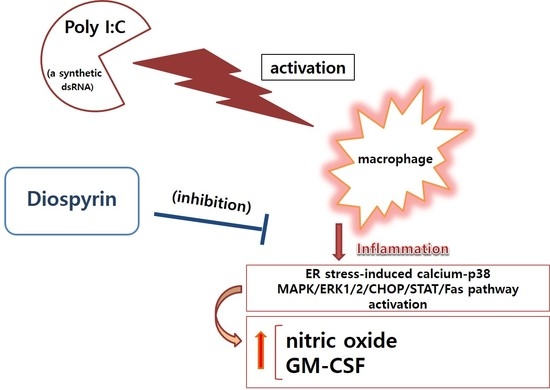
Diospyrin Modulates Inflammation in Poly I:C-Induced Macrophages via ER Stress-Induced Calcium-CHOP Pathway
Abstract
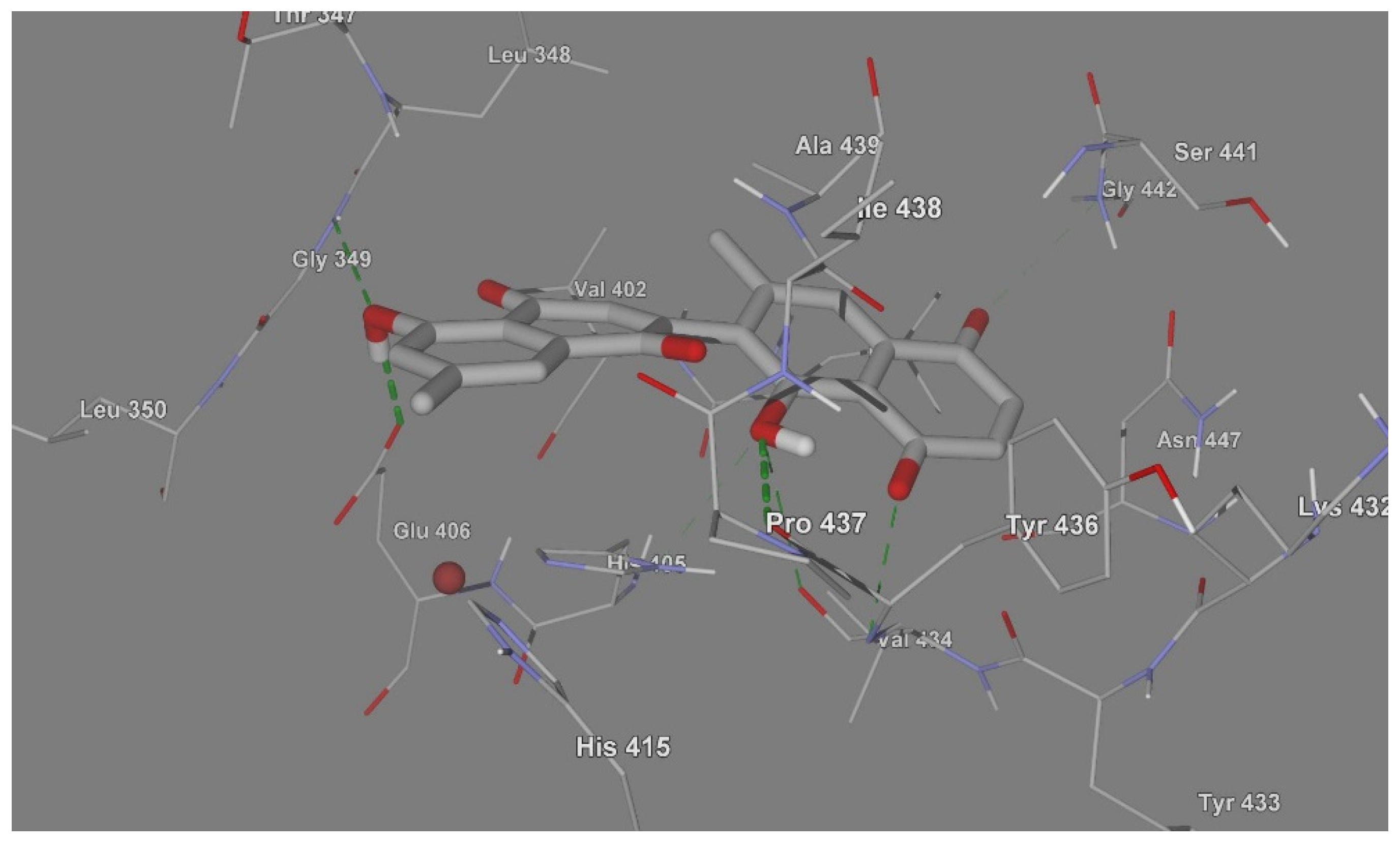
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. NO Production
2.4. Calcium Release
2.5. Cytokines Production
2.6. Quantitative Polymerase Chain Reaction
2.7. Flow Cytometry Assay
2.8. Statistics
3. Results
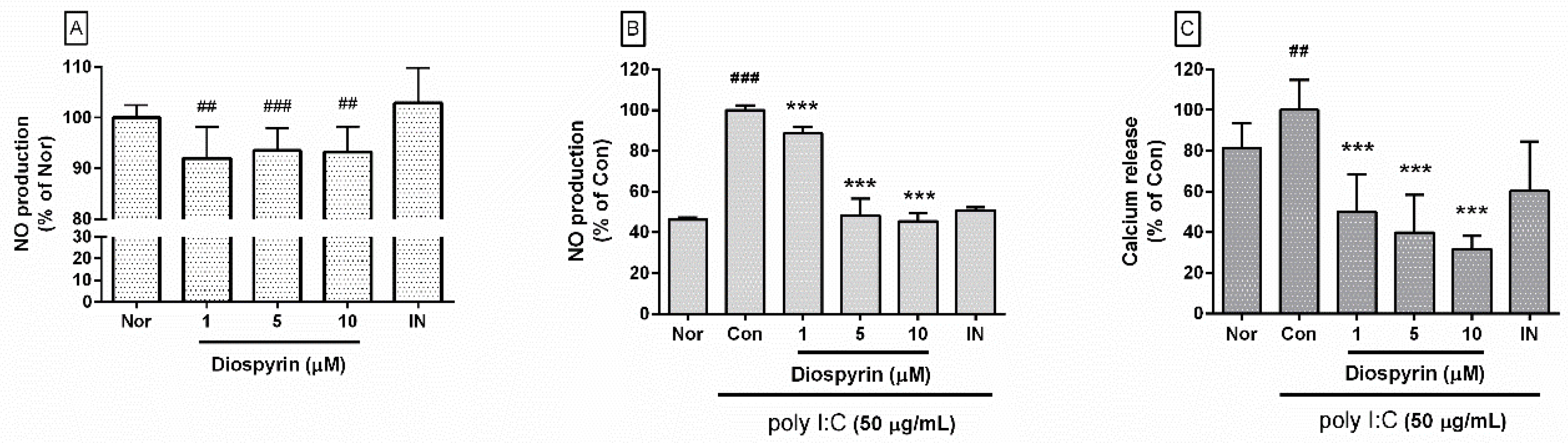
3.1. NO Production from RAW 264.7
3.2. Calcium Release in RAW 264.7
3.3. Cytokine Production from RAW 264.7
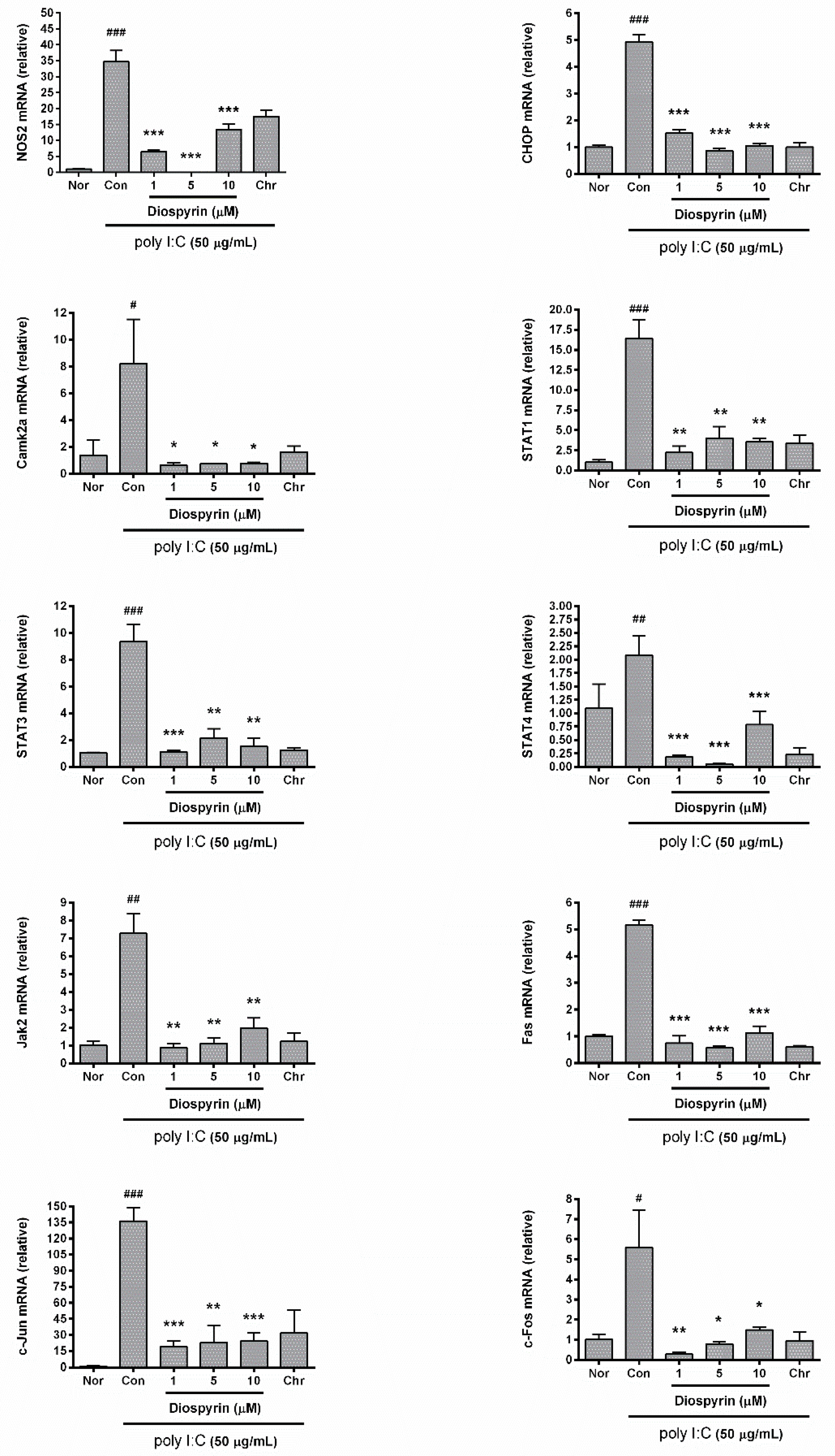
3.4. mRNA Expression of NOS2, CHOP, Camk2a, STAT1, STAT3, STAT4, Jak2, Fas, c-Jun, and c-Fos
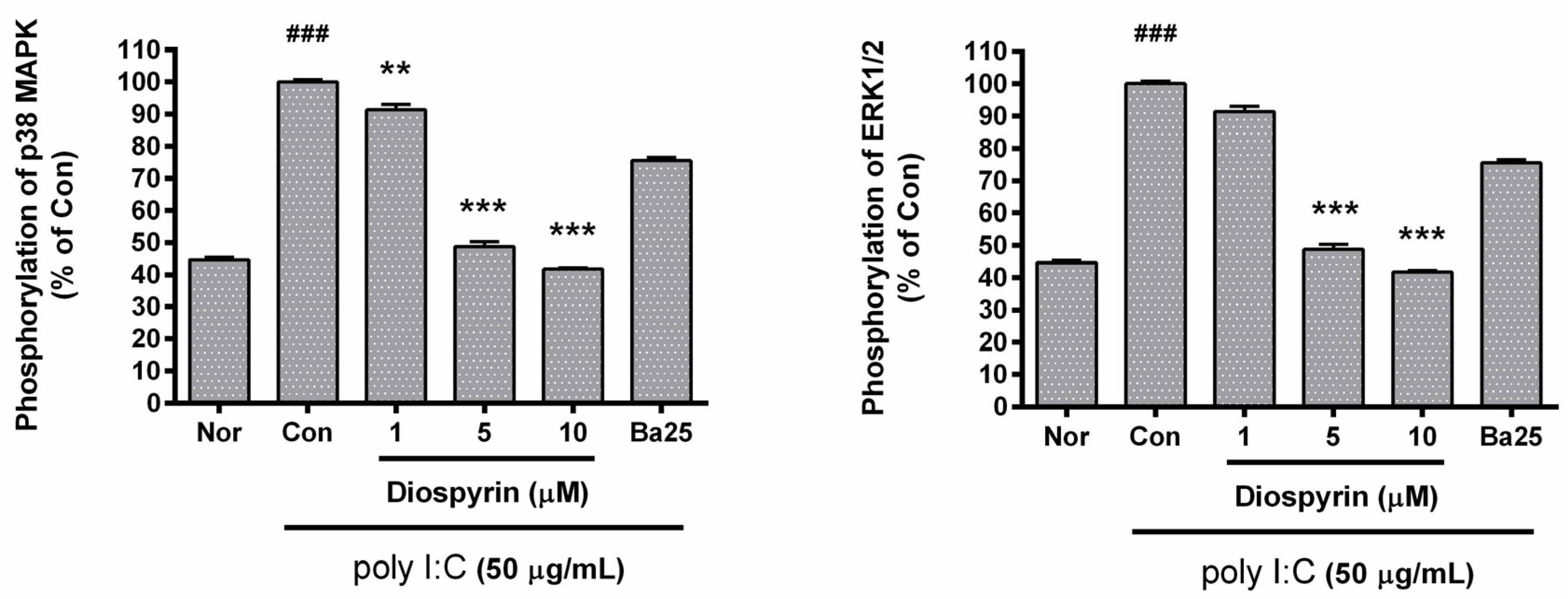
3.5. Phosphorylation of p38 MAPK and ERK1/2 in RAW 264.7
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goff, W.L.; Bastos, R.G.; Brown, W.C.; Johnson, W.C.; Schneider, D.A. The Bovine Spleen: Interactions among Splenic Cell Populations in the Innate Immunologic Control of Hemoparasitic Infections. Vet. Immunol. Immunopathol. 2010, 138, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W. Eicosanoids and the Endogenous Control of Acute Inflammatory Resolution. Int. J. Biochem. Cell Biol. 2010, 42, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Immunology. Neutrophil-Macrophage Communication in Inflammation and Atherosclerosis. Science 2015, 349, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Liao, F.; Lin, T.N.; Liu, F.T. Galectins and Neuroinflammation. Adv. Neurobiol. 2014, 9, 517–542. [Google Scholar] [PubMed]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J.; et al. Resveratrol Inhibits LPS-Induced MAPKs Activation Via Activation of the Phosphatidylinositol 3-Kinase Pathway in Murine RAW 264.7 Macrophage Cells. PLoS ONE 2012, 7, e44107. