Abstract
Food waste has emerged as a critical worldwide concern, resulting in environmental deterioration and economic detriment. Bio-based natural polymer coatings and films have emerged as a sustainable solution to food preservation challenges, particularly in reducing postharvest losses and extending shelf life. Compared to their synthetic counterparts, these polymers, such as chitosan, starch, cellulose, proteins, and alginate, are derived from renewable sources that are biodegradable, safe, and functional. Within this context, this review examines the various bio-based natural polymer coatings and films as biodegradable, edible alternatives to conventional packaging solutions. It examines the different fabrication methods, like solution casting, electrospinning, and spray coating, and incorporates antimicrobial agents to enhance performance. Emphasis is placed on their mechanical, barrier, and antimicrobial properties, their application in preserving fresh produce, how they promote food safety and environmental sustainability, and accompanying limitations. This review highlights the importance of bio-based natural polymer coatings and films as a promising, eco-friendly solution to enhancing food quality, safety, and shelf life while addressing global sustainability challenges.
1. Introduction
Globally, 1.3 billion tons of food are lost or wasted annually, accounting for roughly one-third of the food produced for human consumption [1]. The United Nations Food and Agriculture Organization (FAO) reported that fruit and vegetable waste accounts for about 60% of all food categories [2]. This waste includes by-products generated during processing, which typically range from 25% to 30% of the total product volume [1]. In addition, approximately 20% of food waste is generated during production, 1% during processing, 19% during distribution, and 60% at the consumer level [3]. Thus, various factors contribute to food waste. These include shrinkage during cooking, supply chain issues, strict consumer standards, and environmental factors like weather and soil runoff [4]. Regulatory restrictions also play a role [4]. These contribute to various environmental issues, like greenhouse gas emissions during food production, storage, transportation, and disposal. These wastes also lead to soil degradation, deforestation, and water and air contamination [5].
The consequences of these factors have necessitated a growing interest in extending the shelf life of food products, which has consequently prompted the advancement of food preservation techniques [6]. Beyond the primary goals of preservation against physical damage, microbial contamination, and unnecessary waste [7], various additional goals have been identified for each food preservation approach. These include enhancing value-added properties, providing dietary diversity, and mitigating challenges associated with seasonal production [4]. Recent findings have reported a growing demand for safe and healthy food products, with consumers increasingly avoiding artificial additives and preservatives. This trend is reshaping industry practices in food preservation [8]. Moreover, the rising perishability of food items and the necessity for long-distance transportation have intensified the demand for methods to prolong food shelf life [9]. Thus, effective preservation techniques are crucial to ensure food safety and quality throughout preparation, storage, and transportation [7].
Many developing countries have continued to depend on traditional methods of food preservation, such as evaporative cooling, smoking, salting, and sun drying. For instance, about 70% of food produced in Nigeria is processed using these conventional techniques [10]. Additionally, postharvest treatments involving chemical fungicides and synthetic waxes, such as polyethylene and petroleum-based waxes, remain prevalent in several nations to mitigate postharvest losses and extend the shelf life of perishable fruits and vegetables. However, due to their persistent application, these practices pose significant risks to environmental integrity and public health [11]. The constraints associated with agrochemical usage, the demand for healthy foods, and heightened environmental concerns have intensified the search for innovative preservation technologies alternatives [12]. These alternatives must be readily available, cost-effective, renewable, safe, and biodegradable materials [12]. In this context, natural polymers are becoming sustainable alternatives in food packaging, healthcare, energy storage, and electronics due to their safety and biocompatibility [13]. These polymers, encompassing polysaccharides, proteins, and nucleic acids, constitute integral components of biological systems and perform various essential functions [14].
Natural polymers, including cellulose and chitin, are essential for maintaining the structural integrity of cells in both plants and animals, while others confer biological protection against environmental factors [15]. Recent investigations have shown that using these polymers significantly contributes to developing advanced materials, such as films, membranes, coatings, hydrogels, and micro- and nanoparticle systems [16]. This is attributed to their natural abundance, renewability, and inherent negative carbon footprint, as they are derived from renewable resources [17]. These advantages have led to growing interest in sourcing these polymers from unconventional yet sustainable origins. One such emerging and eco-friendly source is fruit waste. Fruit waste offers a promising path for natural biopolymers due to its abundant bioactive compounds such as vitamins, carbohydrates, dietary fibers, lipids, carotenoids, and polyphenols. These compounds have demonstrated efficacy in fabricating films and edible coatings that enhance the shelf life of food products [18]. Notably, these bioactive components are predominantly located in the seeds, skin, rind, and pomace of various fruits.
Therefore, this review seeks to explore and assess different reports on using bio-based natural polymers as an edible coating/film to improve food safety. It aims to examine the efficacy of bio-based natural polymers in mitigating microbiological contamination and spoilage of food products. Additionally, this review will investigate scholarly studies on the mechanical, barrier, and antibacterial properties of these edible coatings and their applicability to various food packaging functions. Finally, it will identify the challenges and limitations of producing and utilizing bio-based edible coatings, including scalability, cost-effectiveness, and regulatory considerations.
2. Bio-Based Natural Polymers
Bio-based polymers are renewable resource materials used in various scientific, industrial, and consumer applications [19]. They have emerged as the most popular and preferred materials in the food sector and food packaging industries [20]. This is due to their lower carbon footprints and the sustainable nature of these materials [21]. Bio-based natural polymers include starch, chitosan, cellulose, chitin, and other polysaccharides and proteins [22]. However, not every polysaccharide can be used as a bio-renewable polymer [23]. These materials have garnered increased attention as packaging materials in the food industry, especially as an edible coating. Edible coatings are considered eco-friendly alternatives to synthetic packaging and are part of the growing demand for sustainable food preservation techniques [24]. They are thin layers of edible materials (mostly bio-based polymers) applied directly to the surface of food products to provide protection and enhance their shelf life [25]. Generally, edible polysaccharide coatings are water-soluble and have poor moisture barrier qualities. However, they have a relatively low O2 permeability. Edible coatings derived from polysaccharides are often applied to fresh, minimally processed fruits and vegetables [26] by creating a modified environment that lowers the rate of respiration [27]. Among polysaccharides, chitin and cellulose are the most significant due to their unique properties and sustainable origins [28]. These polymers exist naturally and have a diverse set of features and applications. Bio-based polymers have grown significantly in recent years in terms of technological advancements and commercial uses. This section will explore commonly used bio-based polysaccharide materials as edible coatings/films.
2.1. Chitosan-Based Edible Coatings/Films
Chitosan is a natural amino polysaccharide composed of numerous reactive functional groups, derived economically from marine waste materials, particularly the exoskeletons of seafood [29]. It is characterized as a cationic polymer that exhibits biocompatibility and non-toxicity [30,31]. Due to its numerous advantages, including its abundance, non-toxicity, film-forming ability, and capacity to carry active substances, chitosan has remained one of the most widely utilized polysaccharide-based coating materials [32]. Moreover, chitosan possesses antibacterial and antioxidant activities, making it suitable for food preservation applications [33]. The structural configuration of chitosan allows for numerous modifications through its hydroxyl and amino groups. These enhance its properties, enabling the formation of compounds with other polysaccharides [34].
It can be transformed into edible films by dissolving in diluted acid solutions, such as acetic and hydrochloric acid [35,36]. Some literature reports have revealed that chitosan edible coating can prevent postharvest infections on various fruits [37]. However, the antibacterial, antioxidant, mechanical, and conditioning properties of pure chitosan films have been deemed insufficient in practical applications [38]. Nevertheless, the United States Food and Drug Administration (FDA) recognizes chitosan as a Generally Recognized as Safe (GRAS) food ingredient [39]. Thus, it is underscored as an appropriate food packaging agent, leading to several studies on chitosan-based films and coatings for food preservation. For example, various fruits and vegetables, including papaya [40], tomatoes [41], mangoes [42], carrots [43], pomegranates [32], and bananas [44], have shown improved storage stability and extended shelf life when coated with chitosan-based films. Edible films and coatings have also been documented to exhibit significant antibacterial and antioxidant properties, prolonging the shelf life of fruits and vegetables, attributed to their capacity to scavenge free radicals, contributing to their antioxidant activity [45]. Compared to other polysaccharides, lipids, and protein-based coatings and films, chitosan-based coatings and films show greater promise because of their solubility [34]. Chitosan is soluble in glacial acetic acid [46], compared to cellulose, which is insoluble in water and only sparingly soluble in most polar solvents. Additionally, edible coatings/films made of chitosan have good mechanical and barrier qualities. Other documented examples of its application in the literature, such as films and edible coatings, have been summarized in Table 1.

Table 1.
A few examples of chitosan-based edible coating/film on food applications.
2.2. Starch-Based Coating/Films
Starch is one of the most abundant biopolymers, serving as the principal energy storage in many plants, including cereals (e.g., wheat, rice, maize), tubers (e.g., potatoes), roots (e.g., cassava), and legumes [54]. According to the literature report [55], this naturally occurring, biodegradable polymer can be utilized to develop functional food packaging materials due to its widespread availability in raw crops and unique physicochemical properties.
Among eco-friendly natural materials, starch-based materials are the most studied and used [56]. This is mainly due to its low cost, availability, organoleptic (flavorless, tasteless, odorless), optical (transparent, colorless), and barrier (carbon dioxide and oxygen permeability) qualities [57]. These characteristics are perfect for film formation, but the starch biopolymer’s mechanical qualities and water vapor permeability (WVP) are poor due to its hydrophilic nature [54,58].
Starch comprises two polysaccharides, amylose and amylopectin [59], and trace quantities of lipids and proteins [60]. The amylose gives starch its ability to form films; the molecular size of this linear chain polymer, which consists of α-(1-4)-anhydroglucose units, fluctuates between 200 and 800 kg/mol [61,62]. On the other hand, amylopectin is a highly branched polymer of 20–30 glucose moieties, linked by an α-(1-6) glycosidic branch of a short α-(1-4) chain. Its molecular weight is extremely high (5000–30,000 kg/mol) [63]. However, starch molecules are insoluble in water; hydrogen bonds cause this insolubility. Starch must be gelatinized in extra water for a good and consistent film-forming solution [64]. Gelatinization is when heating with water causes starch granules to swell, release amylose, and break apart their crystal structures, leading to a thick, gooey solution. This transition is essential for producing consistent film-forming solutions [64]. As gelatinized starch cools, it undergoes retrogradation (a process in which amylose and amylopectin chains realign and form hydrogen bonds). This molecular reordering leads to increased viscosity and the formation of a gel-like structure [65,66]. The distinctive phase transition behaviors directly affect the structural and functional characteristics of starch-based polymers. These inherent properties often result in materials with insufficient mechanical strength and barrier performance [67,68].
Therefore, enhancing the mechanical and barrier characteristics of starch-based packaging materials is essential. To improve the mechanical properties and lower the hydrophilic properties of starch-based packaging, it was proposed to incorporate mineral nanofillers into the polymer matrix instead of macrofillers [69]. This nanocomposite approach offers superior property enhancement while maintaining the biodegradable nature of the packaging. The industrial application of starch, particularly in the food packaging industry, benefits from its diverse character. It protects against moisture, oxygen, and microbial contamination in foods like fresh produce [70], dairy, and bakery products [71]. Some documented examples of its application in the literature, such as films and edible coatings, are summarized in Table 2.

Table 2.
Some examples of starch-based edible coating/film applications on food products.
2.3. Cellulose-Based Coatings/Films
Cellulose is among the most abundant organic polymers on Earth and plays a critical role in the structure of algae, green plants, and the cell walls of oomycetes [77]. Various sustainable sources, such as wood pulp and kenaf fiber, are utilized in cellulose production [78]. Up to 99% of the material can be bio-based [79]. Recently, it has attracted significant attention due to its inherent biodegradability. Its availability, renewability, biocompatibility, and abundance in the natural environment have further contributed to its increasing popularity [20,80,81,82]. Its mechanical strength is high compared to the plastic-based material [83]. It comprises millions of β (1 → 4) linked D-glucose units [84].
In the food industry, cellulose films are extensively used as edible packaging materials [21]. They demonstrate exceptional thermal stability while serving as an effective carrier for antioxidant and antimicrobial compounds [85]. Their UV-blocking capability and non-toxic properties make them particularly valuable for postharvest applications. These characteristics collectively enhance the preservation of nutritional quality and extend the shelf life of fresh produce [81]. These films demonstrate high efficacy in packaging a range of products. However, their lack of waterproof properties makes them unsuitable for cold storage or for containing wet food items [86]. It exhibits a high capacity for water absorption and demonstrates poor interfacial adhesion, thereby limiting its effectiveness [81].
Moreover, pure cellulose-based hydrogels often exhibit limitations in flexibility and functionality because of cellulose’s rigid crystalline structure and strong hydrogen bonding among glucose units, leading to a highly ordered and inflexible material [87]. Cellulose-derived hydrogels are typically synthesized from water-soluble cellulose derivatives such as methylcellulose (MC), ethyl cellulose (EC), and carboxymethylcellulose (CMC) through physical and/or chemical cross-linking methods [88].
Consequently, to address these limitations, it is essential to combine cellulose biopolymers with other polysaccharides (e.g., pectin, chitosan, or starch) to develop robust and sustainable films or coatings. Furthermore, incorporating bioactive compounds such as polyphenols, antimicrobials, and essential oils may improve their bioactive properties [81]. This synergistic combination enhances the composite’s performance by introducing antioxidant, antimicrobial, or pH-responsive properties while maintaining the structural integrity of the cellulose base. Examples of cellulose-based coating applications in the food sector are detailed in Table 3.

Table 3.
A few examples of cellulose-based edible coating/film applications on food products.
2.4. Alginate-Based Coating/Film
Alginate, a hydrophilic biopolymer with well-documented colloidal properties, is widely applied in food, printing, beverage, and pharmaceutical sectors as a multifunctional additive for thickening, stabilizing, emulsifying, chelating, encapsulating, and forming gels, films, and membranes [96]. It is derived from brown sea algae (Phaeophyceae), consisting of two uronic acids, β-D-mannuronic acid and α-L-guluronic acid [97]. This biopolymer is characterized by its affordability, homogeneity, transparency, water solubility, biocompatibility, and non-toxicity. These properties make it suitable for a diverse range of cell lines; however, it is noted for its limited mechanical strength [98].
Among biopolymers, alginate has been identified as particularly promising for applications demanding high oxygen barrier properties [99]. Alginate-based films and coatings have demonstrated considerable potential for encapsulating active or functional food ingredients, such as medicinal additives, tissue engineering materials, and enzymes [100]. Alginate coatings, in isolation, exhibit a deficiency in antioxidant and antifungal properties. They have shown efficacy in preserving the postharvest quality of various fruits, including peaches [101], sweet cherries [102], and tomatoes [103], effectively delaying the ripening process and extending the shelf life of fruits and vegetables [104]. Additional applications of alginate-based edible coatings/films are summarized in Table 4. However, alginate-based edible coatings/films are more susceptible to moisture damage because of their inherent hydrophilicity [105]. This drawback can lead to reduced barrier properties, increased water vapor permeability, and faster degradation of the coated product. Researchers have explored various strategies to improve moisture resistance, including cross-linking agents, hydrophobic additives, and nanocomposites [106]. Incorporating essential oils has proved to be an effective method of improving moisture [107]. Essential oils enhance hydrophobicity and contribute to active packaging by inhibiting microbial growth, thereby extending the shelf life of perishable foods [108].

Table 4.
Applications of alginate-based edible coating/films in food preservation.
2.5. Protein-Based Edible Coating/Film
Proteins are essential organic macromolecules composed of α-amino acids linked by peptide bonds, forming their primary structure [116,117]. The polypeptide backbone provides the framework for protein folding, which establishes the secondary structure, including α-helices, β-sheets, loops, and bends, stabilized by intramolecular interactions [118,119]. The tertiary structure arises from the overall three-dimensional arrangement of the protein, driven by interactions between the side chains of amino acids [119]. Some proteins also form quaternary structures, assembling multiple polypeptide chains into defined molecular aggregates. These aggregates are often reversible, loosely bound, and exhibit specific geometric arrangements [120].
Protein structure is critical in film formation as it governs the protein’s ability to interact with itself and other components [121]. Proteins are classified as fibrous or globular, each type serving distinct roles. Fibrous proteins, which are water-insoluble, are the primary structural components of animal tissues. In contrast, globular proteins are soluble in water and aqueous solutions of acids, bases, or salts, enabling diverse functional roles in biological systems [122,123]. The physicochemical properties of proteins are determined by the sequence and distribution of amino acid residues along the polymer chain [124]. Proteins are often modified using acids, bases, solvents, or heat to enhance their suitability for film production. These treatments enable the formation of durable systems by extending protein chains and promoting hydrogen, ionic, and covalent bonding. The uniform distribution of polar groups along the polymer chain further enhances the likelihood of intermolecular interactions critical for film strength [125].
The degree of chain-to-chain interactions influences the mechanical properties of protein-based films, such as strength and permeability. Films with stronger bonds exhibit lower permeability to vapors, liquids, and gases, making them practical barriers. Protein-based films are particularly adept at blocking oxygen, even at low relative humidity levels [126]. Commonly used proteins for edible film and coating production include whey, wheat gluten, gelatin, maize zein, casein, and soy protein. These materials offer functional and nutritional benefits, making them suitable for direct consumption [127]. A summary of applications of protein-based edible coatings/films is presented in Table 5.

Table 5.
Protein-based edible coating/films and their applications in food products.
2.6. Comparative Evaluation of Commonly Used Natural Biopolymer Properties
Following a detailed analysis of individual bio-based natural polymers, we summarize and compare their key properties to evaluate their suitability for food preservation. These polymers show different structural characteristics and barrier functionalities, antimicrobial efficacy, and practical applications in food systems [134,135]. Table 6 summarizes key biopolymers’ barrier properties, antimicrobial efficacy, and food applications. Among natural polymers, chitosan is remarkable for its intrinsic antimicrobial activity and strong barrier-forming capacity, which allows its application in food preservation [136,137]. Unlike polymers such as starch and cellulose, which do not possess inherent antimicrobial activity but are interesting because of their good film-forming and barrier properties after adding bioactive agents [138,139,140]. Alginate and protein-based films are suitable for food systems applications, particularly when reinforced with antimicrobials or plasticizers [141,142,143]. This comparison is valuable when choosing biopolymers for food preservation purposes, either to reduce oxygen transfer, increase microbiological barriers, or improve the texture of packaged food. Thus, the table in this context summarizes how these biopolymers align with specific preservation needs in food packaging systems.

