Blending Tradition and Technology: A Celery–Parsley–Turmeric Formulation for Functional Ingredient Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Determination of the Chemical Composition of Samples
2.4. Analysis of Sensory Compliance Quality and Descriptive Sensory Analysis
2.4.1. Preparation of Samples for Sensory Analysis
2.4.2. Sensory Analysis
2.5. Determination of Sample Colour
2.6. Mineral Composition
2.7. Extraction Process
2.8. Total Phenolics (TP) Content
2.9. Antioxidant Activity Tests
2.10. HPLC Analysis
2.11. Determination of Minimal Inhibitory Concentration Against a Wide Range of Microorganisms
2.12. Health-Promoting Properties
2.13. Statistical Analysis
2.13.1. Correlation Analysis and Principal Component Analysis (PCA)
2.13.2. Standard Scores (SS) Evaluation
2.13.3. Artificial Neural Network (ANN) Analysis
3. Results and Discussion
3.1. Proximate Composition, Compliance of Sensory Quality and Colour Parameters of Samples
3.2. Total Phenolics and Antioxidative Activities of Samples
3.3. Statistical Analysis
3.3.1. Standard Scores for Product Blends
3.3.2. Correlation Analysis of Antioxidative Parameters
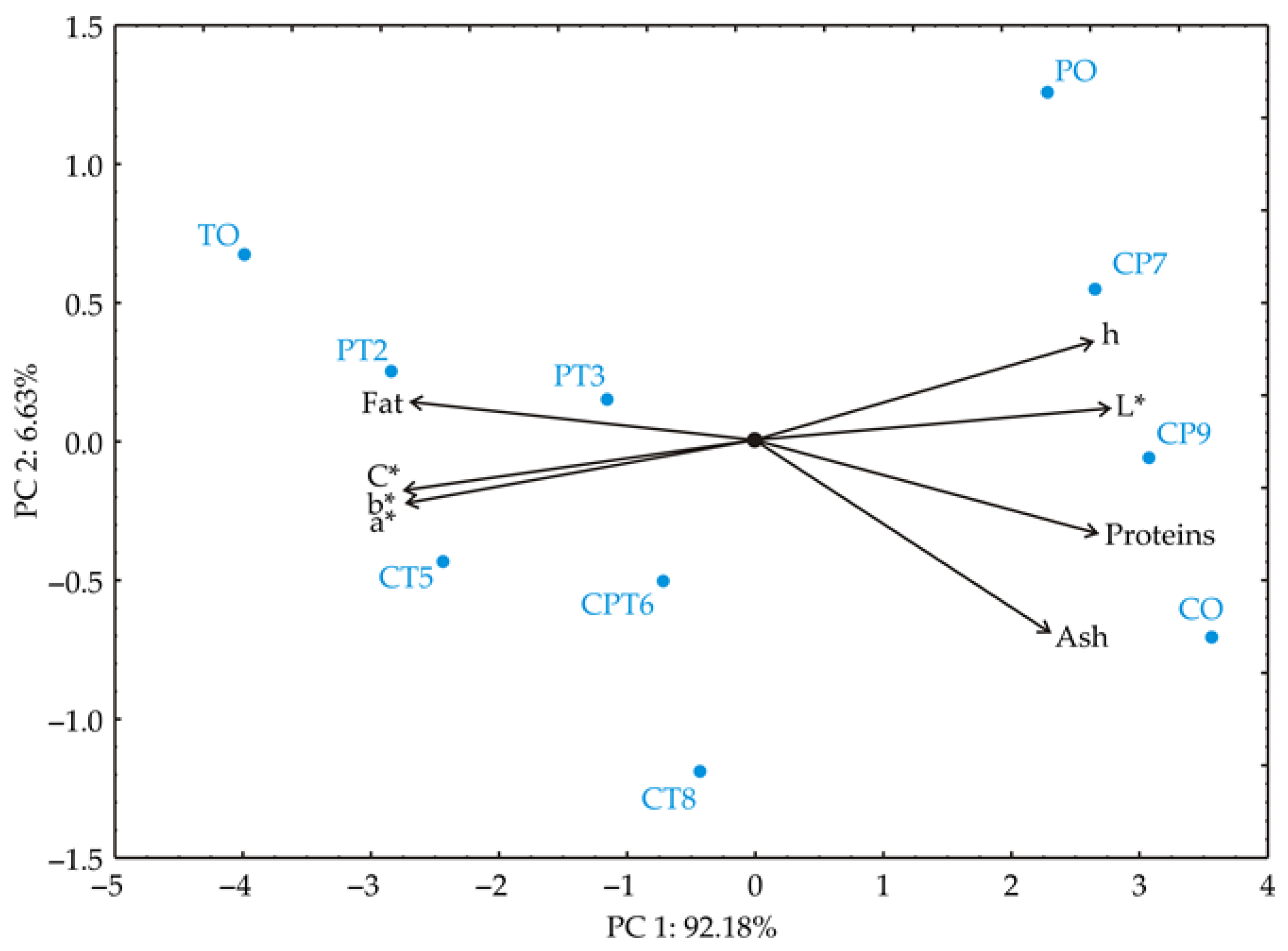
3.3.3. Principal Component Analysis (PCA)
3.3.4. ANOVA
3.3.5. Artificial Neural Network (ANN) Model
3.4. HPLC Analysis of Phenolic Compounds in Optimal Blend
3.5. Mineral Composition of Optimal Blend
3.6. Antimicrobial Properties of Optimal Blend
3.7. Health-Improving Potentials of the CPT6 Sample
3.8. Application Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by Turmeric, the Golden Spice: From Kitchen to Clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 297–318. ISBN 978-3-030-57415-4. [Google Scholar]
