Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Gel Formation
2.4. Color Analysis of Freeze-Dried Maqui Berries and Gels Obtained
2.5. Examination of the Gel Texture
2.6. Evaluation of pH Value of Gels
2.7. Organoleptic Gel Evaluation
2.8. Total Phenolic Content
2.9. Antioxidant Activity
2.9.1. Ability to Quench ABTS Radicals
2.9.2. Ability to Quench DPPH Radicals
2.9.3. Ferric Reducing Power (FRAP)
2.9.4. Metal Chelating Activity (CHEL)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Color Values of Freeze-Dried Maqui Berries and Gels Obtained
3.2. Texture of Gels with Maqui Berries
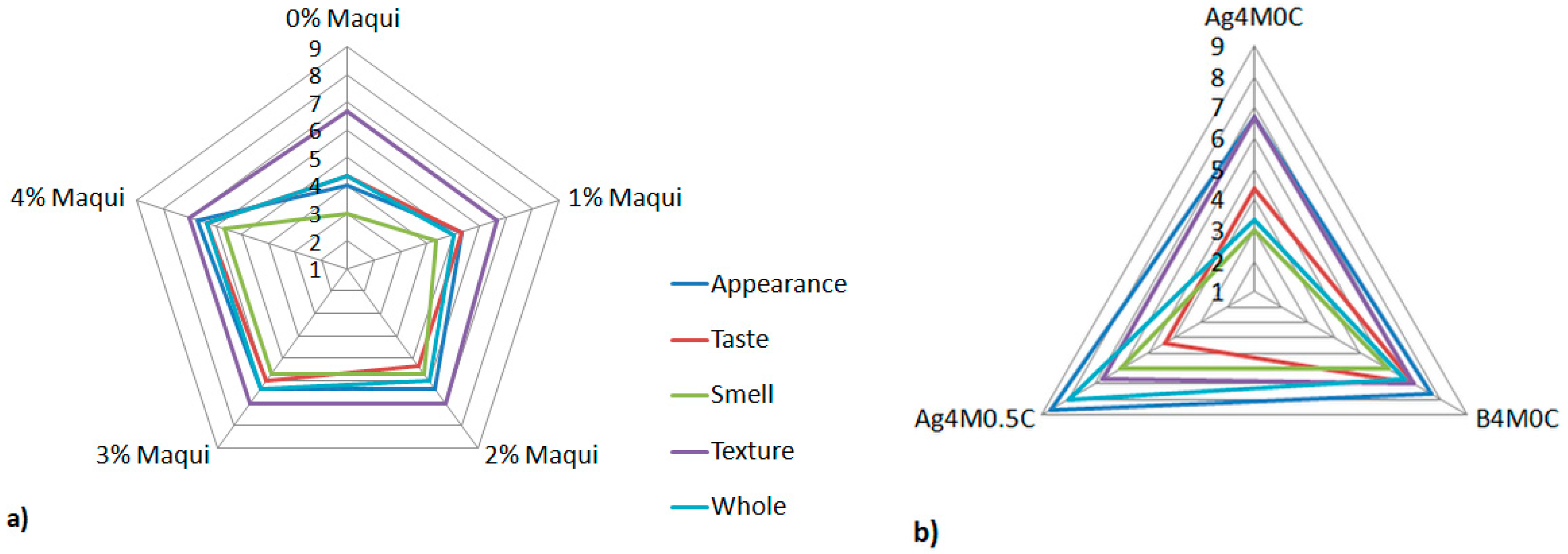
3.3. Sensory Evaluation of Gels with Maqui Berries
3.4. Total Phenolic Content and Antioxidant Activity of Gels with Maqui Berries
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ditlevsen, K.; Sandøe, P.; Lassen, J. Healthy food is nutritious, but organic food is healthy because it is pure: The negotiation of healthy food choices by Danish consumers of organic food. Food Qual. Pref. 2019, 71, 46–53. [Google Scholar] [CrossRef]
- Vasileska, A.; Rechkoska, G. Global and Regional Food Consumption Patterns and Trends. Procedia Soc. Behav. Sci. 2012, 44, 363–369. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Dirnböck, T.; Greimler, P.; Lopez, P.; Stuessy, T.F. Predicting Future Threats to the Native Vegetation of Robinson Crusoe Island, Juan Fernandez Archipelago, Chile. Conserv. Biol. 2003, 17, 1650–1659. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.; Leighton, F. Juice and phenolic fractions of the berry Aristoteliachilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sinerio, J.; Shene, C. Extracts of Maqui (Aristoteliachilensis) and Murta (UgnimolinaeTurcz.): Sources of Antioxidant Compounds and r-Glucosidase/r-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Céspedes-Acuña, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristoteliachilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristoteliachilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; Gonzalez de Mejia, E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vacciniumfloribundum and Aristoteliachilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Neon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui [Aristoteliachilensis (Mol.) Stuntz]. Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sears, B. Anti-inflammatory Diets. J. Am. Coll. Nutr. 2015, 34, 14–21. [Google Scholar] [CrossRef]
- Romanucci, V.; D’Alonzo, D.; Guaragna, A.; Di Marino, C.; Davinelli, S.; Scapagnini, G.; Di Fabio, G.; Zarrelli, A. Bioactive Compounds of Aristotelia chilensis Stuntz and their Pharmacological Effects. Curr. Pharm. Biotechnol. 2016, 17, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristoteliachilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential ofmaqui berry (Aristoteliachilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Misle, E.; Garrido, E.; Contardo, H.; Gonzalez, W. Maqui [Aristoteliachilensis (Mol.) Stuntz]-the Amazing Chilean Tree: A Review. J. Agric. Sci. Technol. 2011, 1, 473–482. [Google Scholar]