Table 6.
Comparative overview of the major biopolymers based on barrier and antimicrobial properties in food applications.
2.7. Other Bio-Based Coating/Film Polymers
In addition to conventional biopolymers such as chitosan, starch, cellulose, alginate, and proteins, several other bio-based polymers and synthetic bio-alternatives have potential for coating and film applications in sustainable food preservation. These include poly (lactic acid) (PLA), polyhydroxyalkanoates (PHAs) like polyhydroxybutyrate (PHB), and novel developments such as non-isocyanate polyurethanes (NIPUs). These materials offer promising avenues due to their biodegradability, renewable origin, and physicochemical properties compatible with food contact applications.
Poly (lactic acid) (PLA), a synthetic biopolymer synthesized from lactic acid via ring-opening polymerization [151,152], is recognized for its superior optical clarity and biodegradability. Additionally, PLA exhibits favorable mechanical properties, moisture resistance [153], and exceptional transparency and printability [151]. It has also shown potential as a barrier for hydrophobic aroma compounds [154]. Additionally, PLA is recyclable, making it an environmentally friendly material [153]. Despite its relatively high production cost, PLA has gained traction in food packaging due to its processability and end-of-life compostability [155].
Similarly, polyhydroxyalkanoates (PHAs), specifically PHB and its copolymer PHBV, are bacterially synthesized from biomass [156] and exhibit semi-crystalline structures with good barrier and thermal properties [157]. However, due to their brittleness and narrow processing window, materials like PHB and PHB/V are unsuitable for technical applications such as deep-drawing films or injection molding in the food industry [158]. Also, their lack of high chemical recyclability has limited their potential [159]. To enhance their flexibility and toughness, they are often blended with other polymers or plasticizers for broader usability [156].
NIPU is a sixth widely used polymer that is synthesized from polycyclic carbonates and amines without the use of hazardous isocyanates [151,160,161]. In response to health and environmental concerns associated with traditional polyurethane (PU) synthesis, non-isocyanate polyurethane (NIPU) has garnered attention as a safer alternative [162], due to its excellent mechanical and physical properties. They are widely used in adhesives, seals, elasticity, rigid foams, and high-performance coatings [161]. Though still under development, NIPUs align with green chemistry principles and present reduced toxicity during manufacturing and application [163]. Despite their advantages, NIPUs face technical challenges such as slow reaction rates and lower molecular weights [164], which can affect their performance. Researchers have addressed these issues using reactive additives, catalysts, and optimized monomer structures to enhance polymerization efficiency [151,165]. Hybrid NIPUs (H-NIPUs), which combine NIPUs with other polymers like epoxies or acrylates, have shown improved mechanical properties and greater application potential [166].
The integration of these emerging polymers with natural biopolymers or nanofillers further enhances their mechanical, antimicrobial, and barrier properties, facilitating their application in advanced food packaging systems [167,168]. However, challenges such as production scalability, cost-efficiency, and regulatory compliance remain key limitations to their widespread adoption [169]. As research advances, the combination of synthetic bio-based polymers with plant or microbial-derived additives could yield next-generation materials that bridge the gap between performance and sustainability in active and intelligent packaging. Table 7 provides a comparative summary of the currently available synthetic bio-based polymers.

Table 7.
Other notable bio-based polymers for food coating/films.
3. Fabrication Techniques
Several fabrication techniques have been employed to produce diverse biopolymer-based films and coatings, each influencing the material’s mechanical barrier and antimicrobial properties [176]. Notable examples of these techniques include solvent casting, electrospinning, extrusion, and layer-by-layer assembly [177]. These methods offer distinct advantages depending on the intended application, as advancements in this field continue to enhance the performance of bio-based polymer films [178]. The fabrication techniques discussed in this section are limited to those relevant to the formation of films and edible coatings specifically for food packaging applications.
3.1. Solution Casting
Solution casting is a popular material science and engineering manufacturing technique for producing thin films and membranes [179]. This method entails dissolving a substance in a solvent to create a homogeneous solution, which is poured or cast onto a flat surface, where the solvent evaporates, leaving a solid layer of the substance [180], as seen in Figure 1. Solvent casting is preferred over other technologies, such as salt leaching, spin coating, microfluidic spinning, and 3D printing, because of its low cost, simplicity, practicality, and ability to produce durable films with adequate mechanical properties and uniformity [181]. However, the qualities of materials generated through solvent casting might vary greatly across production batches due to environmental variables, which can impede manufacturing [182]. Furthermore, ensuring sterility across all manufacturing phases is challenging and might lead to batch contamination.
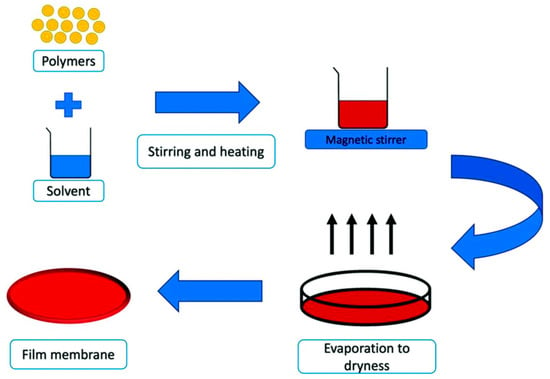
Figure 1.
Schematic diagram of the solution casting process [183]. Reproduced with permission from MDPI under the Creative Commons (CC BY) Copyright © 2023 license. Licensee MDPI.
This method has been extensively applied in the fabrication of biopolymer-based films such as chitosan, gelatin, pectin, and alginate. For instance, chitosan is frequently blended with plasticizers like glycerol to improve its flexibility and mechanical properties [184]. Gelatin films, known for their excellent oxygen barrier properties, exhibit improved strength and thermal stability when combined with starch or alginate [185]. Alginate films, prized for their biocompatibility, can be cross-linked with calcium ions to enhance water resistance and film strength [186,187]. Pectin-based films are often reinforced with starch to improve mechanical and moisture barrier properties [188].
3.2. Electrospinning
Electrospinning is an outstanding technique often used for creating continuous polymer fibers with controlled diameters and morphologies [189]. These nanofibers are deposited onto a collector surface, forming a fibrous mat or film with unique structural and functional characteristics [189]. This method is especially valuable for food packaging applications due to the high surface-area-to-volume ratio of electrospun nanofibers, which enhances their ability to incorporate and release active agents [190]. Furthermore, electro-spun nanofibers have more distinct properties, such as tunable chemical composition, Fiber diameter, pore structure, and morphologies (dense and hollow), which can be obtained by adjusting the following factors: Electrospinning solution properties such as composition, viscosity, conductivity, and surface tension [189,190]. Notably, electrospinning can thus be performed under ambient conditions without high temperatures, extensive chemical reactions, or complex equipment, making it a cost-effective and scalable method. Nevertheless, environmental parameters such as solution temperature and humidity, for inorganic materials, and post-treating parameters such as heating rates and temperatures significantly affect product structure [191]. Electrospinning setups generally consist of three major components: a syringe pump containing the polymer solution, a high-voltage DC power supply, and a collector [192]. When a high voltage is applied to the polymer solution, electrostatic repulsion overcomes surface tension, forming a Taylor cone (a pointed structure that forms at the tip of the needle or nozzle [193]) and ejecting a fine polymer jet. As this jet travels toward the collector, it undergoes stretching and thinning, solidifying into nanofibers upon solvent evaporation [194]. Figure 2 illustrates the setup and working principle of the electrospinning technique.

Figure 2.
A schematic demonstration of the electrospinning process and its key components [195]. Reproduced with permission from MDPI under the Creative Commons (CC BY) Copyright © 2022 license.
Compared to alternative methodologies, electrospinning technology is characterized by its simplicity, ease of control, and low cost, allowing it to be easily scaled up to generate diverse nanomaterials [191]. However, a notable challenge arises from the proximity of the collector to the charged polymer solution, which hinders complete solvent evaporation before the fiber jet arrives at the collector. This phenomenon results in a loosely structured web. Furthermore, the partial evaporation of solvent causes the fibers to adhere to both the collector and one another, thereby complicating the removal process [192]. Various electrospinning configurations can alter the morphology and structure of electrospun fibers, thereby enhancing protection against active agents and improving mechanical and barrier properties in active packaging systems [196]. Ref. [197] investigated the application of electrospinning to produce protein-based encapsulating structures utilizing whey protein isolate (WPI) without the incorporation of organic solvents or exposure to high temperatures. Their findings demonstrated that WPI could yield membranes possessing desirable attributes such as biodegradability, transparency, and odorlessness. These properties render WPI-based electrospun films advantageous for extending the shelf life of various food products by providing an effective protective layer.
3.3. Spray Coating
Spray coating is spraying a polymer solution or suspension onto a substrate, forming a thin film as the solvent evaporates [198,199]. In this technique, a nozzle rapidly sprays tiny solution droplets across the preheated substrate [200]. The most widely used method is pneumatic spraying, which delivers the droplets via a fast-moving gas stream. The spray coating involves four main steps. First, droplets are generated through a process called atomization. These droplets then land and merge on the substrate, forming a wet film. Finally, the substrate undergoes annealing to produce the final film [201]. A schematic diagram of the entire coating process is shown in Figure 3. This approach enables homogeneous coating across wide regions and intricate shapes [202]. It is widely used to produce thin films for electrical devices, coatings, and protective layers. Spray coating techniques offer tremendous potential for large-scale production because these methods have no limitations in substrate size and low polymer use, promising to substitute the standard process, which is the spin coating method [203]. However, Spray coating poses challenges, such as increased film thickness and roughness [85]. Recent research has thus focused on optimizing the morphology of active layers using additives, solvent combinations, and post-thermal annealing [204], high-boiling-point solvents [205], and spray-coating methods [206].
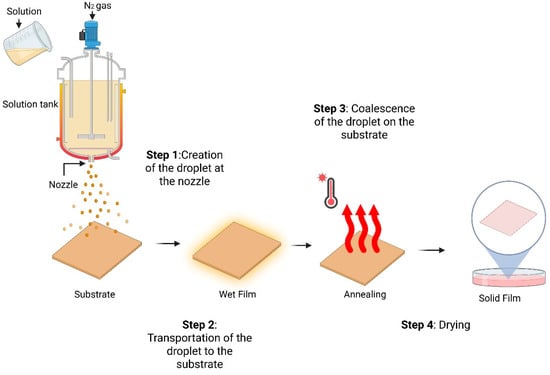
Figure 3.
Schematic diagram of spray coating.
3.4. Extrusion
Extrusion is a continuous and high-throughput manufacturing technique widely employed for shaping thermoplastic polymers into films, sheets, and other functional structures. It is one of the oldest and most important polymer transformation processes, particularly suited for biopolymer film production in food packaging applications [207]. Following the report by [208], as illustrated in Figure 4, raw materials, including natural polymer powders and nanoparticles, are introduced into the extruder. As the mixture traverses the heated barrel of the extruder, the applied heat softens the materials, rendering them pliable for subsequent processing. The rotating screws within the extruder facilitate the blending of the heated components, exerting pressure and shear forces to ensure a uniform distribution of nanoparticles throughout the polymer matrix. The molten material is subsequently extruded through a die, forming thin sheets or films. The extruded material is then cooled by air or water, allowing the film to solidify into its final shape while preserving its structural integrity [209]. The solidified sheets are cut to the requisite dimensions and shapes before packaging as food-safe wrapping or containers. This method offers high production rates and the capacity to fabricate complex shapes [210]. Additionally, edible films can be produced from consumable materials utilizing dry processes, such as extrusion. Extrusion has demonstrated efficacy in generating films with desirable characteristics, including mechanical properties, thermal stability, and antibacterial activity [211]. The extrusion process often yields films exhibiting adequate mechanical properties and robust thermal stability [212]. However, some extruded films have been identified as exceptions, displaying inferior mechanical and oxygen barrier properties. For instance, ref. [213] developed films based on potato peel starch that were plasticized with glycerol; these films demonstrated high transparency, enhanced mechanical properties, improved thermal stability and flexibility, and increased biodegradation in seawater and soil, rendering them suitable for food packaging applications.

Figure 4.
Schematic diagram of an extrusion process [214]. Reproduced with permission from MDPI under the Creative Commons (CC BY) Copyright © 2022 license.
3.5. Compression Molding
Compression molding is a closed molding process used to produce various composite goods [215]. It remains one of the earliest industrial procedures developed for plastics and is sometimes referred to as “matching die molding” [215,216]. This method puts a pre-measured quantity of polymer or biopolymer composite material into a heated mold cavity. This material is then compressed using a matching mold plate under pressure and heat until it takes the shape of the mold. After forming, the resulting product is cooled and demolded, forming a solid film or component with the desired geometry [217] as illustrated in Figure 5. This approach is particularly suited for producing large, complex parts with superior mechanical strength and dimensional stability. Unlike injection molding, it typically incurs lower tooling costs and reduces material waste since it eliminates the need for runners, sprues, or gates [218]. Compression molding has various advantages over injection molding, including lower costs and fewer waste materials, whereas injection molding requires runners, sprues, and gates. Furthermore, the compression molding technique has a comparatively high production rate [219] because the mold cycle time is only a few minutes long [220]. For example, a starch–hydroxypropyl methylcellulose-based film was obtained by compression molding [221], and the addition of HPMC to starch significantly improved the tensile strength and elongation at the break of the films. Also, the films exhibited favorable mechanical properties, making them suitable for various packaging applications where flexibility and strength are crucial.
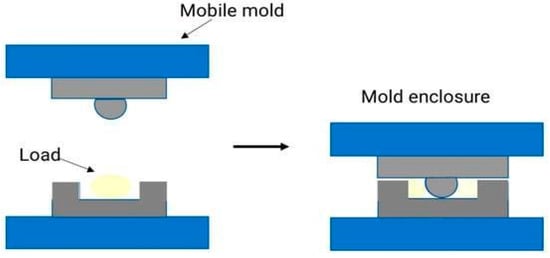
Figure 5.
Schematic diagram for the compression molding process [219]. Reproduced with permission from MDPI under the Creative Commons (CC BY) Copyright © 2023 license.
3.6. Three-Dimensional Printing
3D printing, also known as additive manufacturing, is the process of building products layer by layer from digital models [222]. It is a multidisciplinary technology that combines advanced machinery, computer science, numerical control systems, and materials science [223]. Recent advances in robotics have increased the importance of 3D printing in various industries, including automotive, medicine, textiles, civil engineering, military, and food [224]. It was initially industrialized for modeling plastics but has now been adapted for use in the food sector [225]. When used in food, this additive manufacturing technology is referred to as food-layered manufacturing [226]. This technology allows for the design and fabrication of food personalized to individual health needs and physical activities by controlling the quantity of printing material and nutritional content [227]. Various 3D printing processes, such as fused deposition modeling (FDM), color-jet printing, and selective laser sintering (SLS), use a variety of materials and technologies to produce complicated geometries with high precision (Figure 6) [228], with FDM (Figure 6A) being popular in small businesses due to it being the most affordable. This process involves pushing materials through nozzles at high temperatures and pressures, layering them individually [228]. This technology is commonly used for prototyping, biomedical implants, and custom production [229]. A study by [230] demonstrated that blending PLA with agricultural waste enhanced the film’s mechanical properties, making it more suitable for flexible packaging applications.
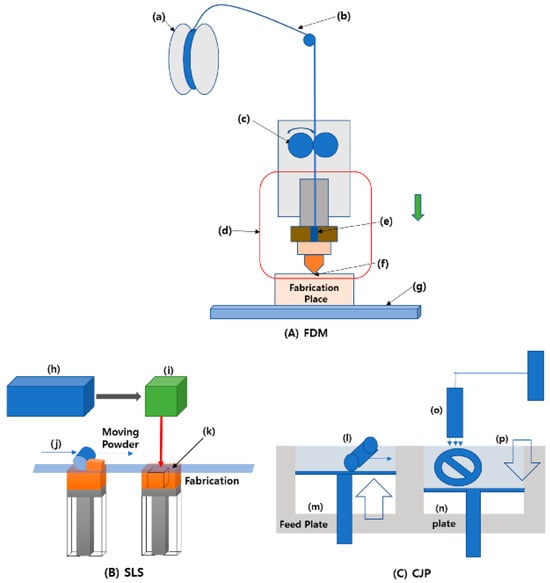
Figure 6.
Schematic diagram of 3D printing machinery. Diagram Label (A): Fused Deposition Modeling (FDM): (a) Coil reel; (b) Plastic filament; (c) Driving motor; (d) Extruder; (e) Molten paste chamber; (f) Nozzle tip; (g) FDM printer bed. Diagram Label (B) Selective laser sintering (SLS): (h) Laser; (i) Scanner system; (j) Roller; (k) Power bed. Diagram Label (C) Color-jet printing: (l) Roller; (m) Powder; (n) Build; (o) Color binder header; (p) Modeling part [228]. Reproduced with permission from MDPI under the license of Creative Commons (CC BY) Copyright © 2021.
3.7. Summary of Fabrication Techniques for Biopolymer-Based Films
Fabrication technique selection is essential because it governs structural, mechanical, and functional properties of biopolymer films/coatings by dictating their molecular interactions [219]. Solution casting, 3D printing, electrospinning, spray coating, molding, and extrusion represent key materials processing techniques with unique operational principles, advantages, and limitations [231,232,233,234]. These methods can be used in diverse fields such as biomedical, engineering, and pharmaceutical [235,236]. While these techniques have been explained in detail in the previous section, Table 8 summarizes their operational principles, advantages, and limitations associated with each method, facilitating a more informed selection based on specific application needs in food packaging and related fields.

Table 8.
Principles, advantages, and limitations of the fabrication techniques.
3.8. Nanocomposite Approaches
Nanocomposites are materials that have a nanoscale structure and improve the macroscopic qualities of products [251]. Nanocomposites represent sophisticated materials synthesized by integrating a matrix, commonly a polymer [252], with nanoscale reinforcing agents to enhance mechanical strength, thermal resilience, and electrical conductivity. These reinforcing agents may be nanoparticles, nanofibers, or nanoplates embedded within the matrix at the nanoscale [190]. Polymer nanocomposites can generally be categorized into three types: (i) intercalated nanocomposites, (ii) flocculated nanocomposites, and (iii) exfoliated nanocomposites [253]. At least one of the filler dimensions is on the nanoscale [254]. Nanocomposites have been reported to have varying degrees of property enhancements [255]. The production of nanocomposites involves adding nanoscale fillers, such as nanoparticles, nanotubes, or nanoclays, to a polymer matrix to improve its properties [256]. These materials exhibit better mechanical, thermal, and electrical properties than their bulk counterparts [257,258]. The poor mechanical and barrier qualities of some bio-based packaging materials, relative to conventional materials, can be enhanced by the integration of nanoparticles [259]. Solution mixing, melt blending, and in situ polymerization are some of the methods used to create nanocomposites [255]. Using composites that incorporate nanoparticles offers advantages over using bare nanoparticles alone, as they enhance monitoring capabilities and improve treatment processes [260]. In creating these composites, nanoparticles are combined with other materials, such as metals, polymers, or both organic and inorganic substances, leading to improved performance and functionality [261]. Nanocomposites have been reported to exhibit varying degrees of property enhancements (Table 9).