- Arsenov, D.; Župunski, M.; Pajević, S.; Nemeš, I.; Simin, N.; Alnuqaydan, A.M.; Watson, M.; Aloliqi, A.A.; Mimica-Dukić, N. Roots of Apium Graveolens and Petroselinum Crispum—Insight into Phenolic Status against Toxicity Level of Trace Elements. Plants 2021, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Kathiresan, C.; Srinivasappa, K.N. 17—Celeriac. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Saxton, UK, 2006; pp. 313–316. ISBN 978-1-84569-017-5. [Google Scholar]
- Ravindran, P.N. Parsley, Oregano, Thyme and Marjoram. In Handbook of Spices in India: 75 Years of Research and Development; Ravindran, P.N., Sivaraman, K., Devasahayam, S., Babu, K.N., Eds.; Springer Nature: Singapore, 2024; pp. 3185–3231. ISBN 978-981-19372-8-6. [Google Scholar]
- Shelef, L.A.; Naglik, O.A.; Bogen, D.W. Sensitivity of Some Common Food-Borne Bacteria to the Spices Sage, Rosemary, and Allspice. J. Food Sci. 1980, 45, 1042–1044. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Latinović, S.; Vasilišin, L.; Pezo, L.; Lakić-Karalić, N.; Cvetković, D.; Ranitović, A.; Brunet, S.; Cvanić, T.; Vulić, J. Impact of Drying Methods on Phenolic Composition and Bioactivity of Celery, Parsley, and Turmeric—Chemometric Approach. Foods 2024, 13, 3355. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Lawless, L.J.R.; Hottenstein, A.; Ellingsworth, J. The Mccormick Spice Wheel: A Systematic and Visual Approach to Sensory Lexicon Development. J. Sens. Stud. 2012, 27, 37–47. [Google Scholar] [CrossRef]
- ISO 13299; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- ISO 4121; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. ISO: Geneva, Switzerland, 2003.
- ISO 6658; Sensory Analysis—Methodology—General Guidance. ISO: Geneva, Switzerland, 2017.
