Abstract
Different techniques that require specific conditions are used to increase long-term stability and facilitate the transportation of products. Solid microbial formulation gained significant attention in the scientific world for several applications due to its benefits, mainly for agriculture. The extensive applications in the agricultural area, especially in the protection as a biopesticide and in the nutrition as a biofertilizer, have expanded knowledge on the production of solid bioproducts to keep up with developments in the community. Recent scientific works have disclosed different techniques, increased yields, and optimized parameters and other related procedures to produce solid microbial formulations with quality. However, the optimal protocol for solid microbial preparations differs between species and strains. The mechanisms underlying the protection and damage during drying methods and storage are, unfortunately, not clearly understood. Therefore, the current review highlights the state of the art of freeze and spray drying, both physical methods that are applied in microorganism formulations. Additionally, the study highlights the stresses these systems are exposed to during the drying process, as well as the strategies employed to ensure their stability throughout processing and storage. In summary, the information in this review provides a theoretical basis for the selection of these relevant technologies, according to the requirements demanded to obtain a sustainable bioinput.
1. Introduction
According to the FAO (Food and Agriculture Organization of the United Nations), sustainable agriculture involves factors such as conservation of soil, water, and animal and plant genetic resources, environmental conservation, and the use of appropriate techniques [1]. Due to population growth and increased demand for food and other natural resources, sustainable agriculture has become a current and extremely relevant theme. The United Nations World Food Program estimates that agrarian yields must increase by 70–100% to feed the projected global population in 2050. The technological advancements made by technological inventions such as genetically engineered crops and new pesticidal composites, attained by modern row crop husbandry, which has led to never-before-seen crop yields, are extraordinary [2].
To extensively increase the worldwide use of pesticides, fertilizers, and water would not only be economically infeasible for the vast majority of the world but would have negative environmental consequences. Therefore, meeting global agricultural yield prospects by just scaling up the current high-input agricultural systems employed in most of the advanced world is simply not possible. There is thus a critical need for improved techniques in the art of increasing crop performance and identifying advantageous traits to desired plant species [3].
The emergence of cost-effective materials and production processes suitable for sustainable agriculture resulted in a notable rise in the development and utilization of microencapsulated products in the last decade. The use of microbial products as agricultural agents has been accepted as an alternative green strategy to maintain crop productivity and safety while reducing the application of chemical fertilizers and pesticides [4]. The market for agricultural biologicals—biopesticides, biofertilizers, and biostimulants—is growing rapidly with global agriculture. Projections indicate that the global biopesticides market will reach USD 15.66 billion by 2029, whereas the combined market for biofertilizers and biostimulants is anticipated to attain USD 5.2 billion by 2028 and USD 7.6 billion by 2029, respectively. In terms of values, CAGR over 10% was attained during the forecast period [5].
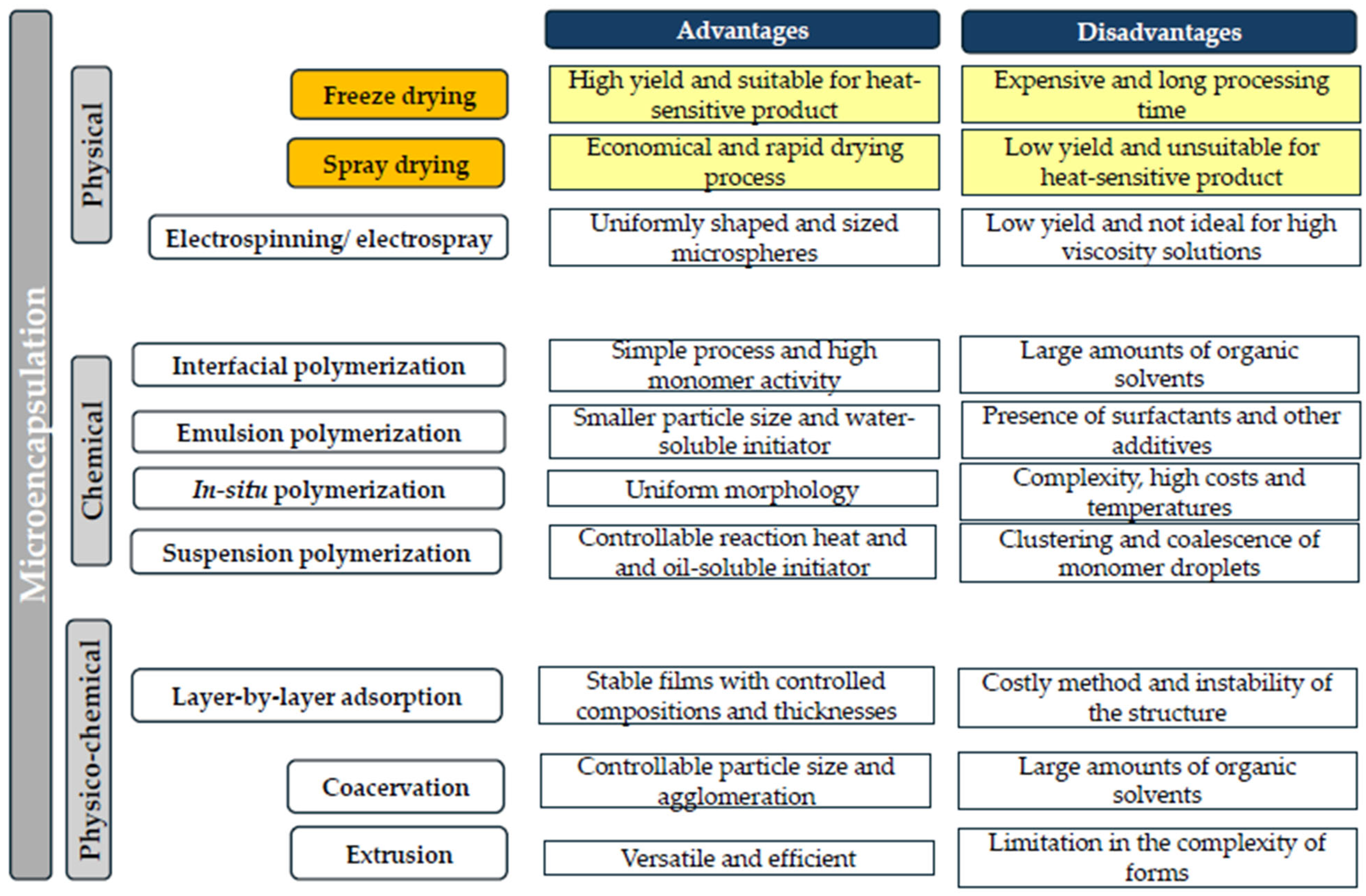
Encapsulation is an emerging technology for combating soil-borne pathogens and abiotic stress and also for promoting green agricultural projects [6]. The preservation of the structure and function of biological materials during long-term storage at high temperature and humidity is of essential importance to the agroindustry [7]. Besides the increased stability of these active materials, the microencapsulation process carries other benefits, such as reduced storage space and transport costs. Currently, a wide range of encapsulation techniques, classified according to the synthesis mechanism, are used (Figure 1). Some examples are emulsification, extrusion, electrospinning, electrospray, coacervation, layer-by-layer assembly, freeze drying, and spray drying [8]. Among these encapsulation techniques, the last two are commonly used for preserving microorganisms [9,10,11].
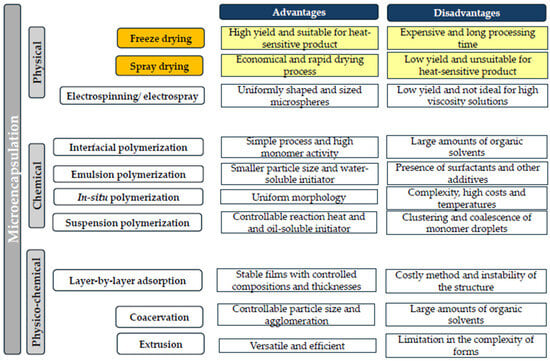
Figure 1.
Classification and advantages and disadvantages of major microencapsulation techniques.
Freeze and spray drying are simple but well-studied preservation methods to develop solid formulations and products for biological control [12]. Freeze drying is suitable for heat-sensitive compounds due to continuous processing at low temperature, while spray drying is advantageous because it is time-saving and flexible and produces smaller-sized droplets [13]. Freeze drying is considered a relatively mild dehydration method in which cells are initially incorporated into a matrix to protect them during freezing and drying. Spray drying involves the atomization of a liquid matrix in a hot air chamber, causing rapid water evaporation and forming dry particles [14]. The powder products from these techniques are stable and can be stored for a long time, which has good commercial value for the shelf life of products [15].
In general, although research on the development of innovative biological control strategies, formulations, and processing techniques is already well-established, emerging approaches in these areas are expected to significantly enhance performance and promote greater adoption and commercialization of microbial-based products. Based on this context, the current review is focused on solid microbial formulation in the agricultural field from different microbial strains. The emphasis is given to present a brief role of freeze and spray drying processes.
