1. Introduction
In the last century, food fermentation evolved from a predominantly empirical practice to a technology-driven discipline based on microbial physiology, biochemical engineering, and advanced process control. Historically, fermentation was a preservation method, lowering pH, generating antimicrobial metabolites, and extending the shelf life of perishable products such as milk, fruit juices, meats, and cereals [
1]. Nowadays, fermentation extends far beyond preservation, enabling the development of products with distinctive sensory profiles, enhanced nutritional value, and targeted functional properties, including probiotic, postbiotic, bioactive, and nutraceutical benefits [
2].
Despite these advances, conventional fermentation workflows, including upstream (raw material preparation and microbial inoculation), midstream (fermentation and metabolic modulation), and downstream (stabilization, refining, and packaging), remain constrained by challenges such as prolonged processing times, susceptibility to contamination, and the degradation of thermolabile compounds when heat-based microbial inactivation is applied. Traditional pasteurization and sterilization, while effective for ensuring safety, can compromise flavor, color, texture, and the bioavailability of heat-sensitive nutrients. These drawbacks have fueled interest in alternative, sustainable processing methods capable of improving product quality, reducing energy and water use, and maintaining safety standards without the detrimental effects associated with high-temperature treatments.
NTTs, including US, PEF, HPP, CP, and PL, have emerged as versatile tools to address limitations inherent to conventional fermentation processes. Applied strategically at different stages of the bioprocess, NTTs can enhance mass transfer and nutrient availability during upstream preparation, modulate microbial metabolism and metabolite synthesis in midstream fermentation, and stabilize or refine products in downstream operations without compromising heat-sensitive bioactive compounds.
From a sustainability perspective, the potential of NTTs goes beyond energy and water efficiency. By extending shelf life and valorizing by-products into value-added ingredients, these technologies contribute to food waste reduction and circular economy strategies. Moreover, their ability to replace chemical-intensive processes supports greener manufacturing and lowers the carbon footprint of food systems. Collectively, these benefits align with broader sustainable development goals, shifting food bioprocessing from preservation-focused approaches toward multifunctional, resource-efficient, and environmentally responsible biomanufacturing systems capable of meeting both consumer demands and planetary boundaries [
3,
4].
Recent studies have demonstrated that NTT-assisted fermentations can improve yields, reduce processing time, and enhance the biofunctional profile of products in diverse matrices, from dairy and plant-based beverages to fermented fruits, vegetables, and by-products [
5,
6,
7]. An US-processed kombucha exhibited a 19% increase in sucrose consumption during fermentation, higher yields of gluconic, propionic, and isobutyric acids, and an altered SCOBY cellulose fiber structure, suggesting enhanced bioactive potential and fermentation efficiency [
8]. Cold plasma processing improved the fermentability of rice and corn bran fibers, increasing glucose diffusion by up to ~22% and enhancing SCFA production (1.5–2.0× higher than controls). Probiotic growth was also stimulated, with prebiotic activity scores rising up to ~1.8-fold compared to untreated fibers [
9]. Overall, while PL remains less mature in active fermentation than technologies like HPP or PEF, its advantages in surface and packaging hygiene, combined with its low resource demand and compatibility with clean-label strategies, make it a promising downstream tool.
Together, these findings demonstrate that NTTs are not limited to preservation but can actively drive microbial metabolism and product functionality. However, translating these laboratory-scale advances into industrial practice remains challenging due to issues of scalability, standardization, and regulatory acceptance. Addressing these barriers requires integrated approaches combining mechanistic understanding, pilot-scale validation, computational modeling, and life cycle assessment to ensure both technological efficacy and environmental sustainability [
10].
Unlike previous reviews that primarily focus on the use of NTTs for microbial inactivation and food preservation, this review emphasizes their emerging role as abiotic modulators in food fermentation. Here, we specifically examine how US, PEF, HPP, CP, and PL can be strategically integrated across upstream, fermentation, and downstream stages of food bioprocessing. By addressing their capacity to modulate microbial physiology, enhance metabolite synthesis, and influence the production of bioactive compounds, the perspective shifted from preservation to process intensification and functional enrichment. Moreover, this review complements the discussion with a bibliometric analysis of the recent scientific literature, providing a broader overview of research trends and industrial readiness. This dual approach not only highlights current technological opportunities and challenges but also establishes the unique contribution of this review in positioning NTTs as enablers of next-generation, sustainability-oriented fermentation systems.
2. State of the Art
The application of NTTs in food fermentation remains fragmented due to the heterogeneity of food matrices and the lack of methodological standardization, which hinder broad comparisons and data synthesis. The effectiveness of NTTs depends strongly on variables such as matrix composition, microbial species involved, and technology-specific parameters (e.g., US frequency, PEF strength, pressure range in HPP). As a result, direct comparisons across studies are challenging, and research that systematically evaluates multiple technologies under comparable conditions is still limited. Most published studies also emphasize microbial inactivation for preservation, whereas fewer explore the subtler role of NTTs in modulating microbial metabolism and enhancing fermentation performance. Finally, the relatively early stage of some technologies and the scarcity of large-scale validation data make it difficult to draw robust conclusions about their feasibility and long-term contribution to sustainable food systems.
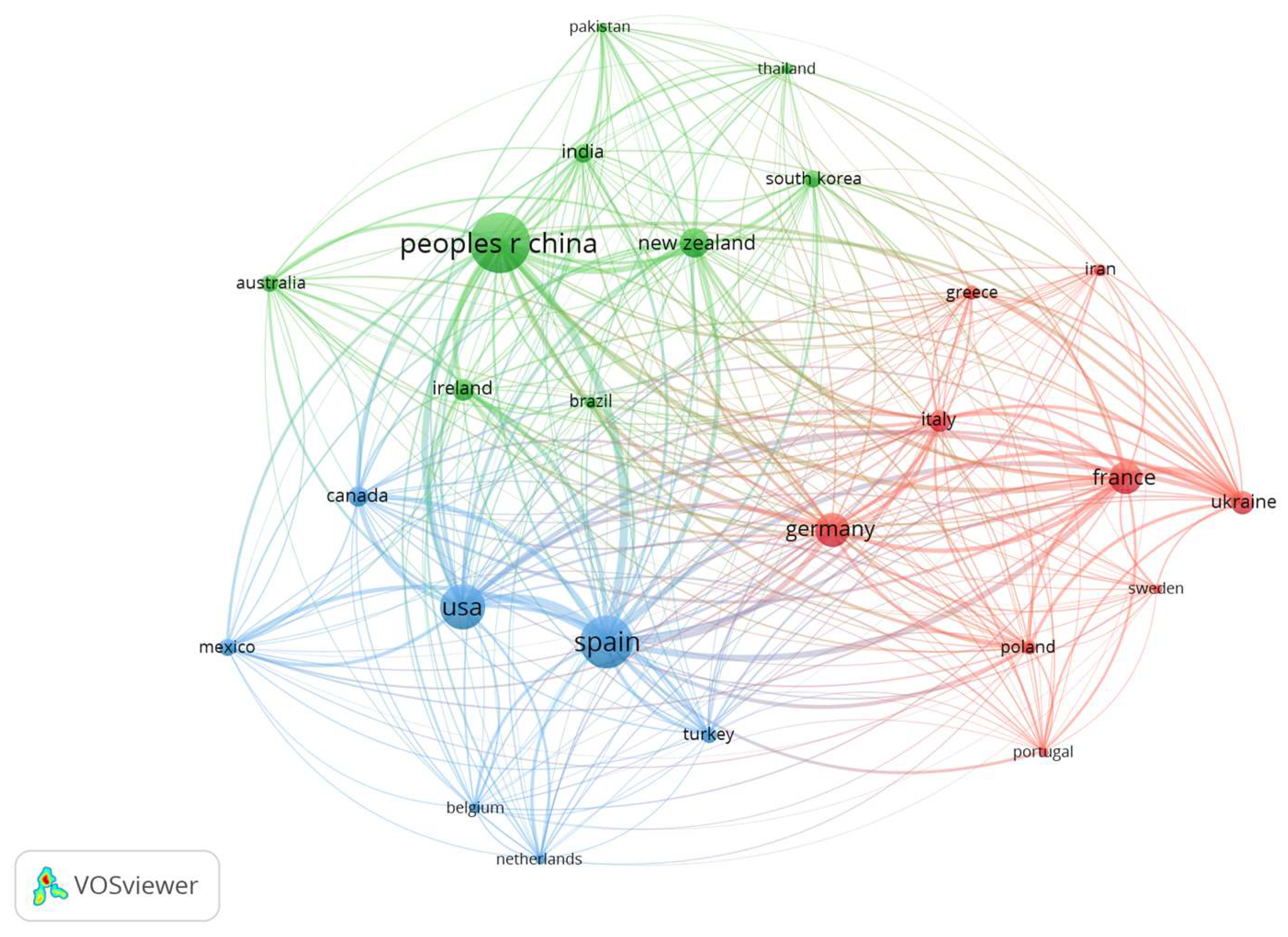
To contextualize the application of NTTs in food fermentation, a bibliometric analysis was performed in August 2025 using the Web of Science database. The search applied the “all fields” filter with the keywords fermentation AND ultrasound OR pulsed electric fields OR high pressure processing OR cold plasma OR pulsed light, restricted to original research articles in the Food Science & Technology category. A total of 14,951 documents were retrieved, ordered by relevance, and the first 1000 were analyzed using VOSviewer (version 1.6.20).
Keyword co-occurrence analysis (minimum frequency: 50) identified 23 high-relevance terms, with pulsed electric fields, inactivation, and quality ranking highest. PEF, HPP, and US were the most frequently represented technologies, indicating their dominant role in the field.
Figure 1 illustrates the keyword co-occurrence network, where color clusters reflect distinct research domains and the overlay visualization highlights recent thematic growth in ultrasound, bioactive compounds, impact, and food matrix.
Bibliographic coupling by country revealed contributions from 69 nations, with 25 surpassing 10 publications, grouped into three major clusters. China, Italy, Spain, and the United States emerged as the most prolific producers (
Figure 2). The size of nodes correlates with publication output, while link thickness indicates the strength of collaborative networks.
Segmenting the search by technology, while maintaining the same inclusion criteria and using technology name AND fermentation, enabled a more targeted view of each approach. For US, 401 documents were identified. Co-occurrence analysis (≥10 occurrences) emphasized antioxidant activity, optimization, growth, extraction, and phenolic compounds as dominant terms, with recent focus shifting toward functional properties, antioxidant, and bioactive compounds. The most associated raw materials (milk, yogurt, and wine) highlight the prevalence of beverage-related studies.
For PEF, 120 documents were retrieved, with 28 keywords above the frequency threshold. Common terms included extraction, inactivation, red wine, anthocyanins, and impact, while recent attention has turned toward bioactive compounds, antioxidant activity, and quality. Strong associations were observed with grape, wine, and orange juice.
HPP yielded 484 documents, from which 65 keywords (≥10 occurrences) emerged. Broadly represented terms included quality, temperature, inactivation, physicochemical properties, and antioxidant activity, with protein, antioxidant activity, and sensory properties featuring more prominently in recent years. Raw materials most cited were protein, milk, beer, wine, and yogurt.
CP showed a markedly smaller research base, with only 18 documents. The analysis identified 191 keywords (≥1 occurrence), including impact, apple, metabolism, shelf-life, and temperature. More recent terms (allergenicity, digestibility, and volatility) suggest emerging safety and quality assessment priorities. Associated raw materials included apple, grape, proteins, beverage, and jujube juice.
PL produced 35 documents, with 53 keywords (≥2 occurrences). Dominant terms included inactivation, color, shelf-life, non-saccharomyces, and optimization, while storage, stability, and mutagenesis emerged more recently. Commonly studied matrices were red wine, beer, and apple.
