Roasting Temperature as a Factor Modifying the Caffeine and Phenolic Content of Ethiopian Coffee
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Processing
2.2. Determination of the Phenolic Compounds
2.3. Determination of the Caffeine
2.4. Statistical Analysis
3. Results and Discussion
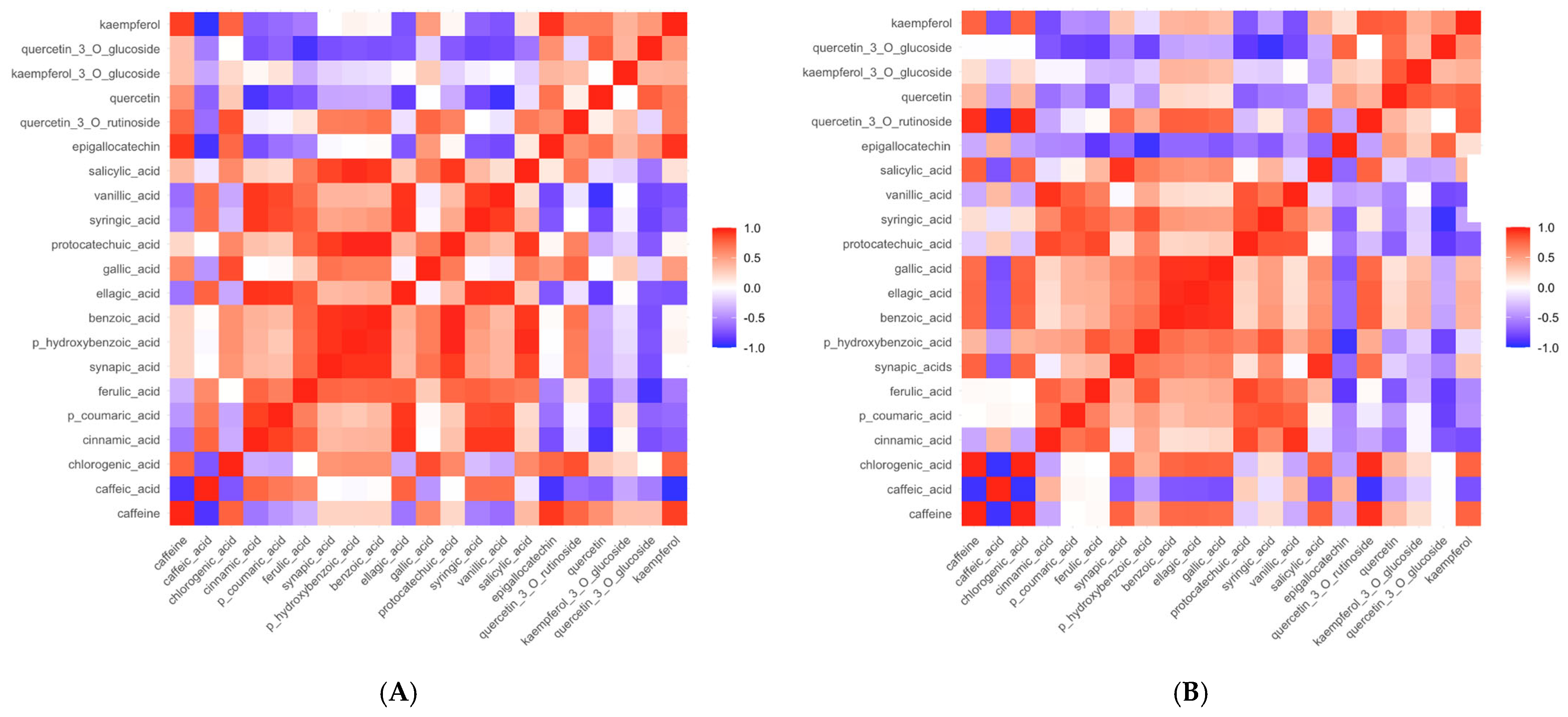
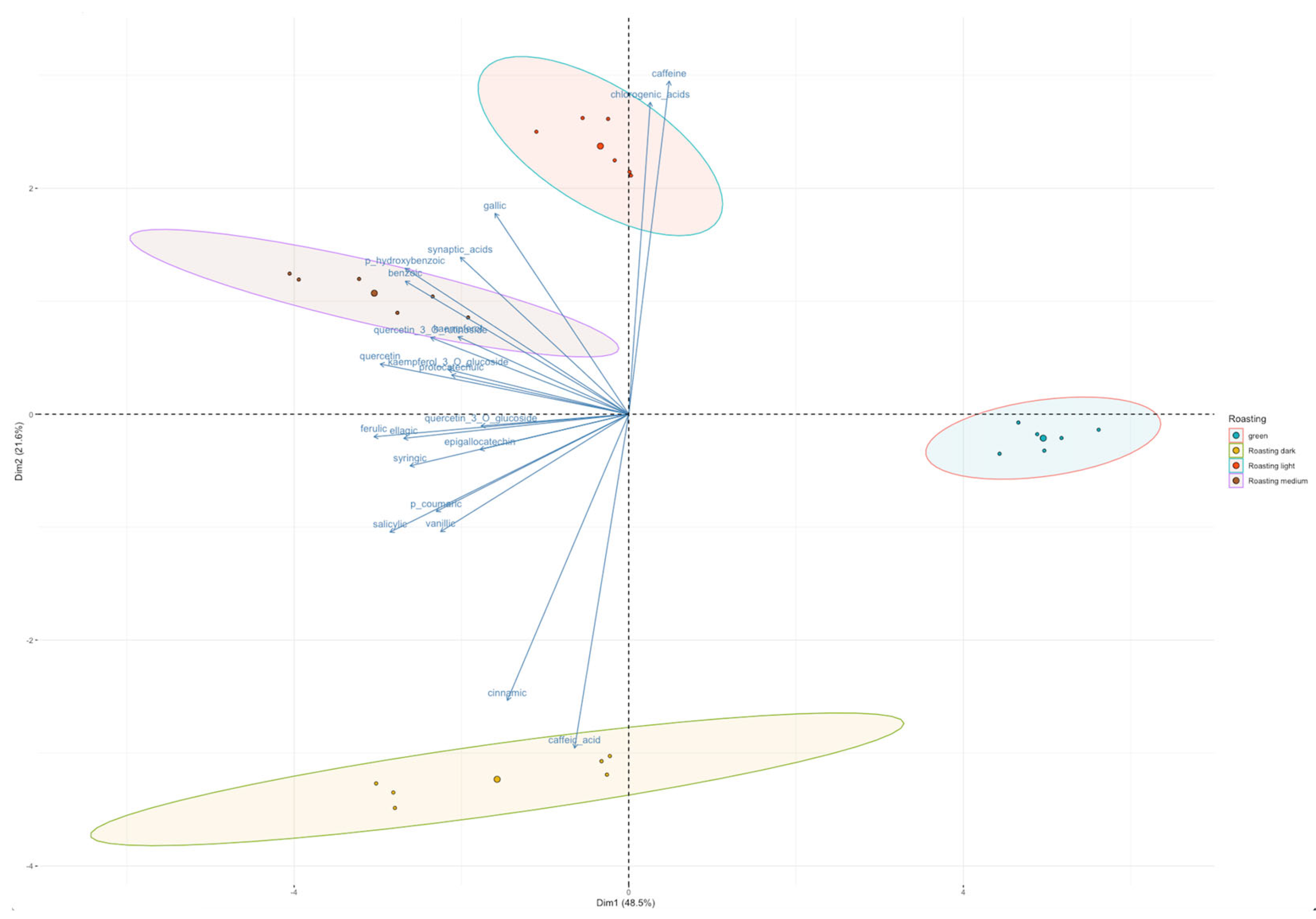
3.1. Content of Bioactive Compounds
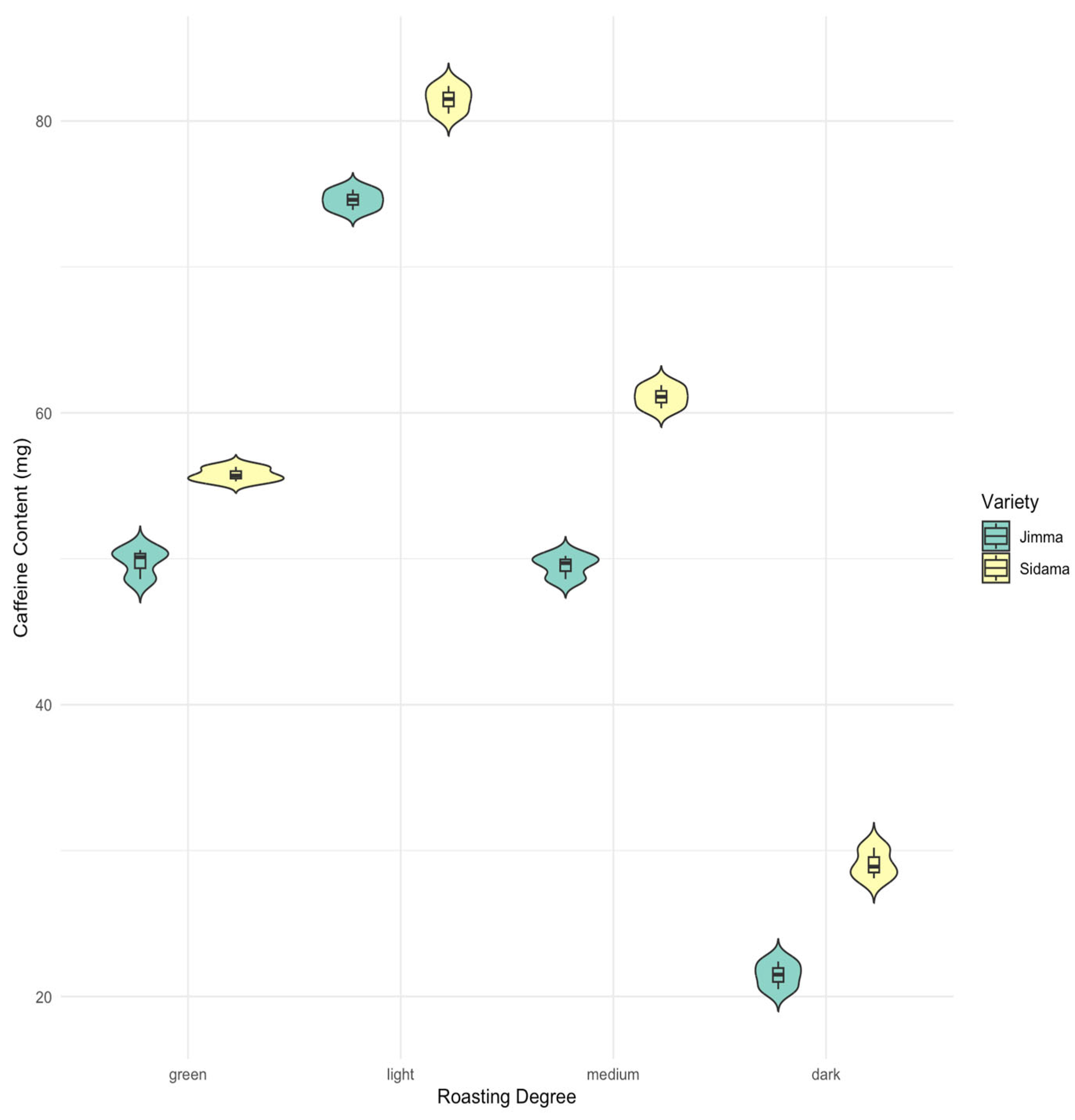
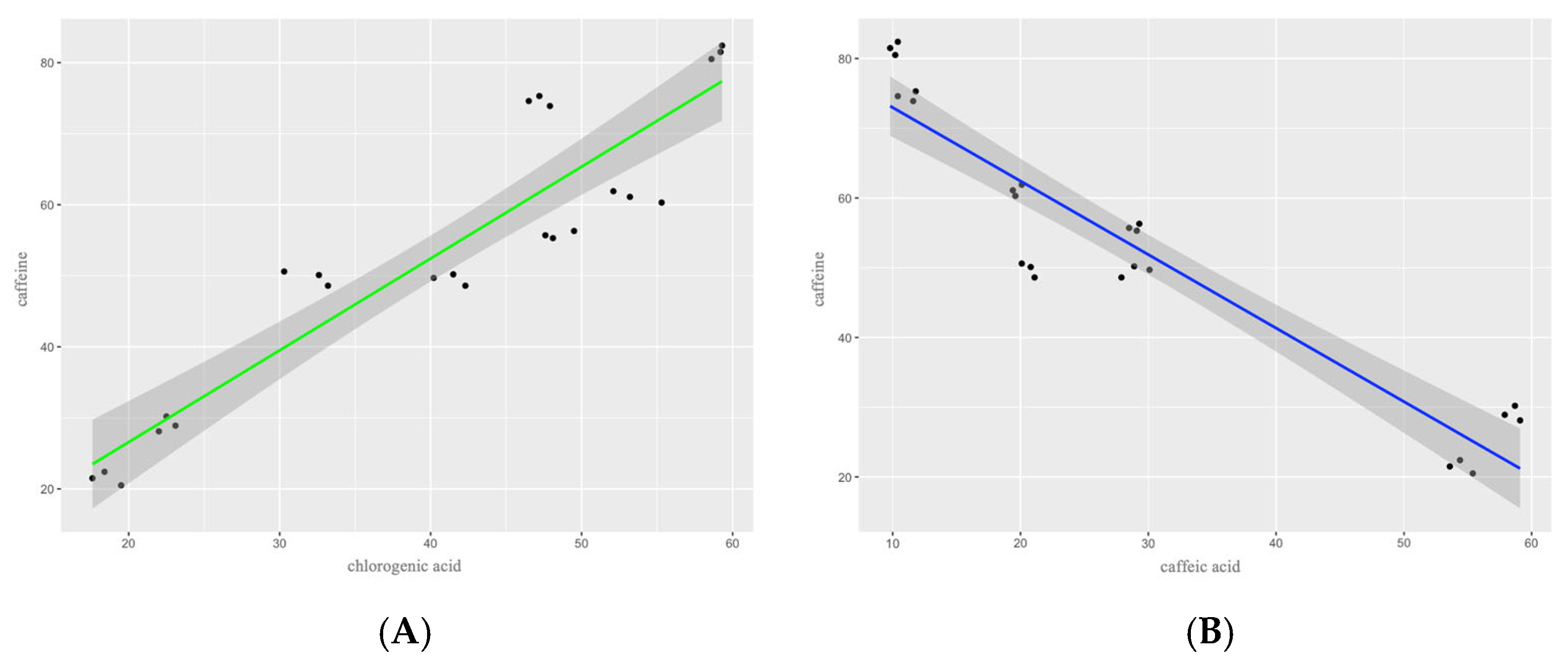
3.2. Caffeine Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Ho, C.T. Polyphenolic Chemistry of Tea and Coffee: A Century of Progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef] [PubMed]
- Bolka, M.; Emire, S. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Celeghini, R.M.S.; Debien, I.C.N.; Nogueira, G.C.; Meireles, M.A.A. Phenolic Compounds in Coffee Compared to Other Beverages. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 137–142. [Google Scholar]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Peng, X.; Gao, Y.; Huang, Y.; Li, X.; Su, H.; Qiu, M. Effect of roasting degree of coffee beans on sensory evaluation: Research from the perspective of major chemical ingredients. Food Chem. 2020, 331, 127329. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Oliveira, R.C.S.; Agresti, P.C.M.; Augusti, R. A preliminary evaluation of the effect of processing temperature on coffee roasting degree assessment. J. Food Eng. 2009, 92, 345–352. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.T. Physicochemical Characteristics of Roasted Coffee. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 247–254. [Google Scholar]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Opitz, S.; Smrke, S.; Goodman, B.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef]