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-Driven Assembly of the RIP1-RIP3 Complex Regulates Programmed Necrosis and Virus-Induced Inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of Double-Stranded RNA and Activation of NF-kappaB by Toll-Like Receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Ray, S.; Hazra, B.; Mittra, B.; Das, A.; Majumder, H.K. Diospyrin, a Bisnaphthoquinone: A Novel Inhibitor of Type I DNA Topoisomerase of Leishmania Donovani. Mol. Pharmacol. 1998, 54, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Shahidullah, A.; Lee, J.-Y.; Kim, Y.-J.; Halimi, S.M.A.; Rauf, A.; Kim, H.-J.; Kim, B.-Y.; Park, W. Anti-Inflammatory Effects of Diospyrin on Lipopolysaccharide-Induced Inflammation Using RAW 264.7 Mouse Macrophages. Biomedicines 2020, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, W.; Yi, D.K. Immunostimulatory Effects of Gold Nanorod and Silica-Coated Gold Nanorod on RAW 264.7 Mouse Macrophages. Toxicol. Lett. 2012, 209, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, W. Anti-Inflammatory Effect of Quercetin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.J.; Lee, J.Y.; Kim, D.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effect of Baicalein on Polyinosinic(-)Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effects of Angelica Sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, C. Topoisomerase I Poisons and Suppressors as Anticancer Drugs. Curr. Med. Chem. 2000, 7, 39–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarrajan, S.; Lulu, S.; Arumugam, M. Computational Evaluation of Phytocompounds for Combating Drug Resistant Tuberculosis by Multi-Targeted Therapy. J. Mol. Model. 