The bibliometric trends indicate a balanced research focus on microbial safety, product stability, and functional enhancement. The temporal evolution in
Figure 1 suggests a gradual shift from preservation-oriented studies toward investigations optimizing process parameters and enhancing nutritional and sensory quality. The concentration of outputs in major hubs (
Figure 2) highlights strong research capacity but also points to geographic asymmetries, with underrepresentation from regions that could provide unique raw materials and traditional fermentation practices.
The bibliometric analysis revealed an increasing number of publications on NTTs in food fermentation, with US, PEF, and HPP dominating the research landscape. This predominance reflects their relatively high level of industrial maturity and regulatory acceptance. HPP, for example, has already achieved broad commercialization in the food sector as a non-thermal pasteurization tool, which facilitates its exploration in fermentation contexts. Similarly, PEF and US benefit from extensive research in both food preservation and bioprocess enhancement, making them attractive for industrial adoption due to existing infrastructure, validated equipment, and scalable protocols.
In contrast, CP and PL remain underrepresented in the fermentation literature. Their limited penetration depth (PL) and highly reactive chemistry (CP) pose challenges in dense or turbid matrices typical of fermentation systems. Furthermore, regulatory hurdles, particularly the classification of CP as a radiation-based technology in some regions, slow down their path toward industrial implementation [
4]. These factors explain their lower visibility in the bibliometric landscape, despite their promising laboratory-scale results.
Another observation concerns the food systems studied. Most research focuses on liquid fermentations (e.g., dairy, beverages, plant-based proteins), where process monitoring and energy distribution are easier to control. Solid-state fermentations, despite their relevance for traditional foods and upcycled by-products, remain underexplored in the context of NTTs. Expanding research into these systems could open new avenues for valorizing agro-industrial residues, enhancing bioactive production, and supporting circular economy models in line with SDGs 12 and 13.
Taken together, these findings highlight not only the current research priorities but also the knowledge gaps that must be addressed to guide the broader industrial adoption of NTTs. While HPP, PEF, and US are closer to market readiness, future innovation will depend on diversifying applications, optimizing less explored technologies, and extending their use to underrepresented food systems.
The significant drop in publication numbers when refining searches by individual technology is likely due to terminology variability, lower industrial adoption for certain NTTs, and relevant studies indexed outside Food Science & Technology.
Future research should address two main priorities: Industrial optimization and standardization, clear operational protocols with full parameter disclosure (e.g., intensity, duration, specific energy, temperature, frequency, pressure) to enable reproducibility and cross-study comparison; “head-to-head” trials applying different NTTs to identical matrices to objectively assess performance; and Environmental and sustainability assessment, quantifying energy use, carbon footprint, and resource efficiency to ensure technological gains are not offset by environmental costs.
Combining NTTs may yield synergistic benefits in microbial control, metabolic modulation, and bioactive retention. Coupling these strategies with multi-omics approaches and advanced monitoring could accelerate the development of scalable, efficient, and sustainable fermentation processes.
In sum, the field exhibits both maturity in some technologies and clear knowledge gaps in others. Bridging these will require harmonized methodologies, expansion into underexplored food systems, robust environmental metrics, and integrated technological solutions for industrial applications.
3. NTTs Overview
NTTs encompass a range of physical processing methods that operate without applying high temperatures to achieve a technological goal. Their relevance to food fermentation lies in their ability to enhance microbial performance, reduce processing time, and preserve heat-sensitive compounds, all while enabling tailored control over the fermentation process [
1,
2]. However, while their advantages are widely reported, it is equally important to acknowledge potential limitations, trade-offs, and unresolved controversies that may affect their industrial adoption.
Beyond their role in food safety, these methods can impose controlled abiotic stress on microbial cells, triggering adaptive responses that influence membrane permeability, enzyme activity, and metabolic pathway regulation. Such responses may be leveraged to stimulate the synthesis of bioactive metabolites or improve substrate utilization efficiency, aligning with the goals of precision fermentation and sustainable production [
5].
NTTs can exert either non-lethal or lethal effects on microorganisms depending on treatment intensity and food matrix conditions. At non-lethal levels, processing such as US and PEF induce transient membrane permeabilization, enhancing nutrient uptake, metabolite exchange, and fermentation kinetics. Similarly, CP and PL can trigger stress responses that stimulate secondary metabolism and bioactive compound release. By contrast, when operated at higher intensities or prolonged exposure, these same technologies achieve irreversible microbial inactivation, supporting downstream stabilization and safety assurance. HPP exemplifies this dual role, with moderate pressures modulating microbial activity while commercial-scale treatments ensure effective decontamination.
3.1. Ultrasound
US technology employs high-frequency acoustic waves, typically between 20 and 100 kHz, to induce acoustic cavitation in liquid systems. The collapse of cavitation bubbles generates highly localized “hotspots” with transient extreme temperatures, elevated pressures, and intense shear forces [
11,
12,
13,
14]. These phenomena simultaneously drive physical effects, such as turbulence, particle size reduction, homogenization, and enhanced mass transfer, alongside chemical effects, including vapor-phase dissociation and the generation of reactive species. Cavitation-induced micro-convection can also modify enzyme conformations, influencing substrate accessibility and catalytic activity [
11,
15]. Cavitation can also induce lipid oxidation, off-flavor formation, and loss of volatile compounds in aroma-sensitive foods. Moreover, uneven acoustic distribution in large fermenters can represent a barrier to reproducibility at large scale.
The effects of US are strongly dependent on acoustic intensity. High-intensity treatments (10–1000 W/cm
2) are effective for microbial disruption and inactivation. In contrast, low-intensity applications (<10 W/cm
2) can stimulate microbial activity and enhance bioprocess performance without causing substantial cellular damage. Although the mechanisms underlying such stimulation remain under investigation, improvements in gas–liquid mass transfer, such as increased gas absorption, and solid–liquid mass transfer, such as accelerated solute dissolution, are recognized as major contributing factors [
16,
17]. In both cases, acoustic cavitation plays a central role: microbubbles formed during the rarefaction phase expand and collapse during compression, releasing localized shock waves. The resulting pressure oscillations can function as abiotic stressors, promoting microbial growth, proliferation, and metabolic reprogramming [
12].
In microbial systems, these effects may disrupt cell aggregates, improve nutrient and metabolite diffusion, and transiently permeabilize cell membranes through a process known as sonoporation. At non-lethal intensities, US has been shown to accelerate fermentation kinetics and boost the biosynthesis of valuable metabolites, including exopolysaccharides (EPS) and organic acids [
18,
19]. However, process outcomes are highly sensitive to operational parameters, such as frequency, amplitude, duty cycle, and medium viscosity, posing challenges for standardization in industrial-scale operations [
20].
Frequency selection is particularly critical, as it dictates cavitation dynamics. Higher frequencies shorten compression–rarefaction cycles, limiting bubble expansion and thereby reducing cavitation intensity. Conversely, lower frequencies favor the formation of larger bubbles that collapse more violently, releasing greater localized energy. Similarly, higher vibrational amplitudes are beneficial when treating viscous media, as they help overcome matrix cohesive forces [
20]. In practical applications, US can be applied directly, using a sonotrode immersed in the product, or indirectly, via a water bath, with the method selected based on the desired energy transfer efficiency and uniformity of treatment [
21].
3.2. Pulsed Electric Fields
PEF technology delivers short, high-voltage pulses, typically 10–80 kV/cm with microsecond to millisecond durations, to liquid or pasty foods positioned between electrodes. The applied field induces charge separation across microbial cell membranes, increasing transmembrane potential beyond a critical threshold (~0.8–1 V) and triggering electroporation: the formation of nanoscale pores in the membrane lipid bilayer [
22,
23].
The effectiveness of PEF processing is influenced by a combination of factors, including process parameters such as pulse duration, field strength, and number of PEFs; environmental conditions such as pH, organic acids, and food constituents; and biological conditions, such as the microbial growth phase and whether the bacteria are Gram-negative or Gram-positive [
24]. Lethal PEF processing, or irreversible electroporation, uses a high electrical field strength to permanently damage microbial cell membranes, leading to cell death and microbial inactivation, achieving >5 log reductions in vegetative pathogens, effects widely exploited for non-thermal microbial inactivation in beverages and other fluid foods [
25,
26].
Conversely, non-lethal PEF processing, or reversible electroporation, uses a lower electric field strength to temporarily permeabilize cell membranes without causing irreversible damage, a state from which the cell can recover [
27,
28].
Corynebacterium glutamicum submitted to PEF at 6 kV/cm for 1–3 ms, with pulses applied 2 to 5 times, resulted in membrane permeabilization but did not affect cell viability. The viability of the cells was maintained because the gaps between pulses allowed the membrane to reseal and restore its integrity [
29]. This is in contrast to lethal conditions, such as those that caused permanent damage and cell death in
Lactobacillus plantarum treated at a much higher intensity of 25 kV/cm for 2.3 µs [
30]. Non-lethal conditions are purposefully chosen to enhance specific bioprocesses, such as increasing nutrient uptake and metabolite synthesis, rather than microbial inactivation. In fermentation, this has been linked to accelerated growth, enhanced metabolic fluxes, and increased synthesis of secondary metabolites [
27,
28]. The choice between these two regimes is critical and depends on the specific application and food matrix characteristics, as factors like food conductivity and viscosity can impact the uniformity of the electric field and, consequently, the processing efficacy.
PEF treatments operate at near-ambient bulk temperatures, preserving thermolabile nutrients and volatile compounds, and are readily adaptable to continuous-flow processing. Key operational variables include electric field strength, specific energy input, pulse frequency, and electrode design [
31]. Advantages include extremely short treatment times, low cumulative heat load, and compatibility with industrial-scale continuous systems. Limitations remain in high capital costs, reduced efficacy in viscous or particulate-rich matrices, and the need for robust electrical safety and precise process control. Electrode fouling, non-uniform field distribution in heterogeneous matrices, and the risk of undesired electrochemical reactions that may lead to byproduct formation may difficult scalability and increase operational complexity. By selecting parameters that favor either reversible or irreversible electroporation, PEF can function as both a metabolic stimulator and a non-thermal preservation tool, making it a versatile candidate for integrated food fermentation strategies.
3.3. High Pressure Processing
HPP subjects food systems to uniform isostatic pressures, typically between 100 and 600 MPa, using a water-based medium as the pressure-transmitting fluid [
32]. The pressure is applied instantaneously and evenly throughout the product, regardless of size or geometry, ensuring consistent processing. Following Le Chatelier’s principle, pressure favors reactions that reduce system volume, primarily affecting non-covalent interactions such as hydrogen bonds and hydrophobic associations [
33]. These molecular alterations can modify protein conformation, alter enzyme activity, and affect membrane integrity without significant heating, thereby preserving vitamins, pigments, volatile aroma compounds, and other quality attributes.
The impact of HPP is highly pressure dependent. At high pressures (>300 MPa), vegetative microbial cells and certain enzymes are irreversibly inactivated, enabling “cold pasteurization” that extends shelf life while retaining fresh-like sensory and nutritional properties [
34]. At non-lethal pressures (5–100 MPa), however, HPP can act as a controlled abiotic stressor, inducing stress-response pathways, upregulating specific genes, and redirecting microbial metabolism toward the production of secondary metabolites [
35]. These effects can be harnessed in fermentation processes to modulate growth kinetics, metabolite distribution, and product quality.