- Mestanza, M.; Mori-Culqui, P.L.; Chavez, S.G. Changes of polyphenols and antioxidants of arabica coffee varieties during roasting. Front. Nutr. 2023, 10, 1078701. [Google Scholar] [CrossRef]
- Frankowski, J.; Przybylska-Balcerek, A.; Graczyk, M.; Niedziela, G.; Sieracka, D.; Stuper-Szablewska, K. The Effect of Mineral Fertilization on the Content of Bioactive Compounds in Hemp Seeds and Oil. Molecules 2023, 28, 4870. [Google Scholar] [CrossRef]
- PN-ISO 10095:1997; Coffee—Determination of Caffeine Content: A Method Using High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 1997.
- Dąbrowska-Molenda, M.; Szwedziak, K.; Zabłudowska, Ż. Analysis of caffeine content in selected types of coffee. Postępy Tech. Przetwórstwa Spożywczego 2019, 2, 69–71. [Google Scholar]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi Mde, T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical composition and antioxidant capacity of coffee pulp. Ciência Agrotecnologia 2018, 42, 307–313. [Google Scholar] [CrossRef]
- Farah, A. Coffee Constituents. In Coffee; Wiley: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Data on coffee composition and mass spectrometry analysis of mixtures of coffee related carbohydrates, phenolic compounds and peptides. Data Brief. 2017, 13, 145–161. [Google Scholar] [CrossRef]
- Erskine, E.; Gültekin Subaşı, B.; Vahapoglu, B.; Capanoglu, E. Coffee Phenolics and Their Interaction with Other Food Phenolics: Antagonistic and Synergistic Effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A.R. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of methylxantines, trigonelline, nicotinic acid and nicotinamide contents in brews of green and processed Arabica and Robusta coffee beans—Influence of steaming, decaffeination and roasting processes on coffee beans. LWT 2020, 125, 109344. [Google Scholar] [CrossRef]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the Antioxidant Activities and Volatile Compounds of Coffee Beans Obtained Using Digestive Bio-Processing (Elephant Dung Coffee) and Commonly Known Processing Methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Lu, J.; Hu, G.; Qiu, M. Ent-kaurane diterpenoids from the cherries of Coffea arabica. Fitoterapia 2019, 132, 7–11. [Google Scholar] [CrossRef]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Crozier, A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Dixon, R.A. The ins and outs of plant specialized metabolite gene organization. Proc. Natl. Acad. Sci. USA 2025, 122, e2504934122. [Google Scholar] [CrossRef]
- Oussou, K.F.; Buyukkurt, O.K.; Guclu, G.; Kelebek, H.; Selli, S. Chlorogenic acids in different coffees. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2025; pp. 239–252. [Google Scholar]
- Tarigan, E.B.; Wardiana, E.; Hilmi, Y.S.; Komarudin, N.A. The changes in chemical properties of coffee during roasting: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012115. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Wang, Y.; Tang Ho, C. Effects of Naturally Occurring Phenolic Compounds in Coffee on the Formation of Maillard Aromas. In Nutrition, Functional and Sensory Properties of Foods; The Royal Society of Chemistry: London, UK, 2013; pp. 98–110. [Google Scholar]