- ISO 8586; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef]
- MacLeod, G.; Ames, J.M. Volatile Components of Celery and Celeriac. Phytochemistry 1989, 28, 1817–1824. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The Effect of Water Deficit Stress on the Growth, Yield and Composition of Essential Oils of Parsley. Sci. Hortic. 2008, 115, 393–397. [Google Scholar] [CrossRef]
- Binello, A.; Grillo, G.; Barge, A.; Allegrini, P.; Ciceri, D.; Cravotto, G. A Cross-Flow Ultrasound-Assisted Extraction of Curcuminoids from Curcuma longa L.: Process Design to Avoid Degradation. Foods 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant Activity of Commercial Soft and Hard Wheat (Triticum aestivum L.) as Affected by Gastric pH Conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzym. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 30th ed.; CLSI: Wayne, PA, USA, 2020; ISBN 978-1-68440-067-6. [Google Scholar]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A Comprehensive Antioxidant and Nutritional Profiling of Brassicaceae Microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef]
- Brlek, T.; Pezo, L.; Voća, N.; Krička, T.; Vukmirović, Đ.; Čolović, R.; Bodroža-Solarov, M. Chemometric Approach for Assessing the Quality of Olive Cake. Fuel Process. Technol. 2013, 116, 250–256. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Contribution of Bacterial Cells as Nucleation Centers in Microbiologically Induced CaCO3 Precipitation—A Mathematical Modeling Approach. J. Basic. Microbiol. 2021, 61, 835–848. [Google Scholar] [CrossRef]
- Šovljanski, O.; Tomić, A.; Pezo, L.; Ranitović, A.; Markov, S. Prediction of Denitrification Capacity of Alkalotolerant Bacterial Isolates from Soil—An Artificial Neural Network Model. J. Serbian Chem. Soc. 2020, 85, 1417–1427. [Google Scholar] [CrossRef]
- Šovljanski, O.; Saveljić, A.; Aćimović, M.; Šeregelj, V.; Pezo, L.; Tomić, A.; Ćetković, G.; Tešević, V. Biological Profiling of Essential Oils and Hydrolates of Ocimum Basilicum Var. Genovese and Var. Minimum Originated from Serbia. Processes 2022, 10, 1893. [Google Scholar] [CrossRef]
- Srinivasan, K. Role of Spices Beyond Food Flavoring: Nutraceuticals with Multiple Health Effects. Food Rev. Int. 2005, 21, 167–188. [Google Scholar] [CrossRef]
- Olayinka, I.O. Proximate, Mineral Composition and Phytochemical Analyses of Turmeric (Curcuma longa) Powder. Niger. J. Anim. Prod. 2023, 874–878. [Google Scholar]
- Enemor, V.H.A.; Ogbodo, U.C.; Nworji, O.F.; Ezeigwe, O.C.; Okpala, C.O.; Iheonunekwu, G.C. Evaluation of the Nutritional Status and Phytomedicinal Properties of Dried Rhizomes of Turmeric (Curcuma longa). J. Biosci. Med. 2020, 8, 163–179. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Finimundy, T.C.; Polyzos, N.; Pinela, J.; Ivanov, M.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. The Bioactivities and Chemical Profile of Turnip-Rooted Parsley Germplasm. Horticulturae 2022, 8, 639. [Google Scholar] [CrossRef]