Table 9.
Nanofillers used in nanocomposite-based food packaging.
4. Properties of Bio-Based Natural Polymer Coatings/Films
Bio-based natural polymer coatings and films have emerged as potential solutions in a variety of industries due to their sustainable and environmentally friendly properties [23]. These materials, generated from renewable sources such as plants, animals, and microbes, have inherent biodegradability, which reduces environmental effects when compared to their petroleum-based counterparts [268]. However, barriers to the widespread adoption of bio-based natural polymer coatings and films include high costs and poor mechanical qualities [269]. To overcome these constraints and maximize their potential, researchers are continuously investigating novel structural configurations and processing methodologies [270]. This section summarizes an overview of the qualities of bio-based natural polymer coatings/films, providing light on their mechanical, barrier (gas and moisture), thermal stability, optical properties, antimicrobial activity, and environmental impact, as well as possible applications in a variety of industries.
4.1. Mechanical Properties
Mechanical properties are essential determinants of the performance and suitability of natural bio-based polymer coatings [271]. These properties, alongside unique physical, chemical, or biological design criteria, are necessary to ensure product safety and high quality for consumers. Over the past decade, various materials have been employed to develop biodegradable polymer composites aimed at enhancing their mechanical properties [272]. The incorporation of inorganic fillers into these polymers can significantly influence the mechanical characteristics and hydrophobicity of the resulting composites, which are critical for biomedical applications [273].
Mechanical properties encompass a range of factors that affect a material’s response to external forces and mechanical stresses. Key mechanical properties considered for natural bio-based polymer coatings include elongation at break, adhesion strength, flexural strength, modulus, coefficient of friction, hardness, impact resistance, and tensile strength.
- Elongation at break quantifies how much a material can stretch before breaking, with higher elongation values signifying increased ductility and flexibility, which are advantageous for coatings subjected to elongation or deformation [274].
- Adhesion strength refers to the bonding force between the coating and the substrate surface. According to literature reports, the tensile strength of composite films decreases with poor adhesion, underscoring the necessity for robust adhesion to prevent delamination or separation of the coating from the substrate, thereby ensuring long-term durability and performance [275,276].
- Tensile strength represents the maximum stress a material can withstand before failure under tension, indicating the coating’s ability to endure deformation under such conditions [277].
These mechanical properties are influenced by polymer synthesis, blends, additives, chemical modifications, or combinations thereof, as well as processing techniques. It is also crucial to evaluate the effects of low temperatures (e.g., frozen products stored at −18 °C) and humidity (e.g., packaging saturated with water due to high condensation, chopped fruits, etc.) on mechanical properties [278]. Naturally sourced materials present a viable option for food packaging, given their capacity for partial or complete biodegradation. However, these natural polymers often exhibit higher costs and limited mechanical strength. Therefore, it is essential to investigate diverse structural arrangements to achieve the desired product characteristics [279].
4.2. Barrier Properties (Gas and Moisture)
Packaging plays a vital role in safeguarding products from the external environment [280]. The packaging material should possess outstanding barrier properties to prevent the passage of various substances like moisture, gases, and lipids through the packaging wall [281]. It is widely acknowledged that quality packaging plays a pivotal role in promoting sustainability, driving technological advancements, and minimizing food loss by preventing the transfer of moisture and oxygen between products and their surroundings [282]. Barrier materials are essential for maintaining product quality, safety, and durability by preventing oxygen and water transfer, which are primary causes of food spoilage, making airtight seals and superior barriers key strategies for extending shelf life [283]. These materials find applicability across diverse sectors. The barrier film market, valued at USD 34.1 billion in 2023, is expected to reach USD 45.1 billion by 2028, driven by traditional plastics like PET, LDPE/HDPE, and PP, which pose environmental concerns [282].
4.3. Thermal Stability
Thermal stability refers to how well a material’s properties, such as oxidation resistance, structure, composition, and mechanical strength, remain unchanged or adapt to temperature changes [284,285]. These properties include oxidation, structure, composition, mechanical properties, and more [284]. Thermal stability addresses how temperature influences adhesive curing, the impact of high temperatures, and fire damage during production [286]. Various methods are employed to analyze the thermal characteristics of a substance. One such method is thermogravimetric analysis (TGA), which evaluates the alteration in mass, temperature of thermal degradation, and thermal stability of composite materials [287]. Moreover, thermal properties hold equal significance in structural uses like heat transfer from one end to another, the capacity to withstand loads at temperatures, material response, and maintaining dimensions at high temperatures [286].
The thermal characteristics of biopolymers are important in deciding their appropriateness for manufacturing and usage as a coating substance [288]. Polymers derived from renewable sources, such as PFA, demonstrate advantages like high thermal stability. Thermal analysis allows researchers to identify the exothermic crystallization peak, which provides insights into and helps model the crystallization behavior of new bio-based polyesters [289]. As the thermal stability of a nanostructure decreases, the coating material undergoes a transition to a typical crystalline form due to grain enlargement. This process causes the nanostructure to break down and leads to the formation of new crystalline phases [284]. Consequently, nanocomposite coatings lose their unique properties when exposed to elevated temperatures, resulting in changes to the film’s overall characteristics.
4.4. Optical Properties
Optical characteristics describe how materials intermingle with light; these characteristics govern how light is absorbed, transmitted, reflected, or scattered by a substance [290]. Scattering can alter the purity and appearance of films and coatings. The refractive index influences light bending, which is important for lens design and optical applications [291]. Opacity is critical in applications like protective coatings and food packaging [292]. The transmittance and reflectance of bio-based films are important considerations in determining their appropriateness for various applications. Reflectivity influences both the appearance and energy efficiency of materials; high reflectivity can reduce heat absorption [293]. Another important optical attribute of bio-based films is their capacity to absorb ultraviolet rays [294]. Absorbance properties are vital for food packaging, as UV light can destroy sensitive materials [295].
Chitosan and its derivatives exhibit effective UV-blocking qualities, which can be linked to the polymer’s inherent structural characteristics [296]. Incorporating natural extracts, such as green tea or turmeric, into biopolymer matrices can also improve UV protection due to their antioxidant characteristics [297]. Color is important in applications like packaging because it influences consumer perception [298]. The composition of bio-based films and any added substances can affect their hue. Natural pigments that provide extra health advantages, such as carotenoids from carrots or anthocyanins from berries, can add color to food [299,300]. The product’s hue influences both its visual appeal and its capacity to shield its contents from light exposure [301].
Natural polymers, including chitosan, cellulose, gelatin, and starch, have distinct structural features that affect their optical qualities [302]. Chitosan films, for example, are well-known for their transparency and UV-blocking properties, which are attributed to the presence of amino and hydroxyl functional groups that can interact with light [303]. Similarly, cellulose-based films have good light transmission, which is useful for packaging applications that need product visibility [304]. For bio-based natural polymer coatings and films to function well in a variety of applications, their optical characteristics are essential [81,305].
4.5. Antimicrobial Activity
The antimicrobial properties of bio-based natural polymer coatings and films have garnered significant attention due to their importance in applications related to food packaging, such as agricultural products, and biomedical devices, by preventing microbiological growth while also benefiting the environment [306]. Natural polymers, including chitosan, cellulose, and gelatin, have inherent antibacterial capabilities due to their biochemical composition [307]. Unlike other antimicrobial polymers, chitosan offers several advantages, including its natural origin and abundance, as well as its effective antimicrobial properties [283,308]. It is also safe for use in tissue engineering, drug delivery, wound dressing, food, and plant protection applications [309,310], having been approved by both the FDA and the EU [311].
In addition, chitosan has strong mechanical, barrier, and film-forming qualities; as a result, food packaging can greatly benefit from its use [283]. Chitosan, a derivative of chitin, contains amino groups that can break microbial cell membranes, resulting in cell death [312,313]. Thus, chitosan has drawn a lot of attention in active food packaging applications [314] due to its exceptional film-forming ability and antimicrobial qualities through electrostatic exchanges and interactions that disrupt the microbial cell and ultimately cause cell death through proper production of DNA or RNA [315]. Many natural polymers can gradually release antimicrobial compounds, offering long-term protection from infections [316].
Bio-based coatings with antibacterial characteristics are especially useful in the food industry. They can increase shelf life by preventing the growth of rotting organisms and diseases [317]. For example, chitosan-based films have been proven to minimize microbial contamination in fruits and vegetables, extending their storage life [318]. The addition of essential oils, such as oregano or thyme, to these films has been reported to increase their antibacterial activity [319]. Bio-based natural polymers are used in biomedical applications for wound dressings and drug delivery systems due to their biocompatibility and antibacterial qualities [309]. Gelatin-based films or coatings, for example, have been shown to have antibacterial properties against fungi, yeast, or common pathogens such as Staphylococcus aureus and Escherichia coli [320,321]. These films can help improve healing and avoid infections. Despite the potential antibacterial qualities of bio-based coatings, problems persist. Material stability, antibacterial activity variability, and food application regulatory approval are all important considerations [322].
More research is needed to improve the durability and effectiveness of these coatings by novel modifications such as nanotechnology and the addition of new bioactive substances [316]. Bio-based natural polymer coatings and films have thus demonstrated significant antimicrobial activity, making them valuable for food packaging and biomedical applications [323]. Nevertheless, continued research and development are needed to further enhance their efficacy and expand their practical uses, contributing to safer and more sustainable materials.
5. Enhancement Strategies
To improve the effectiveness of bio-based natural polymer coatings and films for food preservation, various approaches have been explored to enhance the application of these materials. These strategies focus on improving the mechanical barrier and antimicrobial properties of the films. Some of these approaches are discussed in this section.
5.1. Incorporation of Antimicrobial Agents
The inclusion of antimicrobial compounds such as essential oils and nanoparticles into diverse products is a promising approach to combating microbial contamination [259,324]. Their use in food packaging, medical gadgets, and other materials increases product safety while extending shelf life and improving health outcomes [325]. Nevertheless, several studies are still needed to optimize their use and ensure safety and efficacy in various applications [324]. Recently, numerous reports have been conducted on the use of natural biopolymers such as chitosan, organic acids, and essential oils [326]. Despite being a biopolymer for food packaging, chitosan has no appreciable antibacterial and equivocal antioxidant properties [327]. Hence, property enhancement is required as the antibacterial activity of chitosan could broaden its applications in food packaging.
An edible coating with antibacterial properties has the potential to avoid contamination in food products [328]. Consequently, materials such as essential oils (EOs) can be added to the edible film to improve its antibacterial capabilities [108]. Essential oils are hydrophobic liquids made up of volatile aromatic chemicals that have a variety of properties, including antibacterial, antioxidant, anti-inflammatory, analgesic, anti-depressive, and calming [329]. They are well-known in the pharmaceutical and medical fields, as well as the gastronomy and food packaging industries. The essential oils are derived from various parts of aromatic plants such as wood, roots, barks, seeds, peel, fruit, leaves, flowers, and entire plants [330]. The Food and Drug Administration (FDA) has certified essential oils (EOs), which are natural and highly efficient antimicrobials against bacteria and fungi, for use in food such as GRAS (generally recognized as safe) [331].
Additionally, some nanoparticles (like silver and ZnO nanoparticles) have recently gained attention as effective and safe materials for enhancing food packaging due to their antibacterial properties [332]. Their high surface area-to-volume ratio improves their contact with microbial cells, resulting in effective microbial suppression [333]. Silver nanoparticles are used in textiles, medical equipment, and wound dressings to help prevent infections and improve healing [328]. Similarly, other nanoparticles, like zinc oxide and titanium dioxide, have antibacterial properties and are employed in comparable applications [334]. The inclusion of antimicrobial compounds such as essential oils and nanoparticles into diverse products is a promising approach to combating microbial contamination [335]. Nevertheless, it is necessary to optimize their use and ensure their safety and efficacy in various applications. Several studies involving the use of these materials are summarized in Table 10.

Table 10.
The use of antimicrobial agents on bio-based polymers.
5.2. Crosslinking Methods
Crosslinking is a critical step that has a substantial impact on the mechanical and physicochemical properties of bio-printed constructions, as well as the biological behavior of loaded live cells [342]. Crosslinking techniques are often employed to enhance the barrier characteristics and mechanical integrity of biopolymer-based polymers utilized in food applications [26], making it crucial for developing resilient, sustainable packaging that is biodegradable and environmentally benign. There are different types of crosslinking, such as covalent, ionic, or physical, with covalent being the most stable [343].
Ionic interaction is a widely used method for crosslinking hydrogels in 3D bioprinting by adding multivalent cations to the polymer solution [342]. It allows for rapid crosslinking under mild conditions and physiological pH. However, it has limitations, such as weaker mechanical properties and the potential release of metal ions into the body [342]. Ionic interaction is commonly used for the 3D bioprinting of sodium alginate, an algae-derived anionic polysaccharide [344]. Carboxylic groups of adjacent polymer chains can bind with Mn+, resulting in an ionically crosslinked gel network [345]. The physicochemical and mechanical properties of alginate gels are influenced by the concentration of polymer solution [346]. The type of ionic crosslinker also affects material printability [347].
Calcium is a popular ionic crosslinker due to its high solubility in aqueous conditions, which results in quick gelation [348]. Due to the quick deposition of calcium ions outside the gel, the final 3D-printed structure may be less stable [349]. Adding ions to the polymer solution in 3D bioprinting must be controlled to ensure structural stability, as ionic crosslinkers can impact cell viability and proliferation [350]. While they protect cells from harsh conditions, they can also create non-physiological environments, reducing cell viability. Various cell types, including fibroblasts, myoblasts, endothelial cells, chondrocytes, and Schwann cells, have been incorporated into alginate solutions [342]. Excessive Ca2+ levels can be toxic and harm cell membranes [349].
Photo crosslinking is another notable crosslinking approach that is particularly important in 3D printing applications, as several 3D printing firms have used this crosslinking approach to manufacture their products [351]. Photo crosslinking is an efficient approach for transforming photocurable bioinks into 3D architectures [352]. It is inexpensive and can be done at room temperature, which saves electricity [353]. Other 3D bioprinting methods include stereolithography, digital light processing, laser-assisted approaches, and volumetric bioprinting [354]. These approaches use photo initiators to crosslink bioinks via chain-growth, step-growth, or redox-based processes. Additionally, thermal crosslinking, achieved by heating or chilling a polymer solution, is a simple method suitable for heat- or cold-resistant polymers, especially during 3D printing. However, it has a longer gelation time and lacks precise control over the degree of crosslinking [342]. Several studies involving the use of these materials are summarized in Table 11.

Table 11.
Application of bio-based natural polymer crosslinked coatings on food products.
5.3. Surface Modification Techniques
Numerous surface modification processes provide a range of properties, such as enhanced barrier properties, mechanical stability, and antimicrobial resistance, which are crucial for food packaging applications [359]. For a certain type of coating, choosing a suitable coating technique is necessary as it necessitates meticulous evaluation of mechanical stability, corrosion resistance, biocompatibility, and material properties since each approach presents distinct alternatives to create consistent coatings at room temperature [360]. It has also been used to deposit calcium orthophosphate coatings, which can have antimicrobial effects [361,362,363]. This method is frequently carried out using aqueous solutions, like wet-chemical deposition [361].
On the other hand, thermal spray coatings offer another method to enhance the mechanical and barrier properties of packaging films. There are various thermal spray processes, including flame spraying and plasma spraying, which provide dense coatings with improved adhesion and less porosity [243]. These processes can be particularly useful for creating robust, durable packaging materials. Plasma spraying is ideal for situations requiring higher density and lower porosity, ensuring better protection [364].
Micro-arc oxidation (MAO) is another technique used to improve surface properties such as corrosion resistance and wear resistance [365]. This technique has been effective in improving the properties of packaging materials that need to withstand harsh environments or maintain food quality for extended periods. Lastly, sol-gel coating is a chemical process that may produce films at low temperatures, making it ideal for coating sensitive bio-based materials [366]. The process uses liquid precursors, allowing for the formation of thin films without high energy consumption or degradation of sensitive components [367].
Sol-gel coatings are particularly beneficial for food packaging because they enhance barrier properties while maintaining the environmental benefits of low-energy consumption and minimal pollution [368]. In summary, various surface modification techniques such as electrodeposition, thermal spraying, MAO, and sol-gel coating are critical enhancement strategies in food packaging. These methods can significantly improve the mechanical strength, barrier properties, and antimicrobial functionality of bio-based packaging films, ensuring better food preservation and extending shelf life. Several studies involving the use of these materials are summarized in Table 12.

Table 12.
Surface modification methods on natural bio-based polymers.
6. Application for Food Preservation
As already established in previous sections, bio-based natural polymer coatings and films have gained significant attention in food preservation due to their ability to extend the shelf life of food products while maintaining quality and safety and being eco-friendliest to the environment [374,375,376]. These eco-friendly materials, thus, act as protective barriers against external factors like moisture, oxygen, and microbial contamination [377]. These materials function by creating a modified atmosphere around the food, which in turn minimizes oxidation, spoilage, and dehydration, and ultimately ensures that food products retain their nutritional value and sensory characteristics for longer periods [378]. Furthermore, the incorporation of active agents, such as antimicrobials and antioxidants, enhances the preservation properties of these films, making them ideal for various fresh and processed foods [379]. With growing consumer demand for sustainable packaging solutions, bio-based polymer coatings offer a promising approach to reducing food waste while supporting environmental sustainability. Their application thus ranges from fresh produce to different types of meats.
6.1. Fresh Produce
In contemporary agricultural practices, fruits and vegetables are recognized as essential produce due to their nutritional properties, such as dietary fiber, vitamins, minerals, etc. [380]. The preservation of postharvest quality is critical due to their high water content and respiratory activity [381]. Following harvest, fruits and vegetables naturally undergo a biological process known as respiration, during which the respiration rate elevates, leading to further maturation and eventual senescence [382]. During the packing and transit phases, these agricultural products are subjected to various physical stressors [383]. In this context, bio-based natural polymer coatings and films play a significant role by regulating the microenvironment, mitigating postharvest quality degradation, and extending shelf life due to their non-toxic properties. Table 13 details commonly utilized bio-based natural polymers, including polysaccharides such as gums, pectin, starch, chitosan, alginate, and cellulose [384].
These materials are typically translucent and edible, demonstrating excellent gas barrier properties conducive to maintaining freshness [288]. Although protein-based films are known to be moisture-sensitive, they are widely used owing to their robust mechanical properties. Additionally, lipids are incorporated into these films due to their hydrophobic characteristics, which help minimize water loss in fresh produce [385]. However, lipid films alone lack sufficient mechanical strength for standalone applications, necessitating their combination with other materials. Notably, the inclusion of lipids enhances the moisture barrier properties of the films [386]. Lastly, mucilage, although less well-known, is a promising substance derived from various plant species. The mucilage from Opuntia ficus-indica has garnered attention for its exceptional nutritional attributes, including high concentrations of calcium and potassium, excellent water retention capacity, and bioactive phytochemical properties.
These characteristics render it a favorable candidate for utilization in the food industry [387]. Mucilage-based coatings have the potential to effectively retain moisture and prolong shelf life [388]. For instance, coatings derived from Opuntia ficus-indica mucilage are particularly effective in preserving the quality of banana fruit and extending its shelf life [389]. Similarly, ref. [390] reported that cactus mucilage coatings enriched with ascorbic acid were highly efficient in maintaining the quality of roasted pecan nuts during storage. More examples of the application of edible coating/film on fruits and vegetables are in Table 13.