In microbial systems, moderate pressures can transiently permeabilize membranes, enhancing nutrient uptake, while pressure-induced changes in enzyme conformation can alter catalytic efficiency. Such mechanisms have been exploited in dairy, alcoholic, and functional fermentations to improve yogurt viscosity and adjust the ethanol-to-byproduct ratio in yeast fermentations [
36,
37,
38,
39,
40].
Despite its versatility, industrial integration of HPP into fermentation workflows remains limited by high capital costs, batch-mode operation, and packaging constraints. Moreover, process outcomes are sensitive to operational variables, target pressure, holding time, initial temperature, and matrix composition, complicating standardization and scale-up. Additionally, while generally considered non-thermal, pressure-induced conformational changes in proteins can sometimes alter texture and sensory perception. Innovations in reactor design, including semi-continuous or continuous high-pressure systems, could mitigate these limitations and enable HPP’s broader use as both a preservation tool and a fermentation modulator in minimally processed, high-quality food production [
10].
3.4. Cold Plasma
CP, also referred to as non-thermal or atmospheric cold plasma (ACP), is a partially ionized gas produced under atmospheric or low-pressure conditions that contains a mixture of electrons, ions, neutral species, reactive oxygen (ROS) and nitrogen species (RNS), UV photons, and charged particles [
41]. Unlike thermal plasmas, where all particles reach thermal equilibrium at thousands of Kelvin, CP selectively energizes electrons while keeping the bulk gas near ambient temperature, making it suitable for heat-sensitive biological systems.
Generation of CP involves applying electrical energy to a working gas such as air, oxygen, nitrogen, argon, or their mixtures, resulting in gas ionization and the formation of RONS, including ozone (O
3), hydroxyl radicals (·OH), nitric oxide (NO), and nitrogen dioxide (NO
2). These species drive the antimicrobial, enzyme-modulating, and biomolecule-modifying effects of CP. Plasma can be applied in three modes: direct (plasma directly contacting the sample), semi-direct (separated by a barrier such as a mesh), and indirect (via a plasma-treated medium, e.g., water) [
42]. Common plasma generation methods include dielectric barrier discharge (DBD), plasma jets (PJ), corona discharge (CD), gliding arc discharge (GAD), and radiofrequency (RF), with DBD and PJ being the most widely used in food and bioprocessing applications due to their operational stability and scalability [
4,
43].
At the cellular level, CP action is primarily mediated by oxidative stress, membrane lipid modification, and targeted macromolecule alteration. In microbial cultures, RNS can disrupt lipid bilayers, oxidize proteins, and damage nucleic acids, leading to inactivation or modulation of metabolic pathways [
2]. Controlled non-lethal exposure can trigger antioxidant defense mechanisms, upregulate stress-response genes, and reprogram metabolism toward increased biosynthesis of pigments, polyphenols, and other secondary metabolites [
44,
45,
46]. Nonetheless, concerns persist regarding the potential toxicity of residual plasma-generated compounds, as well as challenges in standardizing treatments across different plasma sources and food matrices.
3.5. Pulsed Light
PL is a non-thermal preservation technology that uses intense, short-duration pulses of broad-spectrum light, ranging from ultraviolet to infrared, produced by xenon flash lamps. The emission spectrum includes germicidal UV-C wavelengths (200–280 nm), which are primarily responsible for microbial inactivation through DNA damage, notably the formation of pyrimidine dimers that inhibit replication [
47]. Each pulse delivers high peak power from microseconds to milliseconds, enabling rapid surface treatment with minimal bulk heating, thereby preserving the nutritional and sensory quality of heat-sensitive foods [
48].
The efficacy of PL is determined by pulse fluence (J/cm
2), number of pulses, distance from the light source, and product optical properties. Its limited penetration depth, typically less than 1 mm, makes it particularly suitable for surface decontamination of solid foods and treatment of optically clear liquids. In complex matrices, shadowing effects and turbidity can reduce effectiveness, necessitating optimization of product positioning and light exposure geometry [
47].
Beyond microbial inactivation, non-lethal PL treatments have been reported to induce physiological and metabolic changes in microorganisms. In fermentation systems, for example, Kwaw et al. [
49] observed that applying PL to lactic-acid-fermented mulberry juice increased phenolic and flavonoid concentrations, total anthocyanins, and antioxidant activities. These enhancements were attributed to stress-induced metabolic activation, suggesting that PL can be tailored to modulate biochemical pathways and improve functional attributes in fermented foods. Intense light pulses may trigger photodegradation of pigments, vitamins, or other heat- and light-sensitive compounds, thereby compromising product quality if process parameters are not carefully optimized.
The integration of PL technology into existing processing infrastructure makes it a highly advantageous solution for industrial food applications. Thus, PL can be strategically positioned both as a downstream intervention to ensure microbial safety and product stability, and as a modulatory tool during fermentation to enhance the biosynthesis of bioactive compounds. Advantages include rapid processing times, low energy demand, and the absence of chemical residues. However, its industrial deployment still faces challenges such as shallow penetration, line-of-sight requirements, and potential photochemical degradation of sensitive biomolecules.
In summary, while each NTT has distinct operational principles and interaction modes with microbial systems, they share the potential to function as precision tools for modulating fermentation performance. Understanding the interplay between physical process parameters, microbial physiology, and target product characteristics is essential to unlock their full potential in industrial food fermentation.
4. Applications of NTTs in Food Fermentation
The integration of NTTs into food fermentation systems can be strategically implemented across three main stages of the bioprocess: upstream (pre-treatment of raw materials or microbial inocula), midstream (during active fermentation), and downstream (post-fermentation stabilization or refinement) (
Figure 3). Each stage offers specific intervention points where physical stimuli can be harnessed to optimize microbial function, guide metabolic fluxes, and modulate the biochemical profile of the final product. However, the benefits of NTTs must be carefully weighed against their operational demands, treatment-specific limitations, and biological variability across substrates and microbial strains.
4.1. Upstream Applications of NTTs
In the upstream phase, NTTs are strategically employed prior to fermentation with the dual objective of modifying substrate structure or inducing physiological priming in inoculated microorganisms. Mechanical disruption of plant tissues by US or HPP, for example, facilitates the release of bound phenolics, oligosaccharides, and other bioactive compounds that enrich the fermentation matrix [
1,
5]. These compounds not only act as carbon and energy sources but also serve as modulators of microbial gene expression, influencing metabolic fluxes and fermentation dynamics. Similarly, PEF enhances substrate permeability and solute diffusion, increasing nutrient accessibility and enzymatic digestibility, an effect particularly beneficial for fibrous or lignocellulosic matrices. However, excessive intensity or uncontrolled application can introduce drawbacks such as off-flavor generation from free radical formation (US), electrode fouling and uneven field distribution (PEF), or excessive energy demand (HPP).
When applied to starter cultures, non-lethal NTTs function as hormetic stimuli, activating stress-response pathways that enhance cell robustness, membrane integrity, and metabolic efficiency during subsequent fermentation [
2]. These effects, however, are strain-dependent and may vary according to treatment intensity, duration, and waveform. Overstimulation can impair viability, destabilize microbial balance in mixed cultures, or induce undesired metabolic pathways. The interplay between operational parameters and intrinsic microbial characteristics, such as cell wall architecture and membrane fluidity further complicates the optimization of such treatments. In addition, the formation of reactive oxygen (ROS) and nitrogen (RNS) species during CP or the photodegradation risk posed by PL raises concerns about potential toxicity and nutrient losses, requiring careful calibration. Consequently, predictive modelling and omics-based profiling of microbial responses are essential for refining upstream NTT protocols.
4.1.1. Ultrasound in Upstream
Recent advances in non-thermal processing have highlighted ultrasound as a promising tool to improve both the efficiency and quality of fermented products. In dairy applications, ultrasound-assisted fermentation of buffalo yogurt at 25 kHz (nominal power 450 W; volumetric power 38 W/L) using five transducers achieved a reduction in fermentation time of up to 2 h (25%). It accelerated the fermentation rate by up to 41%. These moderate sonication treatments enhanced rheological properties by disrupting fat globules and disaggregating proteins, thereby optimizing gel network development. When combined with a 5% (
w/
v) sucrose addition, ultrasound produced a synergistic effect, leading to higher sucrose retention and further improvement in texture and structure. However, extended sonication (3 h or continuous application) adversely affected yogurt microstructure, likely due to excessive disruption of the gel network during acidification [
50].
Ultrasound pre-treatment enhances fermentation efficiency in plant-based substrates by disrupting cell walls and increasing the release of soluble sugars and bioactive compounds [
51]. In Cantaloupe melon juice fermented with
Lacticaseibacillus casei B-442, sonication eliminated the lag phase observed in untreated juice and reduced fermentation time by ~30% [
52]. This effect was accompanied by viable cell counts exceeding 8 log CFU/mL within eight hours, indicating improved substrate accessibility and growth conditions. Similar responses have been reported in other fruit-based fermentations, although the magnitude of improvement depends on the matrix, microbial strain, and processing parameters. Taken together, these dairy and fruit-based systems demonstrate that the mechanism of ultrasound—cell disruption and enhanced mass transfer—is consistent across matrices, but the balance between stimulation and overprocessing depends strongly on intensity and exposure time.
Comparable outcomes have been observed in plant-based systems, reinforcing the cross-sector potential of ultrasound in fermentation. In probiotic beverages from white finger millet (
Eleusine coracana L. var. KMR 340) fermented with
Lacticaseibacillus rhamnosus, ultrasound treatment increased antioxidant activity, viable cell counts, total phenolic and flavonoid concentrations, and reduced particle size [
53]. These findings suggest that ultrasound not only accelerates fermentation but also enhances functional and nutritional attributes, supporting its application as a versatile non-thermal technology capable of improving product quality, preserving bioactive compounds, and meeting consumer demand for minimally processed functional foods.
4.1.2. Pulsed Eletric Fields in Upstream
The rationale for PEF application follows a similar logic, aiming to prepare the raw material and the microbial consortium for optimal fermentation performance. In liquid substrates such as fruit juices, plant extracts, and whey-based formulations, PEF selectively inactivates spoilage microorganisms while preserving native enzymes and sensory characteristics [
6,
25]. This targeted microbial reduction lowers the risk of contamination in downstream stages and, when applied at non-lethal intensities, can precondition starter cultures for improved stress tolerance [
27]. Beyond its sanitizing effect, mild PEF exposure enhances membrane permeability, facilitating nutrient uptake and the release of intracellular metabolites, which translates into accelerated fermentation kinetics and increased yields of polyols and phenolics [
23,
25]. These benefits parallel those observed for ultrasound, indicating that different NTTs can converge on improved mass transfer and metabolic activation.
The treatment with low-intensity PEF (1 kV/cm, 800–1600 µs) was applied to modulate yogurt fermentation using milk with different fat contents. The results demonstrated a significant reduction in fermentation time compared to the control sample (untreated with PEF), particularly for the treatment at 1 kV/cm for 1600 µs with milk containing 2.8% fat. The treated samples also exhibited higher lactose consumption (ranging from 1.6% to 3.1%) and greater lactic acid production (7.2%) [
54]. The application of low-intensity PEF to the initial yogurt culture was explored regarding its acidification capacity in reconstituted skim milk medium, resulting in an average acceleration of milk acidification in approximately 12 min, along with a significantly faster decrease in oxidation/reduction potential, inducing oxidative stress [
55].
When viewed together, these studies demonstrate that non-lethal PEF not only accelerates acidification in dairy matrices but also stimulates oxidative stress responses, which could explain improvements in fermentation kinetics and metabolite yields.