- Zamora-Gasga, V.M.; Bello-Perez, L.A.; Ortiz-Basurto, R.I.; Tovar, J.; Sayago-Ayerdi, S.G. Granola bars prepared with Agave tequila ingredients: Chemical composition and in vitro starch hydrolysis. LWT Food Sci. Technol. 2014, 56, 309–314. [Google Scholar] [CrossRef]
- Leal-Diaz, A.M.; Santos-Zea, L.; Martinez-Escobedo, H.C.; Guajardo-Flores, D.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Effect of Agave americana and Agave salmiana Ripeness on Saponin Content from Aguamiel (Agave Sap). J. Agric. Food Chem. 2015, 63, 3924–3930. [Google Scholar] [CrossRef]
- Muniz-Marquez, D.B.; Contreras, J.C.C.; Rodriguez, R.; Mussatto, S.I.; Wong-Paz, J.E.; Aguilar, T.C.N. Influence of thermal effect on sugar composition of Mexican Agave syrup. CyTA J. Food 2015, 13, 607–612. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urias-Silvas, J.E. Thermal properties of agave fructans (Agave tequilana Weber var. Azul). Carbohydr. Polym. 2012, 87, 2671–2676. [Google Scholar] [CrossRef]
- Phillips, K.M.; Carlsen, M.H.; Blomhoff, R. Total Antioxidant Content of Alternatives to Rafined Sugar. J. Am. Diet. Assoc. 2009, 109, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pintor-Jardines, A.; Arjona-Roman, J.L.; Totosaus-Sanchez, A.; Severiano-Perez, P.; Gonzalez-Gonzalez, L.R.; Escalona-Buendia, H.B. The influence of agave fructans on thermal properties of low-fat, and low-fat and sugar ice cream. LTW Food Sci. Technol. 2018, 93, 679–685. [Google Scholar] [CrossRef]
- Ellis, A.L.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. The effect of sugars on agar fluid gels and the stabilisation of their foams. Food Hydrocol. 2019, 87, 371–381. [Google Scholar] [CrossRef]
- Sugar Reduction. Sugar Reduction Achieving the 20%; Public Health England Publications Gateway Number 2016677; Public Health England: London, UK, 2017. [Google Scholar]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophys. 2019, 33, 217–225. [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Biernacka, B.; Dziki, D. Gluten-free crispbread with freeze-dried blackberry: Quality and mineral composition. CYTA J. Food 2019, 17, 841–849. [Google Scholar] [CrossRef]
- Szczesniak, A.S.; Bertrand, J.H. Application of the General Foods Texturometer to Specific Food Products. J. Texture Stud. 1975, 6, 117–138. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and antioxidant property of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011, 112, 1577–1582. [Google Scholar]