Table 13.
Application and research findings of specific bio-based natural polymer coatings/films on fruits and vegetables.
Table 13.
Application and research findings of specific bio-based natural polymer coatings/films on fruits and vegetables.
| Fruits and Vegetables | Edible Coating/Film Used | Outcomes | References |
|---|---|---|---|
| Pomegranate | Chitosan-based coating | Delayed fruit metabolic changes, higher antioxidant properties, maintained quality and reduced the fruit’s susceptibility to physiological disorders. | [298,391,392] |
| Arabic Gum and starch-based | The overall quality of the fruit was maintained, and shelf life was extended. | [393] | |
| Mango | Chitosan coating | Shelf life was extended, and the overall quality of the fruit was improved. | [42,394] |
| Banana | Opuntia ficus-indica mucilage coating | Extended the banana’s shelf life and preserved its quality. | [389] |
| Arabic Gum and chitosan | Coating efficiently maintained the quality of the bananas, preventing weight loss and preserving fruit firmness, and extended shelf life up to 33 days. | [395] | |
| Starch coating | The starch-based edible coating effectively delayed ethylene production, reduced respiration rates, and slowed chlorophyll degradation, helping to retain fruit firmness and reduce weight loss. This improved the commercial value of bananas and extended their shelf life by 12 days. | [396] | |
| Guava | Chitosan and alginate-based coating | Enhance the quality of fruit and retain the nutritional parameters. | [397] |
| Arabic Gum-based edible coating. | These applications reduced weight loss, decay, and Rhizopus rot while increasing marketability and delaying fruit softening. They preserved chlorophyll, vitamin C, and acidity, and slowed changes in TSS and TSS/acid ratios. Overall, the coatings significantly extended the shelf life compared to untreated fruits. | [398] | |
| carboxymethyl cellulose-based edible coating | The treatment effectively delayed weight loss and decay in guava fruit while maintaining firmness, sugars, acidity, ascorbic acid, phenol content, and sensory quality for up to 12. | [399] | |
| Mandarins | Arabic Gum–Zinc Oxide Nanoparticles Composite Coating | Gum Arabic enriched with ZnO-NPs effectively extended the storage period of ‘Kinnow’ mandarins by reducing rind disorders, minimizing metabolic rate and electrolyte leakage, and preserving fruit texture and overall quality. | [400] |
| Chitosan-based | The coating preserved bioactive compounds and organic acids effectively. | [47,401] | |
| Carboxymethyl cellulose coating | Effectively preserved the postharvest quality of “Kinnow” mandarins, improved membrane integrity and increased the activity of antioxidant enzymes. | [402] | |
| Alginate-based edible coating | Extended the shelf life after harvest and preserved the quality of the fruit. | [403] | |
| Pecan nuts | cactus mucilage coatings | Coatings effectively preserved the quality of microwave-roasted pecan nuts during storage, maintaining key physicochemical and phytochemical properties, reducing enzyme activities, and enhancing antioxidant properties. | [390] |
| Pear | Chitosan-based coating | The coating extended the post-harvest life of ‘Yali’ pears by reducing weight loss and maintaining quality. | [52] |
6.2. Meat and Seafood
The shelf life of some foods can be drastically shortened without the use of chemical additives or preservation methods; raw meat and seafood are especially susceptible [404]. Their high moisture and nutritional content, delicate connective tissues, and neutral pH all contribute to their perishability by providing the ideal conditions for microbial development and spoiling [405]. The initial microbial load and the preservation techniques used have a significant impact on shelf life [406,407]. Any physical, chemical, or sensory change that makes food unsuitable for human eating is referred to as food spoiling [408]. Microbial metabolism frequently causes this degradation, which breaks down dietary ingredients and results in unfavorable alterations in texture, appearance, and odor [409]. Microbial contamination is frequently caused by the natural microbiota of animals as well as environmental and handling factors during production [410]. This bacterial growth can lead to slime production, unpleasant smells, and structural deterioration, all of which can compromise the product’s quality and safety [411].
Factors such as vitamin E levels, fatty acid composition, and prooxidants like free iron significantly influence lipid oxidation, resulting in the formation of compounds that compromise color, nutritional value, and overall quality [412]. The breakdown of proteins, lipids, and carbohydrates, which occurs even at low temperatures (5 °C) due to enzymatic autolysis, leads to meat softening, discoloration, and the promotion of microbial growth and spoilage [413]. Globally, about 3.5 billion kg of poultry and meat are wasted annually across retail, foodservice, and consumer levels, causing major economic and environmental impacts. Microbial spoilage is a leading cause of this meat degradation [411]. Therefore, effective preservation of raw meat and seafood is crucial to prevent the loss of this nutritionally rich natural resource [414]. Recent research has primarily focused on the use of bio-based natural polymer coatings and films to maintain the quality of fresh meat and seafood, as shown in Table 14. Among these, chitosan-coated films have emerged as the most widely utilized bio-preservation films in meat products [354]. For example, ref. [415] studied the usage of an edible coating made of eugenol emulsion mixed with chitosan, and the findings showed that the coating helped preserve the meat’s red color for 14 days and prevented deterioration. Even after 14 days of storage, the meat’s pH, total microbial count, and TVB-N levels remained within acceptable quality criteria, suggesting that the shelf life was increased from 6 to 14 days. More examples in Table 14.

Table 14.
Application of bio-based natural polymer coatings/films on meat or seafood.
6.3. Bakery and Confectionery Products
Bakery products constitute one of the most significant staple foods consumed daily by individuals across the globe [422]. In many industrialized nations, biscuits represent a substantial segment of the confectionery industry, and their consumption is similarly experiencing rapid growth in emerging economies [423]. However, the impact of confectionery products on economic, environmental, and health sustainability is comparatively underrepresented; for instance, in developed countries, bread accounts for nearly half of the daily recommended carbohydrate intake [424]. Bakery items, such as cakes and bread, typically exhibit a short shelf life of three to five days at room temperature in the absence of preservatives [425]. During this period, they undergo various physical, chemical, and microbial changes. Physical and chemical alterations lead to a decline in freshness, texture, and flavor, while microbial spoilage caused by the growth of bacteria, yeast, and mold adversely affects their visual appeal [426]. The emergence of off-flavors and mycotoxins in bread products because of microbiological spoilage poses significant public health risks and results in considerable financial losses for both the baking industry and consumers [427].
To mitigate these challenges, preservation methods are essential; however, the most employed preservation techniques have demonstrated several notable disadvantages, including condensation issues, inadequate penetration capabilities, and high costs [4,7]. Advancements in food packaging technology have facilitated the development of active packaging systems, which encompass antimicrobial packaging, antioxidant packaging, moisture absorbers, ethanol emitters, and carbon dioxide emitters. Recent innovations in active packaging include the creation of nanomaterial packaging utilizing metallic nanoparticles, and edible coatings/films containing proteins [75], each of which presents distinct advantages and disadvantages. Several studies in bio-based natural polymer coatings/films for bakery products are summarized in Table 15.

Table 15.
Application of bio-based natural polymer coatings/films on bakery and confectionery products.
6.4. Dairy Products
Dairy products are recognized for their substantial nutritional value and are extensively incorporated into balanced dietary regimens [434]. In addition to supporting bone health, these products offer several other health benefits, including positive effects on gastrointestinal health and immune function [435]. However, dairy items such as milk, cheese, butter, and eggs are prone to spoilage [436]. Therefore, effective preservation methods are essential to enhance their shelf life, maintain quality, and ensure safety [437,438]. In response to these challenges, a variety of preservation strategies have been developed, encompassing both traditional and innovative techniques [439]. Among these, natural wax-coated papers have emerged as a promising alternative packaging material, providing the necessary barrier properties to be in direct contact with food products, including confectionery, dairy, and baked goods. For example, ref. [440] discovered that after 14 days of using the coated paper in cheese packaging, the cheese shelf life improved. Other examples of bio-based natural polymer coatings/films on dairy products are summarized in Table 16.

Table 16.
Application of bio-based natural polymer coatings/films on dairy products.
6.5. Ready-to-Eat Meals
Ready-to-eat meals are defined as food items that have undergone minimal processing and can be consumed directly from the packaging without any additional preparation [446]. These meals encompass a variety of categories, including salads, ice creams, salty seafood, fresh-cut fruits and vegetables, and meat products. In recent decades, there has been a notable increase in the attention and popularity of ready-to-eat meals as consumers have become increasingly aware of the importance of freshness, convenience, and nutritional value in their food choices [447,448]. The ready meals market has demonstrated robust growth in recent years, expanding from USD 184.38 billion in 2024 to a projected USD 201.82 billion in 2025, representing a compound annual growth rate (CAGR) of 9.5% [449]. It is expected to experience significant growth, reaching USD 303.09 billion by 2029 with a compound annual growth rate (CAGR) of 10.7%.
However, food items, particularly those that are freshly made, are highly susceptible to spoilage due to factors such as enzymatic browning, moisture loss, and microbial growth [450]. These processes can adversely affect the texture, color, and flavor of fruits [451]. Given the expansion of the ready-to-eat food market, there is a pressing need for innovative preservation techniques that can extend the shelf life of minimally processed foods while ensuring their quality and safety [259]. Effective control of spoilage mechanisms necessitates the utilization of appropriate packaging materials [452]. A promising approach for preserving ready-to-eat foods, which balances sustainability and usability, involves the application of edible coatings/films [453,454]. This method provides a partial barrier against carbon dioxide, moisture, and oxygen, facilitates the transfer of additives, prevents the loss of volatile compounds, and can occasionally assist in the synthesis of aroma volatiles [455,456]. Other examples are summarized in Table 17.