4.1.3. High Pressure Processing in Upstream
In the pre-fermentation stage, non-lethal high-pressure treatments (5–100 MPa) can be applied to raw materials or inoculated media to modulate microbial physiology before active fermentation begins. Such treatments have been shown to upregulate stress-response genes, enhance nutrient accessibility through pressure-induced membrane permeabilization, and selectively inactivate competing microflora without damaging the target starter cultures [
56]. In dairy matrices, pre-treatment of milk by moderate HPP has improved yogurt texture and reduced syneresis due to whey protein denaturation and casein micelle disruption [
57]. Similarly, Lopes et al. [
36] reported that pressures of 10–30 MPa, combined with temperature variations, increased lactic acid yield to 70–75% compared with about 40% under atmospheric pressure.
Plant-based applications exhibit similar advantages: the impact on rheological parameters and the effects on soy proteins were investigated by Chen et al. [
58] by subjecting soy milk to HPP prior to fermentation. HPP processing reduced protein particle size, protein dispersibility index, and zeta potential. In addition, it induced modifications in the three-dimensional structure of the proteins, increasing hydrophobic interactions and disulfide bonds. These changes resulted in improved rheological properties and gel texture, as well as an increase in water-holding capacity from 45.4% to 61.3% [
58]. HPP-treated apple juice (200 MPa for 10 min) also retained superior color, phenolic content, and antioxidant activity compared to pasteurized controls (85 °C for 15 min), while achieving a
Lactiplantibacillus plantarum survival rate of 97.37% under simulated gastric conditions [
59].
Using HPP as a biotechnological tool, the study by Bravim et al. [
60] demonstrated that a mild, non-lethal pressure treatment on a wild
Saccharomyces cerevisiae strain significantly enhances ethanol production and stress tolerance during fermentation. The research identified that HHP over-expresses specific genes, particularly SYM1, which directly contributes to this enhanced fermentative capacity and improved stress tolerance. This finding validates HHP as an effective method for optimizing fermentation processes in industries like cachaça and biofuel production.
Such results illustrate the broader principle that physical structuring of substrates and microbial conditioning can act synergistically to improve fermentation outcomes.
4.1.4. Cold Plasma in Upstream
CP integrates into this continuum of upstream interventions for raw material pretreatment and inoculum conditioning. By inactivating spoilage and pathogenic microorganisms on cereal grains, fruit pulp, and other substrates without significant thermal degradation of sensitive nutrients, CP creates a more selective environment for desired fermentation strains [
4,
41]. Additionally, mild oxidative stress generated during CP exposure can prime starter cultures for enhanced metabolic activity. In cereal- and pulse-based matrices, CP treatments have been shown to reduce anti-nutritional factors while improving fermentation performance [
2]. In cereal- and pulse-based substrates, CP can reduce microbial contaminants and degrade anti-nutritional factors, improving fermentation performance [
61].
CP treatments have been reported to improve the functional quality of fermentation substrates by altering surface chemistry and structural properties. For instance, dietary fibers obtained from rice and corn bran exhibited up to ~22% higher glucose diffusion after plasma exposure, which in turn led to 1.5–2.0× increases in SCFA release during in vitro fermentation [
9]. Moreover, probiotic growth was stimulated, with prebiotic activity scores increasing up to 1.8-fold compared to untreated fibers, highlighting CP’s potential as a pre-fermentation enhancer.
Similarly, the impact of CP treatment on the prebiotic potential of Spirulina (
Arthrospira platensis powder) was investigated by Pina-Pérez et al. [
62]. Spirulina samples were exposed to an atmospheric pressure cold plasma device with surface microdischarge, using air as the working gas, for 5 min at effective discharge powers of 1.1, 1.7, 2.2, and 3.3 W. The probiotics
Limosilactobacillus reuteri and
L. rhamnosus GG were employed in the fermentation process. Spirulina treated at 3.3 W for 5 min and fermented with
L. rhamnosus exhibited the highest prebiotic activity, which can be attributed to the ability of cold plasma to degrade macromolecules, such as the complex polysaccharides present in Spirulina.
Cold plasma also exhibited a selective inactivation of Gram-negative bacteria responsible for nitrite production in pickles, as reported by Wei et al. [
63] using DBD plasma (40 kV, 60 s). This selectivity is associated with structural differences between Gram-positive and Gram-negative bacteria, including the thicker cell wall of Gram-positives and the presence of an outer membrane in Gram-negatives.
Plasma-activated water (PAW) extends these benefits to sanitation of equipment and raw material surfaces, representing a sustainable alternative to chlorine-based disinfectants [
64,
65].
4.1.5. Pulsed Light in Upstream
In upstream processing, PL is mainly applied for surface decontamination of raw materials such as fruits, vegetables, and cereal grains. High-intensity pulses in the UV-C range effectively inactivate surface-resident spoilage organisms and pathogens without significant heat buildup, extending raw material shelf life and reducing microbial load prior to fermentation [
48,
49]. For liquid substrates with low turbidity, PL can also reduce initial microbial counts while preserving native pigments, enzymes, and flavor compounds.
Kwaw et al. [
49] demonstrated that applying PL to lactic-acid-fermented mulberry juice significantly increased total phenolics, flavonoids, anthocyanins, and antioxidant capacity compared to untreated controls. Such effects suggest the potential for PL to enhance secondary metabolite production, adjust flavor profiles, and improve functional properties of fermented beverages.
PL (1 pulse/s, 0.52 J/cm
2 for 200 s) was applied as a pre-treatment to Ginkgo biloba leaves intended for fermentation into dark ginkgo tea. The application of PL, combined with fermentation, resulted in a reduction of ginkgolic acids, toxic compounds, and an increase in flavonoid content and antioxidant capacity of the tea. These effects were attributed to the decarboxylation of ginkgolic acids and the denaturation of endogenous enzymes [
66]. Similarly, PL has also been employed as a pre-treatment for grapes, aiming to reduce the population of yeasts and bacteria on the fruit surface, thereby favoring fermentation by selected starter cultures [
67].
PL combined with UV light was employed to induce mutagenesis in a lactic acid bacterium of interest for its ability to convert inorganic selenium (potentially toxic) into organic forms and selenium nanoparticles. This approach led to the development of the
Limosilactobacillus fermentum Ln-9 strain, which exhibited a 3-fold increase in selenium accumulation compared to the original strain. Of the accumulated selenium, 96.34% was present as selenium nanoparticles (SeNPs), which are highly bioavailable and predominantly localized outside the cell. The strain maintained stable morphology, good gastrointestinal tolerance, and demonstrated enhanced antioxidant capacity, features that underscore its potential for applications in the food industry [
68].
When compared across these examples, PL’s applicability lies less in accelerating fermentation itself and more in shaping the microbial ecology and enhancing functional compound production. Yet, its shallow penetration, dependency on line-of-sight exposure, and risks of photodegradation of sensitive compounds remain major barriers for broader adoption in dense fermentations.
Overall, while US and PEF converge on mechanisms of transient membrane permeabilization that accelerate fermentation and enhance metabolite yields, HPP demonstrates unique strengths in textural refinement and microbial modulation under moderate pressure, albeit with scalability and cost trade-offs. CP distinguishes itself by simultaneously enhancing fermentability through substrate modification and exerting selective antimicrobial activity, though concerns remain about process standardization and potential byproduct toxicity. PL, in turn, shows promise as a downstream or adjunct tool, primarily shaping microbial ecology and functional attributes rather than directly accelerating fermentation. Taken together, these findings emphasize that although each NTT offers distinctive benefits, their adoption must be guided by a critical balance between efficacy, scalability, cost, and potential limitations, positioning them as complementary rather than competing tools in the design of sustainable fermentation systems.
4.2. Fermentation Applications
The midstream stage, corresponding to active microbial metabolism, is arguably the most delicate and least explored phase for NTTs integration. Here, real-time modulation of microbial behavior is possible but poses considerable technical and biological challenges. US, when applied intermittently, can improve homogeneity in viscous broths, disrupt metabolite concentration gradients, and stimulate enzymatic activity, accelerating biochemical conversions and improving product titers [
7]. However, indiscriminate application may compromise the viability of beneficial microbes, such as
Lactobacillus spp., especially when applied without tailored pressure profiles or protective matrices. This trade-off highlights the central challenge of downstream NTT integration: balancing microbial safety with the preservation of functional and sensory quality.
The primary hurdle in midstream NTTs application remains technological: continuous or pulsed treatments demand bioreactor modifications, energy delivery systems, and feedback-controlled operation. Few industrial systems currently support such integration, and the lack of real-time biomarkers for cellular stress or metabolic state limits process responsiveness. Therefore, advances in bioprocess monitoring and modular reactor design are essential to unlock midstream NTT deployment at scale.
4.2.1. Ultrasound in Fermentation
US has been widely investigated as a midstream intervention capable of modulating microbial metabolism and metabolite production. In kombucha, high-intensity US (113 W/cm
2 for 7–15 min) increased sucrose utilization by 19% and enhanced gluconic acid yield (6.74 g/L vs. 4.17 g/L in controls), while boosting antioxidant capacity. These effects are attributed to US-induced cell membrane permeabilization, selective stimulation or attenuation of microorganisms, and microstructural changes in the SCOBY, which together promoted a fermentation profile with improved bioactive potential [
8]. These results parallel the work of Coloma et al. [
69], who showed that moderate ultrasonic intensities (100 W) were more effective in stimulating yeast growth than higher levels, indicating the presence of an optimal “energy window.”
Similarly, Hashemi et al. [
70] reported that short sonication (≤5 min) in Bakraei juice enhanced lactic acid and antioxidant activity, but longer treatments reversed the benefits, reducing microbial viability and functionality.
Application of low-intensity ultrasound during the initial 12 h of
Hanseniaspora sp. cider fermentation has been shown to accelerate biomass accumulation while reducing both the final ethanol concentration (≈0.55%
v/
v decrease) and ethanol production rate, in parallel with increased glucose consumption. Such a metabolic response suggests a preferential redirection of carbon flux toward cell growth and CO
2 release rather than ethanol synthesis. These effects are plausibly linked to improved substrate uptake and oxygen transfer, coupled with potential enhancement of enzymatic activity via micro-convective effects that promote mass transfer and induce conformational changes in enzymes, thereby influencing substrate affinity. By increasing the effective availability of substrates, US may stimulate metabolic efficiency and biomass formation; however, detailed enzyme kinetics analyses remain necessary to elucidate the mechanisms involved and optimize the industrial application of this technology [
12].
Despite these positive reports, microbial responses to US are highly variable, depending on both the microorganism and the sonication parameters. Intermittent US at 25 kHz and 300 W/m
3 throughout
S. cerevisiae fermentation more than doubled ethanol yield, whereas continuous sonication under similar conditions had no such effect [
24]. Likewise, a 10% duty cycle at 35 kHz and 1.48 W/cm
2 increased ethanol productivity fourfold in
S. cerevisiae MTCC 170 [
25]. In contrast, direct US (23–32 W/L) and indirect US (1.4 W/L) treatments have been shown to reduce yeast performance, slowing glucose consumption and ethanol production [
26]. Such variability underscores the multifactorial nature of US effects, which are shaped by microbial physiology, treatment conditions, and the growth phase targeted, complicating mechanistic understanding and calling for further research.
In lactic-acid-fermented mulberry juice (LFMJ), the application of ultrasonication (28 kHz, 60 W, 15 min), pulsed light (1.213 J/cm
2/pulse, 360 μs, 3 Hz, 4 s), and their sequential combination was found to enhance bioactive compound levels and antioxidant potential markedly. All non-thermal treatments significantly increased total phenolics, flavonoids, anthocyanins, DPPH• scavenging activity, ABTS•
+ scavenging activity, and reducing power compared with untreated controls. Among the individual techniques, ultrasonication produced a greater (
p < 0.05) improvement in phenolic content and antioxidant capacity than pulsed light alone. When applied sequentially, pulsed light followed by ultrasonication (PUT) yielded the most pronounced enhancements, indicating that this combined approach holds strong potential for industrial-scale processing of LFMJ [
49].