- van der Werf, R.; Marcic, C.; Khalil, A.; Sigrist, S.; Marchioni, E. ABTS radical scavenging capacity in green and roasted coffee extracts. LWT Food Sci. Technol. 2014, 58, 77–85. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Mastrocola, D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Duarte SMda, S.; Abreu CMPde Menezes HCde Santos MHdos Gouvêa, C.M.C.P. Effect of processing and roasting on the antioxidant activity of coffee brews. Ciência E Tecnol. Aliment. 2005, 25, 387–393. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Mengesha, D.; Retta, N.; Woldemariam, H.W.; Getachew, P. Changes in biochemical composition of Ethiopian Coffee arabica with growing region and traditional roasting. Front. Nutr. 2024, 11, 1390515. [Google Scholar] [CrossRef] [PubMed]
- Oestreich-Janzen, S. Chemistry of Coffee. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1085–1117. [Google Scholar]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of bioactive compounds during roasting process of coffee beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef] [PubMed]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Ferraz, M.B.; Farah, A.; Iamanaka, B.T.; Perrone, D.; Copetti, M.V.; Marques, V.X.; Vitali, A.A.; Taniwaki, M.H. Kinetics of ochratoxin A destruction during coffee roasting. Food Control 2010, 21, 872–877. [Google Scholar] [CrossRef]


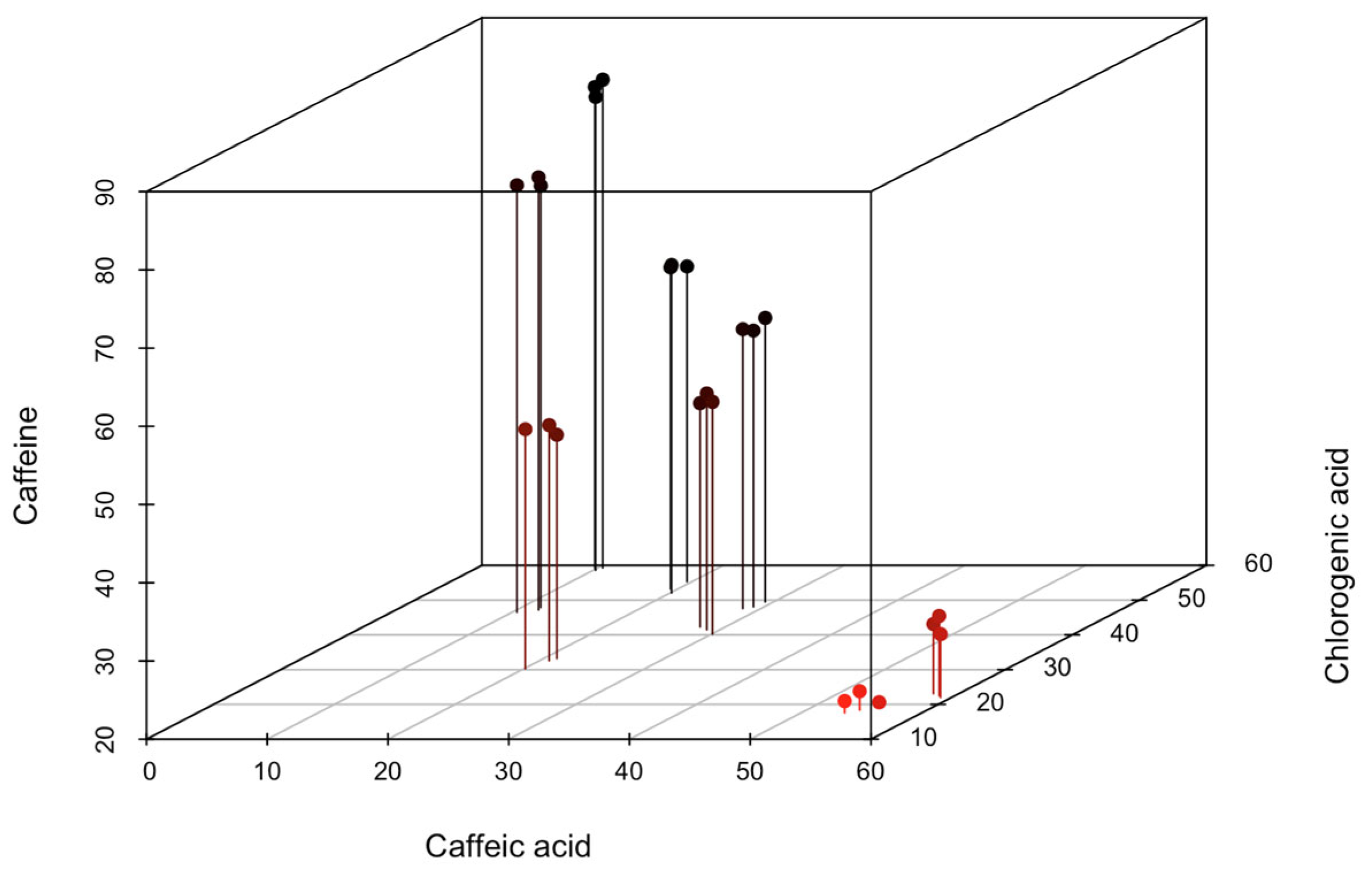
| Jimma | Sidama | |||||||
|---|---|---|---|---|---|---|---|---|
| Green | Light | Medium | Dark | Green | Light | Medium | Dark | |
| Total polyphenol | 127,64 ± 0.83 a (126.73–128.34) | 173.88 ± 1.44 ab (172.25–174.97) | 169.48 ± 1.47 abc (167.78–170.33) | 156.03 ± 0.82 bc (155.08–156.51) | 158.01 ± 1.76 abc (156.45–159.92) | 191.59 ± 1.85 abc (189.65–193.34) | 182.19 ± 0.92 abc (181.22–183.06) | 169.80 ± 1.40 c (168.19–170.69) |
