Ultrasound-Assisted Extraction Enhances Enzymatic Activity and Thermal Stability of Bovine Pancreatin: Effect of pH and Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of pH and Temperature on AA
2.1.1. Temperature on AA
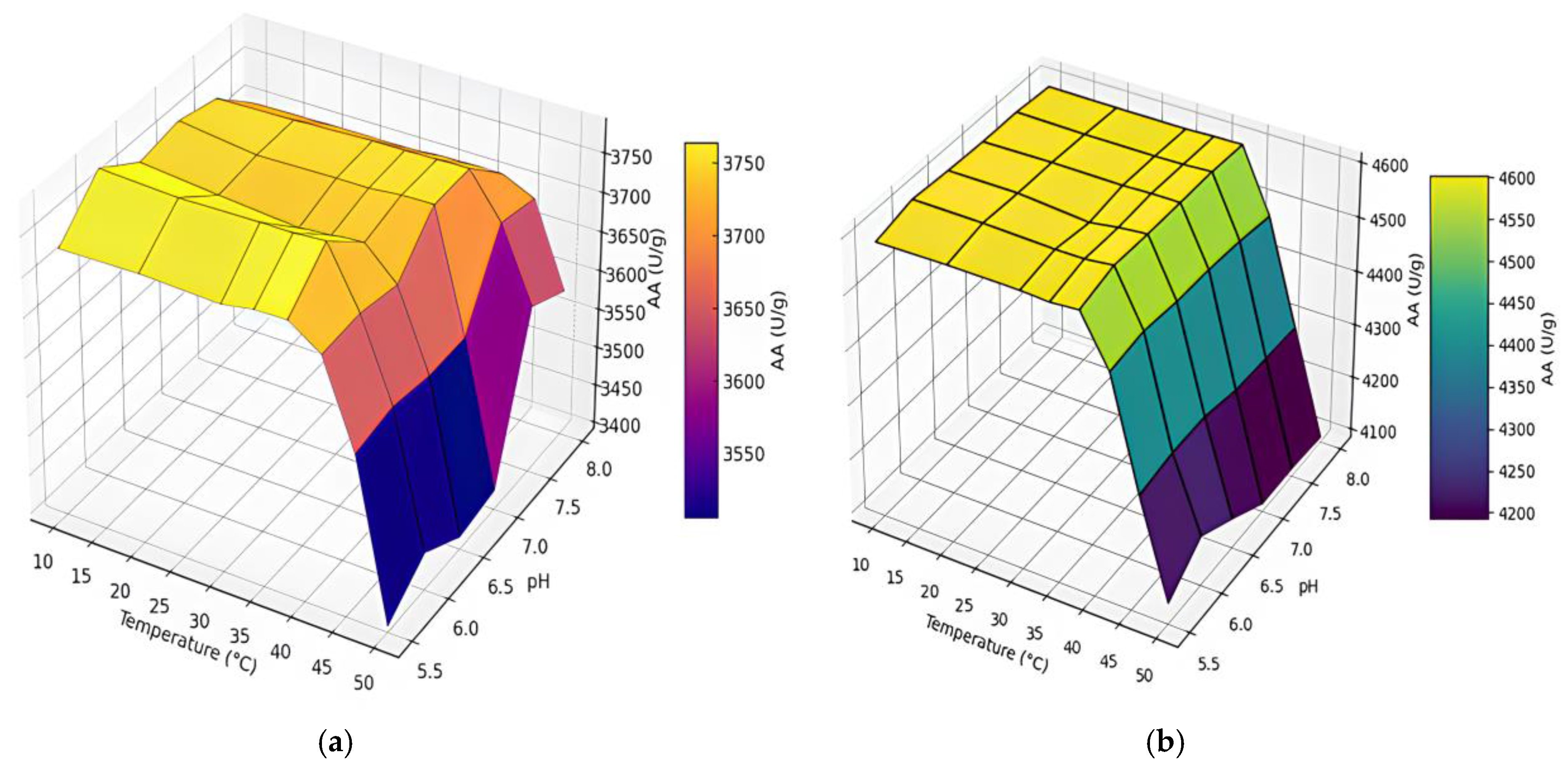
2.1.2. pH and Temperature on AA
2.2. Effect of pH and Temperature on PA
2.2.1. Temperature on PA
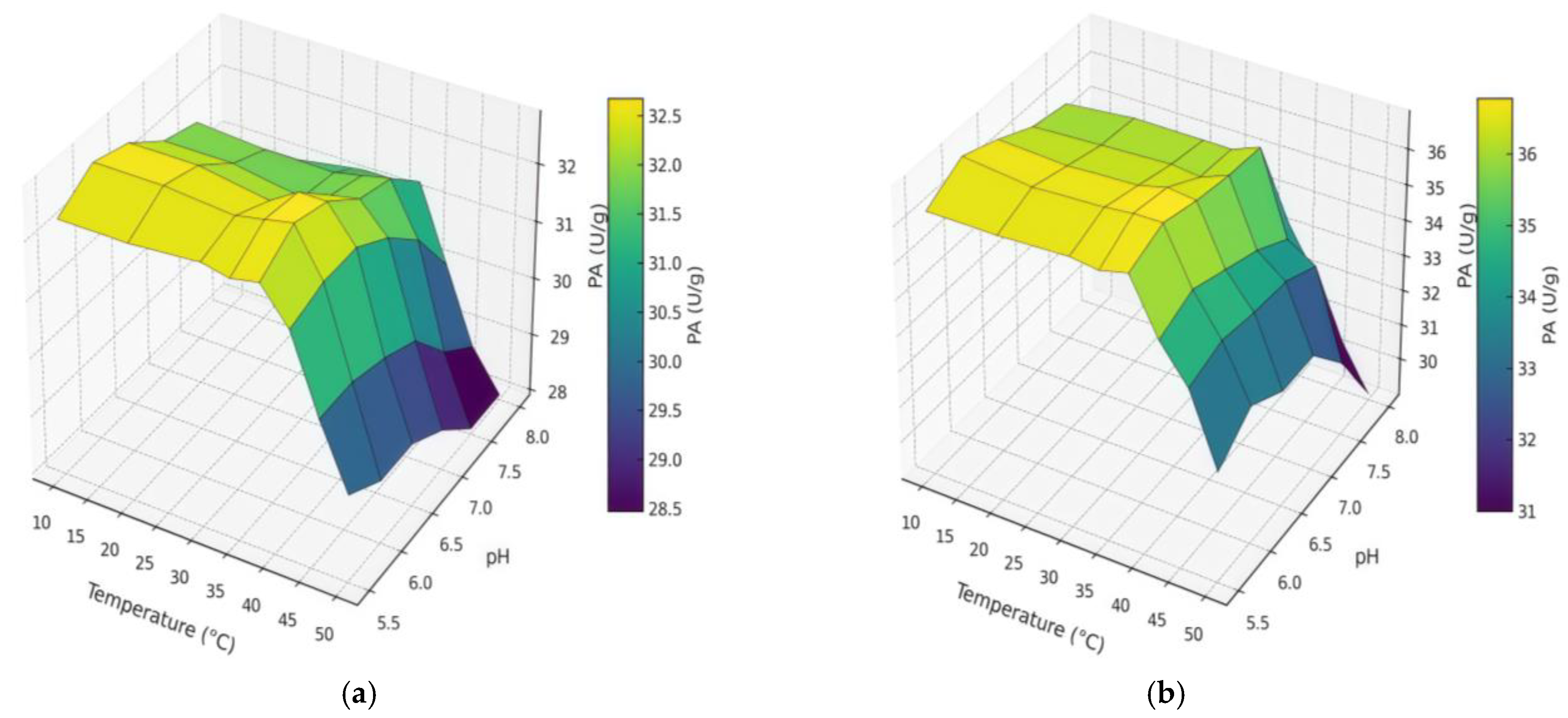
2.2.2. pH and Temperature on PA
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Sample Preparation
3.3. Thawing Loss
3.4. Determination of AA
3.5. Determination of PA
3.6. pH and Color Measurement
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- Ahmad, I.Z.; Tabassum, H.; Ahmad, A.; Kuddus, M. Food enzymes in pharmaceutical industry: Perspectives and limitations. In Enzymes in Food Technology; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 41–62. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.K.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [CrossRef]
- Motta, J.F.G.; de Freitas, B.C.B.; Almeida, A.F.; Martins, G. Use of enzymes in the food industry: A review. Food Sci. Technol. 2023, 43, e106222. [Google Scholar] [CrossRef]
- Kumar, A.; Dhiman, S.; Dhewa, T. Microbial enzymes and major applications in the food industry: A concise review. Food Prod. Process. Nutr. 2024, 6, 85. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Dietary bioactive compounds and human health: The role of bioavailability. Nutrients 2025, 17, 48. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Academic Press: London, UK, 2013. [Google Scholar]
- Pandey, R.; Negi, S. Enzymatic hydrolysis of starch, protein, and fat: Structure, function and applications. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–206. [Google Scholar]
- Zakowiecki, D.; Edinger, P.; Hess, T.; Paszkowska, J.; Staniszewska, M.; Romanova, S.; Garbacz, G. Effect of compaction pressure on the enzymatic activity of pancreatin in directly compressible formulations. Pharmaceutics 2023, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Rydzewska, G.; Domínguez-Muñoz, J.E. Kreon® (Creon®) vs. Lipancrea®: In Vitro Comparison of Two Encapsulated Pancreatin Preparations. Pharmaceuticals 2022, 15, 1570. [Google Scholar] [CrossRef]