The production of alcoholic beverages has emerged as one of the most promising areas for the application of US-assisted fermentation. In the case of ethanol synthesis by
S. cerevisiae, the use of intermittent sonication at a specific energy input of 300 W/m
3 and a frequency of 25 kHz resulted in a twofold increase in ethanol yield [
71]. Comparable results were obtained by Lanchun et al. [
72], who applied US at 24 kHz and 2 W for 1 s pulses, interspersed with 15 s intervals, over a 30 min period during the exponential phase of
S. cerevisiae growth, achieving a 33% increase in cell proliferation. The magnitude and direction of US effects appear to depend strongly on the growth phase during which the energy is applied. For instance, exposure of
S. cerevisiae cultures to US energy levels of 330–360 W·s/m
3 during the lag phase reduced its duration by approximately 1 h relative to untreated controls, whereas higher inputs (>850 W·s/m
3) extended the lag phase and inhibited growth. In
S. cerevisiae K-7 (Sake yeast), low-intensity US not only promoted biomass accumulation but also enhanced glucose consumption and ethanol production, with the strongest effects observed when irradiation was applied during the exponential phase [
73]. These effects were associated with increased intracellular uptake, likely due to reversible membrane permeabilization, which may facilitate nutrient transport and metabolic activity. The growth-promoting effect was frequency dependent, and no significant benefits were observed when treatment was applied during the stationary phase.
Together, these studies reinforce that ultrasound operates within a narrow “stimulation window” in which physical and biochemical enhancement is maximized while avoiding detrimental stress responses. Excessive power or exposure time can shift the effect from stimulatory to inhibitory, underscoring the need for precise optimization. Moreover, industrial adoption may face engineering challenges, such as retrofitting ultrasonic transducers directly into fermenters which requires mechanical adaptation potentially increasing capital costs and complicating process scalability [
74].
4.2.2. Pulsed Electric Field in Fermentation
PEF has been explored as a dynamic tool to transiently increase membrane permeability and metabolite secretion. Nonetheless, microbial susceptibility varies with growth phase: cells in exponential growth may be more vulnerable than those in stationary phase, complicating uniform process control [
75].
During active fermentation, non-lethal PEF intensities are exploited for metabolic stimulation and mass transfer enhancement. Reversible electroporation increases membrane permeability, enabling faster nutrient uptake and metabolite excretion. This can accelerate fermentation rates and boost yields of valuable products [
23,
27]. PEF has also been applied intermittently to modulate stress-response pathways, promoting the synthesis of bioactive secondary metabolites, pigments, and flavor compounds without significant cell mortality.
The application of PEF during the logarithmic growth phase of
Lactiplantibacillus plantarum growth enhanced metabolite production during watermelon juice fermentation. Increases of 19% in L-lactic acid, 6.8% in D-lactic acid, and 15% in acetic acid were observed compared to the control, with these effects being dependent on the applied voltages (L-lactic acid: 5.0 kV, 700 pulses; D-lactic acid: 4.5 kV, 700 pulses; acetic acid: 4.5 kV, 1000 pulses). Notably, cell viability was not affected by the treatment [
30].
PEF treatment of
L. rhamnosus and
Lacticaseibacillus paracasei (1 ms pulses at 5 kV/cm) was shown to increase lactic acid production by 10% after 24 h of fermentation. The process also led to the release of peptides/proteins from the bacteria, a crucial step for obtaining postbiotics. While PEF did not alter the susceptibility of
L. rhamnosus to antibiotics, a decrease was observed for
L. paracasei, highlighting that the effects are strain-dependent and linked to PEF-induced stress and recovery mechanisms. The study concludes that PEF has a promising role in producing postbiotics by carefully tuning treatment parameters to enhance the functionality of lactic acid bacteria [
76].
In contrast to US, PEF’s primary role midstream lies in reversible electroporation, which allows faster nutrient flux and targeted metabolite secretion. The trade-off lies in its strong dependency on growth phase, with exponential cells more vulnerable to over-stress. Thus, while both US and PEF act through membrane modulation, PEF emphasizes electroporation-driven metabolite excretion, whereas US relies more on micro-convective and mechanical effects.
4.2.3. High-Pressure Processing in Fermentation
In lactic acid bacteria fermentations, HPP has been shown to influence organic acid production profiles, exopolysaccharide synthesis, and flavor compound generation, enabling targeted modulation of sensory and functional attributes. These effects arise from pressure-driven changes in enzyme conformation, membrane transport, and intracellular signaling pathways.
The effects of HPP (100 MPa) were observed in the inhibition of lactic acid bacteria growth when applied during the extended fermentation phase (180 min) of yogurt. However, upon returning to atmospheric pressure, the fermentative microorganisms resumed their normal metabolic activity, highlighting the potential of HPP as a fermentation control tool, functioning analogously to an “on/off” switch [
77]. Complementarily, the combined application of HPP and moderate temperature (15 MPa/32 °C) during kefir fermentation resulted in a faster fermentation process, with higher titratable acidity and greater lactic acid concentration, indicating the potential of HPP to stimulate microbial metabolism [
78].
Compared to US and PEF, which stimulate mass transfer continuously, HPP offers intermittent control over fermentation dynamics. Its ability to pause microbial activity and then resume normal metabolism makes it distinct as a bioprocess regulator rather than a stimulator. When combined with mild temperature, HPP synergistically accelerates fermentation, suggesting that pressure-driven enzymatic modulation can complement the electroporation or shear forces seen in other NTTs.
4.2.4. Cold Plasma in Fermentation
Controlled non-lethal CP exposure can act as a metabolic modulator, functioning as an abiotic stressor that stimulates the biosynthesis of secondary metabolites such as pigments, exopolysaccharides, or aroma compounds. Studies have shown that ROS and RNS generated by CP can transiently permeabilize cell membranes and alter redox homeostasis, leading to upregulation of stress-response pathways [
79] When precisely dosed, these effects can accelerate fermentation kinetics, enhance yields of target metabolites, and even steer microbial consortia toward favorable compositional shifts. However, maintaining uniform plasma exposure in liquid fermentations poses challenges, particularly in high-viscosity matrices, making reactor design a critical factor for scalability [
43].
During active fermentation, carefully controlled CP exposure can shift metabolic pathways and enhance the biosynthesis of high-value metabolites. For example, non-lethal dielectric barrier discharge (DBD) treatment of
S. cerevisiae cultures altered membrane permeability and enzyme activity, leading to increased ethanol productivity and changes in volatile compound profiles [
80]. Similarly, CP stimulation of lactic acid bacteria has been linked to higher exopolysaccharide (EPS) production and modified organic acid ratios, effects attributed to oxidative stress-induced metabolic reprogramming [
2].
Unlike HPP, which temporarily halts microbial activity, CP imposes oxidative stress that reprograms metabolism, stimulating the synthesis of secondary metabolites such as pigments, aroma compounds, and exopolysaccharides. In this respect, CP aligns more closely with PEF, in that both induce stress-response pathways, but CP uniquely leverages ROS/RNS chemistry to alter microbial physiology.
4.2.5. Pulsed Light in Fermentation
No studies have reported the application of pulsed light during fermentation. This can be attributed to the primary mode of action of pulsed light, which relies on high-intensity, short-duration pulses capable of inducing photobiological effects and microbial inactivation. Due to its strong antimicrobial and surface-sterilizing properties, pulsed light is predominantly applied in post-fermentation stages, aiming to ensure microbial safety and product stability, rather than during active microbial growth, when it could compromise the viability and metabolic activity of fermenting microorganisms.
These studies show that fermentation NTT applications share unifying mechanisms of reversible stress induction and enhanced mass transfer. US and PEF primarily act by transient membrane permeabilization, while HPP and CP function through pressure or oxidative stress-induced reprogramming of metabolic pathways. PL, by contrast, lacks practical midstream applications due to its antimicrobial intensity.
The challenge for industrial adoption lies in identifying intensity optimal processing parameters that maximize metabolic stimulation without crossing lethal thresholds. Synergistic combinations (e.g., US+PL) and modular reactor designs represent promising avenues, but variability across strains, substrates, and operational scales underscores the need for standardized protocols and deeper mechanistic insights.
4.3. Downstream Applications
In downstream processing, NTTs serve to stabilize, refine, or valorize fermentation products. HPP is particularly valued for its ability to reduce microbial loads and inactivate spoilage enzymes while preserving heat-sensitive flavor compounds and nutrients, attributes critical in clean-label formulations and probiotic products [
1]. However, indiscriminate application may compromise the viability of beneficial microbes, such as
Lactobacillus spp., especially when applied without tailored pressure profiles or protective matrices.
4.3.1. Ultrasound in Downstream
Ultrasound has demonstrated utility in extracting intracellular bioactives such as peptides, polysaccharides, and pigments from fermented biomass. Additionally, it can improve physical properties like texture and emulsion stability in liquid fermented products [
5]. Nevertheless, its impact on volatile compound retention and possible oxidation during cavitation must be considered, particularly in aroma-sensitive formulations. One of the challenges in the development of probiotic-containing foods is the occurrence of over-acidification or post-acidification, characterized by an uncontrolled drop in pH during storage. This phenomenon can compromise sensory quality and consumer acceptance, as well as limit product shelf life. Compared to traditional heat or enzymatic attenuation, US offers a more targeted and tunable approach, as shown in rice beverages and model systems with
L. reuteri [
81,
82,
83]. These studies converge in demonstrating US as a dual-function tool: a microbial activity modulating tool while simultaneously influencing texture and metabolite release.
A promising strategy to address this issue is the attenuation of probiotic metabolism, a process designed to slow acid production without compromising cell viability [
82,
84]. Attenuation can be achieved through several approaches, including spray- or freeze-drying, cell fragilization using lysozyme or solvents, the selection of lactose-negative mutants, and physical methods such as high-pressure homogenization (HPH) [
83].
The concept of attenuated starter cultures was first applied in cheesemaking by Petterson and Sjöström [
85], who used a mild heat treatment (69 °C for 15 s) to accelerate the ripening of semi-hard cheese. This approach promoted a faster release of microbial enzymes, enhancing proteolysis and reducing maturation time. More recently, US has emerged as an alternative attenuation method. US treatments have been applied to counteract excessive acidification by
Lactobacilli in organic rice beverages [
83] and in model systems using
L.reuteri [
82].
The dual role of US in microbial modulation has been well-documented. Depending on the applied frequency and energy, US can either inhibit microbial growth or stimulate it [
86]. These effects arise from two main cavitation phenomena: stable cavitation, occurring under low-intensity conditions where bubbles oscillate without collapsing, and transient (collapse) cavitation, produced at higher intensities when bubbles implode violently, generating extreme localized temperatures and pressures [
87]. At the cellular level, US can induce surface resonance, intracellular shear forces, free radical formation in aqueous environments, and peroxide generation [
88]. Depending on treatment conditions, these mechanisms can lead to microbial inactivation or stimulation, increased growth rates, declumping of cell aggregates, and enhanced membrane permeability [
84].