| Caffeic acid | 20.67 ± 0.51 abc (20.10–21.10) | 11.27 ± 0.76 ab (10.40–11.80) | 28.97 ± 1.10 abc (27.90–30.10) | 54.47 ± 0.90 ac (53.60–55.40) | 28.97 ± 0.42 abc (28.50–29.30) | 10.13 ± 0.31 b (9.80–10.40) | 19.70 ± 0.36 abc (19.40–20.10) | 58.57 ± 0.61 c (57.90–59.10) |
| Chlorogenic acid | 32.03 ± 1.53 abc (30.30–33.20) | 47.20 ± 0.70 abc (46.50–47.90) | 41.33 ± 1.06 abc (40.20–42.30) | 18.50 ± 0.95 a (17.60–19.50) | 48.40 ± 0.98 abc (47.60–49.50) | 59.03 ± 0.38 b (58.60–59.30) | 53.53 ± 1.63 bc (52.10–55.30) | 22.53 ± 0.55 ac (22.00–23.10) |
| Cinnamic acid | 0.50 ± 0.10 a (0.40–0.60) | 1.40 ± 0.30 ab (1.10–1.70) | 2.77 ± 0.15 ab (2.60–2.90) | 9.23 ± 0.15 b (9.10–9.40) | 0.50 ± 0.10 a (0.40–0.60) | 1.73 ± 0.21 ab (1.50–1.90) | 3.63 ± 0.31 ab (3.30–3.90) | 11.20 ± 0.89 b (10.20–11.90) |
| P-coumaric acid | 1.43 ± 0.15 a (1.30–1.60) | 2.23 ± 0.15 abc (2.10–2.40) | 2.40 ± 0.26 abc (2.10–2.60) | 3.23 ± 0.15 abc (3.10–3.40) | 1.40 ± 0.10 ab (1.30–1.50) | 3.00 ± 0.10 abc (2.90–3.10) | 3.47 ± 0.15 bc (3.30–3.60) | 3.43 ± 0.55 c (2.80–3.80) |
| Ferulic acid | 0.47 ± 0.06 a (0.40–0.50) | 1.30 ± 0.20 ab (1.10–1.50) | 2.10 ± 0.10 b (2.00–2.20) | 1.80 ± 0.10 ab (1.70–1.90) | 0.40 ± 0.10 a (0.30–0.50) | 1.33 ± 0.15 ab (1.20–1.50) | 1.80 ± 0.10 ab (1.70–1.90) | 1.57 ± 0.25 ab (1.30–1.80) |
| Synapic acid | 1.50 ± 0.10 ab (1.40–1.60) | 3.23 ± 0.15 a (3.10–3.40) | 4.20 ± 0.10 a (4.10–4.30) | 2.20 ± 0.10 ab (2.10–2.30) | 1.10 ± 0.10 b (1.00–1.20) | 1.53 ± 0.06 abc (1.50–1.60) | 2.20 ± 0.10 bc (2.10–2.30) | 1.00 ± 0.10 abc (0.90–1.10) |