- Legg, E.F.; Spencer, A.M. Studies on the stability of pancreatic enzymes in duodenal fluid to storage temperature and pH. Clin. Chim. Acta 1975, 65, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.T.; Perera, K.I.; Athauda, S.B.P.; Sivakanesan, R.; Kumar, N.S.; Jayasinghe, L. Heat stability of the in vitro inhibitory effect of spices on lipase, amylase, and glucosidase enzymes. Food Sci. Nutr. 2019, 7, 425–432. [Google Scholar] [CrossRef] [PubMed Central]
- Tessier, A.G.; Dombi, G.W.; Bouwman, D.L. Thermostability of purified human pancreatic α-amylase is increased by calcium and human serum albumin. Biochim. Biophys. Acta 1996, 1292, 71–78. [Google Scholar] [CrossRef]
- Sabadini, G.; Mellado, M.; Morales, C.; Mella, J. Arylamines QSAR-Based Design and Molecular Dynamics of New Phenylthiophene and Benzimidazole Derivatives with Affinity for the C111, Y268, and H73 Sites of SARS-CoV-2 PLpro Enzyme. Pharmaceuticals 2024, 17, 606. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; de Souza Claudino, E.; Converti, A.; Porto, T.S. Use of a Sequential Fermentation Method for the Production of Aspergillus tamarii URM4634 Protease and a Kinetic/Thermodynamic Study of the Enzyme. Catalysts 2021, 11, 963. [Google Scholar] [CrossRef]
- Yu, P.; Pan, X.; Chen, M.; Ma, J.; Xu, B.; Zhao, Y. Ultrasound-assisted enzymatic extraction of soluble dietary fiber from Hericium erinaceus and its in vitro lipid-lowering effect. Food Chem. 2023, 6, 100545. [Google Scholar] [CrossRef]
- Peng, P.; Yu, H.; Xian, M.; Qu, C.; Guo, Z.; Li, S.; Zhu, Z.; Xiao, J. Preparation of acetylcholinesterase inhibitory peptides from yellowfin tuna pancreas using moderate ultrasound-assisted enzymatic hydrolysis. Mar. Drugs 2023, 21, 75. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Wang, Y.; Wang, J. Effects of particle size on the extraction and functional properties of enzymes from animal by-products. Food Bioprod. Process. 2019, 117, 1352. [Google Scholar] [CrossRef]
