Abstract
Nuts are nutrient-dense foods recognized for their complex chemical composition and associated health benefits. This review provides a comprehensive overview of the botanical classification, morphology, production, and consumption patterns of key nut species, including walnuts, almonds, pistachios, pecans, peanuts, cashews, bitter kola, and kola nuts. It emphasizes the fatty acid profiles, noting that palmitic acid (C16:0) is the predominant saturated fatty acid, while oleic acid (C18:1) and linoleic acid (C18:2) are the most abundant monounsaturated and polyunsaturated fatty acids, respectively. The review also details various analytical techniques employed for extracting and characterizing bioactive compounds, which are crucial for assessing nut quality and health benefits. Methods such as Soxhlet extraction, solid-phase microextraction (SPME), supercritical fluid extraction (SFE), gas chromatography (GC-FID and GC-MS), and high-performance liquid chromatography (HPLC) are highlighted. Furthermore, it discusses scientific evidence linking nut consumption to antioxidant and anti-inflammatory properties, improved cardiovascular health, and a reduced risk of type 2 diabetes, establishing nuts as important components in a healthy diet. This review underscores the role of nuts as functional foods and calls for standardized methodologies in future lipidomic and volatilomic studies.
1. Introduction
Botanically, nuts are defined as “a dry one-seed fruit that possesses a very hard exterior (pericarp)” [1]. However, nutritional research classifies nuts based on their nutrient composition and edibility; therefore, not only botanical nuts are included in this classification, but also some seeds, legumes, and even drupes that share similar nutrient compositions with true botanical nuts [1]. Some examples of seeds, legumes, and drupes that are nutritionally classified as nuts include almonds, Brazil nuts, cashews, hazelnuts, macadamias, peanuts, pecans, pine nuts, pistachios, walnuts [1]. They have been established to be nutrient-dense and reported to be rich in health-promoting components such as monounsaturated and polyunsaturated fatty acids, essential amino acids, fiber, micronutrients, steroids, and volatile compounds [2,3,4]. According to [5], nuts typically possess less than 7% water content, with their protein content being as high as 13–26% of their total weight; they are very rich in lipids (about 43–76% of total weight) and contain carbohydrates (about 9–30% of total weight) and fiber (7–12% of total weight).
Nuts are commonly consumed globally and are cherished for their nutritional benefits and versatile application in various sectors, such as the food/feed sector [6] and the chemical sector (cosmetics, biofuels, biopolymers, lubricants, surfactants, etc.) [7]. They are processed and consumed in diverse ways globally, and the most common forms of processing and consumption include raw, boiled, or roasted forms, butter or pastes, beverages (milk), and oils. As of 2022, the global nut market was valued at about USD 5.90 billion, and a growth rate of about 5.4% was expected to occur between 2023 and 2030 [8]. The report showed that growth in the nut snacks industry in economies such as China and India played a significant role in the market growth in recent years.
Globally, almonds and walnuts are reported to be the most consumed nuts in 2021, and they account for 31% and 21%, respectively, of total nuts consumed that year [9]. The 2023 report by International Nut showed that cashews ranked third (19%), pistachios ranked fourth (15%), and hazelnuts ranked fifth (11%) in the total nut consumption values of the year 2021 [9]. The top four consuming regions are Asia, Europe, North America, and the Middle East, with consumption rates of 32%, 31%, 20%, and 12%, respectively [9]. North America (the USA specifically) has topped global tree nut production charts since 2018, producing an average of 40% of global tree nuts, with almonds, pistachios, and walnuts being the most widely grown nuts; Türkiye comes second, producing about 11% of global tree nuts [9]. China is the highest producer of peanuts and is responsible for about 35% of global peanuts production, while India and Nigeria rank second and third, respectively, producing 13% and 9% of peanuts globally [9].
While nuts are widely consumed, acknowledged, and celebrated for their nutritional values as shown in Table 1, some debates still exist about how much and how often they should be consumed because, on the one hand, they contain fatty acids that are considered healthy, but on the other hand, concerns have been raised about the impact of their high-lipid content. Some of the health benefits associated with nut consumption include a lowering of LDL cholesterol and improvement of cardiovascular health [10,11,12,13,14,15] and the regulation of glucose levels in the blood, thus reducing the risk of type 2 diabetes [11,16]. However, some studies have reported that some of these benefits are not directly linked to nut consumption, such as those by [17,18]. Though 7 years apart, they could not find a direct association between nut consumption and blood glucose regulation.
The health benefits of nut consumption have been linked to their fatty acids (FAs) and volatile compounds [2,19]. They have been reported to be rich sources of unsaturated fatty acids [19]. The most prevalent fatty acids in nuts are oleic acid, linoleic acid, and α-linolenic acid [2,20,21,22]. Their volatile compounds have been proven to be responsible for their characteristic aromas/flavors, and processing methods such as roasting can degrade fatty acids into volatiles, thus intensifying their flavors but potentially reducing their health benefits [23,24]. However, emerging studies have reported that specific volatiles, such as terpenes and phenols, have antioxidant and antimicrobial properties, and these volatiles are commonly found in nuts and may also be responsible for some of the health benefits nuts possess [25,26,27].
This article aims to give a detailed overview of published information on the methods of determination and health effects of FA and volatiles in tree nuts.
2. Review of Literature
2.1. Tree Nuts
2.1.1. Walnuts
Botanically, walnuts belong to the Juglandaceae family and the Juglans genus. Technically, the walnut is a drupe with a green husk containing a hard shell, which encloses the edible seed (as seen in Figure 1A). The edible seed is wrinkled and resembles a brain (as seen in Figure 1B) [28]. Walnuts are widely cultivated in different regions of the world, and in 2023, global walnut production was estimated to be about 1.2 million metric tons (kernel basis), which is the most significant production volume in about a decade [29]. According to the ICN report [29], China is the largest producer of walnuts, producing about 51% of global production. The second-largest producer is the USA (28%), followed by Chile and Ukraine, responsible for 7% and 3% of global production volumes, respectively. Apart from being the largest producer, China has also been reported to be the largest consumer of walnuts, with a consumption volume of almost 500,000 metric tons in the year 2023. Other top consumers of walnuts are the USA (135,154), Türkiye (60,329), India (34,759), and Italy (32,205) [29]. In the USA and other North American countries, walnuts are primarily consumed as snacks, in breakfast cereals/pastries, and as walnut milk. In Europe, they are mainly consumed in pastries, holiday desserts, and as an essential ingredient in Mediterranean diets. In Asia, especially in countries like China and India, walnuts are a significant feature in dishes, desserts, and also in Ayurvedic medicines. Lastly, in the Middle East, walnut-based sauces and pastes are prominent [30,31]. Walnuts are economically valuable, with their trade representing about 0.0052% of the total world trade, and they make a significant contribution to the economies of major producer countries, mainly through export revenues and employment opportunities along the walnut value chain [32].

Figure 1.
(A) Walnut fruit showing the green husk and hard shell; (B) walnut fruit showing the edible nut inside the hard shell. Source of images: [33,34].
2.1.2. Almonds
Almonds are one of the most widely consumed tree nuts, and they have been reported to be nutritionally rich and used in diverse ways for culinary applications. As such, they have significant economic importance. Botanically, they belong to the Rosaceae family, the Prunus genus, and the species is known as Prunus amygdalus [35]. The almond is a grey-green, ovoid-oblong drupe with an outer hull that holds a hard shell containing the edible seed/pit (as seen in Figure 2A,B) [36]. Almonds are cultivated and produced in a few regions of the world, with global almond production in 2023 being just below 1.5 million metric tons (kernel basis) [29]. The USA is reported to be the largest producer of almonds, accounting for about 79% of global production. Other top producers are Australia (8%) and Spain (6%) [29]. Aside from being the largest producer of almonds, the USA is also the highest consumer, consuming about 345,048 metric tons in 2023. Other top consumers in 2023 include India (about 138,076 metric tons), Spain (about 132,260 metric tons), China (about 89,462 metric tons), and Italy (about 77,308 metric tons) [29]. Almonds are a significant staple in different regions of the world. In North America, they are consumed primarily as snacks, milk (dairy alternative), and butter. They are also a significant ingredient in protein bars and fiber-rich breakfast cereals. In Europe, almonds are used extensively in baked goods, chocolates, and traditional desserts and sweets (e.g., marzipan). In Asia, almonds are commonly used as dairy alternatives, in Ayurvedic medicine, and in desserts and sweets. In the Middle East, they are an essential ingredient in desserts such as nougat and baklava [37]. In 2022, almonds ranked 1678th in world trade with a value of USD 1.65 billion; the top exporter was the USA, exporting about USD 1.22 billion, and the top importer was India, importing about USD 919 million of almonds [38].

Figure 2.
(A) Almond fruit showing the green husk and hard shell; (B) almond fruit showing the edible nut inside the hard shell, along with a blanched nut. Source of images: [39,40].
2.1.3. Pistachio
Pistachio (Pistacia vera) is a commonly consumed and economically viable nut with a distinctive kernel color and flavor. It belongs to the family Anacardiaceae and the genus Pistacia [41,42]. The pistachio is a drupe with its edible kernel (seed) enclosed in a hard endocarp (as seen in Figure 3B), which is itself enclosed in an outer hull (epicarp) (as seen in Figure 3A) [41]. According to the Statistical Yearbook on Nuts, the highest producers of pistachio in 2023 were the USA (63%), Türkiye (17%), and Iran (16%) [29]. The report also stated that the USA had the highest consumption of pistachios and consumed about 270,336 metric tons in 2021. Other top consumers include Türkiye, China, Italy, and Germany, consuming 232,574 metric tons, 97,287 metric tons, 49,026 metric tons, and 41,803 tons, respectively. Though pistachios are consumed in many regions of the world, their forms vary by region. In North America, they are primarily consumed as either salted or roasted snacks and have increasingly been used as a dairy alternative and in confectioneries. Pistachios are very popular in the Mediterranean cuisine and widely used in desserts such as gelato in Europe. In the Middle East, they are often eaten raw or roasted and are a significant ingredient in traditional sweets such as baklava, while in Asia, they are commonly used for desserts such as kheer [43,44]. In 2022, the global trade of pistachios was about USD 2.38 billion, with the USA accounting for about USD 1.75 billion in exports and China for about USD 315 million in imports [45].

Figure 3.
(A) Pistachio fruit showing the hard endocarp enclosed in the outer epicarp; (B) pistachio nut showing the edible nut inside the hard endocarp. Source of images: [46,47].