- Ebru, K.; Fatıma, Y.; Gizem, G.T.; Berna, Ö.Ç.; Afife, M. Quality of Turmeric Powder in Herbal Stores: Pharmacognostical Investigations on Turmeric Powders Obtained from Herbal Stores in Istanbul, Turkey. İstanbul J. Pharm. 2021, 51, 92–97. Available online: https://iupress.istanbul.edu.tr/en/journal/ijp/article/quality-of-turmeric-powder-in-herbal-stores-pharmacognostical-investigations-on-turmeric-powders-obtained-from-herbal-stores-in-istanbul-turkey (accessed on 1 March 2025).
- Parmar, R.G.; Dabhi, M.N.; Rathod, P.J. Effect of Drying Temperature on Proximate Components of Turmeric Rhizome in Tray Dryer: Efecto de La Temperatura de Secado Sobre Los Componentes Proximales Del Rizoma de Cúrcuma En Secadero de Bandejas. S. Fla. J. Environ. Anim. Sci. 2023, 3, 174–181. [Google Scholar] [CrossRef]
- Jeleń, H. Specificity of Food Odorants. In Food Flavors: Chemical, Sensory and Technological Properties; Jeleń, H., Ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-1491-8. [Google Scholar]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive Sensory Analysis: Past, Present and Future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Prakash, O.; Kumar, A.; Tiwari, S.; Bajpai, P. The Versatility of Apigenin: Especially as a Chemopreventive Agent for Cancer. J. Holist. Integr. Pharm. 2024, 5, 249–256. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Kamynina, M.; Sorokin, M.; Zolotovskaia, M.; Koroleva, E.; Kremenchutckaya, K.; Gudkov, A.; Buzdin, A.; Borisov, N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines 2022, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Prasad, S. A Review on Iron, Zinc and Calcium Biological Significance and Factors Affecting Their Absorption and Bioavailability. J. Food Compos. Anal. 2023, 123, 105529. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Odo, E.O.; Ikwuegbu, J.A.; Obeagu, E.I.; Chibueze, S.A.; Ochiaka, R.E. Analysis of the Antibacterial Effects of Turmeric on Particular Bacteria. Medicine 2023, 102, e36492. [Google Scholar] [CrossRef]
- Beshiru, A.; Igbinosa, I.H.; Salami, J.O.; Uwhuba, K.E.; Ogofure, A.G.; Azazi, G.M.; Igere, B.E.; Anegbe, B.; Evuen, U.F.; Igbinosa, E.O. Curcuma longa Rhizome Extract: A Potential Antibiofilm Agent against Antibiotic-Resistant Foodborne Pathogens. Biofouling 2024, 40, 932–947. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Alam, S.; Chowdhury, M.N.R.; Hossain, M.A.; Richi, F.T.; Emon, N.U.; Mohammad, M.; Ahmed, N.; Taher, M.A. Antifungal Potentials of Asian Plants: Ethnobotanical Insights and Phytochemical Investigations. Chem. Biodivers. 2025, 22, e202402867. [Google Scholar] [CrossRef]
- Roney, M.; Huq, A.K.M.M.; Rullah, K.; Zamri, N.B.; Mohd Aluwi, M.F.F. Curcumin, a Bioactive Compound of Turmeric (Curcuma longa) and Its Derivatives as α-Amylase and α-Glucosidase Inhibitors. Cell Biochem. Biophys. 2024, 83, 53–71. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts Its Anti-Hypertensive Effect by Down-Regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Liao, L.; Dong, X.; Hu, X.; Guo, Y.; Du, Z.; Liao, X.; Wang, L. Design, Synthesis, and Antihypertensive Activity of Curcumin-Inspired Compounds via ACE Inhibition and Vasodilation, along with a Bioavailability Study for Possible Benefit in Cardiovascular Diseases. Drug Des. Devel Ther. 2016, 10, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A. Curcumin-Based Fixed Dose Combination Products for Cholesterol Management: A Narrative Review. ACS Pharmacol. Transl. Sci. 2024, 7, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Awari, A.; Kumar, M.; Kaushik, D.; Amarowicz, R.; Proestos, C.; Wahab, R.; Khan, M.R.; Tomasevic, I.; Oz, E.; Oz, F. Proximate Analysis and Techno-Functional Properties of Berberis Aristata Root Powder: Implications for Food Industry Applications. Foods 2024, 13, 2802. [Google Scholar] [CrossRef]
- Long, D.Q.; Doan, T.B.N.; Ton, N.M.N.; Tran, T.T.T.; Le, V.V.M. Quality of Durum Wheat Pasta Fortified with Different Ratios of Turmeric Residue Powder. J. Agric. Food Res. 2024, 16, 101220. [Google Scholar] [CrossRef]
- Bazsefidpar, N.; Ghandehari Yazdi, A.P.; Karimi, A.; Yahyavi, M.; Amini, M.; Ahmadi Gavlighi, H.; Simal-Gandara, J. Brewers Spent Grain Protein Hydrolysate as a Functional Ingredient for Muffins: Antioxidant, Antidiabetic, and Sensory Evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic Bioactives From Plant-Based Foods for Glycemic Control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Salhi, N.A.; Shatreet, B.; Asbah, A.A.; Hawash, Y.H. Assessing the Therapeutic Potential and Safety of Traditional Anti-Obesity Herbal Blends in Palestine. Sci. Rep. 2024, 14, 1919. [Google Scholar] [CrossRef]



| Blend No. | Code | Mass Fraction of Plant Components | ||
|---|---|---|---|---|
| Celery | Parsley | Turmeric | ||
| 1. | TO | 0 | 0 | 1 |
| 2. | PT2 | 0 | 0.333 | 0.667 |
| 3. | PT3 | 0 | 0.667 | 0.333 |
| 4. | PO | 0 | 1 | 0 |
| 5. | CT5 | 0.333 | 0 | 0.667 |
| 6. | CPT6 | 0.333 | 0.333 | 0.333 |
| 7. | CP7 | 0.333 | 0.667 | 0 |
| 8. | CT8 | 0.667 | 0 | 0.333 |
| 9. | CP9 | 0.667 | 0.333 | 0 |
| 10. | CO | 1 | 0 | 0 |
| Blend No. | Code | Plant Component Content (g/100 mL) | ||
|---|---|---|---|---|
| Celery | Parsley | Turmeric | ||
| 1. | TO | 0 | 0 | 0.25 |
| 2. | PT2 | 0 | 0.083 | 0.167 |
| 3. | PT3 | 0 | 0.167 | 0.083 |
| 4. | PO | 0 | 0.25 | 0 |
| 5. | CT5 | 0.083 | 0 | 0.167 |
| 6. | CPT6 | 0.083 | 0.083 | 0.083 |
| 7. | CP7 | 0.083 | 0.167 | 0 |
| 8. | CT8 | 0.167 | 0 | 0.083 |
| 9. | CP9 | 0.167 | 0.083 | 0 |
| 10. | CO | 0.25 | 0 | 0 |
| Sample | Proteins (%) | Ash (%) | Fat (%) | Sensory Compliance Quality Score | L* | a* | b* | C* | h |
|---|---|---|---|---|---|---|---|---|---|
| TO | 4.76 ± 0.08 a | 5.83 ± 0.02 a | 7.88 ± 0.12 j | 4.00 ± 0.74 a | 68.15 ± 1.38 a | 12.72 ± 0.87 d | 62.01 ± 0.34 e | 63.30 ± 0.45 e | 78.41 ± 0.75 a |
| PT2 | 6.77 ± 0.25 b | 6.45 ± 0.11 b | 5.85 ± 0.09 i | 4.42 ± 0.51 a | 70.56 ± 0.28 b | 11.54 ± 0.24 c | 55.83 ± 0.42 d | 57.01 ± 0.39 d | 78.33 ± 0.29 a |
| PT3 | 8.84 ± 0.13 d | 7.09 ± 0.15 c | 3.85 ± 0.05 g | 3.67 ± 0.49 a,b | 74.25 ± 0.54 c | 7.97 ± 0.45 b | 45.55 ± 0.41 c | 46.25 ± 0.48 c | 80.08 ± 0.46 b |