Postbiotics, including non-viable microbial cells, cell fragments, and their bioactive metabolites, have gained increasing attention due to their health-promoting properties. However, conventional thermal inactivation can lead to the degradation of heat-sensitive compounds, thereby reducing their functional potential. NTTs represent viable alternatives, enabling effective microbial inactivation while preserving, or in some cases enhancing, the integrity of bioactive components [
89]. When applied under controlled conditions, ultrasound can act as a targeted physical stressor, inducing cavitational effects that facilitate cell disruption, promote the release of intracellular metabolites, and modulate postbiotic profiles. These effects contribute to improved functionality and stability in food matrices, thereby expanding the scope of postbiotic applications in the functional foods sector [
90]. The study of Nascimento et al. [
91] further illustrates how US can act as a selective inactivation tool for producing postbiotics, preserving ascorbic acid and phenolics while eliminating live cells. Together with animal model evidence, these findings indicate that US-based postbiotic production can preserve nutritional integrity but delivers physiological outcomes distinct from live probiotics, an insight with direct implications for regulatory frameworks and product positioning.
Similarly, studies in animal models have shown that ultrasound-inactivated L. casei postbiotics can efficiently inactivate cells without negatively affecting body weight, food intake, or biochemical parameters. However, unlike live probiotics, these postbiotics did not confer certain typical health benefits: cholesterol levels remained unchanged, and antioxidant enzymes (SOD, CAT) and heat shock proteins were not induced. The main physiological effect was the modulation of intestinal microbiota, particularly an increase in Burkholderiales. Together, these findings highlight that the functional outcomes of postbiotics depend critically on both the inactivation method and the bacterial strain, offering important insights for designing future postbiotic applications.
4.3.2. Pulsed Electric Field in Downstream
High-intensity PEF serves as a non-thermal pasteurization step for beverages such as wine, beer, cider, and functional drinks, achieving >5 log CFU/mL reductions in vegetative pathogens and spoilage yeasts while retaining sensory and nutritional attributes [
25] It can also be integrated with clarification or filtration steps, where electroporation assists in breaking down residual cell aggregates, improving filtration efficiency and product clarity. In some cases, downstream PEF treatments are used to selectively inactivate specific microbial populations while preserving probiotics or other beneficial cultures in functional beverages [
92,
93].
What distinguishes PEF from US in this stage is its precision in selectively inactivating spoilage populations while retaining probiotics, an advantage particularly relevant for functional beverages. This duality—microbial inactivation vs. selective preservation—parallels the strain-dependent responses reported in upstream fermentation and reinforces the importance of fine-tuning intensity thresholds.
4.3.3. High Pressure Processing in Downstream
High-intensity HPP (>300 MPa) is used for “cold pasteurization” of fermented products, extending shelf life while preserving fresh-like sensory and nutritional qualities [
34]. This is particularly relevant for minimally processed, live-culture products where microbial stability must be balanced with probiotic viability. HPP can also stabilize value-added fermented beverages, dairy products, and plant-based alternatives without heat damage, offering clean-label preservation that aligns with consumer demand for natural, additive-free foods.
Fermented milk treated with HPP at 400 MPa for 10 min and subsequently fermented for 48 h demonstrated significant improvements in functional and sensory attributes. The treatment increased angiotensin-converting enzyme inhibitory activity, apparent viscosity, polypeptide concentrations, and aromatic volatile compounds, while enhancing umami and richness perceptions and simultaneously reducing bitterness and astringency. Antioxidant activity was also preserved. These outcomes were associated with pressure-induced protein denaturation and conformational rearrangements, which modulated enzymatic activity and promoted the development of a more stable gel network within the fermented matrix [
94]. Such evidence highlights the role of HPP not only in microbial stabilization but also as a modulator of biochemical and structural transformations that determine product quality.
Unlike US or PEF, which often act by permeabilization, HPP exerts volumetric and isotropic pressure that modifies proteins and enzymes. Studies have consistently shown improvements in texture, viscosity, and sensory complexity, but also reveal trade-offs: excessive pressures (>600 MPa) can severely compromise probiotic viability [
32,
95]. Thus, HPP stands out in downstream applications as a tool that delivers superior product uniformity and sensory attributes, though at the cost of high capital investment and careful optimization to avoid damaging live cultures.
4.3.4. Cold Plasma in Downstream
Cold plasma offers a versatile downstream tool for product stabilization and quality preservation. Post-fermentation treatments, often referred to as cold pasteurization, can effectively inactivate spoilage organisms and pathogens in dairy, meat, plant-based, and functional beverages while minimizing thermal damage to volatile flavor compounds, thermolabile nutrients, and antioxidant capacity [
96,
97]. Distinct from HPP and PEF, CP can also modify surface chemistry, influencing solubility and particle interactions, thus enhancing the functional value of fermented products. Beyond microbial control, CP can modify surface properties of food particles and packaging materials, improving filtration efficiency, facilitating drying, and enhancing the solubility of bioactive compounds through alterations in particle surface chemistry.
In packaging applications, CP treatment of surfaces or headspace gases has been shown to extend shelf life, reduce post-process contamination risk, and improve barrier performance [
98]. This positions CP as a technology that bridges processing and packaging, offering unique sustainability advantages but also facing regulatory hurdles due to concerns over reactive species and byproducts.
Plasma-activated packaging (PAP) technologies are now emerging as complementary strategies, enabling the retention of sensory quality and bioactive stability throughout storage. These combined capabilities position CP as both a preservation technology and a functional quality-enhancement tool in fermented product supply chains.
4.3.5. Pulsed Light in Downstream
PL has established its main niche in downstream processing, particularly as a non-thermal finishing treatment for fermented products. By delivering high-intensity, short-duration pulses of broad-spectrum light (200–1100 nm), PL can rapidly inactivate spoilage and pathogenic microorganisms on product surfaces and packaging materials without inducing bulk heating or compromising sensory quality [
49]. This makes it especially suitable for packaged fermented foods, probiotic beverages, and clear liquid matrices, where microbial safety must be guaranteed without the use of thermal or chemical sanitizers [
47,
48,
99].
A critical consideration in downstream application is balancing microbial inactivation with the preservation of functional attributes. For probiotic and postbiotic formulations, excessive PL exposure could compromise cell viability or alter bioactive compounds, conflicting with regulatory requirements that mandate minimum viable counts for health claims. Therefore, dose optimization, matrix transparency, and treatment geometry are key to ensuring efficacy without impairing product quality. Predictive models integrating microbial inactivation kinetics and optical absorption profiles are emerging tools that can guide this optimization [
100,
101,
102].
From a sustainability perspective, PL offers advantages such as short treatment times, low overall energy demand, and elimination of chemical sanitizers, thereby reducing wastewater generation and effluent toxicity. Compared to resource-intensive methods like HPP, PL operates at lower electrical loads and can be integrated into continuous processing lines with compact equipment, making it more accessible to small and medium enterprises. Furthermore, synergistic combinations of PL with sustainable packaging innovations (e.g., biodegradable or active coatings) are being explored to extend shelf life while aligning with circular economy goals [
103].
However, unlike HPP or PEF, PL has inherent limitations that constrain its broader industrial adoption. These include its shallow penetration depth (<1 mm), dependence on direct line-of-sight exposure, and the risk of photodegradation of sensitive compounds such as vitamins and pigments. Such challenges restrict its application in turbid or dense fermentation matrices and highlight its role as a complementary downstream technology rather than a standalone preservation strategy. Future advances in lamp design, energy modulation, and reactor engineering will be necessary to expand its versatility and reproducibility in large-scale fermentation contexts.
In summary, while PL is less mature than HPP or PEF in fermentation applications, its unique advantages for surface and packaging decontamination, low resource demand, and compatibility with clean-label strategies position it as a promising downstream tool. Its industrial adoption will depend on resolving penetration challenges and integrating treatment protocols with product-specific quality and sustainability targets.
The downstream application of NTTs must strike a balance between microbial inactivation and the preservation of desired functionalities, particularly in the case of probiotic products, where viability is not only a quality attribute but also a regulatory requirement. A tailored, matrix-specific approach, supported by predictive models and empirical validation, is necessary to harness the full potential of NTTs in this final phase of the bioprocess.
Although US, PEF, HPP, CP, and PL differ in their physical principles, their application in food fermentation converges on a set of shared mechanisms. Ultrasound and PEF enhance mass transfer and accelerate fermentation through transient membrane permeabilization, while cold plasma and pulsed light act as abiotic stressors that modulate microbial metabolism and stimulate the release of bioactive compounds. HPP, together with CP and PL, ensures non-thermal microbial stabilization in downstream stages, preserving thermolabile compounds and supporting product safety. Collectively, these technologies reduce reliance on chemical preservatives, shorten processing times, and contribute to more resource-efficient bioprocesses. Framing NTTs under these unifying themes not only underscores their complementary roles across upstream, midstream, and downstream stages but also highlights their strategic relevance in advancing sustainable and innovation-driven food fermentation.
Building on this perspective, the integration of NTTs across upstream, midstream, and downstream operations not only demonstrates their versatility as tools for microbial control and metabolic engineering but also exposes the critical gaps that must be addressed for industrial deployment.
Table 1 summarizes the applications and critical parameters of NTTs at different stages of fermentation, highlighting their practical relevance and operational limitations. Successful adoption will require advances in reactor design for uniform energy delivery, standardized protocols tailored to specific food matrices, and comprehensive sustainability assessments supported by life cycle analysis.
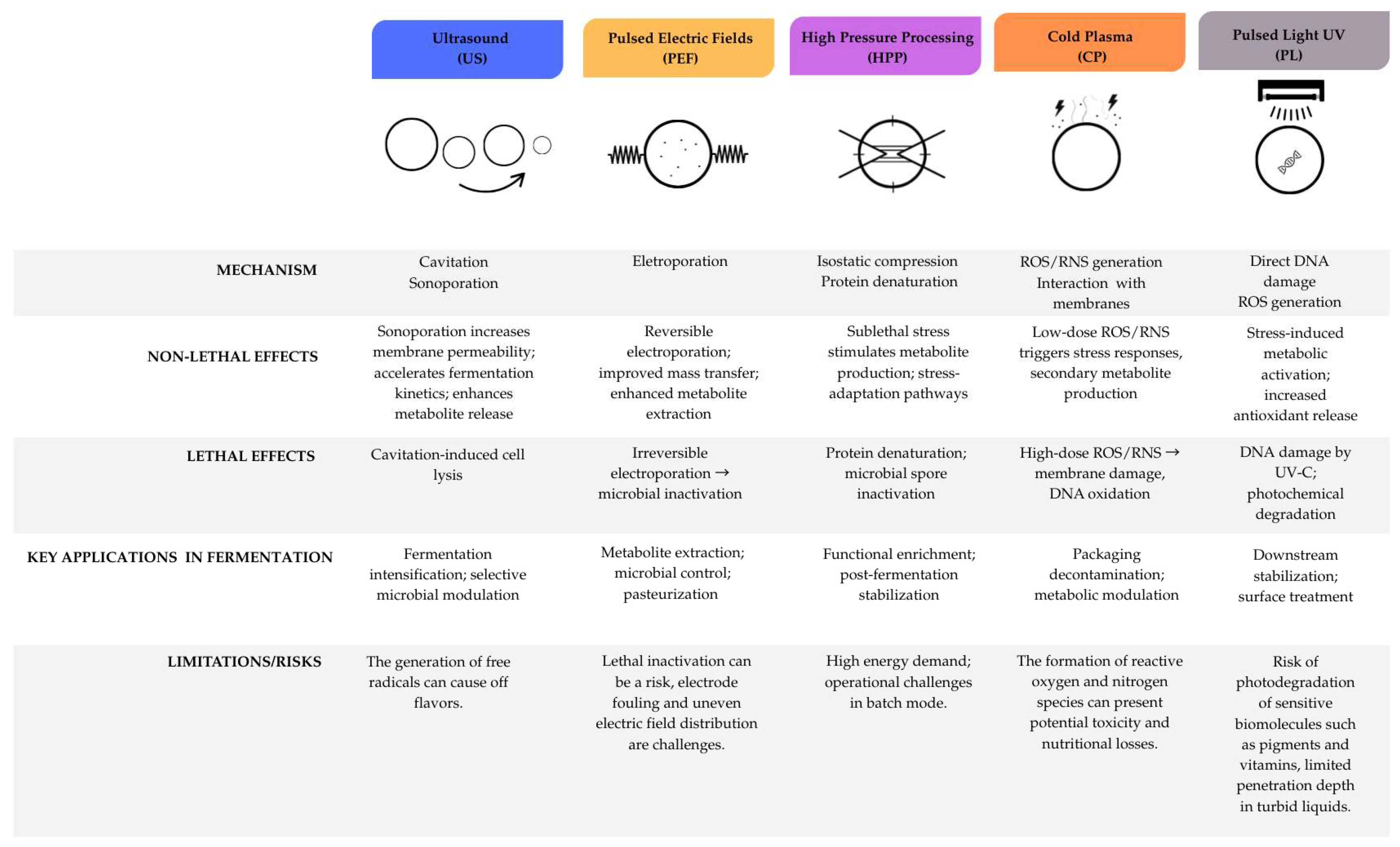
Figure 4 details the mechanism, non-lethal and lethal effects, key applications in fermentation, and limitations/risks associated with each NTT.