2.1.4. Pecan
Though pecans are native to North America, they are also cultivated in other regions and are valued for their characteristic, rich, buttery flavor and nutritional value. Pecans (Carya illinoinensis) belong to the Juglandaceae family and the Carya genus [48,49]. Their fruit is a drupe having a hard brown shell that encloses its edible kernel/seed (as seen in Figure 4A,B) [48,50]. Global production of pecans in 2023 was led by Mexico and closely followed by the USA, with both countries producing 44% and 43% of the total global production, respectively; South Africa comes in third place, supplying about 7% of global production [29]. The USA is reported to be the highest consumer of pecans, with a consumption value of about 90,476 metric tons in 2023. China and Mexico were also top consumers, consuming about 27,181 metric tons and 15,111 metric tons, respectively [29]. Pecans are widely consumed, but the consumption preferences vary by region. In North America, they are mainly consumed as snacks or in baked products such as pie, pralines, and cookies; pecans are also consumed as a dairy alternative (milk and butter) [51,52]. In Asia, especially in China, they are an ingredient in some traditional dishes and snacks [53], while in Europe, they are commonly used in pastries, cakes, and desserts [30].

Figure 4.
(A) Pecan fruit showing the hard brown shell enclosed in the outer epicarp; (B) pecan seed showing the edible nut inside the hard endocarp. Source of images: [54,55].
2.1.5. Peanuts
Peanuts (Arachis hypogaea) are technically legumes but are classified as nuts because of their nutritional similarities to tree nuts. They belong to the Fabaceae family and the Arachis genus [56,57]. Peanut pods develop underground (as seen in Figure 5A), with each pod containing about two to four seeds [56]. Global production in 2023 was close to 50 million metric tons. China was the top producing country, accounting for 38% of the total output globally. India was the second-largest producer, contributing about 13% of the total production, and Nigeria—though the top producer in Africa—ranked third globally, contributing about 9% [29]. Topping the list of consumers is China, consuming about 12,924,297 metric tons in 2023. India follows, consuming 3,535,281 metric tons, and the USA comes in third place, with 1,594,514 metric tons [29]. Peanuts are consumed in different forms globally, mainly raw, roasted, or processed into oil or butter. In the USA, 50% of peanuts are consumed as peanut butter, while the other 50% is consumed in roasted form as snacks, processed into candy/chocolate bars, and used for baking. In Africa, peanuts are the primary ingredients in some traditional soups such as groundnut soup, it is also an essential ingredient in local weaning foods, and they are commonly consumed boiled, roasted or fried as snacks; the oil is also commonly extracted and used for cooking while the residual cake from oil extraction is fried into snacks or used to enrich animal feed; in Europe, it is commonly consumed as peanut butter and peanut-based chocolates while in Asia, it is a staple in curries, stir-fries and some spicy sauces, its oil is also extracted for use in cooking [58,59,60,61]. Due to their versatile uses and high demand for oil and butter, peanuts are economically important. In 2022, global trade in peanuts was valued at about USD 4.13 million, with Argentina as the top exporter (USD 818 million) and China—despite being the largest producer—as the top importer (USD 672 million) [62].

Figure 5.
(A) Peanut pods still attached to the roots of the plant; (B) peanut pods. Source of images: [63,64].
2.1.6. Cashew
Cashews are technically seeds, though they are commonly referred to as nuts, and they are cultivated primarily in tropical regions of the world. Cashews (Anacardium occidentale) belong to the same family as pistachios—the Anacardiaceae family—and the genus Anacardium [65,66,67]. Unlike most seeds, the seed of the cashew is not found inside the fruit but instead grows at the bottom of the fruit and is enclosed in a hard, kidney-shaped shell (as seen in Figure 6A) [65]. In 2023, global production was about 1,170,046 metric tons (kernel basis), and West Africa is responsible for about 55% of that figure, with Côte d’Ivoire being the largest producer in West Africa; India, Cambodia, Eastern Africa, and Vietnam are also notable producers, contributing about 16%, 12%, 7%, and 6% of global production respectively [29]. India is the leading consumer of cashews, consuming about 460,797 metric tons in 2023. Other top consumers include the USA, Germany, China, and the UK, consuming 141,219 metric tons, 51,583 metric tons, 47,013 metric tons, and 27,260 metric tons, respectively, in 2023 [29]. Cashews are consumed in diverse ways globally. In Asia, they are used in curries, stir-fries, sauces, sweets, and snacks. In North America, they are primarily consumed as snacks or in the form of butter and are also included in chocolate and energy bars. In South America, cashews are utilized in beverages and jams such as “caju juice” and other culinary dishes. In Europe, they are commonly consumed in their roasted form as snacks, included in chocolate bars, and, occasionally, used in baked goods. In Africa, cashews are featured in local dishes, desserts such as “bolo polana”, and are also eaten roasted as snacks [68,69]. Due to their versatile usage, cashews are regarded as an economically viable nut. In 2022, the global trade value was about USD 8.29 billion, with Vietnam being both the top exporter and top importer of cashews, exporting about USD 2.8 billion and importing about USD 2.11 billion worth of cashew nuts [70].

Figure 6.
(A) Cashew fruit showing the hard kidney-shaped shell that encloses the nut; (B) processed cashew nut. Source of images: [71,72].
2.1.7. Kola Nut
Kola nut is an economically and culturally important tropical tree species that is most popular for its caffeine-rich seeds. It is native to the western part of Africa, belongs to the Malvaceae family and the genus Cola, and is known botanically as Cola nitida (Vent.) Schott and Endl. [73,74]. The seeds (nuts) are usually pink, white, or red (as seen in Figure 7B), and their high caffeine and theobromine content imparts a bitter taste to them [75,76,77]. Nigeria is the largest producer of kola nut, producing about 50% (188,702 metric tons) of the global production (328,074 metric tons) [78]. Kola nut is mainly consumed traditionally, either in its fresh or dried form, and is used in rites such as wedding ceremonies, religious rites, herbal medicine, and even as a hospitality gesture [75]. Industrially, kola nut is used as a source of caffeine, and, historically, it was the major source of flavor for cola drinks such as Pepsi and Coca-Cola; in the pharmaceutical industry, it is a source of stimulants and antioxidants [75]. The global trade value of kola nuts in 2023 was USD 18.7 million, with Nigeria being the top exporter, exporting about USD 10.7 million worth of kola nuts, and Bahrain being the top importer, importing about USD 10.3 million worth of kola nuts [79].

Figure 7.
(A) Kola nut pods on a tree; (B) edible kola nuts. Source of images: [80,81].
2.1.8. Bitter Kola
The bitter kola is a plant native to West and Central Africa, and it is highly valued for its medicinal, economic, and cultural uses. The plant belongs to the Clusiaceae family and the Garcinia genus, and its species name is Garcinia kola Heckel [82]. Technically, the bitter kola is a seed enclosed in a round, reddish-orange fruit that looks like a small mango (as seen in Figure 8A), and each fruit contains about 1–4 seeds [83]. Annual production and trade estimates of this nut are very limited because its trade is primarily informal, and the information publicly available details production values for specific communities and not on a global scale. For example, according to Ingram and Schure [84], in 2010, the total production and trade value of bitter kola in Cameroon was about USD 249,938, and this value was reported to have increased to USD 660,000 in 2016 [85]. International trade is predominantly among neighboring countries, so most seeds found in markets may originate from a neighboring country, even though the market is in a country that also produces bitter kola [86,87].

Figure 8.
(A) Bitter kola pods on a tree; (B) bitter kola seeds (nut). Source of images: [88,89].

Table 1.
Main Components of the selected nuts.
Table 1.
Main Components of the selected nuts.
| Nut Type | Main Fatty Acids | Key Volatile Compounds | Notable Nutrients | References |
|---|---|---|---|---|
| Almond | Oleic, linoleic, palmitic | Hexanal, benzaldehyde, 2-pentylfuran | Vitamin E, magnesium, fiber, protein | [24,90,91,92] |
| Cashew | Oleic, linoleic, palmitic | Hexanal, 2-heptanone, limonene | Copper, iron, magnesium, protein | [24,92,93,94] |
| Peanut | Oleic, linoleic, palmitic | Hexanal, pyrazines, alcohols | Folate, niacin, protein, magnesium | [24,60,92,95,96] |
| Pistachio | Oleic, linoleic, palmitic | Linalool, hexanal, α-pinene | Vitamin B6, potassium, antioxidants | [24,92,97,98,99,100] |
| Walnut | Linoleic, linolenic, Oleic | Hexanal, methanol, juglone | Omega-3, polyphenols, magnesium | [24,91,92,101,102] |
| Bitter Kola | Linoleic, palmitic, stearic | Camphene, limonene, borneol | Garcinianin, flavonoids, alkaloids, antioxidants | [103,104,105,106,107,108] |
| Kolanut | Oleic, linoleic, stearic | Theobromine, caffeine, limonene | Caffeine, theobromine, tannins | [103,109,110,111] |
| Pecan | Oleic, linoleic, palmitic | Hexanal, hexanol, heptanol, octanal | Manganese, magnesium phosporus, zinc, thiamine | [24,92,112,113] |
3. Fatty Acid and Volatile Compounds Composition of Selected Nuts
3.1. Fatty Acids
Fatty acids have been defined as long, linear carbon chains that form the building blocks of lipids; they characteristically have a methyl group at one end and a carboxyl group at the other end [114,115]. Fatty acids can either be unsaturated or saturated, and unsaturated fatty acids are further classified into two forms: monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) [114]. The most common MUFA found in nuts is Oleic acid (C18:1), and studies have linked it to cardiovascular-protective properties [116,117]. The composition and stability of fatty acids in nuts can be significantly altered by processing methods. Examples of these effects include high-temperature roasting which has been reported to accelerate oxidation particularly of PUFAs [118]. This oxidation in turn leads to a marked increase in lipid oxidation markers such as thiobarbituric acid reactive substances (TBARS) [119]. Another processing method that can affect the fatty acid composition of nuts is frying as the nuts may absorb the frying oil and thus its fatty acid ratio may be altered depending on the type of oil used for frying as oils rich in saturated fatty acids (SFAs) can reduce the ratio of the beneficial MUFAs and PUFAs in the nut and a better alternative could be vacuum frying [120]. Also, the isomerization of PUFAs can occur in the presence of high heat leading to the formation of trans fatty acids that have been linked with cardiovascular diseases [121].
As shown in Table 2, the most abundant fatty acids in nuts are the unsaturated fatty acids oleic acid (C18:1), linoleic acid (C18:2), and the saturated fatty acid called palmitic acid (C16:0). Other common fatty acids include stearic acid (C18:0), linolenic acid (C18:3), and palmitoleic acid (16:1).

Table 2.
Fatty acid composition of the selected nuts.