2. Solid Microbial Formulation
Microbial formulation, or bioformulation, is a combination of a single or consortium of microbial agents (core material) and a carrier material (wall, shell, protector, encapsulated or coating material) that delivers the right amount of active cells at the right time. Based on selected carrier mediums, it is conventionally classified into two types: solid bioformulations and liquid bioformulations. The type of formulation chosen is critical to the success of the application [16]. Solid formulations are the most popular method for encapsulating microorganisms, leading the market for registered products. Solid formulations are inherently stable, unlike liquid ones that change over time due to evaporation and solvent effects, complicating product registration. Solid formulations are less prone to contamination than liquid ones and preserve spores in their natural state, preventing imbibition damage [12]. It comprises granules, microgranules, wettable powders (WPs), wettable granules (WGs)/water dispersible granules (WDGs), and encapsulation within polymeric gels [14].
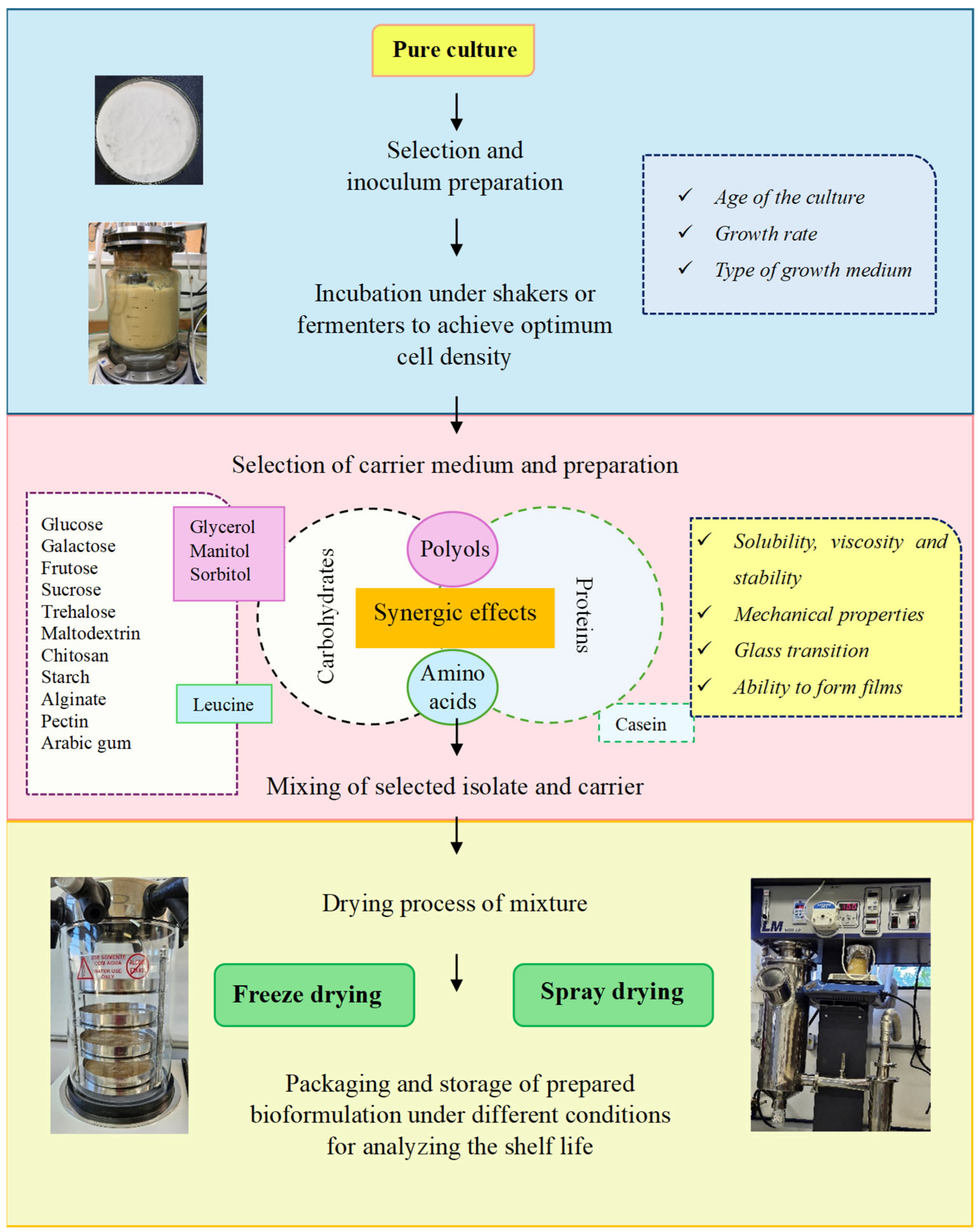
The conventional formulations are made by mixing the beneficial microorganism with an inert carrier such as talc, sawdust, dusts, fly ash, and peat. The arrangement of formulations requires different techniques, such as milling and drying [17,18]. The foundation in the development process of formulation is efficacy, survival, stability during storage and transport, ease of application, better performance in the field, and success of the application under extreme conditions [16]. To overcome the limitations associated with conventional bioformulations, science has advanced toward the development of new formulations. The encapsulation, also called immobilization, is a promising process that has significant advantages over other formulations [17]. The main steps and materials involved in the microencapsulation technologies approached in this review are shown in Figure 2.
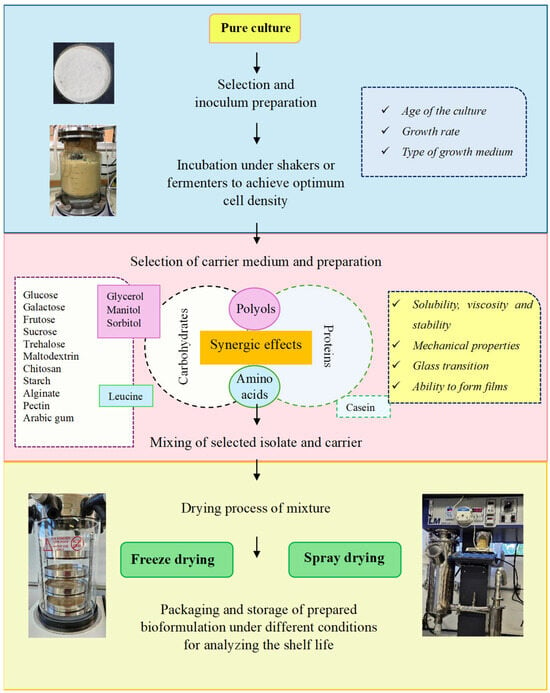
Figure 2.
Steps involved in the development of solid microbial bioformulation.
2.1. Selecting Microorganisms
The strain choice is fundamental to defining the process. Each microorganism is unique; therefore, the processes are not identical. Microorganisms are subject to injury from temperature changes. The nature, causes, and prevention of these injuries are not fully understood. For example, while it is suggested that Gram-positive bacteria need less protection than Gram-negative bacteria, each one likely requires individual consideration. Factors that must be taken into account include the age of the culture, growth rate, type of growth medium, magnitude and rate of temperature decrease, and the subsequent growth pattern following exposure to cold stress. Addition of sugar, skim milk, glycerol, dimethysulfoxide (DMSO), or other cryoprotective agents can stabilize the cultures. Different cryoprotective agents have different effects, and their use is determined empirically [19].
2.2. Selecting Encapsulation Materials
Choosing appropriate encapsulation materials and techniques for specific applications requires careful consideration. The various properties of encapsulation materials affect release kinetics, stability, and compatibility with the substances being encapsulated, requiring extensive experimentation and expertise to identify the best combinations. One of the major challenges in developing microorganism formulations is finding suitable carrier substances. These carriers must ensure high survival rates, maintain dormancy or viability during storage, and be non-toxic to the cells [12]. They should also be economical and easy to use. According to Ali et al. [7], the use of suitable carriers is useful in managing agricultural issues and promoting sustainable agriculture.
During attempts to minimize this damage, types of protectant substances, such as carbohydrates (mono-, di-, and polysaccharides), amino acids, polyols, and proteins, have been explored to evaluate their protective properties [20,21]. Microbial bioengineering involving the use and improvement in encapsulation using polysaccharides has the required potential to address environmental issues and promote green chemistry [7]. The polysaccharides exhibit several useful properties such as good solubility, lower viscosity at higher concentrations, rapid drying, and high glass transition temperature, which make them suitable encapsulation materials [22]. Polyols and disaccharides, like glycerol and trehalose, are the most common osmotic regulators. These substances protect proteins and membranes from damage caused by desiccation stress. Their addition can replace water between lipids, forming a viscous glass matrix that prevents cellular material loss during rehydration [23].