Table 17.
Application of bio-based natural polymer coatings/films on ready-to-eat food.
7. Limitations, Gaps and Future Directions
While bio-based polymers offer clear sustainability benefits, their commercial viability for food packaging remains constrained by several technical limitations [169]. One of the primary challenges lies in their relatively poor mechanical properties when compared to synthetic polymers [459,460,461,462]. Bio-based polymer films exhibit lower tensile strength, limited flexibility, and reduced puncture resistance compared to conventional packaging materials [459,463,464,465]. These limitations significantly constrain their application in scenarios requiring durable packaging, particularly for moisture-sensitive products or heavy food items where structural integrity is vital throughout the supply chain [466]. Additionally, some biopolymers have been documented to show exceptional gas barrier properties, such as effectively blocking oxygen and carbon dioxide to preserve food freshness and prevent oxidation [467]. Nevertheless, they typically perform poorly as moisture barriers due to high water vapor permeability. This severely restricts their use for high-moisture food applications, as the water migration can promote microbial contamination, texture deterioration, and reduced product safety and shelf life [468]
Variability in raw material sources also poses a significant challenge [469]. These biopolymers derived from sources such as plant starch, cellulose, pectin, or animal proteins exhibit natural variations due to growing conditions, seasonal changes, and regional climate differences [470]. Such variability in polymer composition leads to inconsistent film performance, making it challenging to maintain standardized quality across production batches. Consequently, this affects both the reproducibility of manufacturing processes and the reliability of food preservation results. Additionally, susceptibility to microbial degradation poses a major concern [471]. While their biodegradable nature offers clear environmental benefits, their characteristics compromise their functional stability and reduce their effective shelf life during practical use. The industry continues to face the technical challenge of maintaining microbial safety while adhering to clean label standards that exclude synthetic preservatives [270].
The economics of producing natural biopolymers have also emerged as a significant challenge. The production processes of natural biopolymers, including extraction, purification, and formulation, typically sustain higher costs compared to conventional plastic production [472]. These elevated expenses stem from multiple factors: labor-intensive processing methods, relatively low raw material yields, and the requirement for specialized equipment. Such cost structures pose challenges for small and medium-scale producers, potentially limiting their competitiveness in large-scale commercial applications where price sensitivity prevails [473].
In summary, in spite of the advances in the use of natural biopolymers for food packaging, all of the stated concerns continue to create critical research gaps and require further research. Economically, the production, processing, and scale-up costs of these materials are generally higher than those of conventional petroleum-based plastics, which in turn makes cost-efficiency a primary concern for industrial stakeholders [474,475]. Moreover, there is still a need for detailed and comprehensive studies in which long-term performance is evaluated under varying environmental conditions such as temperature, humidity, and mechanical stress. Also, an essential concern is the consumer acceptance profile, which remains a key bottleneck, as perceptions around safety, taste, appearance, and hygiene continue to influence adoption [476,477].
Consequently, addressing these concerns remains a critical hurdle that most likely requires interdisciplinary efforts that will have to focus on polymer formulation optimizations, sustainable and low-cost fabrication technologies, harmonization of regulatory standards, public awareness enlightenment sessions, and possible green market alternatives incentives [478]. These directions hold the potential to significantly advance the scalability, acceptance, and commercial viability of bio-based polymer applications in food systems.
To this end, several approaches are being employed, such as the incorporation of bioactive substances into the polymer matrix, including antioxidants, antimicrobials, and essential oils, to improve functional properties. In addition, most of these additives also enhance the mechanical strength and barrier qualities, prolonging the shelf life of perishable food items, as already documented in the literature [479,480]. Antioxidants such as ascorbic acid and tocopherol can slow down lipid oxidation [481] and color degradation, while antimicrobial peptides or natural extracts like thymol or eugenol can prevent microbial growth on the food surface. Moreover, recent advancements in edible films have embraced the integration of probiotics and other beneficial microorganisms, which not only improve the nutritional value of the packaged food but also contribute to gut health. According to Díaz-Montes and Castro-Muñoz [482], such approaches have opened new avenues in functional packaging, where biopolymers serve as both a physical barrier and a delivery system for health-promoting agents. In addition, comprehensive life cycle assessments to evaluate the environmental impacts across all stages from raw material extraction to disposal are essential. Such analyses are important to substantiate their ecological benefits and inform regulatory frameworks. Concurrently, research should examine consumer perceptions toward edible coatings and biopolymer packaging, addressing safety perceptions and market readiness. Consumer education and awareness campaigns may also be necessary to boost confidence in these emerging technologies and ensure the successful integration of these sustainable packaging solutions.
Therefore, while bio-based polymers demonstrate compelling sustainability advantages for food packaging, their commercial implementation requires multidisciplinary research and innovation to overcome current limitations. By addressing mechanical weaknesses, enhancing functional performance with bioactive agents, and aligning production with economic and consumer expectations, the future of bio-based edible coatings/films can be both environmentally sustainable and commercially successful.
8. Conclusions
This review has examined the extensive range of natural polymers, including chitosan, starch, cellulose, alginate, and mucilage, highlighting their potential as film-forming materials and edible coatings for the preservation of perishable products such as fruits, vegetables, meat, seafood, and ready-to-eat items. Chitosan is particularly distinguished by its exceptional film-forming capacity and inherent antimicrobial properties, rendering it highly suitable for active packaging of perishable foods. In contrast, while starch is widely available and cost-effective, its susceptibility to moisture limits its independent application, necessitating blending with other materials. Similarly, cellulose is noted for its good mechanical strength and gas barrier properties; however, on its own, it lacks the often-required inherent antimicrobial activity, which restricts its functional effectiveness. Valued for its oxygen barrier capabilities and compatibility with bioactive agents, alginate has been utilized in the production of packaging materials for fresh produce. Proteins, due to their nutritional benefits and film-forming capabilities, are increasingly being investigated for use in edible packaging applications. These characteristics have led to a diverse range of ongoing research into these materials. From a commercial viability standpoint, starch-based materials offer the most significant scalability owing to their low cost and widespread availability. In contrast, chitosan and alginate, although functionally superior in certain contexts, encounter challenges related to cost and sourcing. This variability has prompted the exploration of diverse fabrication methods, including solution casting, electrospinning, spray coating, extrusion, and 3D printing, to enhance the structural and functional properties of these bio-based materials. Moreover, the incorporation of additives, such as bioactive agents, including antimicrobials, antioxidants, and probiotics, has augmented the value of these films, improved their preservative properties, and ensured food quality and safety. Despite the numerous advantages of these materials, several limitations persist, particularly regarding film uniformity, mechanical integrity, moisture barrier properties, and production scalability. Additionally, variability in raw material quality and the susceptibility of natural polymers to microbial degradation pose practical challenges for commercial application and long-term storage. To address these significant obstacles, future research should concentrate on optimizing the film-forming process, integrating multifunctional bioactive compounds, and developing low-cost, high-yield manufacturing processes. Furthermore, assessing the environmental impact and consumer perception of these bio-based solutions is essential for promoting their adoption within the food packaging sector. Overall, natural polymer-based coatings represent a viable, sustainable approach for mitigating food spoilage and advancing a circular economy; however, their full potential can only be realized through interdisciplinary innovation and policy-supported implementation.
Author Contributions
Conceptualization, J.O.A., F.S. and O.A.F.; methodology, J.O.A., F.S. and O.A.F.; validation, J.O.A., F.S. and O.A.F.; formal analysis, N.P.M., J.O.A. and F.S.; investigation, N.P.M., J.O.A., F.S. and O.A.F.; resources, O.A.F.; data curation, N.P.M.; writing—original draft preparation, N.P.M.; writing—review and editing, N.P.M., J.O.A., F.S. and O.A.F.; visualization, N.P.M.; supervision, J.O.A. and O.A.F.; project administration, O.A.F.; funding acquisition, O.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based on research supported by the National Research Foundation of South Africa (Ref: SPAR231013155231; CPRR23033088376) and the University Research Committee at the University of Johannesburg.
Data Availability Statement
All data used have been included in this article.
Acknowledgments
We appreciate the Postharvest and Agroprocessing Research Centre for their guidance and support, which was influential in refining this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste. 2011. Available online: https://www.madr.ro/docs/ind-alimentara/risipa_alimentara/presentation_food_waste.pdf (accessed on 2 August 2024).
- Ali Mekouar, M. 15. Food and Agriculture Organization of the United Nations (FAO). Yearb. Int. Environ. Law 2017, 28, 506–520. [Google Scholar] [CrossRef]
- Griffin, M.; Sobal, J.; Lyson, T.A. An analysis of a community food waste stream. Agric. Hum. Values 2009, 26, 67–81. [Google Scholar] [CrossRef]
- Joardder, M.; Masud, M. Food Preservation in Developing Countries: Challenges and Solutions; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Mourad, M. Recycling, recovering and preventing ‘food waste’: Competing solutions for food systems sustainability in the United States and France. J. Clean. Prod. 2016, 126, 461–477. [Google Scholar] [CrossRef]
- Lohita, B.; Srijaya, M. Novel Technologies for Shelf-Life Extension of Food Products as a Competitive Advantage: A Review. In Food Production, Diversity, and Safety Under Climate Change; Advances in Science, Technology and Innovation; Springer: Cham, Switzerland, 2024; Volume Part F2519, pp. 285–306. [Google Scholar] [CrossRef]
- García-Díez, J.; Gonçalves, C.; Grispoldi, L.; Cenci-Goga, B.; Saraiva, C. Determining Food Stability to Achieve Food Security. Sustainability 2021, 13, 7222. [Google Scholar] [CrossRef]
- Shaw, I. Food Safety: The Science of Keeping Food Safe. 2018. Available online: https://books.google.com/books?hl=en7lr=&id=HY1FDwAAQBAJ&oi=fnd&pg=PP14&dq=Food+safety:+the+science+of+keeping+food+safe.+John+Wiley+%26+Sons.&ots=P8foopVnRj&sig=B9aa3aUR4Bvw4WQbGLBOtFEkAXc (accessed on 2 August 2024).
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Lupien, O.F. The Role of Traditional Food Processing Technologies in National Development: The West African Experience. academia.edu. 2008. Available online: https://www.academia.edu/download/41261245/Soy_Processing.pdf (accessed on 2 August 2024).
- Devi, L.; Kalita, S.; Mukherjee, A.; Kumar, S. Carnauba wax-based composite films and coatings: Recent advancement in prolonging postharvest shelf-life of fruits and vegetables. Trends Food Sci. Technol. 2022, 129, 296–305. Available online: https://www.sciencedirect.com/science/article/pii/S0924224422003983 (accessed on 2 August 2024). [CrossRef]
- Alzamora, S.M.; Welti-Chanes, J.; Guerrero, S.N.; Gómez, P.L. Rational use of novel technologies: A comparative analysis of the performance of several new food preservation technologies for microbial inactivation. In Novel Technologies in Food Science: Their Impact on Products, Consumer Trends and the Environment; Springer: New York, NY, USA, 2012; pp. 235–260. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Yu, L.; Chen, L.; Eichhorn, S.; Gandini, A. Polymeric materials from renewable resources. In Biodegradable Polymer Blends and Composites from Renewable Resources; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Use of Hen Egg White Lysozyme in the Food Industry. In Egg Innovations and Strategies for Improvements; Academic Press: Cambridge, MA, USA, 2017; pp. 233–242. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. Available online: https://www.sciencedirect.com/science/article/pii/S0144861720305385 (accessed on 2 August 2024). [CrossRef]
- Porta, R.; Sabbah, M.; Di Pierro, P. Biopolymers as food packaging materials. Int. J. Mol. Sci. 2020, 21, 4942. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in barrier coatings and film technologies for achieving sustainable packaging of food products—A review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Pan, Y.; Farmahini-Farahani, M.; O’hearn, P.; Xiao, H.; Ocampo, H. An Overview of Bio-Based Polymers for Packaging Materials. 2016. Available online: www.Bioresources-Bioproducts.com (accessed on 15 August 2024).
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Rajeswari, S. NATURAL POLYMERS: A RECENT REVIEW. World J. Pharm. Pharm. Sci. 2017, 6, 472–494. [Google Scholar] [CrossRef][Green Version]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Draghici, M.C.; Popesu, P.A.; Popa, V.L.; Bujor, O.; Ion, V.A.; Popa, M.E. Latest developments in edible coatings on minimally processed fruits and vegetables: A review. Foods 2021, 10, 2821. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent advances in edible coating of food products and its legislations: A review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review. Int. J. Sci. Res. Mod. Educ. 2016, 1, 188–204. [Google Scholar]
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Xie, T.; Liao, Z.; Lei, H.; Fang, X.; Wang, J.; Zhong, Q. Antibacterial activity of food-grade chitosan against Vibrio parahaemolyticus biofilms. Microb. Pathog. 2017, 110, 291–297. [Google Scholar] [CrossRef]
- Peng, Z.X.; Wang, L.; Du, L.; Guo, S.R.; Wang, X.Q.; Tang, T.T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Koc, B.; Mujtaba, M.; Ilk, S.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Yildiz, A. Diatomite as a novel composite ingredient for chitosan film with enhanced physicochemical properties. Int. J. Biol. Macromol. 2017, 105, 1401–1411. [Google Scholar] [CrossRef]
- Mwelase, S.; Opara, U.L.; Fawole, O.A. Effect of chitosan-based melatonin composite coating on the quality of minimally processed pomegranate aril-sacs during cold storage. J. Food Process. Preserv. 2022, 46, 1–16. [Google Scholar] [CrossRef]
- You, J.; Xie, S.; Cao, J.; Ge, H.; Xu, M.; Zhang, L.; Zhou, J. Quaternized Chitosan/Poly(acrylic acid) Polyelectrolyte Complex Hydrogels with Tough, Self-Recovery, and Tunable Mechanical Properties. Macromolecules 2016, 49, 1049–1059. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Duan, J. Application of Chitosan Based Coating in Fruit and Vegetable Preservation: A Review. J. Food Process. Technol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X15300503 (accessed on 20 August 2024). [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20173222866 (accessed on 20 August 2024). [CrossRef]
- Singh, H.; Bhasin, J.K.; Dash, K.K.; Shams, R.; Shaikh, A.M.; Béla, K. Effect of chitosan based edible coating in management of post harvest losses in Papaya: A comprehensive review. Appl. Food Res. 2024, 4, 100456. [Google Scholar] [CrossRef]
- Paul, S.K.; Sarkar, S.; Sethi, L.N.; Ghosh, S.K. Development of chitosan-based optimized edible coating for tomato (Solanum lycopersicum) and its characterization. J. Food Sci. Technol. 2018, 55, 2446–2456. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Hafiz, I.A. Postharvest quality of Mango (Mangifera indica L.) Fruit as affected by Chitosan coating. Pak. J. Bot. 2009, 41, 343–357. [Google Scholar]
- Viacava, G.E.; Cenci, M.P.; Ansorena, M.R. Effect of Chitosan Edible Coatings Incorporated with Free or Microencapsulated Thyme Essential Oil on Quality Characteristics of Fresh-Cut Carrot Slices. Food Bioproc. Technol. 2022, 15, 768–784. [Google Scholar] [CrossRef]
- Suseno, N.; Savitri, E.; Sapei, L.; Padmawijaya, K.S. Improving Shelf-life of Cavendish Banana Using Chitosan Edible Coating. Procedia Chem. 2014, 9, 113–120. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. Available online: https://www.sciencedirect.com/science/article/pii/S0144861718308075 (accessed on 2 August 2024). [CrossRef]
- Bautista-Baños, S.; Romanazzi, G.; Jiménez-Aparicio, A. Chitosan in the Preservation of Agricultural Commodities. 2016. Available online: https://books.google.com/books?hl=en&lr=&id=rWhBCwAAQBAJ&oi=fnd&pg=PP1&ots=Kt3CV-h4Nz&sig=m-WSmRHgUr1RsHt_5DSUo3zMe_M (accessed on 23 August 2024).
- Jurić, S.; Bureš, M.S.; Vlahoviček-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalšenjak, N.; Bandić, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Jiang, Q.; Xia, W. Effects of chitosan coating combined with essential oils on quality and antioxidant enzyme activities of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Int. J. Food Sci. Technol. 2017, 52, 404–412. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef]
- Lin, L.; Wang, B.; Wang, M.; Cao, J.; Zhang, J.; Wu, Y.; Jiang, W. Effects of a chitosan-based coating with ascorbic acid on post-harvest quality and core browning of ‘Yali’ pears (Pyrus bertschneideri Rehd.). J. Sci. Food Agric. 2008, 88, 877–884. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Fawole, O.A. Effects of chitosan coatings fused with medicinal plant extracts on postharvest quality and storage stability of purple passion fruit (Passiflora edulis var. Ester). Food Qual. Saf. 2022, 6, fyac016. [Google Scholar] [CrossRef]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and Applicability of Starch-Based Films: An Eco-Friendly Approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Farajpour, R.; Emam Djomeh, Z.; Moeini, S.; Tavahkolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein-based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Arcan, I.; Boyacı, D.; Yemenicioğlu, A. The Use of Zein and Its Edible Films for the Development of Food Packaging Materials. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Cano, A.; Jiménez, A.; Cháfer, M.; Gónzalez, C.; Chiralt, A. Effect of amylose:amylopectin ratio and rice bran addition on starch films properties. Carbohydr. Polym. 2014, 111, 543–555. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Li, E. Effects of amylose and amylopectin chain-length distribution on the kinetics of long-term rice starch retrogradation. Food Hydrocoll. 2021, 111, 106239. [Google Scholar] [CrossRef]
- Lu, H.; Zhan, J.; Shen, W.; Ma, R.; Tian, Y. Assessing Starch Retrogradation from the Perspective of Particle Order. Foods 2024, 13, 911. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods 2022, 11, 2879. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The influence of kaolin clay on the mechanical properties and structure of thermoplastic starch films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Majeed, T.; Dar, A.H.; Pandey, V.K.; Dash, K.K.; Srivastava, S.; Shams, R.; Jeevarathinam, G.; Singh, P.; Echegaray, N.; Pandiselvam, R. Role of additives in starch-based edible films and coating: A review with current knowledge. Progress Org. Coat. 2023, 181, 107597. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef]
- Oz, A.T.; Ulukanli, Z. Application of edible starch-based coating including glycerol plus oleum Nigella on arils from long-stored whole pomegranate fruits. J. Food Process. Preserv. 2012, 36, 81–95. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Effect of Gum Arabic and Starch-Based Coating and Different Polyliners on Postharvest Quality Attributes of Whole Pomegranate Fruit. Processes 2022, 10, 164. [Google Scholar] [CrossRef]