2015, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Majee, S.B.; Ghosh, S.; Hazra, B. Apoptosis-Like Death in Leishmania Donovani Promastigotes Induced by Diospyrin and its Ethanolamine Derivative. Int. J. Antimicrob. Agents 2009, 34, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Ghosh, S.; Das Sarma, M.; Sharma, S.; Das, M.; Saudagar, P.; Prajapati, V.K.; Dubey, V.K.; Sundar, S.; Hazra, B. Evaluation of a Diospyrin Derivative as Antileishmanial Agent and Potential Modulator of Ornithine Decarboxylase of Leishmania Donovani. Exp. Parasitol. 2013, 135, 407–413. [Google Scholar] [CrossRef]
- Lall, N.; Meyer, J.J.M.; Wang, Y.; Bapela, N.B.; van Rensburg, C.E.J.; Fourie, B.; Franzblau, S.G. Characterization of Intracellular Activity of Antitubercular Constituents the Roots of Euclea Natalensis. Pharm. Biol. 2005, 43, 353–357. [Google Scholar] [CrossRef]
- Karpuzoglu, E.; Ahmed, S.A. Estrogen Regulation of Nitric Oxide and Inducible Nitric Oxide Synthase (iNOS) in Immune Cells: Implications for Immunity, Autoimmune Diseases, and Apoptosis. Nitric Oxide 2006, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Thiemermann, C.; Vane, J. Inhibition of Nitric Oxide Synthesis Reduces the Hypotension Induced by Bacterial Lipopolysaccharides in the Rat in Vivo. Eur. J. Pharmacol. 1990, 182, 591–595. [Google Scholar] [CrossRef]
- Cooper, P.R.; Lamb, R.; Day, N.D.; Branigan, P.J.; Kajekar, R.; San Mateo, L.; Hornby, P.J.; Panettieri, R.A., Jr. TLR3 Activation Stimulates Cytokine Secretion without Altering Agonist-Induced Human Small Airway Contraction Or Relaxation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L530–L537. [Google Scholar] [CrossRef] [Green Version]
- Antalis, E.; Spathis, A.; Kottaridi, C.; Kossyvakis, A.; Pastellas, K.; Tsakalos, K.; Mentis, A.; Kroupis, C.; Tsiodras, S. Th17 Serum Cytokines in Relation to Laboratory-Confirmed Respiratory Viral Infection: A Pilot Study. J. Med. Virol. 2019, 91, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Crisci, C.D.; Ardusso, L.R.F.; Mossuz, A.; Muller, L. A Precision Medicine Approach to SARS-CoV-2 Pandemic Management. Curr. Treat. Options Allergy 2020. [Google Scholar] [CrossRef]
