Limitation of K2CO3 as a Chemical Agent for Upgrading Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Carbon Precursors and Chemical Activation
2.2. Analytical Methods
3. Results and Discussion
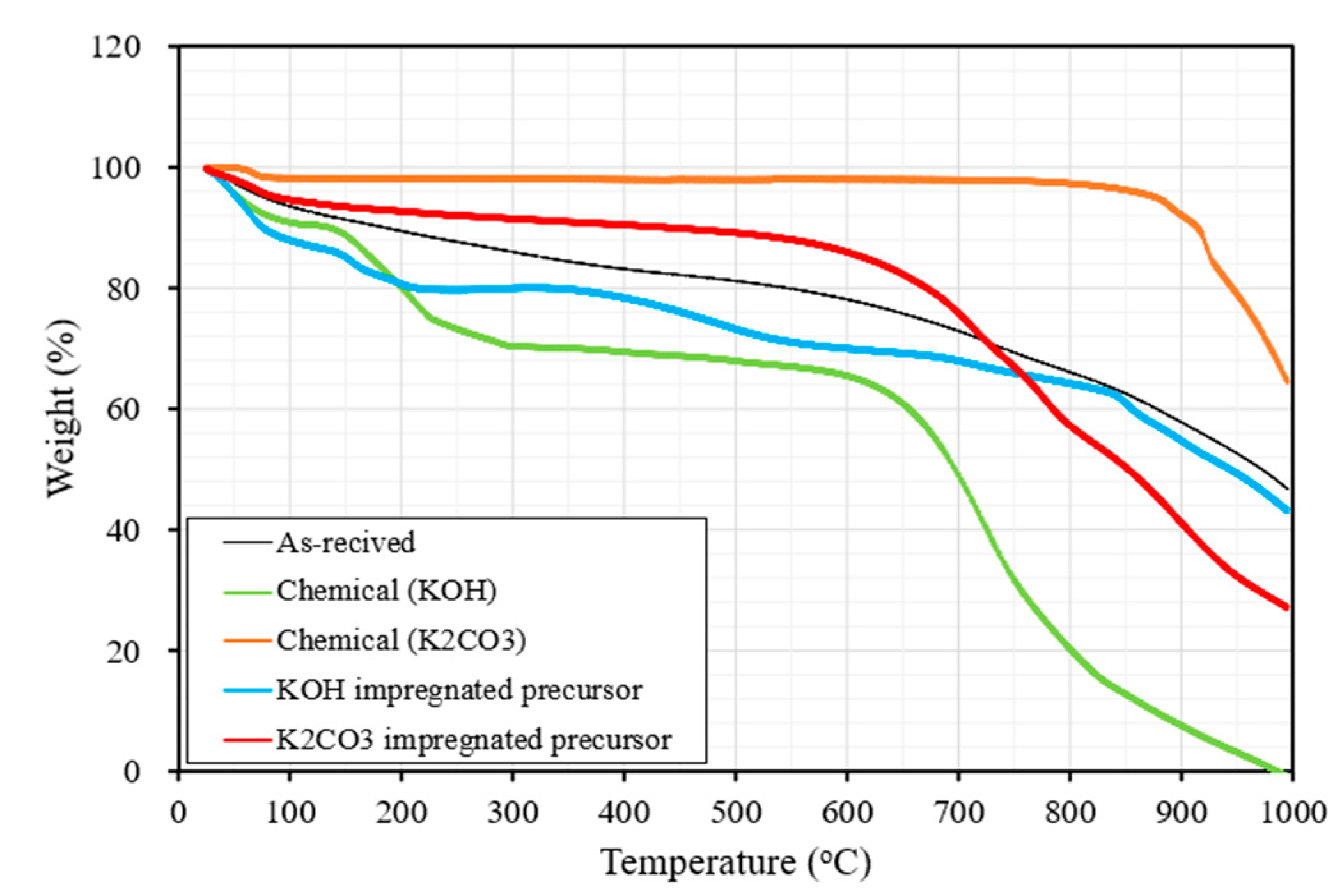
3.1. Changes in Thermal Stability of Chemical Agents
3.2. Changes in Surface Properties of Carbon Precursors Impregnated with KOH and K2CO3
3.2.1. XPS Analysis
3.2.2. XRD Results
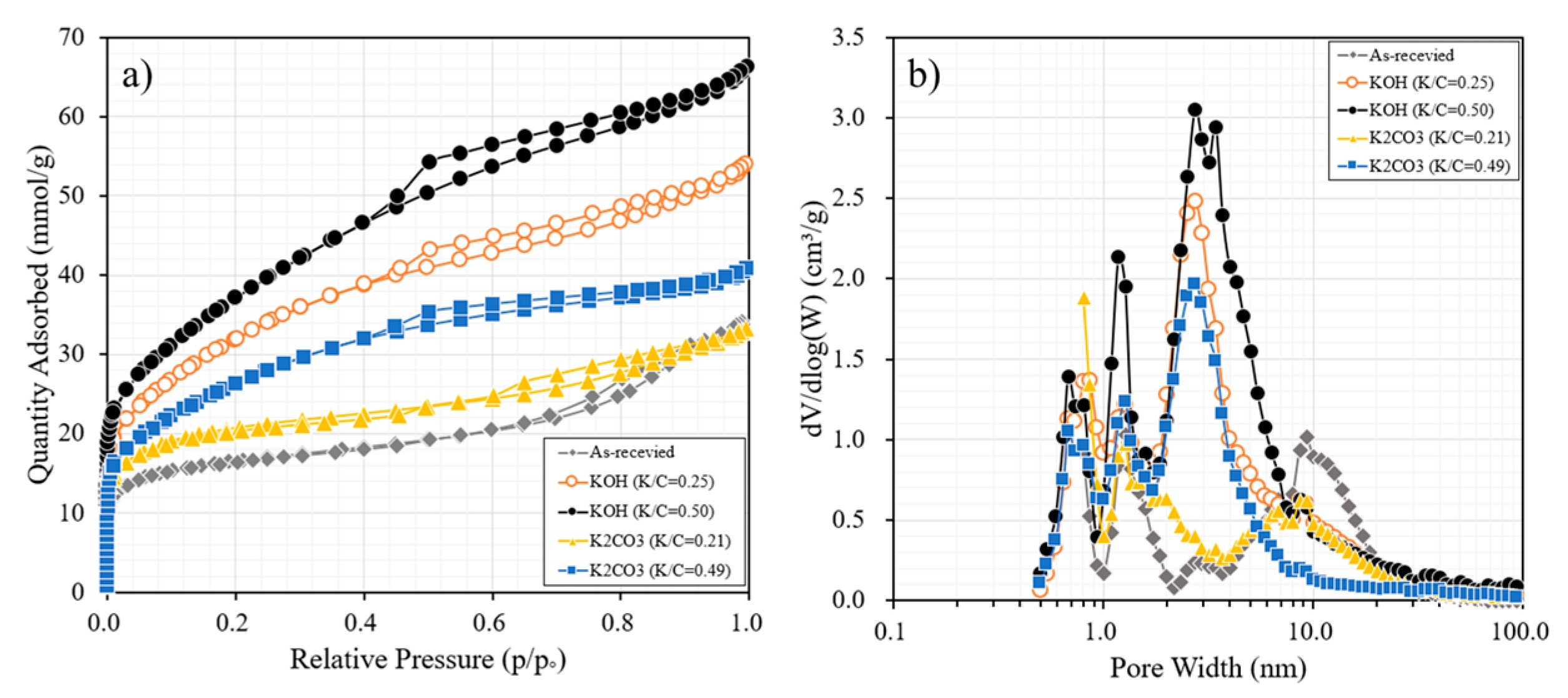
3.3. Chemical Activation with KOH and K2CO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Li, R.; Qiu, J.; Xie, K.; Ling, P.; Yu, M.; Zhang, X.; Zheng, M. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template. Carbon 2012, 50, 4911–4921. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- Activated Carbon Market by Type (Powdered, Granular, others (Pelletized, Bead)), Application (Liquid Phase (Water Treatment, Food & Beverages, Pharmaceutical & Medical)), Gaseous Phase (Industrial, Automotive), Region-Global Forecast to 2021, Activated Carbon Market-Global Trend & Forecasts to 2021. 2017. Available online: www.marketsandmarkets.com/Market-Reports/activated-carbon-362.html (accessed on 9 August 2019).