- Luo, S.; Chen, J.; He, J.; Li, H.; Jia, Q.; Hossen, M.A.; Dai, J.; Qin, W.; Liu, Y. Preparation of corn starch/rock bean protein edible film loaded with d-limonene particles and their application in glutinous rice cake preservation. Int. J. Biol. Macromol. 2022, 206, 313–324. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef]
- Gupta, A.D.; Pandey, S.; Jaiswal, V.K.; Bhadauria, V.; Singh, H. Simultaneous oxidation and esterification of cellulose for use in treatment of water containing Cu(II) ions. Carbohydr. Polym. 2019, 222, 114964. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Khalid, M.; Ratnam, C.T.; Manickam, S.; Walvekar, R.; Hoque, M.E. Sonosynthesis of cellulose nanoparticles (CNP) from kenaf fiber: Effects of processing parameters. Fibers Polym. 2016, 17, 1352–1358. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric Sustainable Materials and Their Emerging Applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Hoque, M.E.; Mahbub, T. Biopolymers for Sustainable Packaging in Food, Cosmetics, and Pharmaceuticals. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–214. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Zhang, D.; Guo, Z. Stable and durable conductive superhydrophobic coatings prepared by double-layer spray coating method. Nanomaterials 2021, 11, 1506. [Google Scholar] [CrossRef]
- Cinelli, P.; Coltelli, M.; Signori, F.; Morganti, P. Cosmetic packaging to save the environment: Future perspectives. Cosmetics 2019, 6, 26. Available online: https://www.mdpi.com/2079-9284/6/2/26 (accessed on 20 August 2024). [CrossRef]
- Shen, D.; Liu, J.; Gan, L.; Huang, N.; Long, M. Green Synthesis of Fe3O4/Cellulose/Polyvinyl Alcohol Hybride Aerogel and Its Application for Dye Removal. J. Polym. Environ. 2018, 26, 2234–2242. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Ma, L.; Zhou, H.; Yu, Y.; Guo, T.; Zhang, Y.; Huang, H. Green pH/magnetic sensitive hydrogels based on pineapple peel cellulose and polyvinyl alcohol: Synthesis, characterization and naringin prolonged release. Carbohydr. Polym. 2019, 209, 51–61. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Awad-Allah, M.A.A.; Abd-Elkarim, N.A.A.; Ahmed, Z.F.R.; Taha, E.M.A. Carboxymethyl Cellulose from Banana Rachis: A Potential Edible Coating to Extend the Shelf Life of Strawberry Fruit. Agriculture 2023, 13, 1058. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT 2005, 38, 617–624. [Google Scholar] [CrossRef]
- Tesfay, S.; Magwaza, L.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. Available online: https://www.sciencedirect.com/science/article/pii/S0304423817305289 (accessed on 1 October 2024). [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Cerqueira, M.A. Active Carboxymethyl Cellulose-Based Edible Coatings for the Extension of Fresh Goldenberries Shelf-Life. Horticulturae 2022, 8, 936. [Google Scholar] [CrossRef]
- Minh, N.P. Synergistic Effect of Methyl Cellulose and Carvacrol Coating on Physicochemical and Microbial Attributes of Mango (Mangifera indica) Fruit in Postharvest storage. J. Pure Appl. Microbiol. 2021, 15, 2280–2287. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of chia seed mucilage/bacterial cellulose edible coating on bioactive compounds and antioxidant activity of strawberries during cold storage. Int. J. Biol. Macromol. 2021, 190, 618–623. [Google Scholar] [CrossRef]
- Han, L.; He, Y.; Wang, S.; Cheng, W.; Ma, L.; Liu, G.; Han, D.; Niu, L. Effects of methyl cellulose-based coating on physiochemical properties and chemical hazards of Chinese fried dough cake during storage. Int. J. Food Sci. Technol. 2021, 56, 4770–4779. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll. 2014, 37, 100–110. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Fata Moradali, M.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ureña, M.; Carullo, D.; Phùng, T.T.T.; Fournier, P.; Farris, S.; Lagorce, A.; Karbowiak, T. Effect of polymer structure on the functional properties of alginate for film or coating applications. Food Hydrocoll. 2024, 149, 109557. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 2008, 88, 1287–1293. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Iversen, L.J.L.; Rovina, K.; Vonnie, J.M.; Matanjun, P.; Erna, K.H.; ‘Aqilah, N.M.N.; Felicia, W.X.L.; Funk, A.A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. [Google Scholar] [CrossRef] [PubMed]
- Luan, Q.Y.; Wang, Y.S.; Chen, Y.; Chen, H.H. Review on improvement of physicochemical properties of sodium alginate-based edible films. J. Food Sci. 2025, 90, e70016. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Bozkurt, F.; Goudjil, M.B.; Tornuk, F. Home-made cheese preservation using sodium alginate based on edible film incorporating essential oils. J. Food Sci. Technol. 2021, 58, 2406–2419. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Luo, W.; Yang, K.; Li, C. Effects of Sodium Alginate Edible Coating with Cinnamon Essential Oil Nanocapsules and Nisin on Quality and Shelf Life of Beef Slices during Refrigeration. J. Food Prot. 2022, 85, 896–905. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Wei, C.; Chen, S.; Ye, X.; Jinhu, T. Development of novel EGCG/Fe loaded sodium alginate-based packaging films with antibacterial and slow-release properties. Food Hydrocoll. 2023, 145, 109032. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, H.; Fu, Y.; Chang, C.; Wu, J. Sodium alginate/gum arabic/glycerol multicomponent edible films loaded with natamycin: Study on physicochemical, antibacterial, and sweet potatoes preservation properties. Int. J. Biol. Macromol. 2022, 213, 1068–1077. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Ummartyotin, S. Influence of a Transparent and Edible Coating of Encapsulated Cannabidiol Nanoparticles on the Quality and Shelf Life of Strawberries. ACS Appl. Mater. Interfaces 2023, 15, 23834–23843. [Google Scholar] [CrossRef]
- Lisboa, M.; Chagas, C.; da Costa, J.C.M.; Rossoni, D.; Mikcha, J.M.G.; de Oliveira Silva, J.V.; Batista, A.; Caetano, W.; Madrona, G.S.; Tonon, L.A.C.; et al. Characterization and effect of edible alginate acidified coatings associated with photodynamics on cheese preservation. Appl. Food Res. 2022, 2, 100113. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- Lee, M.; Rüegg, N.; Yildirim, S. Evaluation of the antimicrobial activity of sodium alginate films integrated with cinnamon essential oil and citric acid on sliced cooked ham. Packag. Technol. Sci. 2023, 36, 647–656. [Google Scholar] [CrossRef]
- Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in food systems—Bionanomaterials, conventional and unconventional sources, functional properties, and development opportunities. Polymers 2021, 13, 2506. [Google Scholar] [CrossRef]
- Pacheco-Gómez, V.; Caballero-Zamora, A.; Martinez-Gonzalez, S.; Prado-Rebolledo, O.; Garcia-Casillas, A. Biochemistry and metabolic pathways of polysaccharides, lipids, and proteins. SciELO Méx. 2021, 11, 1–26. Available online: https://www.scielo.org.mx/scielo.php?pid=S2448-61322021000100205&script=sci_arttext&tlng=en (accessed on 3 October 2024).
- Yan, W.; Zhou, J.; Sun, M.; Chen, J.; Hu, G.; Shen, B. The construction of an amino acid network for understanding protein structure and function. Amino Acids 2014, 46, 1419–1439. [Google Scholar] [CrossRef]
- Dietzen, D.J. Amino acids, peptides, and proteins. In Principles and Applications of Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–380. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, R.I.; Singha, P.; Singh, S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Res. Int. 2021, 148, 110647. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 105, 105664. [Google Scholar] [CrossRef]
- Echers, S.G.; Jafarpour, A.; Yesiltas, B.; García-Moreno, P.J.; Greve-Poulsen, M.; Hansen, D.K.; Hansen, E.B. Targeted hydrolysis of native potato protein: A novel workflow for obtaining hydrolysates with improved interfacial properties. Food Hydrocoll. 2023, 137, 108299. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Bourtoom, T. Review Article Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Abedi, A.; Lakzadeh, L.; Amouheydari, M. Effect of an edible coating composed of whey protein concentrate and rosemary essential oil on the shelf life of fresh spinach. J. Food Process. Preserv. 2021, 45, e15284. [Google Scholar] [CrossRef]
- Robles-Flores, G.d.C.; Abud-Archila, M.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Gutiérrez-Miceli, F.A. Development and Evaluation of a Film and Edible Coating Obtained from the Cajanus cajan Seed Applied to Fresh Strawberry Fruit. Food Bioprocess. Technol. 2018, 11, 2172–2181. [Google Scholar] [CrossRef]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.E.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.; Hanjabam, M.D.; Gudipati, V.; Kannuchamy, N. Protective Effect of Fish Gelatin-Based Natural Antimicrobial Coatings on Quality of Indian Salmon Fillets during Refrigerated Storage. J. Food Process Eng. 2017, 40, e12270. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, J.H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The Effect of Whey Protein-Based Edible Coatings Incorporated with Lemon and Lemongrass Essential Oils on the Quality Attributes of Fresh-Cut Pears during Storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Li, S.; Ren, Y.; Hou, Y.; Zhan, Q.; Jin, P.; Zheng, Y.; Wu, Z. Polysaccharide-Based Composite Films: Promising Biodegradable Food Packaging Materials. Foods 2024, 13, 3674. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible Polymers and Secondary Bioactive Compounds for Food Packaging Applications: Antimicrobial, Mechanical, and Gas Barrier Properties. Polymers 2022, 14, 2395. [Google Scholar] [CrossRef] [PubMed]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- Rashki, S.; Shakour, N.; Yousefi, Z.; Rezaei, M.; Homayoonfal, M.; Khabazian, E.; Atyabi, F.; Aslanbeigi, F.; Safaei Lapavandani, R.; Mazaheri, S.; et al. Cellulose-Based Nanofibril Composite Materials as a New Approach to Fight Bacterial Infections. Front. Bioeng. Biotechnol. 2021, 9, 732461. [Google Scholar] [CrossRef]
- Wardejn, S.; Wacławek, S.; Dudek, G. Improving Antimicrobial Properties of Biopolymer-Based Films in Food Packaging: Key Factors and Their Impact. Int. J. Mol. Sci. 2024, 25, 12580. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers 2025, 17, 1257. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Metha, C.; Pawar, S.; Suvarna, V. Recent advancements in alginate-based films for active food packaging applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Mariod, A.A. Gelatin and Chitosan as Meat By-Products and Their Recent Applications. Foods 2023, 12, 60. [Google Scholar] [CrossRef]
- Qu, T.; Wang, X.; Zhang, F. Antibacterial Food Packaging with Chitosan and Cellulose Blends for Food Preservation. Polymers 2025, 17, 1850. [Google Scholar] [CrossRef] [PubMed]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with Natural Additives as a Potential Food Packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef]
- Wang, L.; Tong, L. Production and Properties of Starch: Current Research. Molecules 2024, 29, 646. [Google Scholar] [CrossRef]
- Franco, T.S.; de Muniz, G.B.; Lomelí-Ramírez, M.G.; Rangel, B.S.; Jiménez-Amezcua, R.M.; Mijares, E.M.; García-Enríquez, S.; Rentería-Urquiza, M. Nanocellulose and Its Application in the Food Industry. Biol. Life Sci. Forum 2023, 28, 2. [Google Scholar] [CrossRef]
- Pournaki, S.K.; Aleman, R.S.; Hasani-Azhdari, M.; Marcia, J.; Yadav, A.; Moncada, M. Current Review: Alginate in the Food Applications. J 2024, 7, 281–301. [Google Scholar] [CrossRef]
- Guan, C.; Wang, C.; Fu, S. Food Protein Nanofibril Gels: From Conditions, Types and Properties to Applications. Foods 2024, 13, 2173. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Bio-based Polymers for Sustainable Packaging and Biobarriers: A Critical Review. BioResources 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Colomines, G.; Ducruet, V.; Courgneau, Ć.; Guinault, A.; Domenek, S. Barrier properties of poly(lactic acid) and its morphological changes induced by aroma compound sorption. Polym. Int. 2010, 59, 818–826. [Google Scholar] [CrossRef]
- Vahabi, H.; Rohani Rad, E.; Parpaite, T.; Langlois, V.; Saeb, M.R. Biodegradable Polyester Thin Films and Coatings in the Line of Fire: The Time of Polyhydroxyalkanoate (PHA)? Prog. Org. Coat. 2019, 133, 85–89. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 242, 130204. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between Degree of Crystallinity, Morphology, Glass Temperature, Mechanical Properties and Biodegradation of Poly (3-Hydroxyalkanoate) PHAs and Their Blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.X. Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A review on the production, properties and applications of non-isocyanate polyurethane: A greener perspective. Prog. Org. Coat. 2021, 155, 106124. [Google Scholar] [CrossRef]
- Catalá, J.; Guerra, I.; García-Vargas, J.M.; Ramos, M.J.; García, M.T.; Rodríguez, J.F. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers 2023, 15, 1561. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Sustainability of Nonisocyanate Polyurethanes (NIPUs). Sustainability 2024, 16, 9911. [Google Scholar] [CrossRef]
- Marturano, V.; Marotta, A.; Salazar, S.A.; Ambrogi, V.; Cerruti, P. Recent advances in bio-based functional additives for polymers. Prog. Mater. Sci. 2023, 134, 101186. [Google Scholar] [CrossRef]
- Bizet, B.; Grau, E.; Asua, J.M.; Cramail, H. Hybrid Nonisocyanate Polyurethanes (H-NIPUs): A Pathway towards a Broad Range of Novel Materials. Macromol. Chem. Phys. 2022, 223, 2100437. [Google Scholar] [CrossRef]
- Al-Oqla, F.; Sapuan, S. Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; CRC Press: Boca Raton, FL, USA, 2020; Available online: https://books.google.com/books?hl=en&lr=&id=L6DhDwAAQBAJ&oi=fnd&pg=PP1&ots=m6DDZmcxiK&sig=CtXP7k02wRhVkEmIy5F0CuAgS44 (accessed on 30 August 2024).
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. R. Soc. Chem. 2021, 11, 40381–40413. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sit, N. A comprehensive review on types and properties of biopolymers as sustainable bio-based alternatives for packaging. Food Biomacromol. 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Katanyoota, P.; Jariyasakoolroj, P.; Sane, A. Mechanical and barrier properties of simultaneous biaxially stretched polylactic acid/thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Polym. Bull. 2023, 80, 5219–5237. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Wang, Y.; Zhao, J.; Ren, W.; Wang, B.; Cai, D. Non-isocyanate polyurethane from sweet potato residual and the application in food preservation. Ind. Crops Prod. 2022, 186, 115224. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Pérez-Álvarez, L.; Hernáez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)-β-cyclodextrin onto polylactic acid (PLLA). Food Hydrocoll. 2019, 88, 228–236. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A. Production of Medium-Chain Length Polyhydroxyalkanoates by Pseudomonas citronellolis Grown in Apple Pulp Waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar] [CrossRef]
- de la Rosa-Ramírez, H.; Dominici, F.; Ferri, J.M.; Luzi, F.; Puglia, D.; Torre, L.; López-Martínez, J.; Samper, M.D. Pentaerythritol and Glycerol Esters Derived from Gum Rosin as Bio-Based Additives for the Improvement of Processability and Thermal Stability of Polylactic Acid. J. Polym. Environ. 2023, 31, 5446–5461. [Google Scholar] [CrossRef]
- Swarupa, S.; Thareja, P. Techniques, applications and prospects of polysaccharide and protein based biopolymer coatings: A review. Int. J. Biol. Macromol. 2024, 253, 131104. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Reddy Nagavally, R. Composite materials—History, types, fabrication techniques, advantages, and applications. In Proceedings of the 29th IRF International Conference, Bengaluru, India, 24 July 2016. [Google Scholar]
- Krstić, M.; Radojević, M.; Stojanović, D.; Radojević, V.; Uskoković, P.; Ibrić, S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite nanotubes for food packaging application: A review. Appl. Clay Sci. 2023, 236, 106856. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kazi, J.; Debnath, M.C. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: Evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother. 2018, 97, 1373–1385. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; Avena-Bustillos, R.J.; Soares, N.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties. A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Chen, Y.; Qiu, D.; Weng, Y. Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin. Gels 2023, 9, 332. [Google Scholar] [CrossRef]
- Abdullah, N.A.S.; Mohamad, Z.; Khan, Z.I.; Jusoh, M.; Zakaria, Z.Y.; Ngadi, N. Alginate based sustainable films and composites for packaging: A review. Chem. Eng. Trans. 2021, 83, 271–276. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Sanchez-García, M.D. Carrageenan polysaccharides for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 594–609. [Google Scholar] [CrossRef]
- Sree, S.; Patra, A.; Arun Prasath, V.; Kambhampati, V.; Xiao, H. Innovations in Electrospinning Techniques for Nanomaterial Synthesis and Its Applications in the Field of Active Food Packaging. J. Futur. Foods, 2025; in press. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Zhu, S. Progress in fabrication of one-dimensional catalytic materials by electrospinning technology. J. Ind. Eng. Chem. 2021, 93, 28–56. Available online: https://www.sciencedirect.com/science/article/pii/S1226086X20304202 (accessed on 23 July 2024). [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Aurazo, F.; Asto, E.; Hurtado, F. Identification and Estimation of the Taylor Cone in Electrospinning Technique Using the Yolo Network. In Proceedings of the 2024 IEEE 31st International Conference on Electronics, Electrical Engineering and Computing, INTERCON 2024, Lima, Peru, 6–8 November 2024; IEEE: Lima, Peru, 2024. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W.; Lin, D.; Li, H. Electrospinning of ceramic nanofibers: Fabrication, assembly and applications. J. Adv. Ceram. 2012, 1, 2–23. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Martins, B.A.; de Albuquerque, P.B.S.; de Souza, M.P. Bio-based Films and Coatings: Sustainable Polysaccharide Packaging Alternatives for the Food Industry. J. Polym. Environ. 2022, 30, 4023–4039. [Google Scholar] [CrossRef]
- Bishop, J.E.; Routledge, T.J.; Lidzey, D.G. Advances in Spray-Cast Perovskite Solar Cells. J. Phys. Chem. Lett. 2018, 9, 1977–1984. [Google Scholar] [CrossRef]
- Patel, M.J.; Baishya, H.; Gupta, R.K.; Garai, R.; Iyer, P.K. Thin Film Solution Processable Perovskite Solar Cell. In Recent Advances in Multifunctional Perovskite Materials; IntechOpen: London, UK, 2022; Available online: www.intechopen.com (accessed on 6 January 2025).
- Colella, S.; Mazzeo, M.; Melcarne, G.; Carallo, S.; Ciccarella, G.; Gigli, G. Spray coating fabrication of organic solar cells bypassing the limit of orthogonal solvents. Appl. Phys. Lett. 2013, 102, 20. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, C.S.; Kim, D.G.; Kim, J.K.; Kim, S.H.; Kang, J.W. Fully spray-coated inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 103, 76–79. [Google Scholar] [CrossRef]
- Girotto, C.; Rand, B.P.; Genoe, J.; Heremans, P. Exploring spray coating as a deposition technique for the fabrication of solution-processed solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 454–458. [Google Scholar] [CrossRef]
- Green, R.; Morfa, A.; Ferguson, A.J.; Kopidakis, N.; Rumbles, G.; Shaheen, S.E. Performance of bulk heterojunction photovoltaic devices prepared by airbrush spray deposition. Appl. Phys. Lett. 2008, 92, 3. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoshikawa, S.; Sagawa, T. Fabrication of bulk heterojunction solar cells by a modified spray-mist coating method. Renew. Energy Power Qual. J. 2013, 11, 366–369. [Google Scholar] [CrossRef]
- Lafleur, P.; Vergnes, B. Polymer Extrusion; John Wiley & Sons: Hoboken, NJ, USA, 2014; Available online: https://books.google.com/books?hl=en&lr=&id=3xqNAwAAQBAJ&oi=fnd&pg=PP6&dq=Polymer+Extrusion&ots=W_PAICAhcN&sig=sXtNG2LY9mqfXiIPqwTw_XakSsE (accessed on 7 January 2025).
- Gaspar-Cunha, A.; Covas, J.A.; Sikora, J. Optimization of Polymer Processing: A Review (Part I—Extrusion). Materials 2022, 15, 384. [Google Scholar] [CrossRef]
- Steel, C.J.; Gabriela, M.; Leoro, V.; Schmiele, M.; Ferreira, R.E.; Chang, Y.K. Thermoplastic Extrusion in Food Processing. In Food Engineering; IntechOpen: London, UK, 2012; Available online: www.intechopen.com (accessed on 10 January 2025).
- Choton, S.; Gupta, N.; Bandral, J.D.; Anjum, N.; Choudary, A. Extrusion technology and its application in food processing: A review. Pharma Innov. J. 2020, 9, 162–168. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: II. Application in bio-based plastics for active packaging. Carbohydr. Polym. 2013, 96, 586–592. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M. Extruded films of blended chitosan, low density polyethylene and ethylene acrylic acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Motsa, N.; Abdillah, A.A. A Comprehensive Characterization of Biodegradable Edible Films Based on Potato Peel Starch Plasticized with Glycerol. Polymers 2022, 14, 3462. [Google Scholar] [CrossRef]
- Pokharel, A.; Falua, K.J.; Babaei-Ghazvini, A.; Acharya, B. Biobased Polymer Composites: A Review. J. Compos. Sci. 2022, 6, 255. [Google Scholar] [CrossRef]