- Teijaro, J.R. Cytokine storms in infectious diseases. Semin. Immunopathol. 2017, 39, 501–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, Z.R.; Corbett, J.A. Macrophage Expression of Inflammatory Genes in Response to EMCV Infection. Biomolecules 2015, 5, 1938–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Park, W. Immunostimulatory Effect of Laminarin on RAW 264.7 Mouse Macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Lee, J.Y.; Han, H.S.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Kim, H.M.; Ko, S.G.; An, H.J.; Lee, Y.J.; et al. Immunomodulatory Effects of Liriope Platyphylla Water Extract on Lipopolysaccharide-Activated Mouse Macrophage. Nutrients 2012, 4, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effects of Oroxylin A on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Exp. Ther. Med. 2016, 12, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Chrysin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Biotechnol. Bioprocess Eng. 2015, 20, 1026–1034. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Choi, Y.K.; Lee, H.L.; Kim, J.; An, H.J.; et al. Inhibitory Effect of Emodin on Raw 264.7 Activated with Double Stranded Rna Analogue Poly I:C. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-Alpha-Converting Enzyme by the Spike Protein of SARS-CoV and ACE2 Induces TNF-Alpha Production and Facilitates Viral Entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pohlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Sakagami, H.; Miwa, N. ACE2: The Key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Palau, V.; Riera, M.; Soler, M.J. ADAM17 Inhibition may Exert a Protective Effect on COVID-19. Nephrol. Dial. Transplant. 2020. [Google Scholar] [CrossRef]