- Li, M.; Liang, Q.; Zhang, Y.; Jiang, X.; Gu, Y.; Song, X.; Wang, X.; Shi, W. Screening of Potential Angiotensin-Converting Enzyme-Inhibitory Peptides in Squid (Todarodes pacificus) Skin Hydrolysates: Preliminary Study of Its Mechanism of Inhibition. Mar. Drugs 2025, 23, 81. [Google Scholar] [CrossRef]
- Xu, K.; Fu, H.; Chen, Q.; Sun, R.; Li, R.; Zhao, X.; Zhou, J.; Wang, X. Engineering thermostability of industrial enzymes for enhanced application performance. Int. J. Biol. Macromol. 2025, 291, 139067. [Google Scholar] [CrossRef]
- Montuori, E.; Martinez, K.A.; De Luca, D.; Ianora, A.; Lauritano, C. Transcriptome Sequencing of the Diatom Asterionellopsis thurstonii and In Silico Identification of Enzymes Potentially Involved in the Synthesis of Bioactive Molecules. Mar. Drugs 2023, 21, 126. [Google Scholar] [CrossRef]
- Razieh, A.; Abdol-Khalegh, B.; Douglas, V.L.; Ahmad, R.K.; Iraj, M.B. Thermal stability and enzymatic activity of RNase A in the presence of cationic gemini surfactants. Int. J. Biol. Macromol. 2012, 50, 1151–1157. [Google Scholar] [CrossRef]
- Abdullahi, N.; Atiku, M.K.; Umar, N.B. The roles of enzyme in food processing–an overview. Fudma J. Sci. 2021, 5, 157–164. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial proteases and their applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, 4th ed.; Wiley-Blackwell: Weinheim, Germany, 2012. [Google Scholar]
- Vera-Salgado, J.; Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; López-García, U.M.; Ragazzo-Sánchez, J.A. Ultrasound-Assisted Extraction of Artocarpus heterophyllus L. Leaf Protein Concentrate: Solubility, Foaming, Emulsifying, and Antioxidant Properties of Protein Hydrolysates. Colloids Interfaces 2022, 6, 50. [Google Scholar] [CrossRef]