- Tatara, R.A. Compression Molding. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; William Andrew Publishing, Elsevier: Norwich, NY, USA, 2024; pp. 389–424. ISBN 978032388667. [Google Scholar]
- Dumont, P.; Martoïa, F.; Orgéas, L. Compression Moulding. In Design and Manufacture of Structural Composites; Harper, L., Clifford, M., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 273–300. ISBN 978-0-12-819160-6. [Google Scholar]
- Fu, H.; Xu, H.; Liu, Y.; Yang, Z.; Kormakov, S.; Wu, D.; Sun, J. Overview of injection molding technology for processing polymers and their composites. ES Mater. Manuf. 2020, 8, 3–23. [Google Scholar] [CrossRef]
- Goodship, V. Introduction to Plastics Recycling. 2007. Available online: https://books.google.com/books?hl=en&lr=&id=ocoQM7cnQTwC&oi=fnd&pg=PA3&dq=%E2%80%A2%09Goodship,+V.+(2017).+Introduction+to+Plastics+Recycling.+Smithers+Rapra.&ots=DPL1WY8gA6&sig=siCc1JcIGbfoHRRqK-tKSAQZHkQ (accessed on 30 July 2024).
- Flórez, M.; Cazón, P.; Vázquez, M. Selected Biopolymers’ Processing and Their Applications: A Review. Polymers 2023, 15, 641. [Google Scholar] [CrossRef]
- Advani, S.; Sozer, E. Process Modeling in Composites Manufacturing. 2002. Available online: https://www.taylorfrancis.com/books/mono/10.1201/9780203910061/process-modeling-composites-manufacturing-suresh-advani-murat-sozer (accessed on 30 July 2024).
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch-hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef]
- Tay, Y.W.D.; Panda, B.; Paul, S.C.; Tan, M.J.; Qian, S.Z.; Leong, K.F.; Chua, C.K. Processing and properties of construction materials for 3D printing. Mater. Sci. Forum 2016, 861, 177–181. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Azzollini, D. Variables affecting the printability of foods: Preliminary tests on cereal-based products. Innov. Food Sci. Emerg. Technol. 2016, 38, 281–291. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D Printing in Food Processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.H.; Hong, G.S. An Overview of 3D Printing Technologies for Food Fabrication. Food Bioprocess. Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- Lee, J. A 3D Food Printing Process for the New Normal Era: A Review. Processes 2021, 9, 1495. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Grad, K.; Wiśniewski, W.; Szulc, J. The Use of Agricultural Waste in the Modification of Poly(Lactic Acid)-Based Composites Intended for 3D Printing Applications. The Use of Toughened Blend Systems to Improve Mechanical Properties. J. Compos. Sci. 2021, 5, 253. [Google Scholar] [CrossRef]
- Cui, J.; Yu, X.; Shen, Y.; Sun, B.; Guo, W.; Liu, M.; Chen, Y.; Wang, L.; Zhou, X.; Shafiq, M.; et al. Electrospinning Inorganic Nanomaterials to Fabricate Bionanocomposites for Soft and Hard Tissue Repair. Nanomaterials 2023, 13, 204. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J. Application of 3D Printing Technology in Dentistry: A Review. Polymers 2025, 17, 886. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Nikodem, A.; Janusz, J.; Jabłoński, A.; Ziąbka, M.; Menaszek, E.; Frankova, J.; Piekarczyk, W.; Fabia, J. 3D Printing and Electrospinning of Drug- and Graphene-Enhanced Polycaprolactone Scaffolds for Osteochondral Nasal Repair. Materials 2025, 18, 1826. [Google Scholar] [CrossRef]
- Akinwamide, S.O.; Abe, B.T.; Akinribide, O.J.; Obadele, B.A.; Olubambi, P.A. Characterization of Microstructure, Mechanical Properties and Corrosion Response of Aluminium-Based Composites Fabricated via Casting. A Review. Int. J. Adv. Manuf. Technol. 2020, 111, 1–23. [Google Scholar] [CrossRef]
- Olatunji, O. Natural Polymers: Industry Techniques and Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kumar, K.; Deepalekshmi, S.; Mariappan, P.; Ahamed, R.M.B.; Ali, M.; Al-Maadeed, S.A. (Eds.) Polymer Nanocomposites in Biomedical Engineering; Lecture Notes in Bioengineering; Springer International Publishing: Cham, Switzerland, 2019; Available online: http://www.springer.com/series/11564 (accessed on 10 March 2025).
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-Based Films and Food Coatings: An Overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research Progress, Models and Simulation of Electrospinning Technology: A Review. J. Mater. Sci. 2022, 57, 10099–10135. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Amin, S.; Panchal, H. A Review on Thermal Spray Coating Processes. Int. J. Curr. Trends Eng. Res. 2016, 2, 514–518. Available online: http://www.ijcter.com (accessed on 10 March 2025).
- Shelar, G.A.; Gaikwad, S.T. Extrusion in Food Processing: An Overview. Pharma Innov. J. 2019, 8, 562–568. Available online: www.thepharmajournal.com (accessed on 10 March 2025).
- Offiah, V.; Kontogiorgos, V.; Falade, K.O. Extrusion Processing of Raw Food Materials and By-Products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2979–2998. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.A. Compression Moulding Technique for Manufacturing Biocomposite Products. Int. J. Appl. Sci. Technol. 2015, 5, 19–25. Available online: https://www.researchgate.net/publication/362593262 (accessed on 12 March 2025).
- Jaafar, J.; Siregar, J.P.; Tezara, C.; Hamdan, M.H.M.; Rihayat, T. A Review of Important Considerations in the Compression Molding Process of Short Natural Fiber Composites. Int. J. Adv. Manuf. Technol. 2019, 105, 3437–3450. [Google Scholar] [CrossRef]
- Li, W.; Feng, T.; Lu, T.; Zhao, F.; Zhao, J.; Guo, W.; Hua, L. Optimization of Compression Molding Parameters and Lifecycle Carbon Impact Assessment of Bamboo Fiber-Reinforced Polypropylene Composites. Polymers 2024, 16, 3435. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A Review of 3D Printing Techniques for Environmental Applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Basha, D.B.; Hussain, S.; Uddin, I.; Gondal, M.A. Critical review on recent developments in conducting polymer nanocomposites for supercapacitors. Synth. Met. 2023, 295, 117326. [Google Scholar] [CrossRef]
- Okpala, C.C. Nanocomposites: An Overview. Int. J. Eng. Res. Dev. 2013, 7, 57–62. Available online: www.ijerd.com (accessed on 12 March 2025).
- Youssef, A.M. Polymer Nanocomposites as a New Trend for Packaging Applications. Polym. Plast. Technol. Eng. 2013, 52, 635–660. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Synthesis and Intercalation Chemistry of Hybrid Organo-Inorganic Nanocomposites. Polym. Sci. Ser. C 2006, 48, 85–111. [Google Scholar] [CrossRef]
- Mittal, V. Characterization Techniques for Polymer Nanocomposites, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer Nanocomposites Having a High Filler Content: Synthesis, Structures, Properties, and Applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Rodriguez, V.M.; Siqqique, S.; Zhu, H. Nanomaterials, Nanofillers, and Nanocomposites: Types and Properties. In Health and Environmental Safety of Nanomaterials: Polymer Nanocomposites and Other Materials Containing Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–37. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Silberbauer, A.; Schmid, M. Packaging Concepts for Ready-to-Eat Food: Recent Progress. J. Packag. Technol. Res. 2017, 1, 113–126. [Google Scholar] [CrossRef]
- Tang, L.; Yang, G.D.; Zeng, G.M.; Cai, Y.; Li, S.S.; Zhou, Y.Y.; Pang, Y.; Liu, Y.; Zhang, Y.; Luna, B. Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Khezri, K.; Mahdavi, H. Polystyrene–Silica Aerogel Nanocomposites by In Situ Simultaneous Reverse and Normal Initiation Technique for ATRP. Microporous Mesoporous Mater. 2016, 228, 132–140. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Improved Barrier and Mechanical Properties of Novel Hydroxypropyl Methylcellulose Edible Films with Chitosan/Tripolyphosphate Nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of Modified Cellulose Nanocrystals on the Barrier and Migration Properties of PLA Nano-Biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized Graphene Sheets for Polymer Nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic Effect of Cellulose and Lignin Nanostructures in PLA Based Systems for Food Antibacterial Packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous Reinforcing and Toughening: New Nanocomposites of Waterborne Polyurethane Filled with Low Loading Level of Starch Nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Malik, G.K.; Mitra, J. Zinc Oxide Nanoparticle Synthesis, Characterization, and Their Effect on Mechanical, Barrier, and Optical Properties of HPMC-Based Edible Film. J. Food Process Eng. 2021, 44, e13566. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of Biopolymer Technology as an Alternative Option for Non-Degradable Plastics and Sustainable Management of Plastic Wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.; Hakim, A. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 45. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite Edible Films and Coatings from Food-Grade Biopolymers. J. Food Sci. Technol. 2018, 55, 3613–3623. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Nurul, S.; Jamil, A.M.; Abdan, K.; Vannini, M.; Sisti, L. A Brief Review on the Influence of Ionic Liquids on the Mechanical, Thermal, and Chemical Properties of Biodegradable Polymer Composites. Polymers 2021, 13, 2597. [Google Scholar] [CrossRef]
- Aničić, N.; Kurtjak, M.; Jeverica, S.; Suvorov, D.; Vukomanović, M. Antimicrobial Polymeric Composites with Embedded Nanotextured Magnesium Oxide. Polymers 2021, 13, 2183. [Google Scholar] [CrossRef]
- Maruddin, F.; Malaka, R.; Baba, S.; Amqam, H.; Taufik, M.; Sabil, S. Brightness, Elongation and Thickness of Edible Film with Caseinate Sodium Using a Type of Plasticizer. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 492, p. 012043. [Google Scholar] [CrossRef]
- Ponnusamy, P.G.; Mani, S. Material and Environmental Properties of Natural Polymers and Their Composites for Packaging Applications. A Review. Polymers 2022, 14, 4033. [Google Scholar] [CrossRef]
- Mondragon, G.; Peña-Rodriguez, C.; González, A.; Eceiza, A.; Arbelaiz, A. Bionanocomposites Based on Gelatin Matrix and Nanocellulose. Eur. Polym. J. 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Kunam, P.K.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Bio-Based Materials for Barrier Coatings on Paper Packaging. Biomass Convers. Biorefin. 2024, 14, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Weber, C. Biobased Packaging Materials for the Food Industry: Status and Perspectives, a European Concerted Action. AGROVOC Database. 2000. Available online: https://agris.fao.org/search/en/providers/122465/records/647235d053aa8c896302456a (accessed on 22 April 2024).
- Figovsky, O.; Shapovalov, L.; Beilin, D.; Leykin, A. Recent Advances in the Development of Non-Isocyanate Polyurethanes Based on Cyclic Carbonates. 2013. Available online: https://www.researchgate.net/publication/261363031 (accessed on 20 March 2025).
- Asim, Z.; Shah, M.U.; Pasha, M.; Qureshi, R.; Kousar, N.; Rehman, A.; Kousar, S. Significance of Sustainable Packaging: A Case-Study from a Supply Chain Perspective. Appl. Syst. Innov. 2022, 5, 117. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2022, 375, 131738. Available online: https://www.sciencedirect.com/science/article/pii/S0308814621027448 (accessed on 20 March 2025). [CrossRef] [PubMed]
- Las-Casas, B.; Arantes, V. From Production to Performance: Tailoring Moisture and Oxygen Barrier of Cellulose Nanomaterials for Sustainable Applications—A Review. Carbohydr. Polym. 2024, 334, 122012. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Raveh, A.; Zukerman, I.; Shneck, R.; Avni, R.; Fried, I. Thermal Stability of Nanostructured Superhard Coatings: A Review. Surf. Coat. Technol. 2007, 201, 6136–6144. Available online: https://www.sciencedirect.com/science/article/pii/S0257897206009509 (accessed on 22 March 2025). [CrossRef]
- Liang, N.; Zhao, Y. A Review on Thermal Stability of Nanostructured Materials. J. Alloys Compd. 2023, 944, 168528. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. Thermal Stability of Natural Fibers and Their Polymer Composites. Iran. Polym. J. 2020, 29, 625–648. [Google Scholar] [CrossRef]
- Väisänen, T.; Das, O.; Lommpo, R.; Laukkanen, A.; Yang, W. A Review on New Bio-Based Constituents for Natural Fiber-Polymer Composites. J. Clean. Prod. 2017, 149, 582–596. Available online: https://www.sciencedirect.com/science/article/pii/S095965261730358X (accessed on 24 March 2025). [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Guigo, N.; Künkel, A.; Müssig, J.; Michel, S.; Pollet, E. Thermal Analysis of Biobased Polymers and Composites. In Thermal Analysis in Practice; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 2; Available online: https://www.sciencedirect.com/science/article/pii/B9780444640628000024 (accessed on 24 March 2025).
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent Semi-Crystalline Polymeric Materials and Their Nanocomposites: A Review. Polym. Eng. Sci. 2020, 60, 2326–2346. [Google Scholar] [CrossRef]
- Gupta, R.; Goddard, N.J. Leaky Waveguides (LWs) for Chemical and Biological Sensing—A Review and Future Perspective. Sens. Actuators B Chem. 2020, 325, 128628. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, F.Y.; Cheung, C.; Chen, X.; Chen, H. Different Effect of Media Opacity on Automated and Manual Measurement of Foveal Avascular Zone of Optical Coherence Tomography Angiographies. Br. J. Ophthalmol. 2021, 105, 812–818. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M. Impact of Metal Nanoparticles on the Mechanical, Barrier, Optical and Thermal Properties of Biodegradable Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2021–2033. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.W. Biopolymer-Based UV Protection Functional Films for Food Packaging. Food Hydrocoll. 2023, 144, 108771. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.H. Implications of Light Energy on Food Quality and Packaging Selection. Adv. Food Nutr. Res. 2012, 67, 25–73. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and Their Derivatives: Antibiofilm Drugs against Pathogenic Bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Wu, M.; Yu, D. Nanofibers-Based Food Packaging. ES Food Agrofor. 2021, 5, 15–25. Available online: https://www.espublisher.com/journals/articledetails/598 (accessed on 23 July 2024). [CrossRef]
- Mwelase, S.; Fawole, O.A. Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions. Plants 2022, 11, 351. [Google Scholar] [CrossRef]
- Bolotov, V.M.; Savvin, P.N.; Komarova, E.V.; Koshevarova, I.B. Effect of Natural Carotenoids and Anthocyanins on Properties of Healthy Food Products. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 052001. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Wiedmann, K.P.; Haase, J.; Bettels, J.; Reuschenbach, C. It’s Not All about Function: Investigating the Effects of Visual Appeal on the Evaluation of Industrial Products Using the Example of Product Color. J. Prod. Brand. Manag. 2019, 28, 15–27. [Google Scholar] [CrossRef]
- Poisson, J.; Zhang, K. Unique Optical Properties of Cellulosic Materials. Acc. Mater. Res. 2024, 5, 920–932. [Google Scholar] [CrossRef]
- Pal, K.; Bharti, D.; Sarkar, P.; Anis, A.; Kim, D.; Chałas, R.; Maksymiuk, P.; Stachurski, P.; Jarzębski, M. Selected Applications of Chitosan Composites. Int. J. Mol. Sci. 2021, 22, 10968. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Z.; Ma, Y.; Liu, H.; Wang, X. Cellulose-Based Films for Food Packaging Applications: Review of Preparation, Properties, and Prospects. J. Renew. Mater. 2023, 11, 1231–1256. [Google Scholar] [CrossRef]
- Nitikornwarakul, C.; Wangpradid, R.; Rakkapao, N. Impact of Molar Composition on the Functional Properties of Glutinous Rice Starch–Chitosan Blend: Natural-Based Active Coating for Extending Mango Shelf Life. Polymers 2024, 16, 1375. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Benabid, F.Z.; Zouai, F.; Benabid, F.Z.; Zouai, F. Algerian Journal of Natural Products. Alger. J. Nat. Prod. 2016, 4, 348–357. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 189, 105634. [Google Scholar] [CrossRef]
- Victor, R.S.D.; Santos, A.M.C.; Sousa, B.V.; Neves, G.A.; Santana, L.N.L.; Menezes, R.R. A review on Chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Zia, K.M. Regulatory Status and Clinical Trials of Chitosan and Derivatives. In Chitosan and Chitosan-Based Materials for Biomedical Applications; Springer: Singapore, 2025; pp. 115–134. [Google Scholar] [CrossRef]
- Giessen, A. Herstellung Wasserfester Funktionaler Nanofasern Durch Elektrospinnen Wässriger Formulierungen. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2009. [Google Scholar]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 125, 107328. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Leng, X.; Zang, J.; Zhao, G. Bio-based antibacterial food packaging films and coatings containing cinnamaldehyde: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef]
- Istiqomah, A.; Prasetyo, W.E.; Firdaus, M.; Kusumaningsih, T. Valorisation of lemongrass essential oils onto chitosan-starch film for sustainable active packaging: Greatly enhanced antibacterial and antioxidant activity. Int. J. Biol. Macromol. 2022, 210, 669–681. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 310, 110762. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Acharya, A.; Hegde, V.; Prakash, B. Rational design of hyperstable antibacterial peptides for food preservation. npj Sci. Food 2021, 5, 9. [Google Scholar] [CrossRef]
- Perumal Pillai, B.; Balasubramaniam, B.; Gupta, R.K.; Tyagi, A. Bio-based materials for antimicrobial films in food applications: Beyond the COVID-19 pandemic era. Oxford Open Mater. Sci. 2023, 3, itad016. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Antimicrobial Packaging for Fresh and Minimally Processed Fruits and Vegetables. In Antimicrobial Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 243–256. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.; Zuhri, M.; Ilyas, R.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: A review. Polymers 2020, 12, 2403. Available online: https://www.mdpi.com/2073-4360/12/10/2403 (accessed on 23 July 2024). [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of Essential Oils: A Review on Their Interaction with Food Components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- El Sawi, S.; Ibrahim, M.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 133–139. Available online: https://www.sciencedirect.com/science/article/pii/S0570178319300053 (accessed on 30 March 2025). [CrossRef]
- Krepker, M.; Shemesh, R.; Danin-Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Garavand, F.; Jafari, S.M. Incorporation of silver nanoparticles into active antimicrobial nanocomposites: Release behavior, analyzing techniques, applications and safety issues. Adv. Colloid. Interface Sci. 2021, 294, 102440. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 72, 132–142. [Google Scholar] [CrossRef]
- Leta, T.B.; Adeyemi, J.O.; Fawole, O.A. Characterization of banana powder-based film reinforced with CNF and Bio-mediated ZnO nanoparticles for potential food applications. Mater. Res. Express 2024, 11, 055301. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of novel active polypropylene based packaging films containing different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Sharma, H.; Ahuja, A.; Sharma, B.; Kulshreshtha, A.; Kadam, A.; Dutt, D. Vapor Phase Antimicrobial Active Packaging Application of Chitosan Capsules Containing Clove Essential Oil for the Preservation of Dry Cakes. Food Bioproc. Technol. 2024, 17, 780–790. [Google Scholar] [CrossRef]
- Yang, S. Characterization of silver nanoparticles. In Polymer Nanocomposites Based on Silver Nanoparticles, Synthesis, Characterization and Applications; Springer: Cham, Switzerland, 2021; pp. 83–107. [Google Scholar] [CrossRef]
- Lv, Y.; Deng, Y.; Wang, M.; Li, C.; Xie, P.; Sun, B.; Yang, Y.; Lang, Y. Effect of chitosan-gelatine edible coating containing nano-encapsulated clove ethanol extract on cold storage of chilled pork. Meat Sci. 2023, 204, 109288. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. Available online: https://www.sciencedirect.com/science/article/pii/S0079670010000912 (accessed on 25 July 2024). [CrossRef]
- Gao, Q.; Kim, B.S.; Gao, G. Advanced Strategies for 3D Bioprinting of Tissue and Organs Analogs Using Alginate Hydrogel Bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef]
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 104, 14–29. [Google Scholar] [CrossRef]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Ye, X.; Wei, L.; Sun, L.; Xu, Q.; Cao, J.; Li, H.; Pang, Z.; Liu, X. Fabrication of food polysaccharide, protein, and polysaccharide-protein composite gels via calcium ion inducement: Gelation mechanisms, conditional factors, and applications. Int. J. Biol. Macromol. 2024, 279, 135397. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Hashmi, S.; Vatankhah-Varnoosfaderani, M.; Stadler, F.J. Effect of H2O and reduced graphene oxide on the structure and rheology of self-healing, stimuli responsive catecholic gels. Rheol. Acta 2016, 55, 163–176. [Google Scholar] [CrossRef]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D bioprinting and photocrosslinking: Emerging strategies & future perspectives. Mater. Sci. Eng. C 2022, 128, 112576. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, W.; Yan, Y. Advances in Photocrosslinkable Materials for 3D Bioprinting. Adv. Eng. Mater. 2022, 24, 2100663. [Google Scholar] [CrossRef]