- Scott, A.J.; O’Dea, K.P.; O’Callaghan, D.; Williams, L.; Dokpesi, J.O.; Tatton, L.; Handy, J.M.; Hogg, P.J.; Takata, M. Reactive Oxygen Species and p38 Mitogen-Activated Protein Kinase Mediate Tumor Necrosis Factor Alpha-Converting Enzyme (TACE/ADAM-17) Activation in Primary Human Monocytes. J. Biol. Chem. 2011, 286, 35466–35476. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 Induces Cleavage of Gasdermin D to Elicit Pyroptosis during Yersinia Infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef] [Green Version]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Goddard, P.J.; Sanchez-Garrido, J.; Slater, S.L.; Kalyan, M.; Ruano-Gallego, D.; Marches, O.; Fernandez, L.A.; Frankel, G.; Shenoy, A.R. Enteropathogenic Escherichia Coli Stimulates Effector-Driven Rapid Caspase-4 Activation in Human Macrophages. Cell Rep. 2019, 27, 1008–1017.e6. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. Hsp70-DnaJ Chaperone Pair Prevents Nitric Oxide- and CHOP-Induced Apoptosis by Inhibiting Translocation of Bax to Mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. 1), E40–E45. [Google Scholar] [CrossRef] [Green Version]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Invest. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M. Regulation of Nitric Oxide Synthesis and Apoptosis by Arginase and Arginine Recycling. J. Nutr. 2007, 137, 1616S–1620S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.S.; Timmins, J.M.; Seimon, T.A.; Sadler, A.; Kolodgie, F.D.; Virmani, R.; Tabas, I. Signal Transducer and Activator of Transcription-1 is Critical for Apoptosis in Macrophages Subjected to Endoplasmic Reticulum Stress in Vitro and in Advanced Atherosclerotic Lesions in Vivo. Circulation 2008, 117, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Name 1 | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NOS2 | TGGAGGTTCTGGATGAGAGC | AATGTCCAGGAAGTAGGTGAGG |