The mixture of numerous compounds of protectant medium (e.g., protein–carbohydrate complex) could result in better protection of microorganisms than a single compound due to additive or synergic protective effects [24]. Proteins are gaining interest from researchers and industry as a wall material for encapsulating bioactive substances due to their extraordinary competitiveness, such as with regard to natural carriers, high renewability, safety, and water holding capacity. Their emulsification, gelation, film-forming, and foaming abilities, as well as their ability to self-associate into complexes, make them promising encapsulation materials [25]. Skim milk powder primarily consists of lactose and soluble whey proteins. These components may act as osmoprotectants and thermal protectants, helping to maintain cell integrity during the spray drying process, akin to non-reducing disaccharides like trehalose and sucrose [26].
In addition to traditional carriers, new compounds are being evaluated. Exopolysaccharides (EPSs) are an important class of microbial biopolymers used as bioemulsifiers in the agricultural area [27]. EPS-producing cells from harsh conditions represented a valuable source of diverse EPSs with novel and unusual features and bioactivities. The cryoprotective and freeze-drying protective activities of the EPS produced by Zygosaccharomyces rouxii were investigated to evaluate its potential industrial applications. The EPS had a stronger protective effect on Lactococcus lactis than trehalose, suggesting that EPS had a vast potential for development and application as a lyoprotectant [28].
2.3. Selecting Microencapsulation Techniques
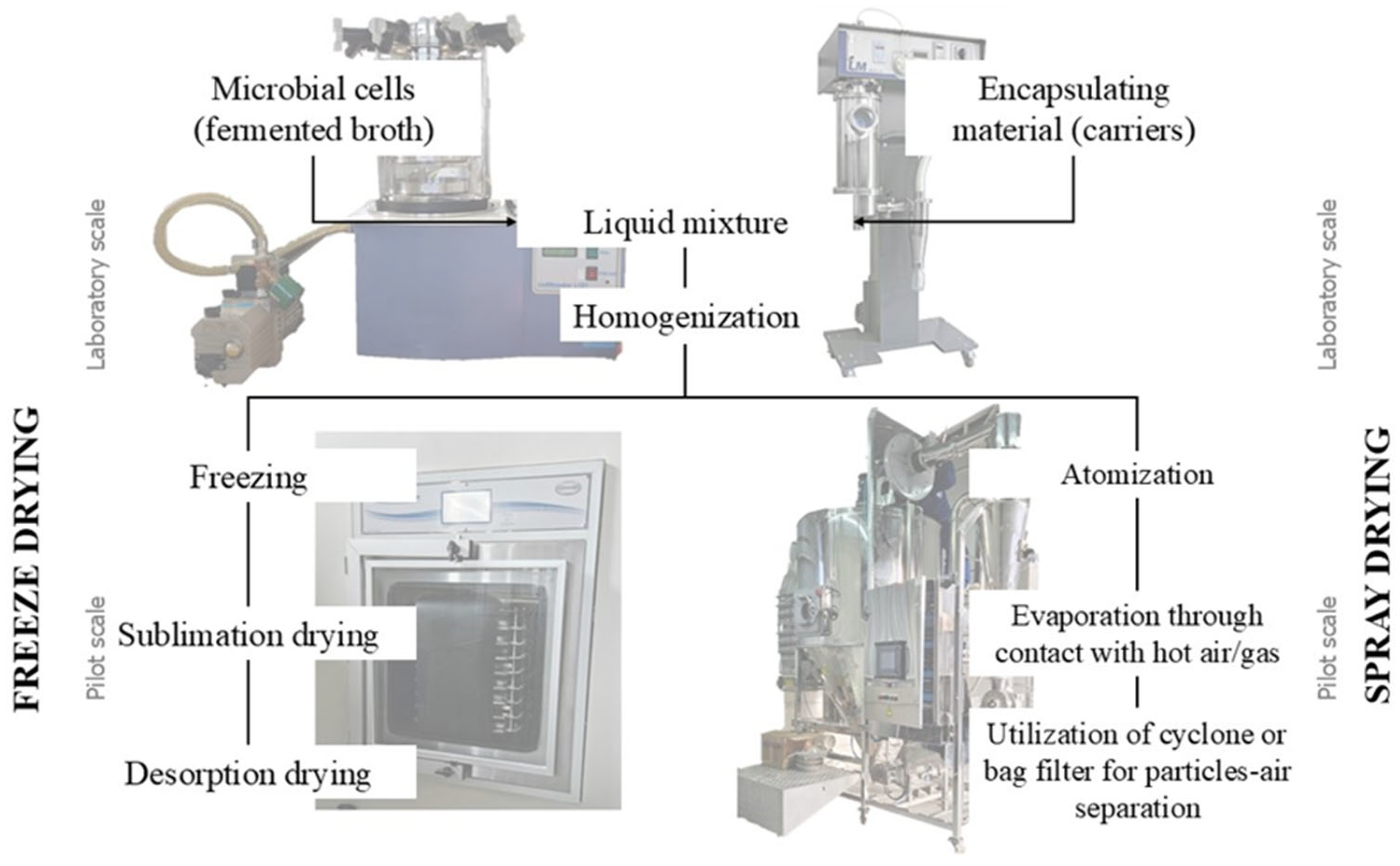
The choice of microencapsulation technique depends on the application, carrier materials, and the target particle size. The production scale, cost, and release mechanism are other relevant factors. As previously reported, the most studied microencapsulated techniques are freeze and spray drying (Figure 3). Some important studies have been summarized in Table 1.
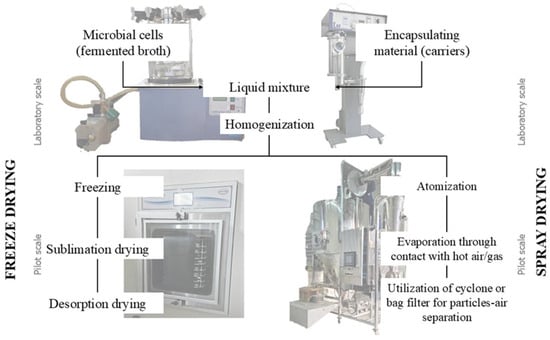
Figure 3.
Comparative chart of freeze and spray drying processes.

Table 1.
A summary of studies conducted to produce freeze- and spray-dried formulations.
2.3.1. Freeze Drying
Freeze drying or lyophilization was, to begin with, carried out in 1890 and the industrial freeze drying started with the production of penicillium and preserved blood plasma. Nowadays, freeze drying is a traditional method to preserve microorganisms [34]. Freeze drying is a three-phase process, in which a material is initially frozen (freezing), and subsequently, the water is removed by sublimation under vacuum (primary drying) and desorptioned (secondary drying) to a level low enough to prevent chemical reactions or biological growth [42]. In short, the freeze-drying process is based on the sublimation mechanism, where solidified vehicle materials are converted directly into the gaseous state without passing through the liquid phase [43]. Freezing is crucial in a freeze-drying cycle as it affects the product’s structure and behavior during drying. Freezing conditions impact the quality and shelf life of freeze-dried products. Primary drying receives the most focus since it can significantly reduce cycle time but may cause defects if not performed correctly. Adjusting heat/mass transfer during primary drying helps control and predict product behavior, unlike the unpredictable freezing process. Maintaining a higher product temperature speeds up primary drying, but it must not exceed the critical limit to ensure quality. Secondary drying removes unfrozen water to reach the desired residual content and is usually simple [44]. The final moisture content of products typically ranges between 0.5% and 3%. Dehydration leads to structural damage in microbial cells, resulting in decreased metabolic activity and reduced cell viability—effects that are especially pronounced when dehydration is performed without the use of matrices or cryoprotective agents [42].
Freeze drying is advised for bioactive compounds sensitive to high temperatures [45]; at the same time, this process can be damaging to many sensitive biological materials. The loss of viability is due to changes in the physical form of membranous lipids, accompanied by the disruption of integrity and liquidity. Improved cell viability and minimal structural damage can be achieved by incorporating simple or complex compounds such as sugars, proteins, amino acids, dietary fibers, and glycerol. These matrix substances primarily act to shield microbial cells by mitigating the damaging effects of dehydration [14,42].
Frequently, the freeze-drying literature pertaining to the stabilization of microorganisms has numerous references to sugars as carriers (mainly disaccharides). Disaccharides can interact with both the phosphate and carbonyl groups of the bilayer polar heads through hydrogen bonds and/or with the methyl group of their hydrophobic part. Sugars also reduce Van der Waals interactions between hydrocarbon chains by elongating the spaces between phospholipid polar heads [43]. Usually, monomers, oligomers, or polymers are employed due to their property of being “glass formers”. This term generally refers to a compound or substance that dissolves easily in solution and does not thicken or polymerize upon contact with water. Such reagents are added to ensure or increase the stability of the bioactive substance during and after the drying process or for long-term storage stability of the dry powder product. These carriers are also called cryoprotectants (freezing stage) and/or lyoprotectants (drying stage) [46]. Due to their influence on the polymer glass transition temperature, they are frequently used to achieve an amorphous form, suitable redispersibility, and stabilization of dried samples [47]. Monosaccharides (e.g., glucose, fructose, and galactose) and disaccharides (e.g., sucrose and trehalose), provided stability thanks to their flexibility, influencing steric hindrance. However, oligo-/polysaccharides (e.g., inulin and dextran) and short-chain sugars may have a synergistic action in cryoprotection and water replacement [48]. Polysaccharides, such as chitosan, pectin, carrageenan, starch, and alginate, are complex substrates that can also be used as encapsulation carriers of bioactive compounds [8]. Nevertheless, rigid polysaccharides are able to interact but leave open gaps. For sugars with high molecular weights and a limited flexibility, these gaps are large compared to the smaller sugars [48]. Other additives such as buffers, bulking agents, tonicity modifiers, and surfactants can also be used during the lyophilization process [49].