3.2. Volatile Compounds
Volatile compounds determine the flavor, aroma, and consumer appeal of nuts. Volatiles are formed during the oxidation of fatty acids because of enzymatic activities or Maillard reactions [162,163,164]. They impact the sensory properties of nuts and are directly related to their appeal for consumption and as processed food/snacks. The major volatiles in nuts include aldehydes (which are derived from the oxidation of lipids and contribute to the fresh, grassy, green, nutty aroma of nuts such as walnuts and almonds), ketones (which are derived from Maillard reactions and the degradation of fatty acids, they provide the buttery, sweet aroma of nuts such as pecans and roasted peanuts), pyrazines (which are products of the Maillard reactions that occur during roasting and are responsible for the roasted-nutty aromas found in roasted almonds and peanuts), esters (which are products of enzymatic reactions and fermentation and contribute to the floral, fruity aroma of nuts such as cashews and pecans), and furans (which are formed from Maillard reactions that happen during thermal processing and are responsible for the toasty caramelized aromas of roasted cashews and walnuts) [102,162,163,165,166]. The formation of volatile compounds is highly dependent on the fatty acid profile of the nut, the processing methods the nut undergoes, and the storage conditions of the nut; therefore, knowledge of these compounds helps improve the storage approach, processing methods, and overall sensory appeal of these nuts. Table 3 below details some of the key volatile compounds identified in our selected nuts from past literature.

Table 3.
Volatile compounds identified in the selected nuts.
4. Analytical Techniques for Studying Fatty Acids and Volatiles
Analytical techniques for studying fatty acids and volatile compounds in nuts often rely on gas chromatography (GC) coupled with mass spectrometry (GC-MS) or flame ionization detection (GC-FID). For instance, protocols for preparing fatty acid methyl esters (FAMEs) have been modified to optimize separation and quantification using GC-FID. These advancements demonstrate the importance of refining methods to achieve comparable results with conventional gravimetric techniques. Such modifications highlight the necessity of standardization to maintain consistency in analytical outcomes [179].
In addition to fatty acid analysis, volatile compound profiling benefits from standardized methodologies. Solid-phase microextraction (SPME) combined with GC-MS is frequently employed to determine volatiles in nuts. This technique offers high sensitivity and efficiency but requires careful calibration to account for matrix effects that may interfere with extraction processes. The use of external calibration or standard addition methods can mitigate these issues, further underscoring the need for uniform practices in analytical procedures [24]. The variability observed in volatile profiles due to agronomic conditions or microbial presence further illustrates the importance of standardized approaches. Without consistent protocols, it becomes challenging to discern whether differences arise from genuine biological variations or methodological inconsistencies. Table 4 below details some techniques that are used for the extraction of fatty acids and volatiles in nuts while Table 5 details techniques that are used for the identification and quantification of the extracted fatty acids and volatiles. An overview of a typical fatty acid and volatile compound analysis workflow is shown in Figure 9.
4.1. Techniques for Fatty Acid Analysis
4.1.1. Gas Chromatography–Mass Spectrometry (GC-MS)
Gas chromatography (GC) is a widely utilized analytical technique for the separation, identification, and quantification of fatty acids and volatile compounds in nuts. Its application is particularly valuable due to its high sensitivity, resolution, and ability to provide detailed structural information about analytes. The method operates by vaporizing the sample and transporting it through a chromatographic column using an inert carrier gas, such as helium or nitrogen. The separation of compounds occurs based on their interaction with the stationary phase of the column and their volatility. The use of GC for fatty acid analysis often involves derivatization steps to enhance volatility and thermal stability. For instance, lipids are typically extracted from nut samples, hydrolyzed to release free fatty acids, and then converted into their methyl ester derivatives (FAMEs) before analysis. This process ensures that the fatty acids can be efficiently separated and detected by GC systems [180,181]. The authors emphasize that this derivatization step is critical for achieving accurate quantitative and structural analysis of lipids. GC coupled with mass spectrometry (GC-MS) is frequently employed for its ability to provide both qualitative and quantitative data. This combination enhances the detection capabilities by allowing not only the separation of compounds but also their identification based on mass spectral data. According to [24], GC-MS is particularly effective for analyzing volatile compounds due to its capacity to handle low concentrations through splitless injection or programmed temperature vaporization (PTV). These methods increase sample capacity when dealing with trace levels of volatiles.
The flame ionization detector (FID) is another common detection system used in GC. It provides excellent sensitivity for routine analyses where specific analytes are targeted using standards. While FID lacks structural elucidation capabilities, it remains a robust choice for quantifying known compounds due to its simplicity and reliability [182]. In contrast, GC-MS offers superior versatility by combining high-resolution separation with detailed molecular characterization [181].
Operational parameters play a significant role in optimizing GC performance. For example, helium is commonly used as a carrier gas at flow rates around 1 mL/min, while injector temperatures are typically set at 250 °C to ensure efficient volatilization of analytes. Oven temperature programs are designed to gradually increase from lower initial temperatures (e.g., 50 °C) to higher final temperatures (e.g., 280 °C), facilitating the separation of compounds with varying volatilities [183]. Such precise control over temperature gradients ensures reproducibility and accuracy in compound identification. The identification of chemical constituents analyzed via GC often relies on matching retention times with authentic standards or comparing spectra against established libraries such as the National Institute of Standards and Technology (NIST) database. This approach enhances confidence in compound identification while minimizing errors associated with manual interpretation [184].
GC also finds applications in monitoring lipid degradation products such as free fatty acids, which serve as indicators of rancidity in nuts. The hydrolysis of triglycerides during storage or processing leads to the formation of free fatty acids, which can be quantified using GC techniques [180]. This capability underscores the importance of GC not only in compositional analysis but also in assessing food quality.
Mass spectrometry (MS) is a highly versatile and widely utilized analytical technique for the characterization of fatty acids in nuts. Its application spans from identifying molecular structures to quantifying lipid components, making it indispensable in nutritional studies [24]. MS operates by ionizing chemical compounds to generate charged particles, which are then separated based on their mass-to-charge ratio (m/z). This separation provides detailed insights into the molecular composition of complex mixtures, such as those found in nut oils and extracts. Gas chromatography coupled with mass spectrometry (GC-MS) is one of the most established methods for lipid analysis. GC-MS combines the separation capabilities of gas chromatography with the structural elucidation power of MS. The process typically involves lipid extraction, hydrolysis, and derivatization to enhance volatility and facilitate chromatographic separation. This approach has proven effective for both quantitative and structural analysis of lipids, including fatty acid methyl esters (FAMEs), which are commonly analyzed forms of fatty acids. The authors indicate that GC-MS is routinely employed due to its ability to provide precise data on lipid profiles.
Another advanced MS technique is the ion mobility spectrometry-mass spectrometry (IMS-MS), which separates lipid ions based on their size, shape, and charge in the gas phase. IMS-MS offers additional structural information through collision cross-section measurements, enabling a deeper understanding of lipid conformations and interactions. This method is particularly useful for studying complex lipid mixtures where traditional techniques may fall short.
4.1.2. High-Performance Liquid Chromatography
High-performance liquid chromatography coupled with electrospray ionization mass spectrometry (HPLC-ESI-MS) has emerged as a popular method for analyzing total lipid extracts or specific lipid fractions. HPLC efficiently separates polar lipids and triglycerides, while ESI-MS facilitates molecular characterization by generating intact ions under soft ionization conditions. This combination allows researchers to study esterified, glycosylated sterols and other nutritionally relevant compounds in nuts. Jardim et al. outline that HPLC-ESI-MS is increasingly favored for its ability to handle diverse lipid classes.
Solid-phase extraction (SPE) techniques integrated with MS have also been explored for separating lipids based on molecular weight or polarity. For instance, hybrid procedures using SPE with aminopropyl-bonded silica gel cartridges have demonstrated high recovery rates for various lipid species, although challenges remain in recovering short-chain fatty acids [181]. These methods highlight the importance of optimizing sample preparation protocols to ensure accurate MS analysis. The choice of MS technique often depends on the specific analytical goals and the nature of the sample matrix. For volatile compounds associated with nut flavor profiles, simultaneous distillation-extraction (SDE) followed by MS analysis can be employed to isolate representative samples while preserving their chemical integrity [185]. Such approaches underscore the critical role of sample preparation in achieving reliable results.
Mass spectrometry’s adaptability extends beyond fatty acid analysis; it is also instrumental in studying volatile organic compounds that contribute to nut aroma and taste. Techniques like tandem mass spectrometry (MS/MS) enable detailed fragmentation studies, providing insights into compound structures that influence sensory properties [186]. Furthermore, semi-quantitative methods based on peak intensity ratios have been applied to assess component concentrations within complex mixtures [187].
4.2. Techniques for Volatile Compound Analysis
4.2.1. Headspace Analysis
Headspace analysis is a widely utilized technique for the study of volatile compounds in various matrices, including nuts. This method is particularly advantageous due to its ability to isolate and concentrate volatile components without requiring extensive sample preparation or the use of solvents. The principle of headspace analysis involves sampling the gaseous phase above a liquid or solid sample, where volatile compounds partition between the sample matrix and the headspace. This partitioning is governed by factors such as temperature, pressure, and the physicochemical properties of the analytes. Techniques like static headspace (SHS) and dynamic headspace (DHS) are commonly employed, with each offering distinct advantages depending on the analytical requirements. Solid-phase microextraction (SPME) is an advanced variation of headspace analysis that has gained significant attention in food analysis due to its solvent-free nature and high sensitivity. SPME employs a coated fiber to absorb volatile compounds directly from the headspace, which are then thermally desorbed into a gas chromatograph for separation and identification. This approach minimizes sample handling and prevents artifact formation, ensuring an accurate representation of the volatile profile [188,189]. Derivatization can also be integrated into SPME to enhance the volatility or reduce the polarity of certain analytes, thereby improving extraction efficiency and detection limits [188]. In nut analysis, headspace techniques are particularly valuable for characterizing aroma-active compounds that contribute to sensory attributes such as flavor and freshness. Volatile compounds in nuts often originate from oxidative pathways involving polyunsaturated fatty acids like linoleic acid. These pathways generate aldehydes, alcohols, ketones, and other volatiles that define nut aromas. For instance, lipoxygenase activity in vegetable oils leads to oxidative degradation products that can be effectively captured using headspace methods [123]. Additionally, highly volatile sulfur-containing compounds have been identified as key contributors to specific aromas in food matrices through similar approaches.
Gas chromatography (GC) coupled with mass spectrometry (MS) is frequently used in conjunction with headspace sampling for the detailed characterization of volatile profiles. The combination of GC-MS allows for both qualitative and quantitative analysis by separating complex mixtures and identifying individual components based on their mass spectra. Techniques such as programmed temperature vaporization (PTV) can be employed when higher sample capacities are required due to low concentrations of volatiles in certain samples [24]. This adaptability makes GC-MS an indispensable tool for studying nut volatiles. Despite its numerous advantages, one limitation of headspace analysis lies in its inability to directly correlate the concentration of volatiles in the gaseous phase with their actual levels in the sample matrix. This discrepancy arises from differences in volatility among compounds, which can lead to variations in their partitioning behavior [189]. However, this challenge can be mitigated by optimizing experimental conditions such as temperature equilibration times or employing calibration strategies.