| CHOP | CCACCACACCTGAAAGCAG | TCCTCATACCAGGCTTCCA |
| Camk2a | AGCCATCCTCACCACTAT | ATTCCTTCACGCCATCATT |
| STAT1 | TGAGATGTCCCGGATAGTGG | CGCCAGAGAGAAATTCGTGT |
| STAT3 | GTCTGCAGAGT TCAAGCACCT | TCCTCAGTCACGATCAAGGAG |
| STAT4 | TTCAGAGCAGCTCAACATGC | GGTGAGGTGACCATCATTGTAG-3 |
| Jak2 | TTGGTTTTGAATTATGGTGTCTGT | TCCAAATTTTACAAATTCTTGAACC |
| Fas | CGCTGTTTTCCCTTGCTG | CCTTGAGTATGAACTCTTAACTGTGAG |
| c-Jun c-Fos | ACTGGGTTGCGACCTGAC AGAGCGGGAATGGTGAAGA | CAATAGGCCGCTGCTCTC TCTTCCTCTTCAGGAGATAGCTG |
| β-Actin | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Khan, I.; Shahidullah, A.; Halimi, S.M.A.; Rauf, A.; Lee, J.-Y.; Kim, Y.-J.; Kim, B.-Y.; Park, W. Diospyrin Modulates Inflammation in Poly I:C-Induced Macrophages via ER Stress-Induced Calcium-CHOP Pathway. Processes 2020, 8, 1050. https://doi.org/10.3390/pr8091050
Kim H-J, Khan I, Shahidullah A, Halimi SMA, Rauf A, Lee J-Y, Kim Y-J, Kim B-Y, Park W. Diospyrin Modulates Inflammation in Poly I:C-Induced Macrophages via ER Stress-Induced Calcium-CHOP Pathway. Processes. 2020; 8(9):1050. https://doi.org/10.3390/pr8091050
Chicago/Turabian StyleKim, Hyun-Ju, Inamullah Khan, Adnan Shahidullah, Syed Muhammad Ashhad Halimi, Abdur Rauf, Ji-Young Lee, Young-Jin Kim, Bong-Youn Kim, and Wansu Park. 2020. "Diospyrin Modulates Inflammation in Poly I:C-Induced Macrophages via ER Stress-Induced Calcium-CHOP Pathway" Processes 8, no. 9: 1050. https://doi.org/10.3390/pr8091050
APA StyleKim, H.-J., Khan, I., Shahidullah, A., Halimi, S. M. A., Rauf, A., Lee, J.-Y., Kim, Y.-J., Kim, B.-Y., & Park, W. (2020). Diospyrin Modulates Inflammation in Poly I:C-Induced Macrophages via ER Stress-Induced Calcium-CHOP Pathway. Processes, 8(9), 1050. https://doi.org/10.3390/pr8091050