Process parameters—including freezing temperature and rate, chamber pressure, annealing steps, and drying temperature—as well as formulation factors such as the choice of cryoprotectants, are critical to the overall success of the process [43]. The freeze drying process has a high production cost and low productivity, is limited by a small sample size and cannot be performed using barrier technology to ensure the sterility of the material, and is very long (more than 24 h), which makes it unsuitable for continuous production. However, the cost of freeze drying and time consumption are some of the main disadvantages [42].
2.3.2. Spray Drying
Microencapsulation using the spray drying process has been used in the food industry since the 1950s. Initially, this process was applied to flavor compounds to protect them from degradation and oxidation, as well as to dry solid suspensions [50]. Spray drying involves atomizing a suspension formulated and spraying it into a heated chamber, which dries the particles for collection. In other words, this is a technique to convert liquid substances into a more practical powder or solid form in one short operation [12,51].
For spray drying encapsulation, the bioactive compound must first be solubilized, emulsified, or dispersed in an aqueous solution containing an encapsulating agent, followed by the suspension or atomization of the mixture into a drying chamber. Atomization can be achieved using various types of atomizers, such as pneumatic atomizers, pressure nozzles, spinning disks, fluid nozzles, and sonic nozzles. After the evaporation of water in the chamber, the dry powder is collected and recovered in a cyclone [50]. Common types of dry collectors include the cyclone separator, the bag filter, and the electrostatic precipitator [52]. Increasing energy to the atomizer reduces droplet size. For a given energy, higher feed rates increase particle size. Particle size also grows with the viscosity and surface tension of the feeding liquid. Depending on the conditions, a very fine powder (10–50 μm) or large size particles (2–3 mm) are produced [50].
Spray drying microencapsulation offers several advantages, including continuity, simplicity, reproducibility, the ability to produce microcapsules with a uniform and controlled size distribution (quality), the wide availability of apparatus, rapid water evaporation, high speed, scalability, and cost-effectiveness [51,53]. This process is a highly significant industrial drying system in use today, which costs over 10 times less than freeze drying, due to the lack of high energy consumption [42].
When it comes to microencapsulation of microorganisms, this technology is not considered an optimal cell immobilization technique due to the high mortality resulting from their simultaneous dehydration and inactivation at high temperatures [54]. In addition to a certain level of heat resistance that microorganisms require to be encapsulated, it is essential to carefully control parameters such as the air inlet temperature (150–220 °C), flow rate, speed, and temperature of the feed material. Additionally, proper cooling during the evaporation of the liquid layer is crucial, ensuring that the droplet temperature remains below 100 °C. This can be monitored by tracking the outlet air temperature, which typically ranges between 50 and 80 °C [51]. While high temperatures can drastically reduce the cellular viability of the encapsulated microorganisms, low temperatures reduce the rate of water evaporation, forming aggregates of microspheres [55]. Furthermore, other drawbacks of this process are the oxidative and osmotic stress [56]. After the drying process, rehydration is considered another critical step in the recovery of spray-dried cells and subsequent application [38].
Carriers that are added before spray drying can improve the recovery of microorganisms by forming a crystalline powder with low water content [38]. Carbohydrates are used extensively due to their ability to form amorphous glassy solids that provide structural support to the carrier material of the delivery system. Among them, maltodextrin has excellent encapsulation properties due to its high solubility, low viscosity, and film-forming ability [57]. Due to the inferior emulsifying capacity of most carbohydrates, they can be used in combination with other carrier materials with good emulsifying capacity, such as gum arabic and modified starches [58,59]. Arabic gum and starches are especially used due to their recognized ability to form spherical particles after the drying process [22]. Other polysaccharides, such as alginate, carboxymethylcellulose, guar gum, and proteins like whey proteins, soy proteins, and sodium caseinate, can be used as an alternative for hydrophobic core material. According to Khem et al. [60], proteins improve the survivability of probiotic strains during spray-drying processes owing to their film-forming ability, which reduces thermal stress and enhances moisture availability. However, the application of these substances is complicated and costly due to their poor solubility in water. This necessitates the increased evaporation of water, resulting in reduced dry matter content and lower concentrations of active ingredients [50].
3. Development Challenges in the Scale-Up Process
Technological challenges limit the growth of the microencapsulation market by affecting accessibility, innovation, and scalability within the industry. The complex nature of microencapsulation technologies requires specialized expertise and equipment, which can be difficult for small- and medium-sized enterprises to access. Additionally, intricate manufacturing processes and the need for specialized equipment maintenance pose further obstacles to widespread adoption and innovation, especially for companies with limited resources [61].
3.1. Scalability of the Microencapsulation Processes
Scaling up microencapsulation processes from laboratory to industrial production requires substantial adjustments to the equipment design, process parameters, and quality control measures. Achieving reproducibility and efficiency at larger scales while maintaining product integrity is a significant technical challenge. Additionally, developing advanced encapsulation techniques to meet specific application needs is an ongoing area of research in microencapsulation [61].
Freeze drying is often seen as a difficult process to design and scale up from the laboratory to industry. To achieve process control and optimization, heat and mass transfer issues must be recognized. The freeze drying process parameters, such as shelf temperature, ramp rate, and chamber pressure, are often determined using a trial-and-error method. This approach can lead to processes that are not optimal, have long processing times, and face scale-up issues. Each step of the freeze-drying process presents unique challenges for process development and scaling [62]. According to Carvalho [63], one of the most significant challenges in scaling up a freeze-drying process is the variation in apparatus characteristics. It is crucial to understand how the sublimation rate and vapor flow are influenced by the equipment design to prevent issues during scale-up.
The work scales of spray driers directly influence the quality and powder yield. The quality of the obtained particles by this method is acceptable, especially on larger scales when the process conditions are optimized. Powders produced at a laboratory scale typically exhibit moderate to low wettability and flowability. These properties are often improved through the combination of spray drying followed by fluidized drying in industrial-scale processes, which results in an increase in particle size [64]. Additionally, the powder yield varies significantly based on the work scales when traditional spray dryers are used. Larger-scale setups tend to provide higher yields because the fraction lost constitutes a smaller portion of the total production volume, whereas laboratory scales typically provide yields in the range of 20–70% [65]. In general, low powder yield is attributed to product loss on the walls of the drying chamber, with quantities remaining consistent. Additionally, fine particles may pass into the exhaust air due to the insufficient separation capacity of the cyclone. However, some separators, such as filter systems, operate efficiently in industrial settings to improve product yield [66]. Industrial-scale drying equipment, which allows for the controlled relative humidity of the air, is available and can be adapted for the dehydration of fungal propagules. This method offers gentle drying suitable for sensitive microbial cells, such as blastospores [26].
3.2. Stability and Viability of Solid Microbial Formulation
Overcoming limitations such as low encapsulation efficiency, poor stability, and limited compatibility with sensitive compounds requires continuous innovation and interdisciplinary collaboration. Some factors, such as appropriate strain and carrier, storage conditions, shelf life, environmental competition and conditions, application method, quality control, and genetic stability, can impact microbial formulation efficiency. Maintaining appropriate storage conditions, such as temperature (4 °C), humidity (3%), and packaging, is essential for preserving the viability and shelf life of the microbes in the formulation. Viability refers to the ability to form colonies (CFUs or colony-forming units) on a nutrient medium suitable for microorganism growth (should not be less than 104 CFU per gram sample), while the shelf life refers to the bioformulation sustenance capacity (usually expected from 6 to 12 months) [38,67].
3.3. Rules, Regulations, and Commercialization
The commercialization of biocontrol products and biofertilizers is growing worldwide, but without effective regulations, their market penetration remains slow. Regulations for biopesticides differ by country and often resemble those for chemicals. The global harmonization of biopesticide regulations would help overcome these barriers and boost commercialization [14]. Consumer anxiety about live microorganisms is another limiting factor for commercialization. Adequate education and awareness to address these issues and support from financial agencies (public and private) should be implemented [54]. Brazil has one of the most advanced rules in the world for registering products for Brazilian agriculture and urban environments. Connected to these regulations are several others that must also be observed by entrepreneurs seeking to legalize their products [68].