4.2.2. Solid-Phase Microextraction (SPME)
Solid-phase microextraction (SPME) is a widely utilized technique for extracting and analyzing volatile compounds, particularly in food matrices such as nuts. This method has gained prominence due to its solvent-free operation, simplicity, and efficiency in isolating analytes from complex samples [170,188]. SPME integrates multiple steps—sampling, extraction, concentration, and sample introduction—into a single streamlined process, which significantly reduces the time and resources required for sample preparation compared to traditional methods like liquid-liquid extraction (LLE) or solid-phase extraction (SPE) [182,188]. The technique involves using a fiber coated with an adsorbent material that selectively binds volatile compounds from the sample matrix. The fiber is exposed to the sample directly or within its headspace, allowing analytes to partition between the sample and the coating on the fiber. Once equilibrium is reached, the fiber is retracted into a protective needle and introduced into an analytical instrument such as gas chromatography–mass spectrometry (GC-MS) for desorption and subsequent analysis [170,189]. This approach eliminates the need for solvents, making it environmentally friendly while minimizing potential interferences caused by solvent residues. The versatility of SPME extends beyond its application in food science; it has been adapted for various matrices and analytical challenges. Its compatibility with cryogenic headspace sampling further enhances its utility in studying thermally sensitive compounds that might degrade under conventional extraction conditions [189].
Additionally, advancements in fiber coatings have expanded their applicability by improving selectivity and durability during repeated use. Despite its advantages, SPME does have limitations. The equilibrium-based nature of the technique means that extraction efficiency can be influenced by factors such as temperature, agitation speed, and matrix composition. These variables must be carefully optimized to ensure reproducibility and accuracy in results [182]. SPME continues to evolve with ongoing research focused on improving fiber materials and expanding its applications across diverse fields. Its role in analyzing fatty acids and volatiles in nuts highlights its importance as an analytical tool and to deepen our understanding of food composition and health-related attributes associated with these bioactive compounds [92,123].
4.2.3. GC-MS for Volatile Profiling
Gas chromatography coupled with mass spectrometry (GC-MS) is a widely utilized analytical technique for the profiling of volatile compounds in nuts and other food matrices. This method combines the separation capabilities of gas chromatography with the identification and quantification strengths of mass spectrometry, making it particularly effective for analyzing complex mixtures of volatiles. The process begins with the extraction of volatile components, which are then introduced into the GC system, where they are separated based on their boiling points and polarity. Subsequently, these separated compounds are ionized and detected by the MS system, allowing for precise structural elucidation and quantification. [181,182].
The application of GC-MS in volatile profiling has been demonstrated across various studies. The dynamic headspace analysis combined with GC-MS was employed to investigate volatiles in different species of Juglans nuts, revealing compositional variations that could serve as taxonomic markers. This approach highlighted significant differences in sesquiterpene hydrocarbons and monoterpenes among species such as J. cineara, J. regia, and C. laciniosa [190]. Furthermore, retention indices calculated using homologous n-alkane hydrocarbons under identical GC-MS conditions have enhanced the reliability of compound identification within complex matrices [191]. The sensitivity of GC-MS allows for detecting key odorants that influence consumer perception. Volatile compounds with odor activity values (OAVs) greater than one are considered detectable by humans and play a crucial role in flavor profiles. In one study focusing on peanuts, 26 volatile compounds were quantified using GC-MS, but only 11 exhibited OAVs above this threshold, underscoring their significance as key contributors to aroma [24]. Similarly, volatile flavor compounds formed during lipid autoxidation processes have been characterized using GC-MS techniques. For example, hexanal—a compound associated with green notes—was identified as a major product during autoxidation reactions involving linoleic acid (LA) and methyl linoleate (ML), demonstrating the utility of GC-MS in tracking oxidative changes in food systems [192]. Sample preparation is an essential step in GC-MS analysis to ensure accurate results. Techniques such as esterification are commonly employed to convert non-volatile fatty acids into more volatile derivatives suitable for GC analysis. Transesterification methods detach fatty acids from triglycerides and transform them into fatty acid methyl esters (FAMEs), which exhibit lower boiling points and reduced polarity compared to their esterified counterparts [182]. The integration of GC-MS into studies on nut composition has provided valuable insights into both nutritional attributes and sensory properties.
4.3. Emerging Analytical Tools
Emerging analytical tools have significantly enhanced the study of fatty acids and volatile compounds in nuts, providing deeper insights into their nutritional composition and health implications. Among these advancements, lipidomics has emerged as a transformative field, offering robust methodologies for tracking lipids in food matrices and raw materials. This approach leverages sensitive analytical platforms to ensure the authenticity and quality of products, which is critical for both research and commercial applications [186]. A literature review reveals that lipidomics not only investigates lipids’ structural and functional dynamics but also introduces innovative solutions to address challenges in food science. Shotgun lipidomics represents one such advancement, enabling precise identification and quantification of triacylglycerides (TAGs), which constitute over 95% of milk fats. This technique segments the spectrum into distinct mass ranges to achieve accurate quantitation, demonstrating its utility in quality control processes for dairy products. While its application in milk analysis is notable, similar methodologies can be adapted for studying nut oils, given their complex lipid profiles. The integration of complementary tools such as gas chromatography (GC), mass spectrometry (MS), nuclear magnetic resonance (NMR), infrared spectroscopy (IR), and ultraviolet spectroscopy (UV) further expands the scope of lipid analysis. Each platform offers unique advantages; for instance, GC excels in separating volatile compounds, while MS provides detailed molecular characterization. Chemometric models and lipid-targeted online databases enhance data interpretation by facilitating pattern recognition and comparative analyses [181]. These tools collectively contribute to a comprehensive understanding of dietary fats.
Volatilomics complements lipidomics by focusing on the volatile components that influence flavor profiles. In walnut oil, aldehydes are identified as major contributors to aromatic volatiles, accounting for approximately 35% of the total volatile content [123]. Advanced techniques such as headspace solid-phase microextraction (HS-SPME) coupled with GC-MS have proven effective in classifying these compounds. For example, studies on peanut oil reveal that roasting temperatures impact aldehyde volatility due to evaporation during heating [170]. Such findings underscore the importance of precise analytical methods in capturing dynamic changes in volatile profiles. Despite these advancements, challenges remain in detecting new volatile flavors due to their increasingly subtle presence.
4.4. Role of Artificial Intelligence in Lipidomics
Artificial intelligence (AI) has emerged as a transformative tool in lipidomics, offering innovative solutions to address the complexities associated with the analysis of lipids and their roles in biological systems. One of the primary applications of AI in lipidomics is in data processing and interpretation. The large volume of data produced by methods like direct infusion MS also referred to as shotgun lipidomics, necessitates robust computational tools for accurate identification and quantification of lipid species. AI algorithms, particularly those based on machine learning, have been employed to enhance the precision of lipid identification by recognizing patterns in spectral data that may be challenging for traditional methods to discern. This capability is especially critical given the structural complexity of lipids, which includes variations in chain length, degree of unsaturation, and functional group modifications.
Furthermore, AI-driven platforms have facilitated the development of comprehensive Lipidomic databases that serve as repositories for experimental data. These databases not only aid researchers in identifying unknown lipid species but also enable cross-comparison between studies, thereby standardizing Lipidomic analyses across different laboratories. For instance, online resources tailored for processing direct infusion MS datasets have streamlined workflows by automating peak alignment and noise reduction tasks. Such advancements reduce manual intervention and improve reproducibility in Lipidomic studies. Another significant contribution of AI lies in its ability to predict the functional implications of lipids based on their compositional profiles. By integrating Lipidomic data with other omics datasets, such as transcriptomics or proteomics, AI models can uncover correlations between specific lipids and physiological or pathological states. This integrative approach has profound implications for understanding how dietary fats influence health outcomes or contribute to disease mechanisms. For example, predictive models can identify biomarkers associated with metabolic disorders or cardiovascular diseases by analyzing patterns within complex datasets.
AI has also enhanced the efficiency of experimental design in lipidomics. Techniques such as supervised learning allow researchers to prioritize specific analytes or pathways for investigation based on prior knowledge or preliminary results. This targeted approach minimizes resource expenditure while maximizing the likelihood of obtaining biologically meaningful insights. Unsupervised learning methods have also been utilized to classify samples into distinct groups based on their lipid profiles without requiring predefined labels. Such clustering techniques are invaluable for exploratory studies where underlying patterns are not yet known. The versatility of AI extends beyond data analysis to include hardware optimization for analytical instruments. Machine learning algorithms have been applied to optimize parameters in high-performance liquid chromatography (HPLC) and gas chromatography (GC) systems coupled with MS detectors. By fine-tuning variables such as flow rates or ionization conditions, these algorithms enhance sensitivity and resolution during lipid extraction and detection processes [188]. This level of optimization ensures that even trace amounts of lipids can be accurately quantified from complex matrices like food samples or biological tissues.
Despite these advancements, challenges remain in fully harnessing AI’s potential within lipidomics. One limitation is the dependency on high-quality training datasets for machine learning models. Incomplete or biased datasets can lead to inaccurate predictions or misclassifications. Efforts are underway to curate more comprehensive datasets that encompass a wider range of lipid species and experimental conditions. Additionally, there is a need for greater transparency in AI algorithms to ensure that their decision-making processes are interpretable by researchers.

Table 4.
Extraction techniques for fatty acids and volatiles in nuts.
Table 4.