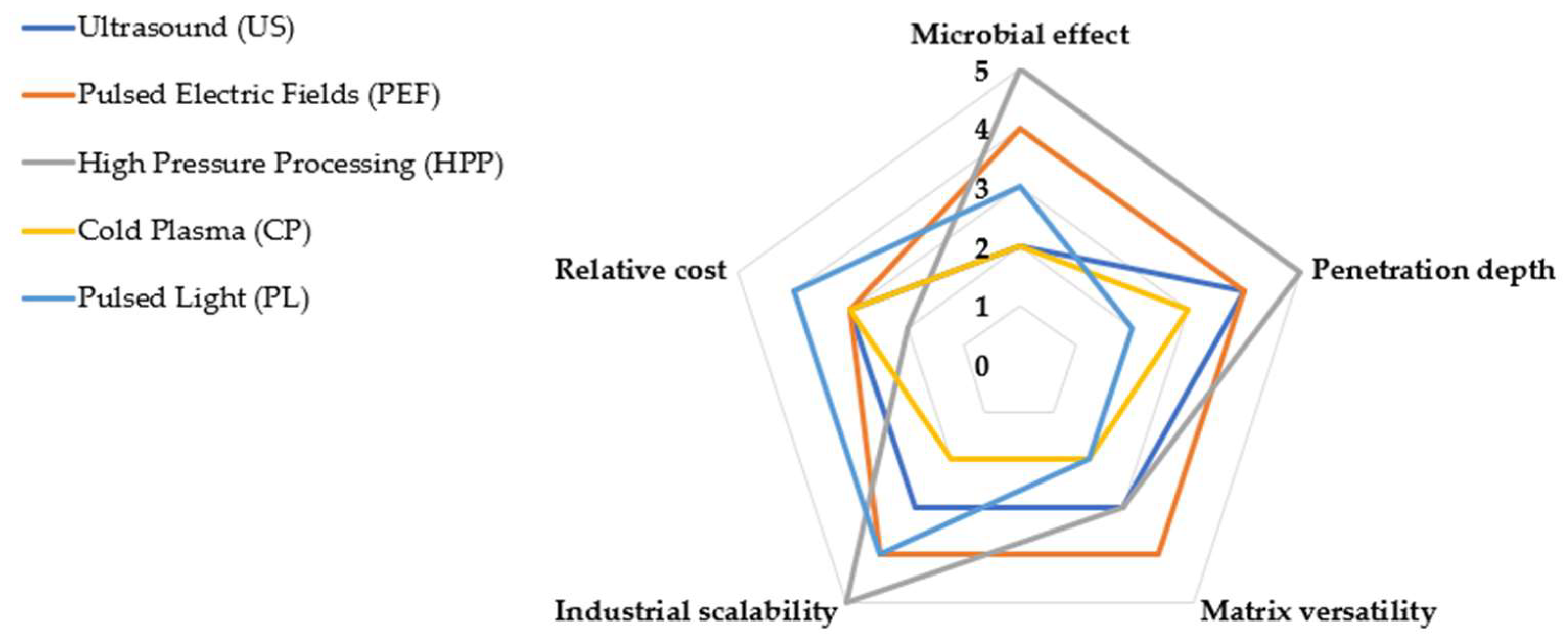
5. Comparative Analysis of NTTs in Food Fermentation
Although NTTs operate at non-lethal and ambient or low temperatures, their impact on microbial physiology, scalability, and cost-effectiveness varies considerably. Choosing the appropriate technology depends on whether the goal is microbial stimulation, selective inactivation, or targeted compound extraction. To provide a coherent comparison, this section discusses NTTs under unifying dimensions: mechanism of action, industrial scalability and maturity, economic considerations, sustainability trade-offs, and process integration.
5.1. Mechanism of Action
NTTs operate at non-lethal or ambient temperatures, their impact on microbial physiology, scalability, and cost-effectiveness varies considerably. Choosing the appropriate technology depends on whether the goal is microbial stimulation, selective inactivation, or targeted compound extraction.
From a microbial modulation standpoint, US and PEF are particularly promising for reversible metabolic enhancement. US induces sonoporation, and PEF induces electroporation, both of which can temporarily increase membrane permeability to accelerate fermentation kinetics and boost secondary metabolite yields, provided process parameters are tightly controlled to prevent irreversible cell damage [
2,
6]. While PEF enables precise control of microbial inactivation and can enhance ethanol productivity in yeast fermentations or facilitate the extraction of intracellular metabolites, its scalability faces obstacles such as electrode fouling, uneven electric field distribution, and high upfront costs. Nevertheless, its energy efficiency and ability to replace heat treatments highlight its potential as a disruptive tool for industrial fermentation processes.
PL, in turn, offers a distinct mode of action through intense, short-duration flashes of broad-spectrum light. While traditionally explored for surface decontamination, recent findings suggest that non-lethal PL exposures can induce stress responses in microorganisms, stimulating metabolic pathways and enhancing the release of phenolics, flavonoids, and antioxidants in fermented matrices. This makes PL a potential modulator of postbiotic and functional compound production. Its major limitations, however, include shallow penetration depth, dependence on direct line-of-sight exposure, and the risk of photodegradation of sensitive compounds, which restrict its broader application in dense fermentation broths. Consequently, PL is currently more relevant for downstream stages such as product stabilization and packaging sanitization rather than direct midstream fermentation control.
CP, by contrast, exerts potent oxidative effects that can stimulate stress-induced metabolite production but can also rapidly inactivate sensitive populations, requiring careful control of exposure time and intensity [
105]. Scalability challenges remain due to variability in generator designs and the need for process standardization.
HPP is valued for its volumetric uniformity and preservation of sensory and nutritional quality, but batch-mode operation and high capital costs limit its integration into continuous fermentation workflows [
10]. PL and UV light remain highly effective for microbial inactivation in transparent or thin-layer systems, yet penetration limitations in turbid fermentation matrices restrict their utility in dense microbial broths [
5].
5.2. Scalability and Industrial Maturity
In terms of industrial adoption, scalability depends on energy efficiency, equipment footprint, and compatibility with existing process infrastructure. HPP and PEF have reached higher commercial maturity in food preservation, offering a smoother transition into fermentation applications. US and CP, while showing great pilot-scale promise, still face engineering challenges in achieving uniform energy distribution at large volumes.
When assessed comparatively, HPP, US, PEF, CP, and PL demonstrate complementary strengths across the bioprocessing chain. HPP excels in delivering consistent, uniform treatment of packaged products regardless of geometry. US is highly effective for enhancing mass transfer and inducing targeted biochemical changes in liquid or semi-liquid matrices, making it well suited for in-line fermentation intensification. PEF provides controlled microbial modulation and extraction efficiency. CP provides a unique reactive chemistry through its mix of reactive oxygen and nitrogen species, enabling precise surface decontamination, enzyme modulation, and selective oxidative effects without significant heating. Industrial maturity remains highest for HPP and PEF, moderate for US, and more emergent for CP and PL. Together, these technologies represent a versatile toolbox whose adoption depends on balancing performance benefits with operational trade-offs such as cost, energy demand, and product-specific limitations.
From a quality standpoint, HPP consistently preserves fresh-like sensory and nutritional attributes; US offers process flexibility and enhanced yields of targeted metabolites; CP enables precise control over microbial and enzymatic activity while maintaining thermolabile compounds.
5.3. Sustainability Profiles
HPP reduces water and packaging waste compared to modified-atmosphere packaging but has high electrical loads if powered by non-renewables. PEF is considered energy efficient and enables solvent-free extraction, aligning with green chemistry principles. US reduces water and energy use in washing and solid-state fermentation but raises concerns over free radical formation and potential off flavors. CP minimizes chemical sanitizers and effluents but raises questions about ROS/RNS toxicity and standardization of safe exposure levels. PL is energy efficient and residue-free, but photodegradation of bioactives remains a risk.
Despite higher energy consumption during the processing step for HPP and PEF, NTTs generally have a carbon footprint comparable to or even lower than conventional thermal methods. This advantage is largely due to their indirect benefits, such as extending product shelf life and reducing food waste, which represents a significant impact on the entire supply chain’s carbon footprint. The strategic application of these technologies therefore balances initial investment challenges with long-term gains in resource efficiency and market value, directly aligning with sustainable development goals.
5.4. Economic Considerations
HPP requires high capital investment and is cost-intensive for SMEs, though it provides strong market acceptance for clean-label products. PEF systems demand lower energy per treatment but face significant upfront costs. US is relatively low-cost and modular, making it more attractive for incremental integration into existing lines. CP requires additional costs for gas handling and safety systems, while PL is among the most affordable but niche in its applications.
The economic viability of NTTs is multifaceted, varying significantly among processing. A techno-economic and life cycle analysis in a case study on orange juice illustrated that while raw material cost is the main factor, HPP and PEF result in higher market selling prices due to substantial capital and operational costs. In contrast, CP and PL demonstrate more competitive costs, approaching those of thermal methods.
5.5. Process Integration
Mapped to process stages, upstream applications include HPP for raw material sanitation, US for pre-treatment and dispersion, and CP for packaging or surface decontamination. Midstream, non-lethal HPP can modulate microbial metabolism, US can intensify fermentation kinetics, and CP can trigger stress responses to boost secondary metabolite synthesis. Downstream, HPP serves as a non-thermal pasteurization step, US assists in clarification or texture refinement, and CP hygienizes packaging and surfaces. Hybrid or sequential use can combine HPP’s uniformity, US’s intensification effects, and CP’s surface chemistry to achieve safety, quality, and sustainability goals that no single method can match.
Despite strong laboratory evidence, scaling NTTs, particularly US and CP, remains challenging. For US, acoustic attenuation and heterogeneous cavitation fields in large fermenters undermine reproducibility. Engineering solutions such as multi-sonotrode arrays, adaptive coupling, or recirculation-based reactors can improve energy distribution but increase operational complexity, maintenance, and costs. CP adoption is similarly hindered by variability in generator design, gas composition, and treatment configuration, compounded by the lack of standardized metrics for reporting key process parameters.