4. Microencapsulation in Agriculture
Encapsulation is an emerging technology that is particularly effective in prolonging the activity of pesticides, enhancing the safety and quality of stored grains [53]. The application of encapsulated fertilizers and pesticides promotes plant health and controls plant diseases [7]. Microencapsulation can reduce waste and improve product efficacy by stabilizing and extending the shelf life of active ingredients. This is particularly true in agriculture, where microencapsulated pesticides and fertilizers can increase crop yields and minimize environmental impact. For example, BASF has quickly adopted microencapsulation technology to enhance agricultural practices. Similarly, Syngenta and other companies are utilizing spray technologies to develop more effective and organic pest repellents [5].
In Brazil, entomopathogenic fungi are predominant in the biopesticides market, representing approximately 50% of registered microbial biopesticides. The majority of these are based on Metarhizium anisopliae and Beauveria bassiana [69]. However, it is often noted that some products exhibit low quality and inconsistent performance in practical applications. This fact is attributed to the majority of entomopathogenic fungi being utilized as unformulated technical concentrates (pure conidia or fungus-colonized substrates, 72.5%), with only a limited number of formulations being available commercially [70]. The most effective approach to applying biocontrol agents in agricultural settings involves developing and preparing appropriate powder formulations. These formulations facilitate their use as seed treatments, particularly for managing seed and root diseases [71]. Table 2 shows an overview of the microencapsulation of microbial cells in the literature, indicating the carrier materials, the bioactive compounds used, and the purpose of the study.

Table 2.
Microencapsulation of microbial cells for agricultural applications.
Metarhizium is a genus of entomopathogenic fungi found globally. It has been studied for its narrow host range, safety, environmental compatibility, and ease of mass production. The mycoinsecticide developed by Bayer (BIO 1020) is an example, containing Metarhizium anisopliae as an active ingredient to target pests from the Coleoptera species [88]. Qiu et al. [82] optimized the production process of microcapsules of M. anisopliae using gelatin and gum arabic as carriers. The authors, in addition to improving the product quality, promoted the development and utilization of microorganism pesticide for Solenopsis invicta control.
Mascarin et al. [26] demonstrated that the blastospore of Beauveria bassiana survived desiccation through spray drying, exhibited good shelf life at 28 °C as a dried formulation when stored under proper conditions, and maintained virulence against whitefly nymphs upon rehydration and application as a spray. Blastospores exhibited excellent survival after spray drying to less than 5% moisture content. The high viability post-spray drying may be partially attributed to the use of skim milk powder as a formulation carrier. The addition of ascorbic acid during spray drying did not enhance desiccation tolerance but increased cell stability (approximately twofold higher half-life). Osmotic regulators, such as glycerol and trehalose, as protectants, have attracted interest as formulation ingredients to preserve viable microbial cells during storage for a long time and to protect the cells during applications in the field [23].
The genus Trichoderma is extensively studied globally as a biological control agent. Their agricultural significance lies in their strong antagonistic activity against soil-borne plant pathogenic fungi, the production of antifungal metabolites, competition for space and nutrients, induction of plant defense responses, mycoparasitic behavior, and their ability to promote plant growth [79]. However, despite numerous studies, significant challenges remain in the large-scale production and commercialization of this product. The primary issue lies in the lack of appropriate formulations that ensure the viability and effectiveness of the biological control agent when applied in the field. This limitation poses a major barrier to the expansion and use of biopesticides in conventional agriculture [81]. An alternative to increase the effectiveness of these products can be achieved by developing formulations containing a mixture of microorganisms. This is a way of reaching different targets or even allowing different mechanisms of action to act simultaneously on the same target (synergism) [68]. Research in culturing multiple organisms in a single fermentation has increased [21].
Bacillus spp. are regarded as highly beneficial biocontrol agents and have been extensively utilized. This genus includes a large group of Gram-positive, rod-shaped, and spore-forming bacteria. They are recognized as one of the most significant genera of bacteria that enhance plant growth and offer biocontrol against both biotic and abiotic stress factors. Additionally, they have demonstrated potential as biofertilizers and biopesticides and produce a wide variety of potential antagonistic compounds [89,90]. Bacillus velezensis has demonstrated effective results in controlling harmful phytopathogens and in the production of biofungicides. Some strains of B. velezensis are capable of promoting plant growth and exhibit antibiotic properties against plant pathogens [91].
Pseudomonas sp. are recognized for their role as natural biological control agents against various plant pathogens. However, challenges such as poor desiccation tolerance and limited shelf stability hinder their commercialization. As non-spore-forming Gram-negative bacteria lacking a cell wall, Pseudomonas species are among the least resilient microorganisms. Slininger et al. [23] explored the potential osmoprotectants of trisaccharide isomelezitose (IMZ) on two strains of Pseudomonas fluorescens used to control maladies of potatoes stored postharvest. The authors reported that IMZ showed potentially superior activity to the structurally related compound melezitose and the currently marketed disaccharide trehalose.
5. Recent Applications of Microencapsulated Materials in Agriculture
The global microencapsulation market is projected to reach USD 13.4 billion by 2026, with a compound annual growth rate (CAGR) of 10.3% expected across all segments from 2023 to 2030. This growth is primarily driven by the expanding range of applications for microencapsulated products across diverse sectors. However, the high costs associated with the microencapsulation process—along with the substantial investment required in research and development—continue to pose significant challenges for many companies seeking to adopt this technology across various industries [92].
In the agricultural sector, microencapsulated pesticides represent the tendency by providing extended effectiveness, minimizing environmental contamination, and reducing health risks for both farmers and consumers. Global agricultural productivity faces threats from weeds, causing 10–15% crop losses annually. Herbicides, which make up 47.5% of the 2 million tons of pesticides used each year, have serious environmental and health impacts. Controlled release formulations via microencapsulation can improve herbicide efficiency at lower doses, addressing these concerns while enhancing safety and cost-effectiveness [61]. Companies like BASF and Syngenta are leveraging this technology to create slow-release pesticides and fertilizers, minimizing consumer health risks while maximizing agricultural output [5].
Brazil stands out on the global scene of sustainable and efficient solutions for agriculture. Currently, there are a total of 6421 patents registered related to bioinputs in the last 20 years, the main components of which are the bacterium Bacillus and the fungus Trichoderma [93]. The National Bioinputs Program, established in 2020, stimulates innovation in the sector, resulting in a notable increase in the number of registered patents. Some of these relevant patents involving methods for increasing crop performance are presented in Table 3. Besides Brazil, the main countries with contributions are the United States, Japan, China, South Korea, Canada, Russia, and Australia.

Table 3.
Patents deposited based on microbial formulations over the last few years.
Thompson et al. [97] created a patent US2014/274691, directed to an inoculum for application to plants, plant seeds, or a plant growth medium, wherein the inoculum comprises an effective amount of a biologically pure bacterial culture disclosed herein and an agriculturally acceptable carrier. The US2019/0194259-A1 invention relates to embedding microorganisms and/or bioactive materials in a protective dry formulation matrix prepared by freeze drying [95].
The microorganisms of the compositions may work synergistically [100]. A reported combination of fungi and bacteria, Trichoderma harzianum and Bacillus amyloliquefaciens, is used to improve plant health, growth, and/or yield. BR112020/014469-A2 relates to the treatment and/or prevention of pathogenic bacterial plant infections using combinations of yeasts and bacteria, Starmerella bombicola, Wickerhamomyces anomalus, Pseudozyma aphidis, Pichia spp., Bacillus amyloliquefaciens, Pseudomonas chlororaphis, Rhodococcus erythropolis, and/or their growth by-products [101].
US2020/0390106-A1 describes the production and use of entomopathogenic fungi for pest control. Specifically, methods for the production of Metarhizium anisopliae and Beauveria bassiana, as well as for their use, and biopesticidal compositions are described [109]. Recently, BR102023/003698-A2 disclosed bioinputs based on fungi Trichoderma harzianum and sophorolipids produced by the fermentation of yeast Starmerella bombicola, used for pest control and plant growth induction. The present invention also refers to a process for sophorolipid production from the utilization of agro-industrial subproducts as drying adjuvants [108].
As seen, significant efforts have been made by the agricultural sector to commercialize certain specialized products, such as plant protection and soil improvement products. However, despite the growing trend in the number of patents among many agrochemical companies, only limited products reach the market [117].
6. Conclusions and Perspectives
The reduction in the use of agrochemicals requires innovative and effective strategies in agriculture. Solid microbial formulations have proven essential, with microencapsulation methods, such as freeze drying and spray drying, being increasingly used to enhance the viability and shelf life of bioactive powders. The choice of carrier material and the definition of processing parameters are crucial, as they directly affect powder recovery and the quality of the final product. The powdered form also facilitates storage and handling. In the context of sustainable agriculture, optimizing microencapsulation processes with consolidated technologies and suitable carriers can make the production of bioactive compounds economically viable. Thus, this manuscript explores the production of microbial bioformulations from freeze drying and spray drying, addressing carrier selection, challenges in development, and recent applications in agriculture.