Extraction techniques for fatty acids and volatiles in nuts.
| Extraction Technique | Principle | Application | References |
|---|---|---|---|
| Soxhlet extraction | Continuous solvent reflux to extract lipids. | Used for total fat extraction before FA analysis. | [193,194,195] |
| Folch/Bligh and Dyer’s method | A biphasic solvent system is used to extract lipids. The nonpolar solvent extracts the lipids, while the polar solvent breaks the bonds between the lipids and water-soluble components, thus allowing more lipids to be extracted. | Used to extract both polar and nonpolar lipids. | [195] |
| Supercritical fluid extraction (SFE) | Uses supercritical CO2 for solvent-free lipid extraction. The supercritical CO2 dissolves and separates the lipids from the sample based on the unique properties of each targeted compound, thus fractionating the compounds as they are extracted. | Used to obtain high-purity lipid extraction without solvent contamination. | [147,195,196,197,198,199,200,201] |
| Solid-phase extraction (SPE) | A solid phase (a sorbent) is used to selectively extract the analyte of interest, such as lipids or volatiles, before eluting them from the sorbent using a suitable solvent. | Used to effectively extract and purify FAs before further profiling. | [202] |
| Headspace solid-phase microextraction (HS-SPME) | Volatile compounds are adsorbed by a coated fiber in the headspace of the sample. | Used to extract volatiles for further profiling. | [24,102,203] |
| Solvent-assisted flavor evaporation (SAFE) | High vacuum is used to extract volatiles while preventing thermal degradation. | Used to extract heat-sensitive volatiles. | [204,205] |
| Steam distillation and solvent extraction (SDE) | Volatiles are distilled with steam and extracted with an organic solvent. | Used to extract volatiles for further profiling. | [132,206,207] |
| Stir bar sorptive extraction (SBSE) | Lipids or volatiles are extracted based on the sorptive extraction principle using a coated stir bar. The stir bar is coated with polymers such as polydimethylsiloxane (PDMS). | Used to extract lipids or volatiles for further profiling. | [208,209] |
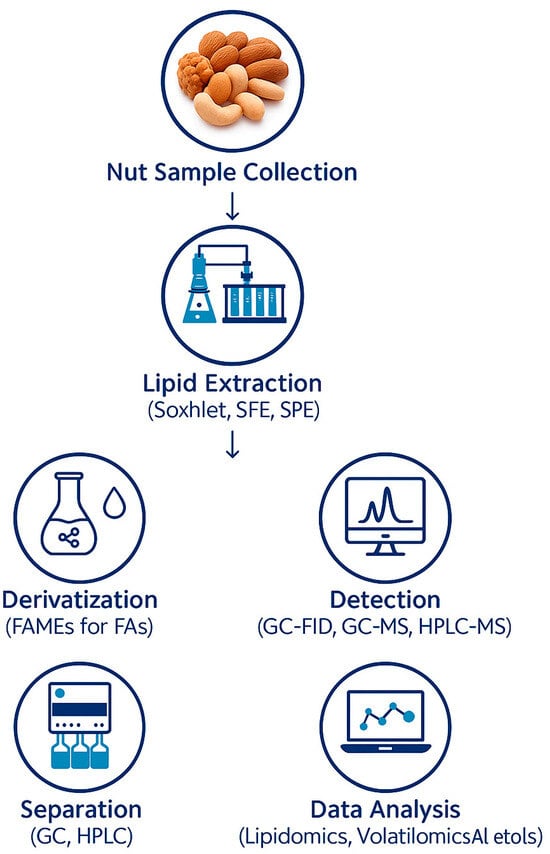
Figure 9.
Overview of fatty acid and volatile compound analysis workflow.

Table 5.
Identification and quantification techniques for the analysis of fatty acids and volatiles in nuts.
Table 5.
Identification and quantification techniques for the analysis of fatty acids and volatiles in nuts.
| Identification and Quantification Technique | Principle | Application | References |
|---|---|---|---|
| Gas chromatography–flame ionization detection (GC-FID) | The lipids are separated based on their boiling points using gas chromatography; then, they are identified and quantified by passing them through a hydrogen flame. The compounds ionize in this flame, and they produce a current based on their concentration. | Used to quantify total and individual fatty acids in nuts. | [20,97,123,147,151,210] |
| Gas chromatography–mass spectrometry (GC-MS) | The volatiles/lipids are separated based on their boiling points using gas chromatography; then, they are identified and quantified using mass spectrometry-based on the mass-to-charge ratio of the compounds. | Used for detailed fatty acid profiling and as the primary method for nut aroma and volatile analysis. | [25,96,100,102,142,145,146,150,157,191,211] |
| High-performance liquid chromatography (HPLC) | Used for liquid samples. The compounds are separated based on their affinities for the column’s stationary phase as they are carried through it using a mobile phase; they are then identified and quantified using a mass spectrometer. | Used to analyze fatty acids without derivatization. | [212,213,214] |
| Gas chromatography–olfactometry (GC-O) | Volatiles are separated using a gas chromatograph and passed through an olfactometric port where trained humans can sniff and identify the odor. | Used for aroma analysis and profiling | [215,216] |
| Proton transfer reaction mass spectrometry (PTR-MS) | Volatiles are detected through the ionization of the compounds using proton transfer from hydronium ions. The ionized compounds are then identified and quantified using a mass spectrometer. | Used to identify and quantify the volatile compounds responsible for the aroma of nuts. | [217,218] |
5. Health Effects
Nuts have been reported to have a high lipid content and are a great source of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). Several decades of experimental, clinical, and epidemiological studies have alluded to the fact that MUFAs, PUFAs, and other components of nuts are positively implicated in lipid metabolism, weight management, improved cardiovascular function, reduced risk of type 2 diabetes, improved brain function, and other metabolic regulations [219,220,221,222]. Vitamins, terpenes, sterols, phenolic compounds, and fiber in nuts have been indicated to possess antioxidant, anti-aging, and anti-inflammatory properties and also positively influence immunological responses, while aldehydes and ketones derived during the roasting process have been reported to be involved in antioxidant defense mechanisms [223,224,225].
Most epidemiological research on the health benefits of nut consumption has focused on cardiovascular diseases (CVD), and most have reported that nut consumption is inversely linked to the risk of CVDs and all-cause mortality in the study populations [6,10,12,14,15,18,226,227,228]. The fact that nuts are known to be energy-dense has created concern about their long-term consumption and their possible effect on weight management; however, there has been no proven direct link between weight gain and nut consumption; rather, studies have demonstrated that nuts have a slightly beneficial effect on adiposity rather than a negative effect [4,229,230,231,232]. The review on the benefits of tree nut consumption on aging and age-related diseases by [233] details the mechanism of action of biologically active compounds found in nuts in relation to aging and age-related diseases.
Table 6 below details the effects of nut consumption on some biological markers of diseases and health conditions as reported by different authors.

Table 6.
Health effects associated with nut consumption.
6. Conclusions
This review critically examined the fatty acid and volatile compound profiles of commonly consumed nuts, along with their analytical methods and health implications. The composition of nuts is influenced not only by genetic factors but also by environmental conditions and postharvest handling, underscoring the importance of standardization in future research. With increasing demand for nuts and nut oils in food, pharmaceutical, and cosmetic industries, it is necessary to improve quality control methods in the postharvest handling of nuts. Volatile profiling and fatty acid analysis, as discussed, serve as powerful tools not only for nutritional assessment but also for verification and adulteration detection. Moving forward, future research should prioritize standardizing analytical protocols, expanding databases of nut volatiles and fatty acids, and exploring the impact of climate change and processing technologies on nut quality. Furthermore, integrating advanced techniques such as metabolomics and machine learning can enhance detection sensitivity and interpretation. Overall, this review provides a foundational reference for researchers and industry stakeholders aiming to improve nut quality, ensure food safety, and deepen scientific understanding of these nutrient-rich commodities.
Author Contributions
Conceptualization, G.A.; methodology, G.A. and O.A.; data curation, O.A.; writing—G.A., O.A., and K.U.; visualization, G.A., O.A., and K.U.; supervision, K.U. All authors have read and agreed to the published version of the manuscript.
Funding
Internal Grant Agency of the Faculty of Tropical AgriSciences, Czech University of Life Sciences Prague, grant number 20243102.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- George, E.S.; Daly, R.M.; Tey, S.L.; Brown, R.; Wong, T.H.T.; Tan, S.Y. Perspective: Is It Time to Expand Research on “Nuts” to Include “Seeds”? Justifications and Key Considerations. Adv. Nutr. 2022, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Curiel-Maciel, N.F.; Arreola-Ávila, J.G.; Esparza-Rivera, J.R.; Luna-Zapién, E.A.; Minjares-Fuentes, J.R.; Sierra-Campos, E.; Meza-Velázquez, J.A. Nutritional Quality, Fatty Acids Content and Antioxidant Capacity of Pecan Nut Fruits from Criolla and Improved Walnut Varieties. Not. Bot. Horti Agrobot. 2021, 49, 12021. [Google Scholar] [CrossRef]
- Bailey, H.M.; Stein, H.H. Raw and Roasted Pistachio Nuts (Pistacia vera L.) Are “good” Sources of Protein Based on Their Digestible Indispensable Amino Acid Score as Determined in Pigs. J. Sci. Food Agric. 2020, 100, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Luna, L. Nuts and Body Weight—An Overview. J. Nutr. Health Sci. 2016, 3, 1. [Google Scholar] [CrossRef][Green Version]
- USDA Food Search | USDA FoodData Central. Available online: https://fdc.nal.usda.gov/food-search?type=Foundation&query=nuts&SFFoodCategory=Nut%20and%20Seed%20Products (accessed on 4 May 2025).