5.6. Critical Parameters
Without harmonized methodologies and deeper understanding, consistent and predictable industrial-scale performance will remain elusive. To address this, critical process parameters must be standardized across technologies and food matrices. These include treatment intensity (e.g., pressure levels in HPP, field strength in PEF, acoustic power in US, plasma energy dose in CP, light fluence in PL), exposure duration, and uniformity of energy distribution within the treated matrix. Additionally, matrix-dependent factors such as optical properties, electrical conductivity, viscosity, and water activity strongly influence treatment efficacy and should be explicitly reported and harmonized. Establishing such standards will improve reproducibility between laboratories, facilitate regulatory approval, and accelerate the industrial adoption of NTTs in food fermentation. To enable a concise comparison of non-thermal technologies, a qualitative radar chart (
Figure 5) was constructed. The five dimensions were selected based on their frequent citation in the literature as critical performance indicators: (i) microbial effect, referring to inactivation capacity or microbial modulation efficiency; (ii) penetration depth, the ability of treatment to act uniformly within the food matrix; (iii) matrix versatility, reflecting the applicability across liquid, semi-solid, and solid substrates; (iv) industrial scalability, considering current commercial adoption and potential for upscaling; and (v) relative cost, accounting for equipment investment and operational expenses. Each technology was scored on a scale of 1–5, with values informed by reported case studies and expert assessments. While these scores are not absolute, they provide a comparative overview of strengths and limitations of each technology in food fermentation contexts.
6. Integration of Non-Thermal Technologies in Food Fermentation into the United Nations Sustainable Development Goals
Acting at strategic points of the bioprocess, ranging from substrate preparation and fermentation control to product stabilization and packaging, these technologies enhance resource efficiency, lower energy and water demand, reduce waste generation, and mitigate greenhouse gas emissions. Such outcomes align strongly with the United Nations Sustainable Development Goals (SDGs), particularly SDG 2 (Zero Hunger), SDG 3 (Good Health and Well-being), SDG 7 (Affordable and Clean Energy), SDG 9 (Industry, Innovation, and Infrastructure), SDG 12 (Responsible Consumption and Production), and SDG 13 (Climate Action), with additional co-benefits for SDGs 6, 14, and 15 (
Figure 6) [
112].
US enhances gas–liquid and solid–liquid mass transfer, shortening fermentation times and increasing yields of metabolites such as exopolysaccharides, bioactive peptides, and organic acids, thereby improving food availability (SDG 2) and maintaining functional properties (SDG 3). In solid-state systems, US reduces wash-water requirements (SDG 6) and, in downstream stabilization, allows for milder microbial control, reducing chemical sanitizer usage and lowering effluent toxicity (SDGs 14, 15). Its relatively modest power requirements support SDG 7, although uneven energy distribution in large-scale systems remains a scalability barrier (SDG 9). Fernandes and Rodrigues [
3] highlighted that ultrasound applied in fruit drying and processing increases product shelf life and nutritional quality (SDGs 2 and 3), reduces losses and chemical usage, promotes resource efficiency, and lowers environmental impact (SDGs 7, 12, 14, 15), while also fostering innovation, productivity, and multi-sectoral partnerships (SDGs 8, 9, 17).
PEF enables selective inactivation of spoilage flora during midstream fermentation, enhancing process stability and reducing contamination risks (SDGs 2, 3). Its influence on microbial metabolism, such as modulating yeast pathways to increase ethanol yield and lower by-product formation, optimizes resource conversion (SDG 12) and reduces energy intensity (SDG 7). Moreover, PEF facilitates low-temperature extraction of polyphenols, carotenoids, and proteins from plant biomass, contributing to industrial innovation (SDG 9) and lowering the carbon footprint relative to solvent-based extraction (SDG 13). Challenges remain in lowering equipment cost and complexity to ensure accessibility across small- and medium-scale producers (SDG 8). Puente-Díaz et al. [
113] and Mukherjee et al. [
114] further linked PEF and HPP to SDGs by highlighting their contributions to safe and nutritious foods, reduced waste, energy efficiency, industrial sustainability (SDGs 2, 3, 7, 9, 12), ecosystem conservation, and efficient recovery of bioactive compounds (SDGs 2, 3, 13, 14, 15).
HPP exemplifies this integration by ensuring microbiological safety in fermented dairy, plant-based beverages, and functional drinks without compromising thermolabile vitamins, phenolics, or probiotic viability. Its capacity to extend shelf life directly supports SDG 2 by reducing post-harvest losses, while lower steam usage compared to thermal pasteurization reduces fossil fuel dependency (SDG 7). In yogurt, kombucha, and vegetable lactic fermentations, HPP facilitates clean-label formulations (SDG 12) and minimizes preservative inputs, thereby reducing environmental burden on aquatic and terrestrial ecosystems (SDGs 14, 15). However, its alignment with SDG 7 depends on access to renewable electricity sources, as high pressures inherently require substantial electrical input.
CP offers versatile downstream applications, functioning as a non-thermal pasteurization tool for fermented products and packaging surfaces. By inactivating pathogens without heat, CP supports SDG 3, while shelf-life extension addresses SDG 2. In fermentation, CP can modulate microbial physiology to enhance the synthesis of secondary metabolites, enriching nutritional profiles. Furthermore, CP reduces water- and energy-intensive sanitation steps (SDG 6, 7) and diminishes toxic wastewater discharge (SDGs 14, 15). Pilot-scale application using cold discharge plasma achieved 49% COD reduction in model industrial wastewater, even eliminating microbes, underlining CP’s capacity for organic degradation and microbial inactivation [
111]. Fernandes and Rodrigues [
4] highlighted the contributions of cold plasma to SDGs, emphasizing its role in food safety, extended shelf life, and production of healthy foods (SDGs 2, 3), reduction of energy consumption and environmental impacts (SDGs 7, 14, 15), promotion of innovation and decent work (SDGs 8, 9), and strengthening of partnerships among industry, academia, and government (SDG 17), with indirect effects on poverty eradication (SDG 1).
PL delivers rapid microbial surface inactivation in seconds without bulk heating, thereby safeguarding food supplies (SDG 2) and preserving bioactive content (SDG 3). In starter culture preparation and equipment sanitation, PL lowers chemical and water usage (SDG 6) and reduces energy consumption (SDG 7). Its compact, relatively low-cost systems support broader industrial deployment (SDG 9), including in decentralized or small-scale processing contexts, although penetration limits in turbid media constrain some applications. The compact and modular nature of these systems makes them easy to integrate into a variety of settings, improving the sanitary conditions of foods in a way that is both efficient and economically sensible [
115,
116].
In essence, NTTs are not merely alternatives to conventional methods; they represent enabling platforms for a systemic transition toward resilient, low-impact, and nutritionally superior food systems. By delivering safety, extending shelf life, reducing losses, and improving resource efficiency, they create a direct technological pathway to the 2030 Agenda of sustainable development. Achieving their full SDG potential, however, requires targeted investments in scale-up engineering, regulatory harmonization, and multi-sectoral partnerships (SDG 17), ensuring equitable access to these innovations across diverse regions and market sizes.
In essence, NTTs are not merely alternatives to conventional methods; they represent enabling platforms for a systemic transition toward resilient, low-impact, and nutritionally superior food systems. By delivering safety, extending shelf life, reducing losses, and improving resource efficiency, they create a direct technological pathway to the 2030 Agenda for sustainable development. Achieving their full SDG potential, however, requires targeted investments in scale-up engineering, regulatory harmonization, and multi-sectoral partnerships (SDG 17), ensuring equitable access to these innovations across diverse regions and market sizes.
7. Research Gaps and Future Directions
Despite the significant advances in the development and application of NTTs in food fermentation, several scientific, technological, and operational gaps still limit their widespread industrial adoption. These gaps span from fundamental mechanistic understanding to engineering scale-up and regulatory harmonization.
At the mechanistic level, deeper insights are needed into the specific biochemical and physiological responses elicited by non-lethal treatments in microbial systems. While technologies such as US, PEF, and CP have shown the capacity to stimulate metabolic pathways and enhance secondary metabolite synthesis, the molecular triggers and regulatory networks involved remain poorly characterized. Advanced omics approaches, integrating transcriptomics, proteomics, and metabolomics, could enable a more predictive understanding of these responses, allowing for the design of treatment processes tailored to specific strains, substrates, and target metabolites.
From an engineering and scale-up perspective, uniform energy delivery remains one of the most critical bottlenecks. For US and CP in particular, heterogeneous energy fields and localized treatment hot spots reduce process reproducibility in larger volumes. This necessitates the development of advanced reactor designs, multi-point energy delivery systems, and real-time process monitoring tools to ensure consistent exposure. Likewise, the integration of NTTs into continuous bioprocessing lines will require modular, adaptable systems that can accommodate variable flow rates, viscosities, and product compositions without compromising performance.
Standardization and process metrology represent another pressing need. Across published studies, critical parameters such as acoustic energy density (US), reactive species concentration (CP), and specific energy input (PEF) are often inconsistently reported, hindering reproducibility and meta-analysis. International consensus on measurement protocols and performance benchmarks would greatly facilitate cross-study comparability and accelerate regulatory approval processes.
In the postbiotic production context, research should focus on optimizing non-lethal treatments to maximize the release and preservation of bioactive compounds, such as peptides, exopolysaccharides, and cell wall components, without degrading their functionality. Hybrid processing strategies, where two or more NTTs are applied sequentially or simultaneously, represent a promising avenue for simultaneously achieving microbial inactivation and bioactive enrichment. However, systematic studies comparing synergistic effects versus single treatments are still lacking.
Finally, a holistic approach integrating sustainability, economic affordability, and regional contexts is essential for NTT development and equitable adoption. While these technologies often require lower bulk temperatures and shorter processing times than traditional methods, their initial capital investment and long-term maintenance costs can present significant barriers for small and medium-sized enterprises. Therefore, explicitly addressing economic affordability through detailed cost-benefit analyses is critical. Furthermore, the viability and environmental impact of NTTs must be evaluated within specific regional contexts. For example, a technology that conserves water may be particularly valuable in arid regions, while a method with a higher energy demand might be less suitable where grid stability is a concern. The successful and equitable integration of NTTs into industrial practice thus requires a nuanced understanding of these varied economic and geographical factors to inform policy decisions and investment strategies.
Despite extensive bibliographic research, the use of NTT in food fermentation is affected by the food matrices’ heterogeneity and the lack of research standardization, which compromises the generalization and synthesis of results. The effect of NTT is highly dependent on variables such as the food matrix, the type of microorganism, and the specific parameters of each technology, making the study’s comparison a challenge. Research that directly compares the effectiveness and feasibility of different technologies for the same application is scarce. In addition, the academic literature, while rich in technical data and potential benefits, often lacks detailed economic analysis and case studies of industrial scalability, which limits the assessment of actual market prospects. Additionally, most published papers on NTT focus on microbial inactivation for preservation, rather than exploring the subtle modulation of microbial metabolism to optimize the fermentation process. Finally, the early stages and the lack of scalability data of some technologies make the volume of data insufficient for robust conclusions about their feasibility and contribution to sustainability.
8. Conclusions
This review showed that NTTs hold significant potential to transform the fermented food industry. By offering a viable alternative to thermal processing, these methods enhance food safety, quality, and functional value. The specific mechanisms of NTTs, such as preserving heat-sensitive compounds, modulating microbial activity, and improving mass transfer, enable benefits that extend beyond the reach of conventional processes.
Despite this potential, the widespread industrial adoption of NTTs still faces key challenges. Future research and development should focus on addressing issues related to uniform energy delivery at scale, standardizing process metrics, and ensuring economic affordability across different regional contexts. A nuanced understanding of the initial capital investment and long-term maintenance costs is critical for facilitating equitable adoption, particularly for small and medium-sized enterprises.
Ultimately, the successful integration of NTTs into next-generation food production will depend on a holistic and multidisciplinary approach. Coordinated efforts between process engineers, scientists, and regulatory bodies are essential to overcome these barriers, unlock the full potential of these technologies, and secure their scalable and sustainable future in the food industry.