- Kęska, P.; Wójciak, K.M.; Stasiak, D.M. Influence of Sonication and Taraxacum officinale Addition on the Antioxidant and Anti-ACE Activity of Protein Extracts from Sous vide Beef Marinated with Sour Milk and after In Vitro Digestion. Molecules 2020, 25, 4692. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Hussain, M.; Qayum, A.; Zhang, X.; Hao, X.; Liu, L.; Wang, Y.; Hussain, K.; Li, X. Improvement in bioactive, functional, structural and digestibility of potato protein and its fraction patatin via ultra-sonication. LWT 2021, 148, 111747. [Google Scholar] [CrossRef]
- Miljanović, A.; Bielen, A.; Grbin, D.; Marijanović, Z.; Andlar, M.; Rezić, T.; Roca, S.; Jerković, I.; Vikić-Topić, D.; Dent, M. Effect of Enzymatic, Ultrasound, and Reflux Extraction Pretreatments on the Yield and Chemical Composition of Essential Oils. Molecules 2020, 25, 4818. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Marszałek, K.; Iqbal, M.A.; Chughtai, M.F.J.; Hu, W.; Barba, F.J.; Bi, J.; Liu, X.; Xu, X. Inactivation and structural changes of polyphenol oxidase in quince (Cydonia oblonga Miller) juice subjected to ultrasonic treatment. J. Sci. Food Agric. 2020, 100, 2065–2073. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Liao, F.-Y.; Su, Y.-L.; Weng, J.-R.; Lin, Y.-C.; Feng, C.-H. Ultrasound–Vortex-Assisted Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Diode Array Detection for Determining UV Filters in Cosmetics and the Human Stratum Corneum. Molecules 2020, 25, 4642. [Google Scholar] [CrossRef]
- Qin, L.; Porfyrakis, K.; Tzanakis, I.; Grobert, N.; Eskin, D.G.; Fezzaa, K.; Mi, J. Multiscale interactions of liquid, bubbles and solid phases in ultrasonic fields revealed by multiphysics modelling and ultrafast X-ray imaging. Ultrason Sonochem. 2022, 89, 106158. [Google Scholar] [CrossRef]
- Dutta, T.K.; Jana, M.; Pahari, P.R.; Bhattacharya, T. The effect of temperature, pH, and salt on amylase in Heliodiaptomus viduus (Gurney) (Crustacea: Copepoda: Calanoida). Turk. J. Zool. 2006, 30, 167–172. Available online: https://journals.tubitak.gov.tr/zoology/vol30/iss2/11 (accessed on 4 July 2025).