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, 2136. [Google Scholar] [CrossRef]
- Hayes, D.G. Oils and Their Use Beyond the Food Industry. In Oil and Oilseed Processing: Opportunities and Challenges; Wiley: Hoboken, NJ, USA, 2021; pp. 119–148. [Google Scholar] [CrossRef]
- GVR. Nuts Market Size, Share & Trends Analysis Report By Product (Almonds, Peanuts, Cashew, Walnuts, Hazelnuts, Pistachios, And Others), By Distribution Channel (Offline, Online), By Region, And Segment Forecasts, 2023–2030; Grand View Research, Inc.: San Francisco, CA, USA, 2022. [Google Scholar]
- INC. Nuts and Dried Fruits Statistical Yearbook 2022/2023; International Nuts and Dried Fruits Counci: Reus, Spain, 2023. [Google Scholar]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut Consumption on All-Cause, Cardiovascular, and Cancer Mortality Risk: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef]
- Chen, G.C.; Zhang, R.; Martínez-González, M.A.; Zhang, Z.L.; Bonaccio, M.; Van Dam, R.M.; Qin, L.Q. Nut Consumption in Relation to All-Cause and Cause-Specific Mortality: A Meta-Analysis 18 Prospective Studies. Food Funct. 2017, 8, 3893–3905. [Google Scholar] [CrossRef]
- De Souza, R.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Ahmed, S.H.; Alhabib, K.F.; Altuntas, Y.; Basiak-Rasała, A.; Dagenais, G.R.; Diaz, R.; et al. Association of Nut Intake with Risk Factors, Cardiovascular Disease, and Mortality in 16 Countries from 5 Continents: Analysis from the Prospective Urban and Rural Epidemiology (PURE) Study. Am. J. Clin. Nutr. 2020, 112, 208–219. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P.M. Nuts and Cardio-Metabolic Disease: A Review of Meta-Analyses. Nutrients 2018, 10, 1935. [Google Scholar] [CrossRef]
- Coates, A.; Hill, A.; Tan, S. Nuts and Cardiovascular Disease Prevention. Curr. Atheroscler. Rep. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, M.J.Z.; Aghasadeghi, K.; Hakimi, H.; Yarmohammadi, H.; Nikaein, F. The Effect of Walnut Oil Consumption on Blood Sugar in Patients with Diabetes Mellitus Type 2. Int. J. Endocrinol. Metab. 2016, 14, e34889. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Hernández-Alonso, P.; Jenkins, D.J.A.; Kendall, C.W.C.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut Consumption and Type 2 Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Am. J. Clin. Nutr. 2021, 113, 960–971. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut Consumption and Risk of Type 2 Diabetes, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef]
- Ros, E.; Mataix, J. Fatty Acid Composition of Nuts–Implications for Cardiovascular Health. Br. J. Nutr. 2006, 96 (Suppl. S2), S29–S35. [Google Scholar] [CrossRef]
- Kesen, S.; Amanpour, A.; Selli, S. Comparative Evaluation of the Fatty Acids and Aroma Compounds in Selected Iranian Nut Oils. Eur. J. Lipid Sci. Technol. 2018, 120, 1800152. [Google Scholar] [CrossRef]
- Levent, O. A Detailed Comparative Study on Some Physicochemical Properties, Volatile Composition, Fatty Acid, and Mineral Profile of Different Almond (Prunus dulcis L.) Varieties. Horticulturae 2022, 8, 488. [Google Scholar] [CrossRef]
- Memon, N.N. Nutritional Characteristics (Fatty Acid Profile, Proximate Composition and Dietary Feature) of Selected Nuts Available in Local Market. Pak. J. Anal. Environ. Chem. 2019, 20, 39–46. [Google Scholar] [CrossRef]
- Murley, T.; Kelly, B.; Adhikari, J.; Reid, W.; Koppel, K. A Comparison of Fatty Acid and Sensory Profiles of Raw and Roasted Pecan Cultivars. J. Food Sci. 2020, 85, 2665–2672. [Google Scholar] [CrossRef]
- Valdés García, A.; Sánchez Romero, R.; Juan Polo, A.; Prats Moya, S.; Maestre Pérez, S.E.; Beltrán Sanahuja, A. Volatile Profile of Nuts, Key Odorants and Analytical Methods for Quantification. Foods 2021, 10, 1611. [Google Scholar] [CrossRef] [PubMed]
- Machova, M.; Bajer, T.; Silha, D.; Ventura, K.; Bajerova, P. Volatiles Composition and Antimicrobial Activities of Areca Nut Extracts Obtained by Simultaneous Distillation-Extraction and Headspace Solid-Phase Microextraction. Molecules 2021, 26, 7422. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Beltran, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Carmen Garrigos, M. Monitoring the Oxidative Stability and Volatiles in Blanched, Roasted and Fried Almonds under Normal and Accelerated Storage Conditions by DSC, Thermogravimetric Analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Antioxidant Potential, Anti-Nutritional Factors, Volatile Compounds and Phenolic Composition of Microwave Heat-Treated Plum (Prunus domestica. L.) Kernels: An Analytical Approach. Br. Food J. 2022, 124, 3236–3256. [Google Scholar] [CrossRef]
- Grierson, A.J.C.; Long, D.G. Flora of Bhutan: Including a Record of Plants from Sikkim, 1st ed.; Royal Botanic Garden: Edinburgh, UK, 1983; Volume 1, ISBN 9780950427010. [Google Scholar]
- INC. Nuts and Dried Fruits Statistical Yearbook 2024; International Nuts and Dried Fruits Council: Reus, Spain, 2024. [Google Scholar]
- Aranceta, J.; Pérez Rodrigo, C.; Naska, A.; Vadillo, V.R.; Trichopoulou, A. Nut Consumption in Spain and Other Countries. Br. J. Nutr. 2006, 96 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Jenab, M.; Sabaté, J.; Slimani, N.; Ferrari, P.; Mazuir, M.; Casagrande, C.; Deharveng, G.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Consumption and Portion Sizes of Tree Nuts, Peanuts and Seeds in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohorts from 10 European Countries. Br. J. Nutr. 2006, 96, S12–S23. [Google Scholar] [CrossRef] [PubMed]
- The Observatory of Economic Complexity Walnuts with Shell (HS: 080231) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/walnuts-with-shell (accessed on 27 January 2025).
- Böhringer, F. Walnut Shell Inside Its Green Husk [Image]; Licensed under CC BY-SA 2.5.20. September 2008. Available online: https://commons.wikimedia.org/wiki/File:Juglans_regia_Echte_Walnussfrucht_2.jpg (accessed on 4 May 2025).
- Toe River Club Black Walnut (Juglans nigra). Available online: https://sites.duke.edu/toeriverclub/flora/trees/ (accessed on 6 June 2025).
- Batsch, A.J.G.K. Prunus Amygdalus. In Beytrage und Entwurfe zur Pragmatischen Geschicte der Drey Natur-Reiche Nach Ihren Verwandtschaften… Gewachsreich; Industrie Comptoir: Jena, Germany, 1801; Volume 1, p. 30. [Google Scholar]
- Rohrer, J.R. Feddes Repert: Prunus dulcis. In Flora of North America; Flora of North America Association: Point Arena, CA, USA, 1967; Volume 9, p. 372. [Google Scholar]
- Nikolaus, J. Almonds in International Cuisines and Cultures. Available online: https://www.confettitravelcafe.com/almonds-in-international-cuisines-and-cultures/ (accessed on 3 February 2025).
- The Observatory of Economic Complexity Almonds with Shell (HS: 080211) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/almonds-with-shell (accessed on 3 February 2025).
- Google Image Result Almond in Shell with Leaves. Available online: https://www.google.com/imgres?imgurl=https://media.istockphoto.com/id/939391834/photo/almonds-on-the-tree.jpg?s%3D612x612%26w%3D0%26k%3D20%26c%3DGhAnH2qteSpMjW84SpBbQiKAmfgJXXo-90MGT5V9yzk%3D&tbnid=q6GCKncdWm0F-M&vet=1&imgrefurl=https://www.istockphoto.com/photos/almonds-in-shell-with-leaf&docid=aJXRDGmkr3B-TM&w=612&h=406&source=sh/x/im/m1/1&kgs=69aa651ebc458546 (accessed on 6 June 2025).
- Leidus, I. Almond. Available online: https://en.wikipedia.org/wiki/Almond (accessed on 6 June 2025).
- WFO. Pistacia vera L. Available online: https://www.worldfloraonline.org/taxon/wfo-0000393766 (accessed on 3 February 2025).
- Kew Science. Pistacia vera L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:70280-1 (accessed on 3 February 2025).
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Mateos, R.; Salvador, M.D.; Fregapane, G.; Goya, L. Why Should Pistachio Be a Regular Food in Our Diet? Nutrients 2022, 14, 3207. [Google Scholar] [CrossRef]
- The Observatory of Economic Complexity Nuts, Edible: Pistachios, Fresh or Dried, in Shell (HS: 080251) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/nuts-edible-pistachios-fresh-or-dried-in-shell (accessed on 4 February 2025).
- Pixabay. Pistachio Seeds Shelled—Free Photo. Available online: https://pixabay.com/photos/pistachio-seeds-shelled-oilseeds-7574180/ (accessed on 28 June 2025).
- Pixabay. Pistachios on Pistachio Tree—Free Photo. Available online: https://pixabay.com/photos/pistachios-pistachio-tree-tree-1540134/ (accessed on 28 June 2025).
- WFO. Carya illinoinensis (Wangenh.) K.Koch. Available online: https://www.worldfloraonline.org/taxon/wfo-0000588763 (accessed on 5 February 2025).
- Wangenheim, F.A.J.; Koch, K.H.E. Carya illinoinensis. In Dendrologie. Bäume, Sträucher und Halbsträucher, welche in Mittel- und Nord-Europa im Freien kultivirt werden. Kritisch Beleuchtet von Karl Koch; Ferdinand Enke: Erlangen, Germany, 1869; Volume 1, p. 593. [Google Scholar]
- Stone, D.E. Dendrologie: Carya illinoinensis. In Flora of North America; Flora of North America Association: Point Arena, CA, USA, 1869; p. 3. [Google Scholar]
- Prasad, R.B.N. Walnuts and Pecans. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 6071–6079. [Google Scholar]
- Carunchia, M.; Wang, L.; Han, J.H. The Use of Antioxidants in the Preservation of Snack Foods. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; pp. 447–474. ISBN 9781782420972. [Google Scholar]
- Du, C. Chinese Consumer Preferences for and Attitudes Towards Pecans; Oklahoma State University: Oklahoma, OK, USA, 2020. [Google Scholar]
- Pixabay. Pecan Toasted Nuts Candied—Free Photo. Available online: https://pixabay.com/photos/pecan-toasted-nuts-candied-nuts-1090266/ (accessed on 28 June 2025).
- Pixabay. Pecan Tree Pecans Agriculture—Free Photo. Available online: https://pixabay.com/photos/pecan-tree-pecans-agriculture-grove-186328/ (accessed on 28 June 2025).
- WFO. Arachis hypogaea L. Available online: https://www.worldfloraonline.org/taxon/wfo-0000174378 (accessed on 11 February 2025).
- Linnaeus, C. Arachis hypogaea. In Species Plantarum; Laurentii Salvii: Stockholm, Sweden, 1753; Volume 2, p. 741. [Google Scholar]
- Kline, M. Manufacturing Foods with Peanut Ingredients. In Peanuts: Genetics, Processing, and Utilization; AOCS Press: Urbana, IL, USA, 2016; pp. 429–445. ISBN 9781630670382. [Google Scholar]
- Rachaputi, R.C.N.; Wright, G. Peanuts, Overview; Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.L. Peanut Composition, Flavor and Nutrition. In Peanuts: Genetics, Processing, and Utilization; AOCS Press: Urbana, IL, USA, 2016; pp. 289–345. ISBN 9781630670382. [Google Scholar]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2015, 53, 31. [Google Scholar] [CrossRef]
- The Observatory of Economic Complexity Ground Nuts (HS: 1202) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/ground-nuts (accessed on 12 February 2025).
- Hassani, N. How to Grow and Care for Peanut Plants. Available online: https://www.thespruce.com/peanut-plant-profile-4797389 (accessed on 6 June 2025).
- FreePik. Peanut Pods Photos—Free Photo. Available online: https://www.freepik.com/photos/peanut-pods (accessed on 28 June 2025).
- WFO. Anacardium occidentale L. Available online: https://www.worldfloraonline.org/taxon/wfo-0000533072 (accessed on 12 February 2025).