- Korea International Trade Statistics. Available online: http://stat.kita.net (accessed on 9 August 2019).
- Zhou, W.; Bai, B.; Chen, G.; Ma, L.; Jing, D.; Yan, B. Study on catalytic properties of potassium carbonate during the process of sawdust pyrolysis. Int. J. Hydrog. Energy 2018, 43, 13829–13841. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y. Structural and adsorption characteristic of potassium carbonate activated biochar. RSC Adv. 2018, 33, 21012–21019. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Monte, V.; Hill, J.M. Activated carbon production: Recycling KOH to minimize waste. Mater. Lett. 2018, 220, 238–240. [Google Scholar] [CrossRef]
- Nowrouzi, M.; Behin, J.; Younesi, H.; Bahramifar, N.; Charpentier, P.A.; Rohani, S. An enhanced counter-current approach towards activated carbon from waste tissue with zero liquid discharge. Chem. Eng. J. 2017, 326, 934–944. [Google Scholar] [CrossRef]
- Yuan, M.; Kim, Y.; Jia, C.Q. Feasibility of recycling KOH in chemical activation of oil-sands petroleum coke. Can. J. Chem. Eng. 2012, 90, 1472–1478. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Hayashi, J.; Horikawa, T.; Takea, I.; Muroyama, K.; Ani, F.N. Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Hayashi, J.; Horikawa, T.; Muroyama, K.; Gomes, V.G. Activated carbon from chickpea husk by chemical activation with K2CO3: Preparation and characterization. Microporus Mesoporus Mater. 2002, 55, 63–68. [Google Scholar] [CrossRef]
- Lu, C.; Xu, S.; Liu, C. The role of K2CO3 during the chemical activation of petroleum coke with KOH. J. Anal. Appl. Pyrolysis 2010, 87, 282–287. [Google Scholar] [CrossRef]
- Adinata, D.; Wan Daud, W.M.A.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, S.Y.; Park, J.E.; Lee, G.B.; Kim, S.; Hong, B.U. Impact of the oxygen functional group of nitric acid-treated activated carbon on KOH activation reaction. Carbon Lett. 2019, 29, 281–287. [Google Scholar] [CrossRef]
- Marsh, H. Activated Carbon, 1st ed; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Hilton, R.; Bick, P.; Tekeei, A.; Leimkuehler, E.; Pfeifer, P.; Suppes, G.J. Mass balance and performance analysis of potassium hydroxide activated carbon. Ind. Eng. Chem. Res. 2012, 51, 9125–9135. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Habibi, R.; Mims, C.A.; Hill, J.M. K2CO3-catalyzed CO2 gasification of ash-free coal: Kinetic study. Energy Fuels 2013, 27, 4875–4883. [Google Scholar] [CrossRef]
- Quyn, D.M.; Hayashi, J.I.; Li, C.Z. Volatilisation of alkali and alkaline earth metallic species during the gasification of a Victorian brown coal in CO2. Fuel Process. Technol. 2005, 86, 1241–1251. [Google Scholar] [CrossRef]