- Fadeel, A.; Moll, B.A.; Jones, R.L. Effect of temperature on the synthesis and secretion of α-amylase in barley aleurone layers. Plant Physiol. 1980, 66, 466–470. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC440655/ (accessed on 4 July 2025). [CrossRef] [PubMed]
- Elmansy, E.A.; Asker, M.S.; El-Kady, E.M.; Sholkamy, E.N.; Awad, M.F. Production and optimization of α-amylase from thermo-halophilic bacteria isolated from different local marine environments. Bull. Natl. Res. Cent. 2018, 42, 31. Available online: https://bnrc.springeropen.com/articles/10.1186/s42269-018-0033-2 (accessed on 4 July 2025). [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Vallés, D.; Furtado, S.; Villadóniga, C.; Cantera, A.M.B. Adsorption onto alumina and stabilization of cysteine proteinases from crude extract of solanum granuloso-leprosum fruits. Process Biochem. 2011, 46, 592–598. [Google Scholar] [CrossRef]
- Sango, D.M.; Abela, D.; McElhatton, A.; Valdramidis, V.P. Assisted ultrasound applications for the production of safe foods. J. Appl. Microbiol. 2014, 116, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Majeed, T.; Lee, C.C.; Orts, W.J.; Tabassum, R.; Shah, T.A.; Jardan, Y.A.B.; Dawoud, T.M.; Bourhia, M. Characterization of a thermostable protease from Bacillus subtilis BSP strain. BMC Biotechnol. 2024, 24, 49. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.D.; Abdul Rahman, N.; Mohd Yusof, H.; Mohd Shariff, F.; Yasid, N.A. Effect of cultural conditions on protease production by a thermophilic Geobacillus thermoglucosidasius SKF4 isolated from Sungai Klah Hot Spring Park, Malaysia. Molecules 2020, 25, 2609. [Google Scholar] [CrossRef]
- Sarker, P.K.; Talukdar, S.A.; Deb, P.; Sayem, S.A.; Mohsina, K. Optimization and partial characterization of culture conditions for the production of alkaline protease from Bacillus licheniformis P003. SpringerPlus 2013, 2, 506. [Google Scholar] [CrossRef]
- Qian, J.; Chen, D.; Zhang, Y.; Gao, X.; Xu, L.; Guan, G.; Wang, F. Ultrasound-Assisted Enzymatic Protein Hydrolysis in Food Processing: Mechanism and Parameters. Foods 2023, 12, 4027. [Google Scholar] [CrossRef]
- Soares, A.d.S.; Duarte Augusto, P.E.; de Castro Leite Junior, B.R.; Nogueira, C.A.; Rufino Vieira, E.N.; Ribeiro de Barros, F.A.; Stringheta, P.C.; Ramos, A.M. Ultrasound assisted enzymatic hydrolysis of sucrose catalyzed by invertase: Investigation on substrate, enzyme and kinetics parameters. LWT Food Sci. Technol. 2019, 107, 164–170. [Google Scholar] [CrossRef]
- Rathnakumar, K.; Kalaivendan, R.G.T.; Eazhumalai, G.; Raja Charles, A.P.; Verma, P.; Rustagi, S.; Bharti, S.; Kothakota, A.; Siddiqui, S.A.; Manuel Lorenzo, J.; et al. Applications of ultrasonication on food enzyme inactivation—Recent review report (2017–2022). Ultrason. Sonochem. 2023, 96, 106407. [Google Scholar] [CrossRef]
- Maric, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brncic, M.; Brncic, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2017. [Google Scholar]
- Thi Thu Tra, T.; Khanh Tien, N.; Van Viet Man, L. Effects of ultrasonication variables on the activity and properties of alpha amylase preparation. Biotechnol. Prog. 2018, 34, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and Biotechnological Aspects of Microbial Proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. Available online: https://www.scirp.org/reference/referencespapers?referenceid=939650 (accessed on 4 July 2025). [CrossRef]
- Li, J.; Sun, C.; Yue, X.; Ma, W.; Wang, Y.; Zhao, J.; Zhu, G.; Bai, Y. Ultrasound-assisted immersion freezing improves the digestion properties of beef myofibrillar protein. Food Chem. X 2024, 22, 102144. [Google Scholar] [CrossRef]
- Patent No. SU651810. Method for Obtaining Pancreatin. USSR; Filed 1979 Mar 15. Russian. Available online: https://patentscope.wipo.int/search/ru/detail.jsf?docId=SU28775124 (accessed on 4 July 2025).