- DeFilipps, R.A.; Krupnick, G.A. The Medicinal Plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef]
- Linnaeus, C. Anacardium occidentale. In Species Plantarum; Laurentii Salvii: Stockholm, Sweden, 1753; Volume 1, p. 383. [Google Scholar]
- Cashews. Real Food Encyclopedia, FoodPrint, GRACE Communications Foundation. 2025. Available online: https://foodprint.org/real-food/cashews/ (accessed on 30 April 2025).
- Azam-Ali, S.H.; Judge, E.C. Small-Scale Cashew Nut Processing; Food and Agricultural Organisation: Rome, Italy, 2001; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/617adfe0-05b6-4bbc-beec-0e310c074b21/content (accessed on 4 May 2025).
- The Observatory of Economic Complexity Fresh/Dried Cashew Nuts (HS: 080130) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/freshdried-cashew-nuts (accessed on 13 February 2025).
- Sabatini. The Process of Planting Cashew Trees (Cashew Nuts). Available online: https://steemit.com/gardening/@sabatini/the-process-of-planting-cashew-trees-cashew-nuts-c9648cc47a355 (accessed on 6 June 2025).
- Molinero Luis Fried Cashew—Free Photo. Available online: https://www.freepik.com/free-photo/fried-cashew_1196998.htm#fromView=search&page=1&position=0&uuid=b645d03b-ac46-4238-831a-2a95f956c648&query=cashew (accessed on 28 June 2025).
- WFO. Cola nitida (Vent.) Schott & Endl. Available online: https://worldfloraonline.org/taxon/wfo-0000614491 (accessed on 11 March 2025).
- Schott, H.W.; Endlicher, S.F.L. Cola nitida. In Meletemata Botanica; Typis Caroli Gerold: Vindobonae, Austria, 1832; p. 36. [Google Scholar]
- Ekalu, A.; Habila, J.D. Phytochemistry, Pharmacology and Medicinal Uses of Cola (Malvaceae) Family: A Review. Med. Chem. Res. 2020, 29, 2089–2105. [Google Scholar] [CrossRef]
- Lateef, A. Cola nitida: Milestones in Catalysis, Biotechnology and Nanotechnology for Circular Economy and Sustainable Development. Biocatal. Agric. Biotechnol. 2023, 53, 102856. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Caffeine: Characterization and Properties. Encycl. Food Health 2016, 2016, 556–572. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Producrs. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 March 2025).
- The Observatory of Economic Complexity Nuts, Edible: Kola Nuts (Cola spp.), Fresh or Dried, Whether or Not Shelled or Peeled (HS: 080270) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/nuts-edible-kola-nuts-cola-spp-fresh-or-dried-whether-or-not-shelled-or-peeled (accessed on 11 March 2025).
- Urban Tropicals. Caffeine Kola Nut Tree (Cola nitida). Available online: https://urbantropicals.com/product/caffeine-kola-nut-tree-cola-nitida/ (accessed on 6 June 2025).
- Neogric. Kola Nut (Cola nitida) Top Exporters & Suppliers (Nigeria & Africa). Available online: https://www.neogric.com/kola-nut-cola-nitida-top-exporters-suppliers-nigeria-africa/ (accessed on 28 June 2025).
- Heckel, É.M. Garcinia kola. J. Pharm. Chim. 1883, 5, 88–89. [Google Scholar]
- WFO. Garcinia kola Heckel. Available online: https://www.worldfloraonline.org/taxon/wfo-0000694394 (accessed on 26 February 2025).
- Ingram, V.; Schure, J. Review of Non Timber Forest Products (NTFPs) in Central Africa; Cameroon: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Awono, A.; Atyi, R.E.A.; Foundjem-Tita, D.; Levang, P. Vegetal Non-Timber Forest Products in Cameroon, Contribution to the National Economy. Int. For. Rev. 2016, 18, 66–77. [Google Scholar] [CrossRef]
- Maňourová, A.; Leuner, O.; Tchoundjeu, Z.; Van Damme, P.; Verner, V.; Přibyl, O.; Lojka, B. Medicinal Potential, Utilization and Domestication Status of Bitter Kola (Garcinia kola Heckel) in West and Central Africa. Forests 2019, 10, 124. [Google Scholar] [CrossRef]
- Onyekwelu, J.C.; Stimm, B. Garcinia kola. In Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie; Wiley: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- The Green Institute. Garcinia kola. Available online: https://greeninstitute.ng/plants/2023/6/19/garcinia-kola (accessed on 28 June 2025).
- Neogric 10 Amazing Uses and Health Benefits of Bitter (Garcinia) Kola You Probably Don’t Know. Available online: https://www.neogric.com/10-benefits-of-bitter-kola-you-probably-dont-know/ (accessed on 28 June 2025).
- USDA. Nuts, Almonds, Whole, Raw—Nutrients. Available online: https://fdc.nal.usda.gov/food-details/2346393/nutrients (accessed on 6 June 2025).
- Savage, G.P.; Dutta, P.C.; McNeil, D.L. Fatty Acid and Tocopherol Contents and Oxidative Stability of Walnut Oils. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 1059–1063. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of Nuts and Their Potential Health Benefits—An Overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- USDA. Nuts, Cashew Nuts, Raw—Nutrients. Available online: https://fdc.nal.usda.gov/food-details/2515374/nutrients (accessed on 6 June 2025).
- Liao, M.; Zhao, Y.; Xu, Y.; Gong, C.; Jiao, S. Effects of Hot Air-Assisted Radio Frequency Roasting on Nutritional Quality and Aroma Composition of Cashew Nut Kernels. LWT 2019, 116, 108551. [Google Scholar] [CrossRef]
- USDA. Peanuts, Raw—Nutrients. Available online: https://fdc.nal.usda.gov/food-details/2515376/nutrients (accessed on 6 June 2025).
- Mexis, S.F.; Kontominas, M.G. Effect of Gamma Irradiation on the Physico-Chemical and Sensory Properties of Raw Shelled Peanuts (Arachis hypogaea L.) and Pistachio Nuts (Pistacia vera L.). J. Sci. Food Agric. 2009, 89, 867–875. [Google Scholar] [CrossRef]
- Hojjati, M.; Noguera-Artiaga, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Effects of Microwave Roasting on Physicochemical Properties of Pistachios (Pistaciavera L.). Food Sci. Biotechnol. 2015, 24, 1995–2001. [Google Scholar] [CrossRef]
- USDA. Nuts, Pistachio Nuts, Raw—Nutrients. Available online: https://fdc.nal.usda.gov/food-details/2515379/nutrients (accessed on 6 June 2025).
- Kelebek, H.; Sonmezdag, A.S.; Guclu, G.; Cengiz, N.; Uzlasir, T.; Kadiroglu, P.; Selli, S. Comparison of Phenolic Profile and Some Physicochemical Properties of Uzun Pistachios as Influenced by Different Harvest Period. J. Food Process Preserv. 2020, 44, e14605. [Google Scholar] [CrossRef]
- Polari, J.J.; Ferguson, L.; Wang, S.C. Pistachio Kernel Composition of “Kalehghouchi”, “Pete 1”, and “Lost Hills” in California. Hortscience 2020, 55, 666–669. [Google Scholar] [CrossRef]
- Zwarts, L.; Savage, G.P.; McNeil, D.L. Fatty Acid Content of New Zealand-Grown Walnuts (Juglans regia L.). Int. J. Food Sci. Nutr. 1999, 50, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lyu, S.; Gao, C.; Si, X.; Wang, K.; Huang, C.; Chen, J.; Huang, J. Comparative Analysis of Aroma Profiles in Walnut, Pecan and Hickory Nuts during the Roasting Process Using E-Nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT 2024, 210, 116810. [Google Scholar] [CrossRef]
- Dah-Nouvlessounon, D.; Adjanohoun, A.; Sina, H.; Noumavo, P.A.; Diarrasouba, N.; Parkouda, C.; Madodé, Y.E.; Dicko, M.H.; Baba-Moussa, L.; Dah-Nouvlessounon, D.; et al. Nutritional and Anti-Nutrient Composition of Three Kola Nuts (Cola nitida, Cola acuminata and Garcinia kola) Produced in Benin. Food Nutr. Sci. 2015, 6, 1395–1407. [Google Scholar] [CrossRef]
- Farombi, E.O.; Owoeye, O. Antioxidative and Chemopreventive Properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health 2011, 8, 2533. [Google Scholar] [CrossRef]
- Adesuyi, A.O.; Elumm, I.K.; Adaramola, F.B.; Nwokocha, A.G. Nutritional and Phytochemical Screening of Garcinia kola. Adv. J. Food Sci. Technol. 2012, 1, 9–14. [Google Scholar]
- Terashima, K.; Aqil, M.; Niwa, M. Garcinianin, a Novel Biflavonoid from the Roots of Garcinia kola. Heterocycles 1995, 41, 2245–2250. [Google Scholar] [CrossRef]
- Iwu, M.; Igboko, O. Flavonoids of Garcinia kola Seeds. J. Nat. Prod. 1982, 45, 650–651. [Google Scholar] [CrossRef]
- Niwa, M.; Ito, J.; Terashima, K.; Aqil, M. Garcipyran, a Novel 6-Aryl-1,2-Benzopyran Derivative from Garcinia kola. Heterocycles 1994, 38, 1927–1932. [Google Scholar] [CrossRef]
- Atawodi, S.E.O.; Pfundstein, B.; Haubner, R.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Content of Polyphenols Compounds in the Nigerian Stimulants Cola nitida ssp. Alba, Cola nitida ssp. Rubra A. Chev, and Cola acuminata Schott & Endl and Their Antioxidant Capacity. J. Agric. Food Chem. 2007, 55, 9824–9828. [Google Scholar] [CrossRef]
- Adeyeye, A.; Ayodele, O.D.; Akinnuoye, G.A. Comparative Study of the Proximate and Fatty Acid Profiles of Cola nitida, Cola acuminata and Garcinia kola. Am. J. Food Sci. Nutr. 2017, 4, 80–84. [Google Scholar]
- Adesanwo, J.K.; Ogundele, S.B.; Akinpelu, D.A.; McDonald, A.G. Chemical Analyses, Antimicrobial and Antioxidant Activities of Extracts from Cola nitida Seed. J. Explor. Res. Pharmacol. 2017, 2, 67–77. [Google Scholar] [CrossRef][Green Version]
- USDA. Nuts, Pecans—Nutrients. Available online: https://fdc.nal.usda.gov/food-details/170182/nutrients (accessed on 6 June 2025).