Author Contributions
Conceptualization, L.L. and M.A.M.; methodology, L.L. and M.A.M.; formal analysis, L.L. and M.A.M.; investigation, L.L. and M.A.M.; resources, L.L.; writing—original draft preparation, L.L.; writing—review and editing, L.L. and M.A.M.; visualization, L.L.; supervision, M.A.M.; project administration, M.A.M.; funding acquisition, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq—150182/2023-6) and by the Foundation for the Support of Research in the State of Rio Grande do Sul (FAPERGS—22/2551-0000398-2, 23/2551-0000146-2, and 22/2551-0001635-9).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAGR | Compound annual growth rate |
| CFU | Colony forming units |
| CMC | Carboxymethyl cellulose |
| DMSO | Dimethyl sulfoxide |
| DWPI | Whey protein isolate denatured |
| EPS | Exopolysaccharides |
| FAO | Food and Agriculture Organization of the United Nations |
| FOS | Fructooligosaccharide |
| HPMC | Hydroxypropyl methylcellulose |
| IMZ | Trisaccharide isomelezitose |
| SA | Sodium alginate |
| WDGs | Water dispersible granules |
| WGs | Wettable granules |
| WPs | Wettable powders |
| WPI | Whey protein isolate |
References
- Food and Agriculture Organization of the United Nations (FAO). Building a Common Vision for Sustainable Food and Agriculture Principles and Approaches; FAO: Rome, Italy, 2014. [Google Scholar]
- Wigley, P.; Turner, S.; George, C.; Williams, T.; Roberts, K.; Sheamus, G.; Lowe, K. Agriculturally Advantageous Microorganisms, Microbial Compositions, and Consortia. Patent CN108430222B, 22 October 2021. [Google Scholar]
- Alford, B.; Wilk, D.; Zhu, H. Agriculturally Beneficial Microbes, Microbial Compositions, and Consortia. Patent AU2023222089A1, 15 August 2024. [Google Scholar]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol Res. 2022, 259, 127016. [Google Scholar] [CrossRef] [PubMed]
- Future Market Insights Inc. Microencapsulation Market Analysis—Size, Share & Forecast 2024–2034. 2024. Available online: https://www.futuremarketinsights.com/reports/microencapsulation-market (accessed on 15 February 2025).
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R.A.; Krishnankutty, R.E. Application of Encapsulated Bacillus licheniformis Supplemented with Chitosan Nanoparticles and Rice Starch for the Control of Sclerotium rolfsii in Capsicum annuum (L.) Seedlings. Curr. Microbiol. 2021, 78, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Cybulska, J.; Frąc, M.; Zdunek, A. Application of polysaccharides for the encapsulation of beneficial microorganisms for agricultural purposes: A review. Int. J. Biol. Macromol. 2023, 244, 125366. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242, 124784. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.B.A.C.; Costa, C.J.M.; Pomella, A.W.V.; Ribeiro, E.J.; Santos, L.D.; Zotarelli, M.F. Evaluation of lethality temperature and use of different wall materials in the microencapsulation process of Trichoderma asperellum conidias by spray drying. Powder Technol. 2019, 347, 199–206. [Google Scholar] [CrossRef]
- Obradović, N.; Volić, M.; Nedović, V.; Rakin, M.; Bugarski, B. Microencapsulation of probiotic starter culture in protein–carbohydrate carriers using spray and freeze-drying processes: Implementation in whey-based beverages. J. Food Eng. 2022, 321, 110948. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Liu, Y.; Wang, Y.; Chen, Y.; Guan, W.; Li, X.; Wang, Y. Improved viability of Lactobacillus plantarum embedded in whey protein concentrate/pullulan/trehalose hydrogel during freeze drying. Carbohydr. Polym. 2021, 260, 117843. [Google Scholar] [CrossRef]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.M.R.; De France, K. Biotechnological development of Trichoderma-based formulations for biological control. Appl. Microbiol. Biotechnol. 2023, 107, 5595–5612. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Shao, Y.; Wang, L.; He, J.; He, Y. Microencapsulation of Monascus red pigments by emulsification/internal gelation with freeze/spray-drying: Process optimization, morphological characteristics, and stability. LWT 2023, 173, 114227. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Progress in Biological Control; Springer: Cham, Switzerland, 2020; Volume 21, pp. 275–293. [Google Scholar]
- Sansone, F.; Esposito, T.; Mencherini, T.; Del Prete, F.; Cannoniere, A.L.; Aquino, R.P. Exploring microencapsulation potential: Multicomponent spray dried delivery systems for improvement of Chlorella vulgaris extract preservation and solubility. Powder Technol. 2023, 429, 118882. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, B. A review of agricultural microbial inoculants and their carriers in bioformulation. Rhizosphere 2024, 29, 100843. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Alenezi, F.N.; Belbahri, L. Recent Advances in Encapsulation Techniques of Plant Growth-Promoting Microorganisms and Their Prospects in the Sustainable Agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Dubey, S.; Sharma, S. “Next-Generation Bioformulations” for Plant Growth Promotion and Stress Mitigation: A Promising Approach for Sustainable Agriculture. J. Plant Growth Regul. 2023, 42, 6741–6759. [Google Scholar] [CrossRef]
- Baker, C.A.; Brooks, A.A.; Henis, J.M. Encapsulation of Biological Material in Non-Ionic Polymer Beads. Patent US5089407A, 18 February 1992. [Google Scholar]
- Han, L.; Pu, T.; Wang, X.; Liu, B.; Wang, Y.; Feng, J.; Zhang, X. Optimization of a protective medium for enhancing the viability of freeze-dried Bacillus amyloliquefaciens B1408 based on response surface methodology. Cryobiology 2018, 81, 101–106. [Google Scholar] [CrossRef]
- Mejía-Avellaneda, L.F.; Suárez, H.; Jiménez, H.; Mesa, L. Challenges and opportunities for the production of lactic acid bacteria inoculants aimed for ensiling processes. Crit. Rev. Biotechnol. 2021, 42, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Slininger, P.J.; Côté, G.L.; Shea-Andersh, M.A.; Dien, B.S.; Skory, C.D. Application of isomelezitose as an osmoprotectant for biological control agent preservation during drying and storage. Biocontrol Sci. Technol. 2021, 31, 132–152. [Google Scholar] [CrossRef]
- Bellali, S.; Bou Khalil, J.; Fontanini, A.; Raoult, D.; Lagier, J.-C. A new protectant medium preserving bacterial viability after freeze drying. Microbiol. Res. 2020, 236, 126454. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cahoon, E.; Zhang, Y.; Ciftci, O.N. Extraction of astaxanthin from engineered Camelina sativa seed using ethanol-modified supercritical carbon dioxide. J. Supercrit. Fluids 2019, 143, 171–178. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Behle, R.W.; Kobori, N.N.; Delalibera, I., Jr. Improved shelf life of dried Beauveria bassiana blastospores using convective drying and active packaging processes. Appl. Microbiol. Biotechnol. 2016, 100, 8359–8370. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Zhang, M.; Che, Y.; Wu, C. A novel exopolysaccharide produced by Zygosaccharomyces rouxii with cryoprotective and freeze-drying protective activities. Food Chem. 2022, 392, 133304. [Google Scholar] [CrossRef] [PubMed]
- Toegel, S.; Salar-Behzadi, S.; Horaczek-Clausen, A.; Viernstein, H. Preservation of aerial conidia and biomasses from entomopathogenic fungi Beauveria brongniartii and Metarhizium anisopliae during lyophilization. J. Invertebr. Pathol. 2010, 105, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Rishabh, D.; Athira, A.; Preetha, R.; Nagamaniammai, G. Freeze dried probiotic carrot juice powder for better storage stability of probiotic. J. Food Sci. Technol. 2023, 60, 916–924. [Google Scholar] [CrossRef]
- Friuli, M.; Nitti, P.; Aneke, C.I.; Demitri, C.; Cafarchia, C.; Otranto, D. Freeze-drying of Beauveria bassiana suspended in Hydroxyethyl cellulose based hydrogel as possible method for storage: Evaluation of survival, growth and stability of conidial concentration before and after processing. Results Eng. 2021, 12, 100283. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Francés, J.; Montesinos, E.; Bonaterra, A. Improvement of a dry formulation of Pseudomonas fluorescens EPS62e for fire blight disease biocontrol by combination of culture osmoadaptation with a freeze-drying lyoprotectant. J. Appl. Microbiol. 2014, 117, 1122–1131. [Google Scholar] [CrossRef]