| P-hydroxybenzoic acid | 2.20 ± 0.10 ab (2.10–2.30) | 4.41 ± 0.10 abc (4.30–4.50) | 5.17 ± 0.04 c (5.12–5.20) | 3.27 ± 0.11 abc (3.20–3.40) | 2.00 ± 0.10 a (1.90–2.10) | 3.37 ± 0.15 abc (3.20–3.50) | 4.54 ± 0.05 bc (4.50–4.60) | 3.23 ± 0.15 abc (3.10–3.40) |
| Benzoic acid | 0.11 ± 0.01 a (0.10–0.12) | 0.72 ± 0.03 ab (0.70–0.75) | 0.85 ± 0.03 b (0.82–0.88) | 0.65 ± 0.02 ab (0.63–0.67) | 0.23 ± 0.01 a (0.22–0.24) | 0.87 ± 0.02 b (0.86–0.89) | 0.67 ± 0.02 ab (0.65–0.69) | 0.49 ± 0.03 ab (0.45–0.51) |
| Ellagic acid | 0.28 ± 0.07 a (0.20–0.33) | 0.45 ± 0.04 ab (0.42–0.49) | 0.64 ± 0.02 ab (0.62–0.66) | 0.75 ± 0.02 b (0.73–0.76) | 0.32 ± 0.03 a (0.28–0.34) | 0.65 ± 0.02 ab (0.63–0.67) | 0.56 ± 0.02 ab (0.54–0.58) | 0.46 ± 0.03 ab (0.43–0.48) |
| Gallic acid | 8.47 ± 0.31 ab (8.20–8.80) | 10.60 ± 0.26 ac (10.40–10.90) | 9.26 ± 0.17 abc (9.10–9.44) | 8.53 ± 0.15 ab (8.40–8.70) | 7.43 ± 0.25 b (7.20–7.70) | 10.67 ± 0.47 c (10.30–11.20) | 10.07 ± 0.21 abc (9.90–10.30) | 9.27 ± 0.32 abc (8.90–9.50) |
| Protocatechuic acid | 0.13 ± 0.06 ab (0.10–0.20) | 0.53 ± 0.06 abcd (0.50–0.60) | 0.87 ± 0.06 ac (0.80–0.90) | 0.27 ± 0.06 abcd (0.20–0.30) | 0.20 ± 0.10 bd (0.10–0.30) | 0.40 ± 0.10 abcd (0.30–0.50) | 0.77 ± 0.06 cd (0.70–0.80) | 0.83 ± 0.06 abcd (0.80–0.90) |
| Syringic acid | 0.30 ± 0.10 a (0.20–0.40) | 0.50 ± 0.10 ab (0.40–0.60) | 0.73 ± 0.06 ab (0.70–0.80) | 0.80 ± 0.10 ab (0.70–0.90) | 0.30 ± 0.10 a (0.20–0.40) | 0.73 ± 0.06 ab (0.70–0.80) | 0.93 ± 0.15 b (0.80–1.10) | 0.77 ± 0.06 ab (0.70–0.80) |
| Vanillic acid | 0.12 ± 0.02 a (0.10–0.13) | 3.30 ± 0.20 abc (3.10–3.50) | 10.53 ± 0.32 abc (10.30–10.90) | 22.40 ± 1.21 b (21.10–23.50) | 0.33 ± 0.06 ac (0.29–0.40) | 4.33 ± 0.21 abc (4.10–4.50) | 10.13 ± 0.50 abc (9.60–10.60) | 19.20 ± 0.79 bc (18.30–19.80) |