| PO | 11.00 ± 0.22 f | 7.74 ± 0.24 d | 1.83 ± 0.03 d | 4.17 ± 0.72 a,b | 82.89 ± 0.20 d | −0.42 ± 0.04 a | 20.81 ± 0.22 b | 20.81 ± 0.22 b | 91.16 ± 0.11 c |
| CT5 | 7.77 ± 0.05 c | 7.70 ± 0.06 d | 5.56 ± 0.05 h | 4.25 ± 0.45 b,c | 70.73 ± 0.31 b | 11.58 ± 0.21 c | 55.81 ± 0.76 d | 57.00 ± 0.77 d | 78.28 ± 0.11 a |
| CPT6 | 9.87 ± 0.12 e | 8.32 ± 0.21 e | 3.55 ± 0.04 f | 3.58 ± 0.51 b,c | 74.79 ± 0.10 c | 7.82 ± 0.08 b | 45.69 ± 0.30 c | 46.36 ± 0.30 c | 80.29 ± 0.11 b |
| CP7 | 11.85 ± 0.10 g | 9.03 ± 0.14 f | 1.54 ± 0.03 c | 3.25 ± 0.45 c,d | 83.06 ± 0.16 d | −0.35 ± 0.10 a | 21.16 ± 0.34 b | 21.17 ± 0.34 b | 90.94 ± 0.26 c |
| CT8 | 10.62 ± 0.15 f | 9.56 ± 0.16 g | 3.30 ± 0.03 e | 3.17 ± 0.58 c,d | 74.55 ± 0.32 c | 8.12 ± 0.18 b | 45.89 ± 0.55 c | 46.61 ± 0.56 c | 79.96 ± 0.16 b |
| CP9 | 12.85 ± 0.05 h | 10.17 ± 0.09 h | 1.28 ± 0.01 b | 2.67 ± 0.49 c,d | 83.75 ± 0.17 d,e | −0.32 ± 0.03 a | 20.38 ± 0.21 a,b | 20.38 ± 0.22 a,b | 90.92 ± 0.09 c |
| CO | 13.88 ± 0.12 i | 11.39 ± 0.15 i | 0.96 ± 0.02 a | 2.75 ± 0.62 d | 84.64 ± 0.33 e | −0.26 ± 0.04 a | 19.27 ± 0.25 a | 19.27 ± 0.25 a | 90.77 ± 0.12 c |
| Sample | Total Phenolics (mg GAE/g) | DPPH (µmol Trolox/g) | ABTS (µmol Trolox/g) | FRAP (μmol Fe2+/g) |
|---|---|---|---|---|
| TO | 23.44 ± 0.93 e | 166.02 ± 4.65 f | 358.78 ± 13.27 e | 333.09 ± 16.45 e |
| PT2 | 16.03 ± 0.04 c | 130.67 ± 2.65 e | 271.80 ± 1.52 d | 257.04 ± 7.33 d |
| PT3 | 9.90 ± 0.14 b | 79.97 ± 3.06 c | 115.68 ± 3.92 b | 150.57 ± 8.23 c |
| PO | 0.77 ± 0.01 a | 1.03 ± 0.01 a | 4.11 ± 0.14 a | 8.06 ± 0.35 a |
| CT5 | 17.46 ± 0.41 d | 105.31 ± 1.97 d | 240.63 ± 8.13 c | 264.65 ± 9.66 d |
| CPT6 | 9.56 ± 0.71 b | 61.99 ± 0.39 b | 104.01 ± 1.61 b | 103.35 ± 8.99 b |
| CP7 | 0.80 ± 0.02 a | 1.09 ± 0.01 a | 5.21 ± 0.50 a | 8.72 ± 0.21 a |
| CT8 | 10.16 ± 0.18 b | 61.80 ± 1.02 b | 111.35 ± 4.81 b | 115.90 ± 2.03 b |
| CP9 | 0.83 ± 0.01 a | 1.61 ± 0.02 a | 7.32 ± 0.15 a | 9.20 ± 0.05 a |
| CO | 0.91 ± 0.02 a | 2.44 ± 0.02 a | 8.07 ± 0.00 a | 10.51 ± 0.31 a |
| Factor | TP | DPPH | ABTS | FRAP | Sens | Proteins | Ash | Fat | L* | a* | b* | C* | h | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Celery (L) | 1 | 0.0012 | 15.89 | 333.3 | 1067.0 | 0.001688 | 0.01081 | 0.00034 | 0.00006 | 0.0170 | 0.0264 | 1.688 | 1.550 | 0.6193 |
| Celery (Q) | 1 | 0.2179 | 248.80 * | 133.7 | 35.0 | 0.000081 | 0.00587 | 0.00269 | 0.00016 | 0.2508 | 0.0042 | 0.485 | 0.506 | 0.0703 |
| Parsley (L) | 1 | 0.0012 | 15.88 | 333.0 | 1067.2 | 0.001592 | 0.01131 | 0.00024 | 0.00007 | 0.0166 | 0.0264 | 1.685 | 1.546 | 0.6197 |
| Parsley (Q) | 1 | 0.4255 | 191.60 | 268.6 | 82.5 | 0.250122 | 0.00023 | 0.00003 | 0.00000 | 0.0921 | 0.0071 | 0.229 | 0.229 | 0.0213 |
| Tumeric (L) | 1 | 0.0028 | 16.97 | 344.2 | 1085.2 | 0.001582 | 0.01243 | 0.00019 | 0.00018 | 0.0138 | 0.0231 | 1.601 | 1.463 | 0.6046 |
| Tumeric (Q) | 1 | 3.1682 | 234.07 * | 2.5 | 897.9 | 0.140465 | 0.00830 | 0.00047 | 0.00000 | 19.9776 * | 23.1595 * | 164.316 | 174.047 * | 57.1695 * |
| Error | 3 | 0.9446 | 62.42 | 685.6 | 765.9 | 0.474610 | 0.01789 | 0.00270 | 0.00160 | 1.8785 | 0.6241 | 13.395 | 13.093 | 5.2461 |