- GOST 34440-2018; Enzyme Preparations for Food Industry. Methods for the Determination of Amylase Activity. Standartinform: Moscow, Russia, 2018; p. 19. Available online: https://allgosts.ru/07/100/gost_34440-2018 (accessed on 4 July 2025).
- GOST 34430-2018; Enzyme Preparations for Food Industry. Methods for the Determination of Protease Activity. Standartinform: Moscow, Russia, 2018; p. 15. Available online: https://meganorm.ru/Index2/1/4293735/4293735117.htm (accessed on 4 July 2025).
- Yuan, D.; Xu, Y.; Kong, B.; Cao, C.; Zhang, F.; Xia, X.; Zhang, H.; Liu, Q.; Zhao, J. Application of seaweed dietary fiber as a potential alternative to phosphates in frankfurters with healthier profiles. Meat Sci. 2023, 196, 109044. [Google Scholar] [CrossRef]
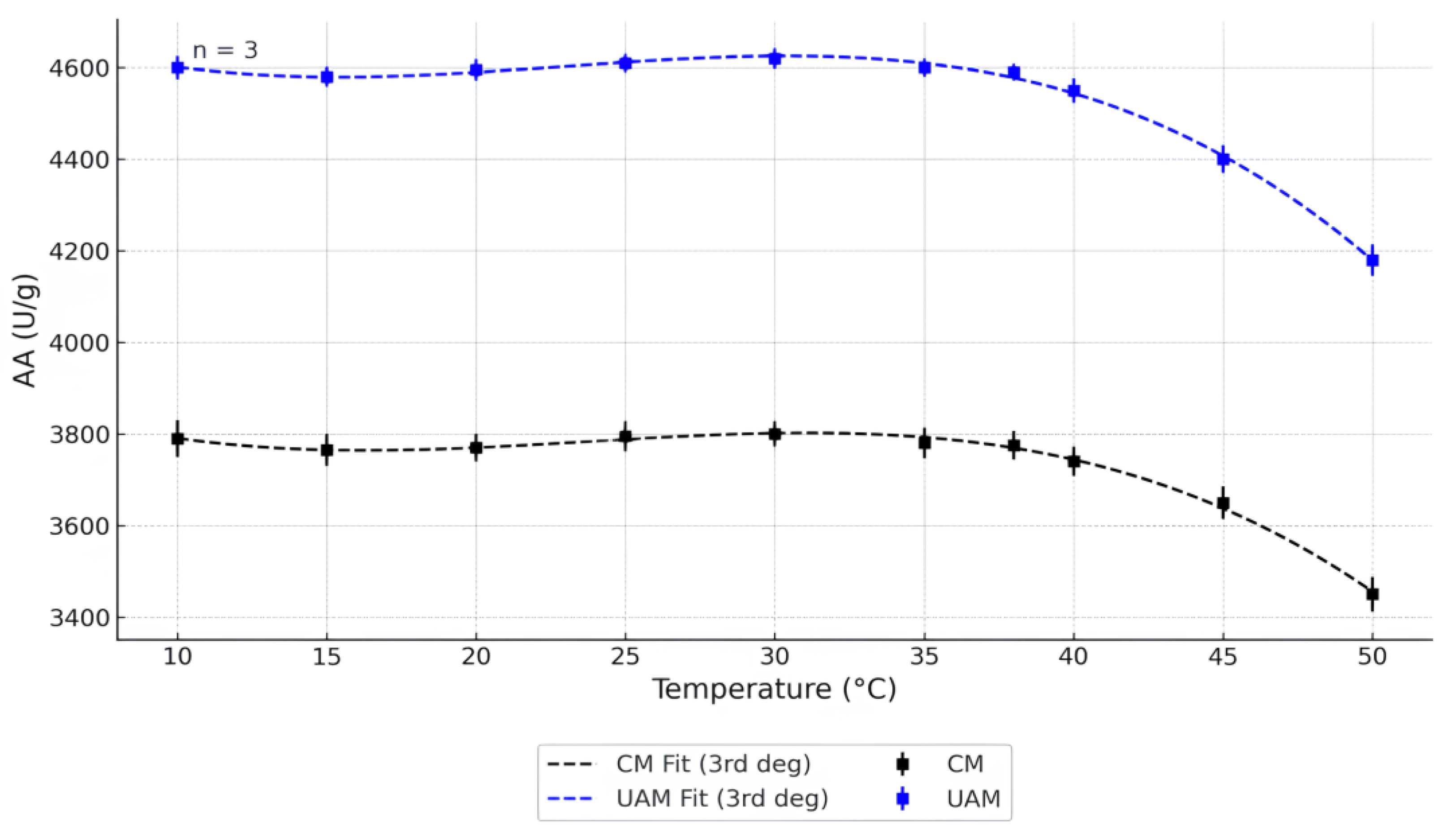


| Source | p-Value | Significant |
|---|---|---|
| Temperature | 1.00 × 10−26 | Yes |
| Method (CM vs. UAM) | 1.21 × 10−52 | Yes |
| Temperature × Method | 0.099 | No |
| Source | p-Value | Significant |
|---|---|---|
| Temperature | 2.30 × 10−16 | Yes |
| Method (CM vs. UAM) | 9.52 × 10−28 | Yes |
| Temperature × Method | 0.85 | No |
| Extraction | pH | Temperature (°C) | AA (U/g) | PA (U/g) |