- Stephan, D.; Da Silva, A.P.M.; Bisutti, I.L. Optimization of a freeze-drying process for the biocontrol agent Pseudomonas spp. and its influence on viability, storability and efficacy. Biol. Control. 2016, 94, 74–81. [Google Scholar] [CrossRef]
- Muñoz-Celaya, A.L.; Ortiz-García, M.; Vernon-Carter, E.J.; Jauregui-Rincón, J.; Galindo, E.; Serrano-Carreón, L. Spray-drying microencapsulation of Trichoderma harzianum conidias in carbohydrate polymers matrices. Carbohydr. Polym. 2012, 88, 1141–1148. [Google Scholar] [CrossRef]
- Liu, C.P.; Liu, S.D. Low-temperature spray drying for the microencapsulation of the fungus Beauveria bassiana. Dry. Technol. 2009, 27, 747–753. [Google Scholar] [CrossRef]
- Horaczek, A.; Viernstein, H. Beauveria brongniartii subjected to spray-drying in a composite carrier matrix system. J. Microencapsul. 2004, 21, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Abadias, M.; Teixidó, N. Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J. Appl. Microbiol. 2012, 112, 954–965. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A novel encapsulation of Streptomyces fulvissimus Uts22 by spray drying and its biocontrol efficiency against Gaeumannomyces graminis, the causal agent of take-all disease in wheat. Pest. Manag. Sci. 2021, 77, 4357–4364. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, M.; Shankar, S.R.M.; Subramani, N.; Vendhahari, B.N.; Ramyadevi, D. Study on spray-drying of Bacillus velezensis NKMV-3 strain, its formulation and bio efficacy against early blight of tomato. Biocatal. Agric. Biotechnol. 2022, 45, 102483. [Google Scholar] [CrossRef]
- Campos, D.C.; Acevedo, F.; Morales, E.; Aravena, J.; Amiard, V.; Jorquera, M.A.; Inostroza, M.G.; Rubilar, M. Microencapsulation by spray drying of nitrogen-fixing bacteria associated with lupin nodules. World J. Microbiol. Biotechnol. 2014, 30, 2371–2378. [Google Scholar] [CrossRef]
- Meena, K.K.; Taneja, N.K.; Ojha, A.; Meena, S. Application of spray-drying and freeze-drying for microencapsulation of lactic acid bacteria: A review. Ann. Phytomed. Int. J. 2023, 12, 706–716. [Google Scholar] [CrossRef]
- Abla, K.K.; Mehanna, M.M. Freeze-drying: A flourishing strategy to fabricate stable pharmaceutical and biological products. Int. J. Pharm. 2022, 628, 122233. [Google Scholar] [CrossRef]
- Tchessalov, S.; Maglio, V.; Kazarin, P.; Alexeenko, A.; Bhatnagar, B.; Sahni, E.; Shalaev, E. Practical Advice on Scientific Design of Freeze-Drying Process: 2023 Update. Pharm. Res. 2023, 40, 2433–2455. [Google Scholar] [CrossRef]
- Muhoza, B.; Yuyang, H.; Uriho, A.; Harindintwali, J.D.; Liu, Q.; Li, Y. Spray-and freeze-drying of microcapsules prepared by complex coacervation method: A review. Food Hydrocoll. 2023, 140, 108650. [Google Scholar] [CrossRef]
- Andreana, I.; Bincoletto, V.; Manzoli, M.; Rodà, F.; Giarraputo, V.; Milla, P.; Arpicco, S.; Stella, B. Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches. Materials 2023, 16, 1212. [Google Scholar] [CrossRef]
- Kumar, S.; Gokhale, R.; Burgess, D.J. Sugars as bulking agents to prevent nano-crystal aggregation during spray or freeze-drying. Int. J. Pharm. 2014, 471, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tonnis, W.F.; Mensink, M.A.; De Jager, A.; van der Voort Maarschalk, K.; Frijlink, H.W.; Hinrichs, W.L.J. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol. Pharm. 2015, 12, 684–694. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K. Recent advances in freeze-drying: Variables, cycle optimization, and innovative techniques. Pharm. Dev. Technol. 2022, 27, 904–923. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials-Physics and Chemistry-New Edition; Pignatello, R., Musumeci, T., Eds.; InTech: Rijeka, Croatia, 2018; pp. 9–35. [Google Scholar]
- Jose, N.; Ray, D.P.; Misra, S.; Nayak, L.; Ammayappan, L. Microencapsulation and nanoencapsulation of fungicidal and insecticidal agents for grain packaging and storage. J. Stored Prod. Res. 2024, 109, 102468. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, L.Y.; Prevóst, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2010, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Gaiani, C.; Edorh, J.M.; Borges, F.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration. Foods 2023, 12, 3117. [Google Scholar] [CrossRef]
- Tatasciore, S.; Santarelli, V.; Neri, L.; Di Mattia, C.D.; Di Michele, A.; Mastrocola, D.; Pittia, P. Microencapsulation of hop bioactive compounds by spray drying: Role of inlet temperature and wall material. Curr. Res. Food Sci. 2024, 8, 100769. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Matos Jorge, L.M.; de Matos Jorge, R.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef] [PubMed]
- Toprakçı, İ.; Güngör, K.K.; Torun, M.; Şahin, S. Spray-drying microencapsulation of plum peel bioactives using Arabic gum and maltodextrin as coating matrix. Food Biosci. 2024, 61, 104824. [Google Scholar] [CrossRef]
- Khem, S.; Woo, M.W.; Small, D.M.; Chen, X.D.; May, B.K. Agent selection and protective effects during single droplet drying of bacteria. Food Chem. 2015, 166, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Custom Market Insights. Global Microencapsulation Market 2024–2033. 2024. Available online: https://www.custommarketinsights.com/report/microencapsulation-market/ (accessed on 15 February 2025).
- Patel, S.M.; Pikal, M.J. Emerging freeze-drying process development and scale-up issues. Aaps Pharmscitech 2011, 12, 372–378. [Google Scholar] [CrossRef]
- de Melo Carvalho, T. Consistent Scale-Up of the Freeze-Drying Process. Ph.D. Thesis, Tecnical University of Denmark, Kongens Lyngby, Denmark, 2018. [Google Scholar]
- Fuchs, M.; Turchiuli, C.; Bohin, M.; Cuvelier, M.E.; Ordonnaud, C.; Peyrat-Maillard, M.N.; Dumoulin, E. Encapsulation of oil in powder using spray drying and fluidised bed agglomeration. J. Food Eng. 2006, 75, 27–35. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Ståhl, K.; Claesson, M.; Lilliehorn, P.; Lindén, H.; Bäckström, K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int. J. Pharm. 2002, 233, 227–237. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial bioformulation: A microbial assisted biostimulating fertilization technique for sustainable agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Bettiol, W.; da Silva, J.C.; de Castro, M.L.M.P. Uso atual e perspectivas do Trichoderma no Brasil. In Trichoderma Uso na Agricultura; Meyer, M.C., Mazaro, S.M., da Silva, J.C., Eds.; Embrapa: Brasília, Brazil, 2019; pp. 21–43. [Google Scholar]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, Í., Jr.; Fernandes, É.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef]
- Oliveira DGPde Lopes, R.B.; Rezende, J.M.; Delalibera, Í., Jr. Increased tolerance of Beauveria bassiana and Metarhizium anisopliae conidia to high temperature provided by oil-based formulations. J. Invertebr. Pathol. 2018, 151, 151–157. [Google Scholar] [CrossRef]
- Kakvan, N.; Heydari, A.; Zamanizadeh, H.R.; Rezaee, S.; Naraghi, L. Development of new bioformulations using Trichoderma and Talaromyces fungal antagonists for biological control of sugar beet damping-off disease. Crop Prot. 2013, 53, 80–84. [Google Scholar] [CrossRef]
- Liu, C.P.; Da Liu, S. Formulation and characterization of the microencapsulated entomopathogenic fungus Metarhizium anisopliae MA126. J. Microencapsul. 2009, 26, 377–384. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, L.; Qin, S.; Li, C. Encapsulation of R. planticola Rs-2 from alginate-starch-bentonite and its controlled release and swelling behavior under simulated soil conditions. J. Ind. Microbiol. Biotechnol. 2012, 39, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-N.; Chen, C.-Y.; Lin, Y.-C.; Chen, M.-J. Formulation of a novel antagonistic bacterium based biopesticide using microencapsulated techniques in fungal disease control. J. Agric. Sci. 2013, 5, p153. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Cheng, J.; Nie, X.; Yu, X.; Zhao, Y.; Wang, W. Microencapsulation of Bacillus subtilis B99-2 and its biocontrol efficiency against Rhizoctonia solani in tomato. Biol. Control 2015, 90, 34–41. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Cellulase enhances endophytism of encapsulated Metarhizium brunneum in potato plants. Fungal Biol. 2018, 122, 373–378. [Google Scholar] [CrossRef]
