.458 | 1.084 |
| 9.5 | 1.00 | 0.467 | 0.214 | 1.572 |
| 10 | 1.06 | 0.518 | 0.387 | 1.226 |
| 12 | 1.30 | 0.404 | 0.405 | 1.190 |
| [CH4] % vol. | P0 = 100 kPa | P0 = 150 kPa |
|---|---|---|
| 7 | 1.21 | 1.12 |
| 8 | 0.79 | 0.90 |
| 9 | 0.64 | 0.66 |
| 9.5 | 0.61 | 0.53 |
| 10 | 0.64 | 0.56 |
| 12 | 2.82 | 2.15 |
| [CH4] % vol | MIE [mJ] at P0 = 100 kPa | MIE [mJ] at P0 = 150 kPa |
|---|---|---|
| 7 | 1.00 | 1.19 |
| 8 | 0.85 | 0.74 |
| 9 | 0.58 | 0.58 |
| 9.5 | 0.52 | 0.54 |
| 10 | 0.55 | 0.61 |
| 12 | 2.02 | -- |
| [CH4] % vol. | −ΔMIE [%] at P0 = 80 kPa | −ΔMIE [%] at P0 = 100 kPa | −ΔMIE [%] at P0 = 150 kPa |
|---|---|---|---|
| 7 | 14.8 | 1.65 | 10.7 |
| 8 | 1.85 | 6.33 | 5.6 |
| 9 | 3.06 | 9.38 | 12.1 |
| 9.5 | 1.98 | 11.5 | 1.89 |
| 10 | 11.1 | 4.69 | 1.79 |
| 12 | -- | 5.36 | 6.38 |
| Pmax/kPa | Reference |
|---|---|
| 700 | Cylinder, V = 10 m3 [51], Cylinder V = 0.17 L, with Φ/h = 1, present data |
| 720 | Sphere, V = 20 L [9] |
| 770 | Sphere V = 20 L [12]; Cylinder, V = 22 L, L/D = 1.15 [14]); Cylinder, V = 5.34 L, L/D = 1.17 [52] |
| 790 | Sphere, V = 20 L [17,18,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodan, M.; Ghicioi, E.; Laszlo, R.; Nalboc, I.; Suvar, S.; Nicola, A. Experimental and Numerical Study of Ignition and Flame Propagation for Methane–Air Mixtures in Small Vessels. Processes 2021, 9, 998. https://doi.org/10.3390/pr9060998
Prodan M, Ghicioi E, Laszlo R, Nalboc I, Suvar S, Nicola A. Experimental and Numerical Study of Ignition and Flame Propagation for Methane–Air Mixtures in Small Vessels. Processes. 2021; 9(6):998. https://doi.org/10.3390/pr9060998
Chicago/Turabian StyleProdan, Maria, Emilian Ghicioi, Robert Laszlo, Irina Nalboc, Sonia Suvar, and Aurelian Nicola. 2021. "Experimental and Numerical Study of Ignition and Flame Propagation for Methane–Air Mixtures in Small Vessels" Processes 9, no. 6: 998. https://doi.org/10.3390/pr9060998
APA StyleProdan, M., Ghicioi, E., Laszlo, R., Nalboc, I., Suvar, S., & Nicola, A. (2021). Experimental and Numerical Study of Ignition and Flame Propagation for Methane–Air Mixtures in Small Vessels. Processes, 9(6), 998. https://doi.org/10.3390/pr9060998