|---|---|---|---|---|
| CM | 6 | 38 | 3785 | 32.8 |
| UAM | 6 | 38 | 4606 | 36.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenenbay, G.; Chomanov, U.; Tursunov, A. Ultrasound-Assisted Extraction Enhances Enzymatic Activity and Thermal Stability of Bovine Pancreatin: Effect of pH and Temperature. Processes 2025, 13, 2511. https://doi.org/10.3390/pr13082511
Kenenbay G, Chomanov U, Tursunov A. Ultrasound-Assisted Extraction Enhances Enzymatic Activity and Thermal Stability of Bovine Pancreatin: Effect of pH and Temperature. Processes. 2025; 13(8):2511. https://doi.org/10.3390/pr13082511
Chicago/Turabian StyleKenenbay, Gulmira, Urishbay Chomanov, and Alibek Tursunov. 2025. "Ultrasound-Assisted Extraction Enhances Enzymatic Activity and Thermal Stability of Bovine Pancreatin: Effect of pH and Temperature" Processes 13, no. 8: 2511. https://doi.org/10.3390/pr13082511
APA StyleKenenbay, G., Chomanov, U., & Tursunov, A. (2025). Ultrasound-Assisted Extraction Enhances Enzymatic Activity and Thermal Stability of Bovine Pancreatin: Effect of pH and Temperature. Processes, 13(8), 2511. https://doi.org/10.3390/pr13082511