- Houghton, P.J.; Raman, A. Methods for extraction and sample clean-up. In Laboratory Handbook for the Fractionation of Natural Extracts; Springer: Boston, MA, USA, 1998; pp. 22–53. [Google Scholar]
- Hashim, N.; Shaari, A.R.; Mamat, A.S.; Ahmad, S. Effect of Differences Methanol Concentration and Extraction Time on the Antioxidant Capacity, Phenolics Content and Bioactive Constituents of OrthosiphonStamineus Extracts. MATEC Web Conf. 2016, 78, 01004. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic. Am. J. Enol. Vitic. 1965, 6, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, F.I.; Chang, C.Y. Antioxidant Properties of the Extracts from Different Parts of Broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Casati, C.B.; Baeza, R.; Sánchez, V. Physicochemical properties and bioactive compounds content in encapsulated freeze-dried powders obtained from blueberry, elderberry, blackcurrant and maqui berry. J. Berry Res. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- GarridoMakinistian, F.; Sette, P.; Gallo, L.; Bucalá, V.; Salvatori, D. Optimized aqueous extracts of maqui (Aristoteliachilensis) suitable for powder production. J. Food Sci. Technol. 2019, 56, 3553–3560. [Google Scholar] [CrossRef]
- Garrido Makinistian, F.; Gallo, L.; Sette, P.; Salvatori, D.; Bucalá, V. Nutraceutical tablets from maqui berry (Aristoteliachilensis) spray-dried powders with high antioxidant levels. Dry. Technol. 2020, 38, 1231–1242. [Google Scholar] [CrossRef]
- Fredes, C.; Montenergo, G.; Zoffoli, J.P.; Gómez, M.; Paz, R. Polyphenol Content and Antioxidant Activity of Maqui (Aristoteliachilensis [Molina] Stuntz) During Fruit Development and Maturation in Central Chile. Chil. J. Agric. Res. 2012, 72, 582–589. [Google Scholar] [CrossRef]
- Romero-González, J.; Shun Ah-Hen, K.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total phenolics, anthocyanin profile and antioxidant activity of maqui, Aristoteliachilensis (Mol.) Stuntz, berries extract in freeze-dried polysaccharides microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef]
- Leyrer, J.; Hunter, R.; Rubilar, M.; Pavez, B.; Morales, E.; Torres, S. Development of dye-sensitized solar cells based on naturally extracted dye from the maqui berry (Aristoteliachilensis). Optic. Mater. 2016, 60, 411–417. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Valentão, P.; Moreno, D.A.; Ferreres, F.; García-Viguera, C.; Andrade, P.B. New beverages of lemon juice enriched with the exotic berries maqui, açaí, and blackthorn: Bioactive components and in vitro biological properties. J. Agric. Food Chem. 2012, 60, 6571–6580. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Huertas, J.P.; Moreno, D.A.; Periago, P.M.; García-Viguera, C. Quality and microbial safety evaluation of new isotonic beverages upon thermal treatments. Food Chem. 2016, 194, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Leyrer, J.; Rubilar, M.; Morales, E.; Pavez, B.; Leal, E.; Hunter, R. Factor Optimization in the Manufacturing Process of Dye-Sensitized Solar Cells Based on Naturally Extracted Dye from a Maqui and Blackberry Mixture (Aristotelia chilensis and Rubus glaucus). J. Electron. Mater. 2018, 47, 6136–6143. [Google Scholar] [CrossRef]
- Rein, M.J. Copigmentation Reactions and Color Stability of Berry Anthocyanins. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2005; p. 87. [Google Scholar]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocol. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Torres, C.A.; Romero, L.A.; Diaz, R.I. Quality and sensory attributes of apple and quince leathers made without preservatives and with enhanced antioxidant activity. Food Sci. Technol. 2014, 62, 996–1003. [Google Scholar] [CrossRef]
- Freses, C.; Paz, R. The powerful colour of the maqui (Aristoteliachilensis [Mol.] Stuntz) fruit. J. Berry Res. 2014, 4, 175–182. [Google Scholar] [CrossRef]
| Type of Sample | Color Parameters | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ||
| Maqui Powder | 16.33 ± 0.33 a | 8.28 ± 0.10 d | 9.57 ± 0.24 e | 12.65 ± 0.24 d,e | 49.14 ± 0.46 g | |
| Amount of agar | 0.5% | 55.12 ± 0.81 g | 2.0 ± 0.12 a | 12.05 ± 0.70 f | 13.15 ± 0.92 e | 81.99 ± 1.21 j |
| 1% | 54.28 ± 0.57 f,g | 2.11 ± 0.17 a,b | 11.97 ± 0.69 f | 12.94 ± 0.85 d,e | 80.19 ± 0.81 i,j | |
| 1.5% | 53.39 ± 0.88 f,g | 2.33 ± 0.29 a,b | 11.85 ± 0.56 f | 12.07 ± 0.61 d | 78.89 ± 0.91 i | |
| 2% | 52.09 ± 0.73 f | 2.41 ± 0.11 b | 11.71 ± 0.62 f | 11.13 ± 0.78 c,d | 75.32 ± 1.11 h | |
| Amount of maqui | 0% | 53.04 ± 0.58 f | 2.33 ± 0.29 a,b | 11.85 ± 0.56 f | 12.07 ± 0.61 d | 78.89 ± 0.91 i |
| 1% | 27.16 ± 0.31 d,e | 4.13 ± 0.16 c | 3.67 ± 0.89 b,c | 5.56 ± 0.49 a | 41.18 ± 1.91 f | |
| 2% | 26 ± 0.68 d | 7.87 ± 0.42 d | 5.58 ± 0.35 d | 9.66 ± 0.94 b | 37.78 ± 1.3 e | |
| 3% | 24.29 ± 0.22 c | 10.43 ± 0.21 e | 4.46 ± 0.39 c | 11.35 ± 0.35 c,d | 23.13 ± 1.43 c | |
| 4% | 22.86 ± 0.39 b | 10.91 ± 0.23 e,f | 2.52 ± 0.22 a | 11.19 ± 0.20 c,d | 13.34 ± 0.87 a | |
| 5% | 22.12 ± 0.48 b | 10.99 ± 0.32 e,f | 2.32 ± 0.24 a | 11.02 ± 0.31 b,c | 12.44 ± 0.99 a | |
| Optimal gels | Ag4M0C | 22.86 ± 0.39 b | 10.91 ± 0.23 e,f | 2.52 ± 0.22 a | 11.19 ± 0.2 c | 13.34 ± 0.87 a |
| Ag4M0.5C | 28.91 ± 0.16 e | 10.5 ± 0.59 e,f | 6.09 ± 0.48 d | 12.16 ± 0.67 d,e | 30.24 ± 1.35 d | |
| B4M0C | 23.24 ± 0.33 b | 11.03 ± 0.19 f | 3.14 ± 0.15 b | 11.46 ± 0.22 c,d | 15.89 ± 0.46 b | |
| Type of Sample | Texture Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Hardness (N) | Elasticity (mm) | Springiness | Cohesiv. | Gummin. (N) | Chewiness (N) | ||