- Zheng, K.; Gu, Q.; Zhou, D.; Zhou, M.; Zhang, L. Recent progress in surgical adhesives for biomedical applications. Smart Mater. Med. 2022, 3, 177–195. [Google Scholar] [CrossRef]
- Li, X.L.; Shen, Y.; Hu, F.; Zhang, X.X.; Thakur, K.; Rengasamy, K.R.; Khan, M.R.; Busquets, R.; Wei, Z. Fortification of polysaccharide-based packaging films and coatings with essential oils: A review of their preparation and use in meat preservation. Int. J. Biol. Macromol. 2023, 242, 124767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, J.; Zhang, R.; Lin, J.; Zhou, M.; Qin, X.; Wang, K.; Zhang, R.; Zhou, Y.; Zhu, Q.; et al. Development of multi-cross-linking, rapid curing, and easy cleaning, edible hydrogels for meat preservation. Food Hydrocoll. 2024, 155, 110186. [Google Scholar] [CrossRef]
- Wen, L.; Liang, Y.; Lin, Z.; Xie, D.; Zheng, Z.; Xu, C.; Lin, B. Design of multifunctional food packaging films based on carboxymethyl chitosan/polyvinyl alcohol crosslinked network by using citric acid as crosslinker. Polymer 2021, 230, 124048. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Rafflisman Zaidi, N.S.; Mah, S.K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Thakare, M.R.; Wharton, J.A.; Wood, R.J.K.; Menger, C. Exposure effects of alkaline drilling fluid on the microscale abrasion-corrosion of WC-based hardmetals. Wear 2007, 263, 125–136. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings—A review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Surface modification of magnesium and its biodegradable alloys by calcium orthophosphate coatings to improve corrosion resistance and biocompatibility. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 2, pp. 151–191. [Google Scholar] [CrossRef]
- Sebastiá, P.; Climent, V.; Feliu, J.M. Ionic Liquids in the Field of Metal Electrodeposition. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhang, J.; Dai, C.S.; Wei, J.; Wen, Z.H. Study on the bonding strength between calcium phosphate/chitosan composite coatings and a Mg alloy substrate. Appl. Surf. Sci. 2012, 261, 276–286. [Google Scholar] [CrossRef]
- Talib, S.R.J.; Saad, S.; Toff, M.R.M.; Hashim, H. Thermal Spray Coating Technology—A Review. Solid State Sci. Technol. 2003, 11, 109–117. [Google Scholar]
- Abbasi, S.; Golestani-Fard, F.; Rezaie, H.R.; Mirhosseini, S.M.M. MAO-derived hydroxyapatite/TiO2 nanostructured multi-layer coatings on titanium substrate. Appl. Surf. Sci. 2012, 261, 37–42. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Ayu, H.M.; Redzuan, N.; Izman, S. Research progress of sol-gel ceramic coating: A review. Mater. Today Proc. 2021, 48, 1849–1854. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 1, 5102014. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Seng, D.H.L.; ZHANG, Z.; Zhang, Z.Q.; Meng, T.L.; Teo, S.L.; Tan, B.H.; Loi, Q.; Pan, J. Influence of spray angle in cold spray deposition of Ti-6Al-4V coatings on Al6061-T6 substrates. Surf. Coat. Technol. 2022, 432, 128068. [Google Scholar] [CrossRef]
- Sridhar, S.; Viswanathan, A.; Venkateswarlu, K.; Rameshbabu, N.; Parthasarathi, N.L. Enhanced visible light photocatalytic activity of P-block elements (C, N and F) doped porous TiO2 coatings on Cp-Ti by micro-arc oxidation. J. Porous Mater. 2015, 22, 545–557. [Google Scholar] [CrossRef]
- Séverin, I.; Lionti, K.; Dahbi, L.; Loriot, C.; Toury, B.; Chagnon, M.C. In vitro toxicity assessment of extracts derived from sol-gel coatings on polycarbonate intended to be used in food contact applications. Food Chem. Toxicol. 2016, 93, 51–57. [Google Scholar] [CrossRef]
- Razavi, R.; Tajik, H.; Moradi, M.; Molaei, R.; Ezati, P. Antimicrobial, microscopic and spectroscopic properties of cellulose paper coated with chitosan sol-gel solution formulated by epsilon-poly-L-lysine and its application in active food packaging. Carbohydr. Res. 2020, 489, 107912. [Google Scholar] [CrossRef] [PubMed]
- Ajermoun, N.; Lahrich, S.; Farahi, A.; Bakasse, M.; Saqrane, S.; El Mhammedi, M.A. Electrodeposition of silver onto carbon graphite and their catalysis properties toward thiamethoxam reduction: Application in food and beverage samples. Heliyon 2020, 6, e05784. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Parente, A.G.; de Oliveira, H.P.; Cabrera, M.P.; de Morais Neri, D.F. Bio-based polymer films with potential for packaging applications: A systematic review of the main types tested on food. Polym. Bull. 2023, 80, 4689–4717. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 32–58. [Google Scholar] [CrossRef]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible Films and Coatings as Carriers of Living Microorganisms: A New Strategy Towards Biopreservation and Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 271–306. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Li, M.; Lin, J. Fruits and vegetables preservation based on AI technology: Research progress and application prospects. Comput. Electron. Agric. 2024, 226, 109382. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Ščetar, M.; Kurek, M.; Galic, K. Trends in fruit and vegetable packaging—A review. Croat. J. Food Technol. Biotechnol. Nutr. 2010, 5, 69–86. Available online: https://hrcak.srce.hr/file/95967 (accessed on 29 August 2024).
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Nechita, P.; Iana-Roman, M.R. Review on polysaccharides used in coatings for food packaging papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Improvement of water barrier property of rice starch-chitosan composite film incorporated with lipids. Food Sci. Technol. Int. 2009, 15, 149–158. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and Lipid-containing Composite Edible Coatings and Films. Food Rev. Int. 2022, 38, 574–597. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Greco, G.; Gentile, C.; Settanni, L.; Inglese, P. Effect of opuntia ficus-indica mucilage edible coating on quality, nutraceutical, and sensorial parameters of minimally processed cactus pear fruits. Agronomy 2021, 11, 1963. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; de Oliveira, K.Á.R.; Queiroga, T.S.; de Souza, E.L. Development and Application of Mucilage and Bioactive Compounds from Cactaceae to Formulate Novel and Sustainable Edible Films and Coatings to Preserve Fruits and Vegetables—A Review. Foods 2024, 13, 3613. [Google Scholar] [CrossRef]
- Shinga, M.H.; Fawole, O.A. Opuntia ficus indica mucilage coatings regulate cell wall softening enzymes and delay the ripening of banana fruit stored at retail conditions. Int. J. Biol. Macromol. 2023, 245, 125550. [Google Scholar] [CrossRef]
- Mukwevho, P.L.; Kaseke, T.; Fawole, O.A. Ascorbic acid-enriched cactus mucilage coatings maintained quality attributes of roasted ‘Wichita’ pecan nuts under accelerated storage conditions. J. Agric. Food Res. 2024, 18, 101301. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012, 130, 267–272. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Optimization of gum arabic and starch-based edible coatings with lemongrass oil using response surface methodology for improving postharvest quality of whole ‘wonderful’ pomegranate fruit. Coatings 2021, 11, 442. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Neeraj; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate-based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; Abou El Ghit, H.M.; Fikry, M.; Bahloul, A.M.E.; Morsy, A.R.; A., L.A.; Abdelaziz, A.M.R.A.; Alhaithloul, H.A.S.; et al. The Effects of a Gum Arabic-Based Edible Coating on Guava Fruit Characteristics during Storage. Coatings 2022, 12, 90. [Google Scholar] [CrossRef]
- Kumar, S.; Baswal, A.K.; Ramezanian, A.; Gill, K.S.; Mirza, A.A. Impact of carboxymethyl cellulose-based edible coating on storage life and quality of guava fruit cv. ‘Allahabad Safeda’ under ambient storage conditions. J. Food Meas. Charact. 2021, 15, 4805–4812. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Fawole, O.A.; Oluwafemi, O.S. Evaluating the Efficacy of Gum Arabic-Zinc Oxide Nanoparticles Composite Coating on Shelf-Life Extension of Mandarins (cv. Kinnow). Front. Plant Sci. 2022, 13, 953861. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Chen, M.; Chen, C.; Chen, Y.; Fu, Y.; Wan, C.; Chen, J. Effects of Chitosan-Based Coatings Enriched with Cinnamaldehyde on Mandarin Fruit cv. Ponkan during Room-Temperature Storage. Coatings 2018, 8, 372. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Ejaz, S.; Hussain, S.; Ercisli, S.; Saleem, M.S.; Sardar, H. Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 2021, 168, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. Available online: https://www.sciencedirect.com/science/article/pii/S0963996918305283 (accessed on 29 August 2024).
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–334. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Dinakarkumar, Y.; Shin, H. A comprehensive overview on the preservation techniques and packaging of processed meat products: Emphasis on natural derivatives. J. King Saud Univ. Sci. 2024, 36, 103032. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Torrieri, E.; Masi, P. Emerging technologies in seafood processing: An overview of innovations reshaping the aquatic food industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13281. [Google Scholar] [CrossRef]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P. Seafood Chilling, Refrigeration and Freezing: Science and Technology. 2015. Available online: https://books.google.com/books?hl=en&lr=&id=12JvCAAAQBAJ&oi=fnd&pg=PA186&ots=eygiGgcHO3&sig=Tr9U5SLojRrtXqpqB1Y6CfBOTvA (accessed on 29 August 2024).
- Ghaly, A.E.; Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Kuwahara, K.; Osako, K. Effect of Sodium Gluconate on Gel Formation of Japanese Common Squid Mantle Muscle. 2003. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20033153745 (accessed on 30 August 2024).
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Srinivasa Gopal, T.K. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Chen, H.; Song, R.; Li, Y. Performance of eugenol emulsion/chitosan edible coating and application in fresh meat preservation. J. Food Process. Preserv. 2022, 46, 16407. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Azadi, A.; Rafieian, F.; Sami, M.; Rezaei, A. Investigating the effects of chitosan/ tragacanth gum/ polyvinyl alcohol composite coating incorporated with cinnamon essential oil nanoemulsion on safety and quality features of chicken breast fillets during storage in the refrigerator. Int. J. Biol. Macromol. 2023, 253, 126481. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, M.; Chitrakar, B.; Adhikari, B.; Yang, C. Effects of nanoemulsion-based chicken bone gelatin-chitosan coatings with cinnamon essential oil and rosemary extract on the storage quality of ready-to-eat chicken patties. Food Packag. Shelf Life 2022, 34, 100933. [Google Scholar] [CrossRef]
- Kim, J.H.; sung Hong, W.; Oh, S.W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, Y.; Sıtkı, M.; Üniversity, K.; Hasanhocaoğlu, H.; Üniversitesi, Y.M. Combined effect of orange peel essential oil and gelatin coating on the quality and shelf life of shrimps. J. Food Saf. Food Qual. 2017, 68, 69–78. [Google Scholar] [CrossRef]
- Dai, W.; Yan, C.; Ding, Y.; Wang, W.; Gu, S.; Xu, Z.; Zhou, X.; Ding, Y. Effect of a chitosan coating incorporating epigallocatechin gallate on the quality and shelf life of bighead carp (Aristichthys nobilis) fillets during chilled storage. Int. J. Biol. Macromol. 2022, 219, 1272–1283. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Manley, D. Biscuit, Cracker and Cookie Recipes for the Food Industry. 2001. Available online: https://books.google.com/books?hl=en&lr=&id=tK2jAgAAQBAJ&oi=fnd&pg=PP1&ots=7HDL5Q2c9s&sig=AmyhYuj9PpInsDjGXMt_xSCYDtM (accessed on 2 September 2024).
- Konstantas, A.; Stamford, L.; Azapagic, A. Evaluating the environmental sustainability of cakes. Sustain. Prod. Consum. 2019, 19, 169–180. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Strategies to extend bread and GF bread shelf-life: From Sourdough to antimicrobial active packaging and nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, I.; Baeva, M.; Popova, A.; Fidan, H.; Goranova, Z.; Milkova-Tomova, I. Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf. Foods 2022, 11, 3831. [Google Scholar] [CrossRef]
- Saraiva, L.E.F.; Naponucena, L.D.O.M.; da Silva Santos, V.; Silva, R.P.D.; de Souza, C.O.; Souza, I.E.G.L.; Mamede, M.E.; Druzian, J.I. Development and application of edible film of active potato starch to extend mini panettone shelf life. LWT 2016, 73, 311–319. [Google Scholar] [CrossRef]
- De Pilli, T. Development of a vegetable oil and egg proteins edible film to replace preservatives and primary packaging of sweet baked goods. Food Control 2020, 114, 107273. [Google Scholar] [CrossRef]
- Grosso, A.L.; Asensio, C.M.; Grosso, N.R.; Nepote, V. Increase of walnuts’ shelf life using a walnut flour protein-based edible coating. LWT 2020, 118, 108712. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Abu, J.O. Shelf Life Extension of Toasted Groundnuts through the Application of Cassava Starch and Soy Protein-Based Edible Coating. Niger. Food J. 2014, 32, 133–138. Available online: www.nifst.org (accessed on 20 April 2025). [CrossRef]
- Mouzakitis, C.K.; Sereti, V.; Matsakidou, A.; Kotsiou, K.; Biliaderis, C.G.; Lazaridou, A. Physicochemical properties of zein-based edible films and coatings for extending wheat bread shelf life. Food Hydrocoll. 2022, 132, 107856. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people (Review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Nasralla, N.N.; Gomah, N.H.; Aly, M.M.; Abdel-Aleem, J.A.; Hammam, A.R.; Osman, D.M.; El-Derwy, Y.M. Compositional characteristics of dairy products and their potential nondairy applications after shelf-life. Curr. Res. Food Sci. 2022, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Bagheripoor, N.; Khoshgozaran-Abras, S.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M.; MollaKhalili, N.; Jazaeri, S. Application of active edible coatings to improve the shelf-life of cheese. Food Sci. Technol. Res. 2018, 24, 949–962. [Google Scholar] [CrossRef]
- Salama, H.H.; Trif, M.; Rusu, A.V.; Bhattacharya, S. Application of Functional and Edible Coatings and Films as Promising Strategies for Developing Dairy Functional Products—A Review on Yoghurt Case. Coatings 2022, 12, 838. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Hamed, Y.S.; Kamel, R.M.; Shawir, S.M.; Sakr, H.; Ali, M.; Ammar, A.; Saleh, M.N.; El Fadly, E.; Salama, M.A.; et al. Enhanced physical properties, antioxidant and antibacterial activity of bio-composite films composed from carboxymethyl cellulose and polyvinyl alcohol incorporated with broccoli sprout seed extract for butter packaging. Int. J. Biol. Macromol. 2024, 255, 128346. [Google Scholar] [CrossRef]
- Sun, R.; Song, G.; Zhang, H.; Zhang, H.; Chi, Y.; Ma, Y.; Li, H.; Bai, S.; Zhang, X. Effect of basil essential oil and beeswax incorporation on the physical, structural, and antibacterial properties of chitosan emulsion-based coating for eggs preservation. LWT 2021, 150, 112020. [Google Scholar] [CrossRef]
- Mahajan, K.; Kumar, S.; Bhat, Z.F.; Naqvi, Z.; Mungure, T.E.; Bekhit, A.E.D.A. Functionalization of carrageenan-based edible film using Aloe vera for improved lipid oxidative and microbial stability of frozen dairy products. Food Biosci. 2021, 43, 101336. [Google Scholar] [CrossRef]
- Dhir, B.; Singla, N. Consumption Pattern and Health Implications of Convenience Foods: A Practical Review. Curr. J. Appl. Sci. Technol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, T.; Jiang, Z.; Zhu, Y. Low temperature preservation for perishable ready to eat foods: Not entirely effective for control of L. monocytogenes. Trends Food Sci. Technol. 2023, 142, 104228. [Google Scholar] [CrossRef]
- Ready Meals Global Market Report 2025, Size and Share by 2034. Available online: https://www.thebusinessresearchcompany.com/report/ready-meals-global-market-report (accessed on 7 May 2025).
- Mwelase, S.; Kaseke, T.; Fawole, O.A. Development and optimization of methylcellulose-based edible coating using response surface methodology for improved quality management of ready-to-eat pomegranate arils. CYTA J. Food 2023, 21, 656–665. [Google Scholar] [CrossRef]
- Liu, C.L.; Hsu, C.K.; Hsu, M.M. Improving the quality of fresh-cut pineapples with ascorbic acid/sucrose pretreatment and modified atmosphere packaging. Packag. Technol. Sci. 2007, 20, 337–343. [Google Scholar] [CrossRef]
- Ayhan, Z. Effect of Packaging on the Quality and Shelf-life of Minimally Processed/Ready to Eat Foods. 2011. Available online: http://www.academicfoodjournal.com (accessed on 30 April 2025).
- Cofelice, M.; Iftikhar, A.; Lopez, F.; De Leonardis, A. Effect of edible coatings on quality parameters and phenol composition of ready-to-eat Salanova lettuce. Eur. Food Res. Technol. 2024, 250, 691–700. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Olivas, G.; Barbosa-Cánovas, G.V.I. Edible Coatings for Fresh-Cut Fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Singla, M.; Pareek, S.; Kumar, N.; Sagar, N.A.; Fawole, O.A. Chitosan-Cinnamon Oil Coating Maintains Quality and Extends Shelf Life of Ready-To-Use Pomegranate Arils under Low-Temperature Storage. J. Food Qual. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Liguori, G.; Greco, G.; Gaglio, R.; Settanni, L.; Gentile, C.; Inglese, P. Mucilage-Based and Calcium Ascorbate Edible Coatings Improve Postharvest Quality and Storability of Minimally Processed Cactus Pear Fruit Stored under Passive Atmosphere. Horticulturae 2023, 9, 1. [Google Scholar] [CrossRef]
- Kozlu, A.; Elmacı, Y. Quince seed mucilage as edible coating for mandarin fruit; determination of the quality characteristics during storage. J. Food Process Preserv. 2020, 44, 11. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Armynah, B.; Tahir, D. Bioplastics Starch-Based with Additional Fiber and Nanoparticle: Characteristics and Biodegradation Performance: A Review. J. Polym. Environ. 2021, 29, 3459–3476. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef]
- Rosseto, M.; Rigueto, C.V.; Krein, D.D.; Balbé, N.P.; Massuda, L.A.; Dettmer, A. Biodegradable Polymers: Opportunities and Challenges. In Organic Polymers; Sand, A., Zaki, E.G., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–18. ISBN 978-1-78984-618-8. [Google Scholar]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Meraldo, A. Introduction to Bio-Based Polymers. In Multilayer Flexible Packaging, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 47–52. [Google Scholar] [CrossRef]
- Singh, S.; Habib, M.; Rao, E.S.; Kumar, Y.; Bashir, K.; Jan, S.; Jan, K. A comprehensive overview of biodegradable packaging films: Part I—Sources, additives, and preparation methods. Discov. Food 2025, 5, 41. [Google Scholar] [CrossRef]
- Hu, S.; Han, L.; Yu, C.; Pan, L.; Tu, K. A Review on Replacing Food Packaging Plastics with Nature-Inspired Bio-Based Materials. Foods 2025, 14, 1661. [Google Scholar] [CrossRef]
- Thomas, S.; Cintil, A.A.; Chirayil, J.; Thomas, B. Handbook of Biopolymers; Springer: Singapore, 2023. [Google Scholar]
- O’Connor, L.J.; Favreau-Farhadi, N.; Barrett, A.H. Use of edible barriers in intermediate moisture food systems to inhibit moisture migration. J. Food Process. Preserv. 2018, 42, e13512. [Google Scholar] [CrossRef]
- Chalk, S. Raw Material Variability 2014. MJH Life Sciences. Available online: https://www.biopharminternational.com/view/raw-material-variability (accessed on 27 May 2025).
- Toma, D.-I.; Manaila-Maximean, D.; Fierascu, I.; Baroi, A.M.; Matei, R.I.; Fistos, T.; Chican, I.E.; Fierascu, R.C. Applications of Natural Polymers in the Grapevine Industry: Plant Protection and Value-Added Utilization of Waste. Polymers 2025, 17, 18. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Zia, K.M.; Akram, N.; Tabasum, S.; Noreen, A.; Akbar, M.U. Processing of bio-based polymers for industrial and medical applications. In Processing Technology for Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–238. [Google Scholar] [CrossRef]
- Jernström, E.; Karvonen, V.; Kässi, T.; Kraslawski, A.; Hallikas, J. The main factors affecting the entry of SMEs into bio-based industry. J. Clean. Prod. 2017, 141, 1–10. [Google Scholar] [CrossRef]
- Fayshal, M.A. Current practices of plastic waste management, environmental impacts, and potential alternatives for reducing pollution and improving management. Heliyon 2024, 10, e40838. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; Ghani, A.A. Expanding policy for biodegradable plastic products and market dynamics of bio-based plastics: Challenges and opportunities. Sustainability 2021, 13, 6170. [Google Scholar] [CrossRef]
- Pakseresht, A.; Ahmadi Kaliji, S.; Canavari, M. Review of factors affecting consumer acceptance of cultured meat. Appetite 2022, 170, 105829. [Google Scholar] [CrossRef]
- Civille, G.V. Food quality: Consumer acceptance and sensory attributes. J. Food Qual. 1991, 14, 1–8. [Google Scholar] [CrossRef]
- Churton, H.; McCabe, B.K. Advancing a food loss and waste bioproduct industry: A critical review of policy approaches for application in an Australian context. Heliyon 2024, 10, e32735. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Idowu, A.M.; Onyeaka, H.N. Recycling Technologies for Biopolymers: Current Challenges and Future Directions. Polymers 2024, 16, 2770. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