| r2 | 0.998 | 0.998 | 0.995 | 0.994 | 0.864 | 1.000 | 1.000 | 1.000 | 0.995 | 0.998 | 0.995 | 0.995 | 0.984 | |
| adj r2 | 0.995 | 0.994 | 0.986 | 0.983 | 0.591 | 0.999 | 1.000 | 1.000 | 0.984 | 0.993 | 0.985 | 0.986 | 0.953 |
| Variable | Network | Training Perf. | Test Perf. | Training Error | Test Error | Training Algorithm | Error Function | Hidden Activation | Output Activation |
|---|---|---|---|---|---|---|---|---|---|
| TP | MLP 3-6-1 | 0.978 | 1.000 | 0.963 | 0.914 | BFGS 20 | SOS | Logistic | Logistic |
| DPPH | MLP 3-9-1 | 0.991 | 1.000 | 26.873 | 3.631 | BFGS 14 | SOS | Tanh | Tanh |
| ABTS | MLP 3-10-1 | 0.953 | 1.000 | 439.146 | 41.002 | BFGS 4 | SOS | Logistic | Identity |
| FRAP | MLP 3-3-1 | 0.980 | 1.000 | 296.648 | 18.806 | BFGS 3 | SOS | Logistic | Identity |
| Sens | MLP 3-5-1 | 0.811 | 1.000 | 0.045 | 0.048 | BFGS 3 | SOS | Logistic | Identity |
| Proteins | MLP 3-7-1 | 0.999 | 1.000 | 0.003 | 0.003 | BFGS 35 | SOS | Exponential | Exponential |
| Ash | MLP 3-10-1 | 0.986 | 1.000 | 0.028 | 0.014 | BFGS 5 | SOS | Identity | Logistic |
| Fat | MLP 3-3-1 | 1.000 | 1.000 | 0.000 | 0.000 | BFGS 187 | SOS | Logistic | Identity |
| L* | MLP 3-4-1 | 1.000 | 1.000 | 0.005 | 0.048 | BFGS 20 | SOS | Tanh | Identity |
| a* | MLP 3-3-1 | 1.000 | 1.000 | 0.000 | 0.018 | BFGS 30 | SOS | Logistic | Identity |
| b* | MLP 3-4-1 | 1.000 | 1.000 | 0.006 | 0.035 | BFGS 53 | SOS | Tanh | Identity |
| C* | MLP 3-9-1 | 1.000 | 1.000 | 0.026 | 0.177 | BFGS 108 | SOS | Tanh | Exponential |
| h | MLP 3-7-1 | 1.000 | 1.000 | 0.004 | 0.017 | BFGS 9 | SOS | Exponential | Logistic |
| Experimental Parameter | Verification Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | RMSE | MBE | MPE | SSE | AARD | r2 | Skew | Kurt | Mean | StDev | Var | |
| TP | 2.516 | 1.484 | 0.326 | 36.322 | 17.613 | 36.322 | 0.963 | 0.050 | −1.511 | 0.326 | 1.547 | 2.395 |
| DPPH | 49.497 | 6.581 | −0.690 | 89.772 | 346.480 | 89.772 | 0.987 | −0.177 | 1.069 | −0.690 | 6.997 | 48.953 |
| ABTS | 906.020 | 28.156 | 1.017 | 94.755 | 6342.139 | 94.755 | 0.945 | 0.739 | 1.642 | 1.017 | 30.081 | 904.837 |
| FRAP | 540.000 | 21.737 | −15.448 | 60.340 | 3779.999 | 60.340 | 0.982 | −0.284 | −1.112 | −15.448 | 16.348 | 267.267 |
| Sens | 0.103 | 0.300 | −0.041 | 5.700 | 0.720 | 5.700 | 0.611 | −0.560 | −0.525 | −0.041 | 0.318 | 0.101 |
| Proteins | 0.007 | 0.079 | −0.010 | 0.672 | 0.050 | 0.672 | 0.999 | 1.627 | 2.534 | −0.010 | 0.084 | 0.007 |
| Ash | 0.040 | 0.187 | −0.046 | 2.099 | 0.281 | 2.099 | 0.976 | −0.406 | −0.371 | −0.046 | 0.194 | 0.038 |
| Fat | 0.000 | 0.010 | 0.001 | 0.267 | 0.001 | 0.267 | 1.000 | 0.866 | 0.114 | 0.001 | 0.010 | 0.000 |
| L* | 0.045 | 0.199 | −0.102 | 0.208 | 0.316 | 0.208 | 0.999 | −0.319 | −2.240 | −0.102 | 0.182 | 0.033 |
| a* | 0.016 | 0.117 | 0.062 | −0.242 | 0.109 | 1.689 | 1.000 | 1.414 | −0.039 | 0.062 | 0.106 | 0.011 |
| b* | 0.033 | 0.170 | 0.048 | 0.399 | 0.232 | 0.399 | 1.000 | 2.170 | 5.598 | 0.048 | 0.175 | 0.030 |