| Amount of agar addition | 0.5% | 7.11 ± 0.31 a | 1.33 ± 0.05 a | 0.39 ± 0.01 a | 0.18 ± 0.01 b | 1.28 ± 0.05 a | 0.50 ± 0.02 a |
| 1.0% | 14.52 ± 0.63 b | 1.74 ± 0.07 b | 0.48 ± 0.02 b | 0.19 ± 0.01 b | 2.76 ± 0.09 b | 1.32 ± 0.081 b | |
| 1.5% | 28.95 ± 0.99 h | 2.02 ± 0.14 c | 0.53 ± 0.04 b,c | 0.20 ± 0.004 b | 5.86 ± 0.22 e,f | 3.14 ± 0.02 f | |
| 2% | 37.82 ± 1.39 i | 2.69 ± 0.11 f | 0.62 ± 0.02 d | 0.10 ± 0.007 a | 3.78 ± 0.18 c | 2.34 ± 0.08 c | |
| Amount of maqui addition | 0% | 28.95 ± 0.62 h | 2.02 ± 0.14 c | 0.53 ± 0.04 b,c | 0.20 ± 0.004 b | 5.62 ± 0.22 e | 3.14 ± 0.02 f |
| 1% | 26.7 ± 0.39 g | 2.10 ± 0.12 c,d | 0.54 ± 0.03 c,d | 0.21 ± 0.008 b | 6.12 ± 0.15 f | 3.04 ± 0.005 e | |
| 2% | 25.24 ± 0.47 f | 2.12 ± 0.11 c,d | 0.55 ± 0.03 c,d | 0.24 ± 0.01 c | 7.83 ± 0.13 h | 3.37 ± 0.001 g | |
| 3% | 23.63 ± 0.34 e | 2.23 ± 0.08 d | 0.57 ± 0.03 c,d | 0.33 ± 0.01 e | 7.29 ± 0.15 g | 4.45 ± 0.003 h | |
| 4% | 21.31 ± 0.40 d | 2.24 ± 0.07 d,e | 0.61 ± 0.04 d | 0.34 ± 0.012 e | 7.24 ± 0.17 g | 4.45 ± 0.06 h | |
| Optimal gels | Ag4M0C | 21.31 ± 0.40 d | 2.24 ± 0.07 d,e | 0.61 ± 0.04 d | 0.34 ± 0.012 e | 7.24 ± 0.17 g | 4.45 ± 0.06 h |
| B4M0C | 24.13 ± 0.89 e,f | 2.25 ± 0.06 d,e | 0.63 ± 0.01 d | 0.35 ± 0.01 e | 8.44 ± 0.27 i | 5.32 ± 0.06 i | |
| Ag4M0.5C | 18.2 ± 0.45 c | 2.37 ± 0.09 e | 0.59 ± 0.05 d | 0.27 ± 0.012 d | 4.91 ± 0.25 d | 2.89 ± 0.03 d | |
| Kind of Sample | TPC (GAEmg/g) | ABTS (EC50 mg/mL) | DPPH (EC50 mg/mL) | CHEL (EC50 mg/mL) | FRAP (EC50 mg/mL) |
|---|---|---|---|---|---|
| Maqui powder | 34.82 ± 2.18 d | 1.37 ± 0.03 a | 3.38 ± 0.23 a | 28.42 ± 1.67 a | 2.48 ± 0.02 a |
| Ag4M0C | 1.92 ± 0.10 c | 27.60 ± 0.65 d | 60.93 ± 4.34 b | 196.60 ± 3.34 b | 31.59 ± 0.10 b |
| Ag4M0.5C | 1.87 ± 0.10 b | 22.60 ± 0.10 b | 58.86 ± 3.93 b | 208.99 ± 4.78 c | 45.00 ± 1.42 c |
| B4M0C | 1.62 ± 0.06 a | 25.14 ± 0.09 c | 65.61 ± 7.05 c | 189.00 ± 4.17 b | 50.48 ± 0.94 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobaszek, P.; Różyło, R.; Dziki, L.; Gawlik-Dziki, U.; Biernacka, B.; Panasiewicz, M. Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar. Processes 2020, 8, 1294. https://doi.org/10.3390/pr8101294
Sobaszek P, Różyło R, Dziki L, Gawlik-Dziki U, Biernacka B, Panasiewicz M. Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar. Processes. 2020; 8(10):1294. https://doi.org/10.3390/pr8101294
Chicago/Turabian StyleSobaszek, Patryk, Renata Różyło, Laura Dziki, Urszula Gawlik-Dziki, Beata Biernacka, and Marian Panasiewicz. 2020. "Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar" Processes 8, no. 10: 1294. https://doi.org/10.3390/pr8101294
APA StyleSobaszek, P., Różyło, R., Dziki, L., Gawlik-Dziki, U., Biernacka, B., & Panasiewicz, M. (2020). Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar. Processes, 8(10), 1294. https://doi.org/10.3390/pr8101294