| Salicylic acid | 3.23 ± 0.25 ab (3.10–3.40) | 4.33 ± 0.32 abc (4.10–4.70) | 5.20 ± 0.36 ac (4.90–5.60) | 3.33 ± 0.15 ab (3.20–3.50) | 4.27 ± 0.15 abc (4.10–4.40) | 5.27 ± 0.21 ac (5.10–5.50) | 4,30 ± 0.26 c (4.10–4.60) | 3.57 ± 0.15 b (3.40–3.70) |
| Epigallocatechin | 0.86 ± 0.04 ab (0.82–0.89) | 0.94 ± 0.02 a (0.92–0.96) | 0.75 ± 0.02 b (0.73–0.76) | 0.62 ± 0.02 b (0.62–0.64) | 0.95 ± 0.04 a (0.92–0.99) | 0.83 ± 0.02 ab (0.81–0.84) | 0.77 ± 0.02 ab (0.76–0.79) | 0.84 ± 0.03 ab (0.82–0.87) |
| Quercetin-3-O-rutinoside | 0.75 ± 0.03 ab (0.72–0.77) | 1.20 ± 0.10 ac (1.10–1.30) | 0.92 ± 0.01 abc (0.91–0.93) | 0.74 ± 0.03 ab (0.71–0.77) | 0.87 ± 0.02 abc (0.85–0.89) | 1.43 ± 0.15 c (1.30–1.60) | 0.95 ± 0.04 abc (0.92–0.99) | 0.73 ± 0.02 b (0.71–0.74) |
| Quercetin | 0.86 ± 0.02 ab (0.84–0.88) | 0.77 ± 0.02 abc (0.71–0.83) | 0.63 ± 0.11 abc (0.54–0.75) | 0.54 ± 0.04 ac (0.51–0.59) | 0.74 ± 0.04 abc (0.71–0.78) | 0.63 ± 0.02 b (0.61–0.64) | 0.52 ± 0.03 c (0.49–0.55) | 0.39 ± 0.03 abc (0.36–0.41) |
| Kaempferol-3-O-glucoside | 0.64 ± 0.03 abc (0.62–0.67) | 1.33 ± 0.15 a (1.20–1.50) | 0.41 ± 0.01 b (0.40–0.42) | 0.74 ± 0.03 ac (0.71–0.77) | 0.53 ± 0.02 abc (0.51–0.55) | 0.98 ± 0.11 ac (0.90–1.10) | 0.47 ± 0.04 bc (0.42–0.50) | 0.62 ± 0.01 abc (0.61–0.63) |
| Quercetin-3-O-glucoside | 2.38 ± 0.20 a (2.16–2.55) | 2.11 ± 0.03 abc (2.08–2.13) | 1.35 ± 0.09 b (1.28–1.45) | 1.72 ± 0.05 abc (1.68–1.77) | 2.44 ± 0.03 ac (2.41–2.47) | 2.07 ± 0.03 abc (2.04–2.10) | 1.34 ± 0.03 bc (1.32–1.37) | 1.44 ± 0.04 abc (1.41–1.48) |
| Kaempferol | 0.95 ± 0.03 abc (0.92–0.98) | 1.45 ± 0.03 a (1.42–1.48) | 0.90 ± 0.02 abc (0.89–0.92) | 0.76 ± 0.02 abc (0.74–0.78) | 0.87 ± 0.02 abc (0.85–0.89) | 1.13 ± 0.06 ab (1.10–1.20) | 0.74 ± 0.03 bc (0.72–0.77) | 0.61 ± 0.03 c (0.58–0.63) |