- Lee, G.B.; Park, J.E.; Hwang, S.Y.; Kim, J.H.; Kim, S.; Kim, H.; Hong, B.U. Comparison of by-product gas composition by activations of activated carbon. Carbon Lett. 2019, 29, 263–272. [Google Scholar] [CrossRef]
- Punsuwan, N.; Tangsathitkulchai, C.; Takarada, T. Low temperature gasification of coconut shell with CO2 and KOH: Effects of temperature, chemical loading, and introduced carbonization step on the properties of syngas and porous carbon product. Int. J. Chem. Eng. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Zhao, C.; CHen, X.; Zhao, C.; Wu, Y.; Dong, W. K2CO3/Al2O3 for capturing CO2 in flue gas from power plant. Part 3: CO2 capture behaviors of K2CO3/Al2O3 in a bubbling fluidized-bed reactor. Energy Fuels 2010, 26, 3062–3068. [Google Scholar] [CrossRef]
- Kanoh, H.; Luo, H. Chapter 4 Alkali-Metal-Carbonate-Based CO2 Adsorbents. In Post-Combustion Carbon Dioxide Capture Materials; The Royal Society of Chemistry: London, UK, 2019; pp. 206–258. [Google Scholar]
- Hartman, M.; Svoboda, K.; Čech, B.; Pohořelý, M.; Šyc, M. Decomposition of potassium hydrogen carbonate: Thermochemistry, kinetics, and textural changes in solid. Ind. Eng. Chem. Rev. 2019, 58, 2868–2881. [Google Scholar] [CrossRef]

| Preparation of Carbon Precursors | K/C Ratio | C 1s (285.15 eV) | K 2p (294.25 eV) | N 1s (401.11 eV) | O 1s (532.73 eV) |
|---|---|---|---|---|---|
| KOH impregnation | 0.5 | 27.1 | 9.17 | N.D | 63.7 |
| K2CO3 impregnation | 0.2 | 77.5 | 0.99 | 0.89 | 20.6 |
| 0.4 | 34.0 | 17.2 | 1.09 | 47.7 | |
| 0.5 | 23.9 | 8.19 | N.D | 67.9 |
| Agent | K/C Ratio | K 2p3/2 | K 2p1/2 | C 1s | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ko (293.0) | KCB (295.7) | Ko (294.5) | KCB (296.6) | C=C (284.4) | C–C (285.9) | C–O (287.1) | C=O (288.5) | COOH (289.7) | ||
| KOH | 0.5 | 49.6 | 15.9 | 25.7 | 8.88 | 51.7 | 22.5 | 7.59 | 10.2 | 7.95 |
| K2CO3 | 0.2 | 53.9 | 11.5 | 25.9 | 8.68 | 71.7 | 16.8 | 5.65 | 3.62 | 2.15 |
| 0.4 | 54.2 | 10.3 | 30.9 | 4.54 | 56.3 | 14.5 | 6.79 | 8.36 | 14.1 | |
| 0.5 | 50.0 | 15.2 | 26.3 | 8.59 | 50.8 | 23.7 | 7.62 | 10.4 | 7.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, G.; Park, J.-E.; Kim, S.-H. Limitation of K2CO3 as a Chemical Agent for Upgrading Activated Carbon. Processes 2021, 9, 1000. https://doi.org/10.3390/pr9061000
Kim J-H, Lee G, Park J-E, Kim S-H. Limitation of K2CO3 as a Chemical Agent for Upgrading Activated Carbon. Processes. 2021; 9(6):1000. https://doi.org/10.3390/pr9061000
Chicago/Turabian StyleKim, Ji-Hyun, Gibbum Lee, Jung-Eun Park, and Seok-Hwi Kim. 2021. "Limitation of K2CO3 as a Chemical Agent for Upgrading Activated Carbon" Processes 9, no. 6: 1000. https://doi.org/10.3390/pr9061000
APA StyleKim, J.-H., Lee, G., Park, J.-E., & Kim, S.-H. (2021). Limitation of K2CO3 as a Chemical Agent for Upgrading Activated Carbon. Processes, 9(6), 1000. https://doi.org/10.3390/pr9061000