| C* | 0.152 | 0.365 | 0.197 | 0.777 | 1.066 | 0.777 | 1.000 | 1.160 | 0.183 | 0.197 | 0.328 | 0.108 |
| h | 0.021 | 0.136 | −0.006 | 0.131 | 0.147 | 0.131 | 0.999 | −0.839 | −0.658 | −0.006 | 0.145 | 0.021 |
| Individual Phenolic Compounds | Phenolic Compound Content (μg/100 g) |
|---|---|
| p-Hydroxybenzoic acid | 85.75 ± 0.11 |
| Gallic acid | 14.04 ± 0.31 |
| Protocatechuic acid | 20.33 ± 0.61 |
| Cinnamic acid | 9.23 ± 0.35 |
| Ferulic acid | 6.34 ± 0.18 |
| Apigenin | 37.56 ± 0.15 |
| Chlorogenic acid | 7.96 ± 0.58 |
| CPT6 Sample | Content (mg/kg) |
|---|---|
| Sodium, Na | 494.64 ± 4.02 |
| Potassium, K | 1407.93 ± 6.77 |
| Calcium, Ca | 2506.66 ± 28.49 |
| Magnesium, Mg | 1211.41 ± 20.35 |
| Phosphorous, P | 3716.50 ± 22.76 |
| Zinc, Zn | 80.27 ± 1.60 |
| Manganese, Mn | 59.76 ± 1.06 |
| Iron, Fe | 37.53 ± 0.24 |
| Microorganism | MIC (mg/mL) |
|---|---|
| Staphylococcus aureus | 100 |
| Bacillus subtilis | 1.56 |
| Listeria monocytogenes | 3.125 |
| Escherichia coli | >100 |
| Salmonella Typhimurium | 3.125 |
| Pseudomonas aeruginosa | >100 |
| Candida albicans | 100 |
| Aspergillus niger | 100 |
| Health-Improving Potential Parameters | CPT6 Sample |
|---|---|
| Antidiabetic activity—α-Amylase-IP (I (%)) | 72.00 ± 1.20 |
| Antidiabetic activity—α-Amylase-IP (mg ACAE/gde) | 6.50 ± 0.31 |
| Antidiabetic activity—α-Glucosidase-IP (I (%)) | 80.00 ± 3.43 |
| Antidiabetic activity—α-Glucosidase-IP (mg ACAE/gde) | 6.80 ± 0.22 |
| Antihypertension activity (% ACE Inhibition) | 62.00 ± 1.10 |
| Antihypercholesterolemic activity (% HMGCR Inhibition) | 55.00 ± 2.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latinović, S.; Šovljanski, O.; Grujić, S.; Pezo, L.; Škrobot, D.; Čanadanović-Brunet, J.; Cvetković, D.; Vasilišin, L.; Lakić-Karalić, N.; Pećanac, B.; et al. Blending Tradition and Technology: A Celery–Parsley–Turmeric Formulation for Functional Ingredient Applications. Processes 2025, 13, 1849. https://doi.org/10.3390/pr13061849
Latinović S, Šovljanski O, Grujić S, Pezo L, Škrobot D, Čanadanović-Brunet J, Cvetković D, Vasilišin L, Lakić-Karalić N, Pećanac B, et al. Blending Tradition and Technology: A Celery–Parsley–Turmeric Formulation for Functional Ingredient Applications. Processes. 2025; 13(6):1849. https://doi.org/10.3390/pr13061849
Chicago/Turabian StyleLatinović, Staniša, Olja Šovljanski, Slavica Grujić, Lato Pezo, Dubravka Škrobot, Jasna Čanadanović-Brunet, Dragoljub Cvetković, Ladislav Vasilišin, Nataša Lakić-Karalić, Biljana Pećanac, and et al. 2025. "Blending Tradition and Technology: A Celery–Parsley–Turmeric Formulation for Functional Ingredient Applications" Processes 13, no. 6: 1849. https://doi.org/10.3390/pr13061849
APA StyleLatinović, S., Šovljanski, O., Grujić, S., Pezo, L., Škrobot, D., Čanadanović-Brunet, J., Cvetković, D., Vasilišin, L., Lakić-Karalić, N., Pećanac, B., Vučić, G., Milošević, M., & Vulić, J. (2025). Blending Tradition and Technology: A Celery–Parsley–Turmeric Formulation for Functional Ingredient Applications. Processes, 13(6), 1849. https://doi.org/10.3390/pr13061849