- Mancera-López, M.E.; Izquierdo-Estévez, W.F.; Escalante-Sánchez, A.; Ibarra, J.E.; Barrera-Cortés, J. Encapsulation of Trichoderma harzianum conidia as a method of conidia preservation at room temperature and propagation in submerged culture. Biocontrol Sci. Technol. 2019, 29, 107–130. [Google Scholar] [CrossRef]
- Aguirre-Güitrón, L.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A. Optimisation of the spray drying process of formulating the post-harvest biocontrol agent Meyerozyma caribbica. Biocontrol Sci. Technol. 2018, 28, 574–590. [Google Scholar] [CrossRef]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the formulation of alginate-gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma sp. formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Qiu, H.L.; Fox, E.G.P.; Qin, C.S.; Zhao, D.Y.; Yang, H.; Xu, J.Z. Microcapsuled entomopathogenic fungus against fire ants, Solenopsis invicta. Biol. Control 2019, 134, 141–149. [Google Scholar] [CrossRef]
- Saberi-Rise, R.; Moradi-Pour, M. The effect of Bacillus subtilis Vru1 encapsulated in alginate—Bentonite coating enriched with titanium nanoparticles against Rhizoctonia solani on bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Meftah Kadmiri, I.; El Mernissi, N.; Azaroual, S.E.; Mekhzoum, M.E.M.; Qaiss, A.E.K.; Bouhfid, R. Bioformulation of Microbial Fertilizer Based on Clay and Alginate Encapsulation. Curr. Microbiol. 2021, 78, 86–94. [Google Scholar] [CrossRef]
- Felizatti, A.P.; Manzano, R.M.; Rodrigues, I.M.W.; da Silva, M.F.G.F.; Fernandes, J.B.; Forim, M.R. Encapsulation of B. bassiana in Biopolymers: Improving Microbiology of Insect Pest Control. Front Microbiol. 2021, 12, 704812. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131. [Google Scholar] [CrossRef]
- Brondi, M.; Florencio, C.; Mattoso, L.; Ribeiro, C.; Farinas, C. Encapsulation of Trichoderma harzianum with nanocellulose/carboxymethyl cellulose nanocomposite. Carbohydr. Polym. 2022, 295, 119876. [Google Scholar] [CrossRef]
- Hamrouni, R.; Regus, F.; Farnet Da Silva, A.M.; Orsiere, T.; Boudenne, J.L.; Laffont-Schwob, I.; Christen, P.; Dupuy, N. Current status and future trends of microbial and nematode-based biopesticides for biocontrol of crop pathogens. Crit. Rev. Biotechnol. 2024, 45, 333–352. [Google Scholar] [CrossRef]
- Prasad, B.; Sharma, D.; Kumar, P.; Dubey, R.C. Biocontrol potential of Bacillus spp. for resilient and sustainable agricultural systems. Physiol. Mol. Plant Pathol. 2023, 128, 102173. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, Y.; Singh, B.K.; Wu, Y.; Munir, S.; He, P. Biocontrol of plant pathogens in omics era-with special focus on Endophytic bacilli. Crit. Rev. Biotechnol. 2023, 44, 562–580. [Google Scholar] [CrossRef]
- Keshmirshekan, A.; de Souza Mesquita, L.M.; Ventura, S.P.M. Biocontrol manufacturing and agricultural applications of Bacillus velezensis. Trends Biotechnol. 2024, 42, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets. Microencapsulation Market. 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/microencapsulation-market-83597438.html (accessed on 28 February 2025).
- dos Santos, J.P.; de Oliveira, A.L.P.; Putti, F.F. Bioinputs in agriculture: Techological overview of biological patents. GeSec 2024, 15, 01–18. [Google Scholar]
- Cutcliffe, C.; Eid, J.S.; Bullard, J.; Schicklberger, M.F.; Cheng, A.; Kolterman, O.; Altman, T. Methods and Compositions Relating to Isolated and Purified Microbes. Patent US20200121738A1, 23 April 2020. [Google Scholar]
- Harel, M.; Drewes, R.; Brian, C.; Artimovich, E. Stable Dry Powder Composition Comprising Biologically Active Microorganisms and/or Bioactive Materials and Methods of Making. Patent US20190194259A1, 27 June 2019. [Google Scholar]
- Harrel, M.; Scarbrow, J.; Drewes, R. Dry Glassy Composition Containing a Bioactive Substance. Patent JP5886763B2, 16 March 2016. [Google Scholar]
- Thompson, B.; Thompson, K.; Angle, B. Plant Growth-Promoting Bacteria and Methods of Use. Patent US20140274691A1, 18 September 2014. [Google Scholar]
- Harrel, M.; Tang, Q.; Drewes, R.; Rice, T.; Carpenter, B.; Raditsis, E. Stabilizing Composition for Biological Materials. Patent KR102062645B1, 6 January 2020. [Google Scholar]
- Ackerson, R.; Cantor, S.; Reap, J.J.; Tang, Q. Stabilizing Methods for Coating Seeds with Biological Materials. Patent AU2017219895, 16 August 2018. [Google Scholar]
- Jonner, P.S.; Palmer, S.; Alibek, K.; Ibragimova, S. Microbial-Based Products to Promote Plant Root and Immune Health. Patent KR20200142081A, 21 December 2020. [Google Scholar]
- Farmer, S.; Alibek, K.; Milovanovic, M.; Ibragimova, S. Materials and Methods for Treating Bacterial Infections in Plants. Patent BR112020014469A2, 1 December 2020. [Google Scholar]
- Bobeck, D.; Pearce, C. Microbial Inoculant Compositions and Uses Thereof in Agriculture. Patent AU2016270813B2, 15 February 2018. [Google Scholar]
- Shakeel, A.H.; Davis, Z.G.; Frank, J.T.; Zomorodi, S.; Pourtaheri, P. Compositions and Methods for Scalable Production and Delivery of Biologicals. Patent US20200267971A1, 27 August 2020. [Google Scholar]
- Bruck, D.; Burns, F.; Presnail, J. Fungal Entomopathogen Biocides and Their Use in Plants. Patent CA2998391C, 12 September 2023. [Google Scholar]
- Tamsil, A.; Blog, S.; Higgins, D. Methods and Compositions for Improving Engineered Microorganisms That Fix Nitrogen. Patent JP7420712B2, 23 January 2024. [Google Scholar]
- Embry, M.; Tarasova, J.; Pidgeon, L.; Gogul, G.; van der Linden, K. Method for Improving Agricultural Production of Poultry by Applying Microbial Consortia or Purified Strains Thereof. Patent CN109874294B, 20 June 2020. [Google Scholar]
- Chiatello, M.L.; Oman, M. Polymer-Based Antimicrobial Composition, and Using Method Thereof. Patent JP2022070983A, 13 May 2022. [Google Scholar]
- Rocha, T.M.; Marcelino, P.R.F.; de Souza, P.V.R.; dos Santos, J.C.; da Silva, S.S. Bioinput Based on T. harzianum and Sophorolipids, Kit, Use, Processes for Preparation of Sophorolipids and the Said Bioinput, and Methods for Inducing Growth and Protection Against Pests. Patent BR 102023003698-8 A2, 10 September 2024. [Google Scholar]
- Farmer, S.; Alibek, K. Materials and Methods for Control of Insect Pests Using Entomopathogenic Fungi. Patent US20200390106A1, 17 December 2020. [Google Scholar]
- Keller, F. Biofertilizer Composition Comprising Thermophosphate Dispersed in Emulsified Oil. Patent BR102022024883A2, 18 June 2024. [Google Scholar]
- De Oliveira, A.L.M.; Moreira, A.A.; de Oliveira, S.M. Method of Immobilization of Plant Growth-Promoting Bacteria on Organic Surfaces. Patent BR102022012809A2, 9 January 2024. [Google Scholar]
- Kellar, K.E.; Kan, Y.; Pelligra, C.; Barnett, E.; Burclev, C.; Vysinski, A.; Leland, J.; Dohan, B.; Fetkhe, M.H.; Trahan, A.D.; et al. Stable Compositions with Inoculant and Methods for Production Thereof. Patent RU2739954C2, 30 December 2020. [Google Scholar]
- Santiago-Ortiz, J.; Williams, T.; Wilk, D.; Zhu, H.; Patri, A.; Hymus, G. Agriculturally Beneficial Microbes, Microbial Compositions, and Consortia. Patent US20220264892A1, 25 August 2022. [Google Scholar]
- Bywater-Ekegard, M. Microbial Compositions and methods for Bioprotection. Patent CA2984075C, 23 January 2024. [Google Scholar]
- Goormachtig, S.; Viaene, T. Means and Methods for Plant Yield Enhancement. Patent US11071302B2, 27 July 2014. [Google Scholar]
- Montenegro, E.A.; Pinedamijangos, W.G.; Dutton-Forrest de Avaros, E.M.; Ramazzini-Santos, H.R.; Garcia-Garonte, I.V.; Vitus Alora, F.; Delgado Hernandez, V.M. An Agricultural Formulation Comprising at Least ONE bacterial Strain Bacillus safoci RGM 2450 and/or Bacterial Strain Bacillus siamensis RGM 2529 and an Agricultural Excipient; Use of Formulations and Methods for Stimulating Growth and/or Increasing Crop Yield and/or Protecting Crops Against Diseases and Pests. Patent CN116724107A, 8 September 2023. [Google Scholar]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).