| Jimma | Sidama | |||||||
|---|---|---|---|---|---|---|---|---|
| Green | Light | Medium | Dark | Green | Light | Medium | Dark | |
| Caffeine | 49.77 ± 1.04 e (48.60–50.60) | 74.60 ± 0.70 b (73.90–75.30) | 49.50 ± 0.82 e (48.60–50.20) | 21.47 ± 0.95 g (20.50–22.40) | 55.77 ± 0.50 d (55.30–56.30) | 81.47 ± 0.95 a (80.50–82.40) | 61.10 ± 0.80 c (60.30–61.90) | 29.07 ± 1.06 f (28.10–30.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzyska-Szczupak, K.; Przybylska-Balcerek, A.; Buśko, M.; Szwajkowska-Michałek, L.; Szablewski, T.; Stuper-Szablewska, K. Roasting Temperature as a Factor Modifying the Caffeine and Phenolic Content of Ethiopian Coffee. Processes 2025, 13, 2037. https://doi.org/10.3390/pr13072037
Rzyska-Szczupak K, Przybylska-Balcerek A, Buśko M, Szwajkowska-Michałek L, Szablewski T, Stuper-Szablewska K. Roasting Temperature as a Factor Modifying the Caffeine and Phenolic Content of Ethiopian Coffee. Processes. 2025; 13(7):2037. https://doi.org/10.3390/pr13072037
Chicago/Turabian StyleRzyska-Szczupak, Katarzyna, Anna Przybylska-Balcerek, Maciej Buśko, Lidia Szwajkowska-Michałek, Tomasz Szablewski, and Kinga Stuper-Szablewska. 2025. "Roasting Temperature as a Factor Modifying the Caffeine and Phenolic Content of Ethiopian Coffee" Processes 13, no. 7: 2037. https://doi.org/10.3390/pr13072037
APA StyleRzyska-Szczupak, K., Przybylska-Balcerek, A., Buśko, M., Szwajkowska-Michałek, L., Szablewski, T., & Stuper-Szablewska, K. (2025). Roasting Temperature as a Factor Modifying the Caffeine and Phenolic Content of Ethiopian Coffee. Processes, 13(7), 2037. https://doi.org/10.3390/pr13072037