- Thewes, F.R.; Both, V.; Thewes, F.R.; Brackmann, A.; Wagner, R.; Ribeiro, S.R.; Ludwig, V.; Rossato, F.P. Pecan Storage: Effects of 1-MCP on the Overall Quality and Volatile Compounds Profile of Shelled and Unshelled Pecans. LWT 2021, 145, 111298. [Google Scholar] [CrossRef]
- Lund, J.; Rustan, A.C. Fatty Acids: Structures and Properties. Encycl. Life Sci. 2020, 1, 283–292. [Google Scholar] [CrossRef]
- Lichtenstein, A.H. Fats and Oils. In Encyclopedia of Human Nutrition; Academic Press: Cambridge, MA, USA, 2013; Volumes 2–4, pp. 201–208. ISBN 9780123848857. [Google Scholar]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health Effects of Oleic Acid and Long Chain Omega-3 Fatty Acids (EPA and DHA) Enriched Milks. A Review of Intervention Studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Oxidative Stability of Tree Nut Oils. J. Agric. Food Chem. 2008, 56, 4751–4759. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic Resources, Chemistry, by-Products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; García-Segovia, P.; Martínez-Monzó, J. Vacuum Frying: An Alternative to Obtain High-Quality Dried Products. Food Eng. Rev. 2011, 3, 63–78. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans Fatty Acids and Cardiovascular Disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- Salcedo, C.L.; Nazareno, M.A. Effect of Phenolic Compounds on the Oxidative Stability of Ground Walnuts and Almonds. RSC Adv. 2015, 5, 45878–45887. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Desamparados Salvador, M.; Gomez-Alonso, S.; Fregapane, G. Characterization of Virgin Walnut Oils and Their Residual Cakes Produced from Different Varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Okatan, V.; Gündeşli, M.A.; Kafkas, N.E.; Attar, Ş.H.; Kahramanoğlu, İ.; Usanmaz, S.; Aşkın, M.A. Phenolic Compounds, Antioxidant Activity, Fatty Acids and Volatile Profiles of 18 Different Walnut (Juglans regia L.) Cultivars and Genotypes. Erwerbs-Obstbau 2022, 64, 247–260. [Google Scholar] [CrossRef]
- Ampofo, J.; Grilo, F.S.; Langstaff, S.; Wang, S.C. Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods 2022, 11, 3151. [Google Scholar] [CrossRef]
- Mu, H.; Gao, H.; Chen, H.; Fang, X.; Zhou, Y.; Wu, W.; Han, Q. Study on the Volatile Oxidation Compounds and Quantitative Prediction of Oxidation Parameters in Walnut (Carya cathayensis Sarg.) Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 16044–16050. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018, 83, 2745–2752. [Google Scholar] [CrossRef]
- Li, H.; Han, J.; Zhao, Z.; Tian, J.; Fu, X.; Zhao, Y.; Wei, C.; Liu, W. Roasting Treatments Affect Oil Extraction Rate, Fatty Acids, Oxidative Stability, Antioxidant Activity, and Flavor of Walnut Oil. Front. Nutr. 2023, 9, 1077081. [Google Scholar] [CrossRef]
- Elmore, J.S.; Nisyrios, I.; Mottram, D.S. Analysis of the Headspace Aroma Compounds of Walnuts (Juglans regia L.). Flavour. Fragr. J. 2005, 20, 501–506. [Google Scholar] [CrossRef]
- Yoshinaga-Kiriake, A.; Takata, D.; Norito, T.; Maki, M.; Mochizuki, S.; Yoshinaga, K. Comparative Evaluation of Fatty Acid Composition, Tocopherols, and Volatile Compounds of Walnut Oil between Juglans mandshurica Maxim. Var. Sachalinensis (Komatsu) Kitam and J. regia L. J. Oleo Sci. 2022, 71, 1743–1748. [Google Scholar] [CrossRef]
- Masoodi, L.; Masoodi, F.A.; Gull, A.; Gani, A.; Muzaffer, S.; Sidiq, M. Effect of γ-Irradiation on the Physicochemical and Sensory Properties of Fresh Walnut Kernels (Juglans regia) during Storage. Food Chem. Adv. 2023, 3, 100301. [Google Scholar] [CrossRef]
- Niu, B.K.; Olajide, T.M.; Liu, H.A.; Pasdar, H.; Weng, X.C. Effects of Different Baking Techniques on the Quality of Walnut and Its Oil. Grasas Y Aceites 2021, 72, e406. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J.; Solar, A.; Zlatic, E. The Influence of Atmosphere on the Oxidation of Ground Walnut During Storage at 20 °C. Food Technol. Biotechnol. 2012, 50, 454–460. [Google Scholar]
- Athaillah, Z.A.; Wang, S.C. Physical and Chemical Characteristics of Walnut (Juglans regia L.) Kernels with Different Skin Lightness. J. Food Sci. 2024, 89, 2730–2746. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.D.; Crowe, T.W.; Johnson, L.A.; White, P.J. Impact of Extraction Method on Yield of Lipid Oxidation Products from Oxidized and Unoxidized Walnuts. J. Am. Oil Chem. Soc. 2002, 79, 453–456. [Google Scholar] [CrossRef]
- Martinez, M.L.; Maestri, D.M. Oil Chemical Variation in Walnut (Juglans regia L.) Genotypes Grown in Argentina. Eur. J. Lipid Sci. Technol. 2008, 110, 1183–1189. [Google Scholar] [CrossRef]
- Martinez, M.L.; Mattea, M.A.; Maestri, D.M. Varietal and Crop Year Effects on Lipid Composition of Walnut (Juglans regia) Genotypes. J. Am. Oil Chem. Soc. 2006, 83, 791–796. [Google Scholar] [CrossRef]
- Mexis, S.F.; Badeka, A.V.; Chouliara, E.; Riganakos, K.A.; Kontominas, M.G. Effect of γ-Irradiation on the Physicochemical and Sensory Properties of Raw Unpeeled Almond Kernels (Prunus dulcis). Innov. Food Sci. Emerg. Technol. 2009, 10, 87–92. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Karaat, F.E. Organic vs conventional almond: Market quality, fatty acid composition and volatile aroma compounds. Appl. Ecol. Env. Res. 2019, 17, 7783–7793. [Google Scholar] [CrossRef]
- Hojjati, M.; Lipan, L.; Carbonell-Barrachina, A.A. Effect of Roasting on Physicochemical Properties of Wild Almonds (Amygdalus scoparia). J. Am. Oil Chem. Soc. 2016, 93, 1211–1220. [Google Scholar] [CrossRef]
- Mexis, S.F.; Kontominas, M.G. Effect of Oxygen Absorber, Nitrogen Flushing, Packaging Material Oxygen Transmission Rate and Storage Conditions on Quality Retention of Raw Whole Unpeeled Almond Kernels (Prunus dulcis). LWT-Food Sci. Technol. 2010, 43, 1–11. [Google Scholar] [CrossRef]
- Mexis, S.F.; Riganakos, K.A.; Kontominas, M.G. Effect of Irradiation, Active and Modified Atmosphere Packaging, Container Oxygen Barrier and Storage Conditions on the Physicochemical and Sensory Properties of Raw Unpeeled Almond Kernels (Prunus dulcis). J. Sci. Food Agric. 2011, 91, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Tietel, Z.; Veksler, N.; Galilov, I.; Melamed, S.; Harel-Beja, R.; Holland, D. A New Israeli Almond “Shefa”: Phytochemical Composition and Response to Roasting Temperature and Duration. Int. J. Food Prop. 2024, 27, 883–896. [Google Scholar] [CrossRef]
- Roxas, A.A.; Marino, G.; Avellone, G.; Caruso, T.; Marra, F.P. The Effect of Plant Water Status on the Chemical Composition of Pistachio Nuts (Pistacia vera L. Cultivar Bianca). Agriculture 2020, 10, 167. [Google Scholar] [CrossRef]
- Polari, J.J.; Zhang, L.; Ferguson, L.; Maness, N.O.; Wang, S.C. Impact of Microclimate on Fatty Acids and Volatile Terpenes in “Kerman” and “Golden Hills” Pistachio (Pistacia vera) Kernels. J. Food Sci. 2019, 84, 1937–1942. [Google Scholar] [CrossRef]
- Demirkoz, A.B.; Karakas, M.; Bayramoglu, P.; Uner, M. Analysis of Volatile Flavour Components by Dynamic Headspace Analysis/Gas Chromatography-Mass Spectrometry in Roasted Pistachio Extracts Using Supercritical Carbon Dioxide Extraction and Sensory Analysis. Int. J. Food Prop. 2018, 21, 972–981. [Google Scholar] [CrossRef]
- Ozturk, I.; Sagdic, O.; Yalcin, H.; Capar, T.D.; Asyali, M.H. The Effects of Packaging Type on the Quality Characteristics of Fresh Raw Pistachios (Pistacia vera L.) during the Storage. LWT-Food Sci. Technol. 2016, 65, 457–463. [Google Scholar] [CrossRef]
- Ling, B.; Yang, X.; Li, R.; Wang, S. Physicochemical Properties, Volatile Compounds, and Oxidative Stability of Cold Pressed Kernel Oils from Raw and Roasted Pistachio (Pistacia vera L. Var Kerman). Eur. J. Lipid Sci. Technol. 2016, 118, 1368–1379. [Google Scholar] [CrossRef]
- Mukwevho, P.L.; Kaseke, T.; Fawole, O.A. Optimisation Using Response Surface Methodology of Quality, Nutritional and Antioxidant Attributes of “Wichita” Pecan Nuts Roasted by Microwaves. Processes 2023, 11, 2503. [Google Scholar] [CrossRef]
- Ribeiro, S.R.; Klein, B.; Machado Ribeiro, Q.; Duarte dos Santos, I.; Gomes Genro, A.L.; de Freitas Ferreira, D.; Janner Hamann, J.; Smanioto Barin, J.; Cichoski, A.J.; Fronza, D.; et al. Chemical Composition and Oxidative Stability of Eleven Pecan Cultivars Produced in Southern Brazil. Food Res. Int. 2020, 136, 109596. [Google Scholar] [CrossRef]
- Alinezhad, M.; Hojjati, M.; Barzegar, H.; Shahbazi, S.; Askari, H. Effect of Gamma Irradiation on the Physicochemical Properties of Pistachio (Pistacia vera L.) Nuts. J. Food Meas. Charact. 2021, 15, 199–209. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Biolatto, A.; Rizzo, S.A.; Pérez, C.D.; Frusso, E.A.; Carduza, F.; Rossetti, L. Oxidative Stability Parameters and Sensory Properties of In-Shell “Stuart” Pecans [Carya illinoinensis (Wangenh.) K.Koch] Stored at Different Temperatures under Non-Accelerated Conditions. Postharvest Biol. Technol. 2021, 179, 111591. [Google Scholar] [CrossRef]
- Juan-Polo, A.; Beltran Sanahuja, A.; Monedero Prieto, M.; Sanchez Reig, C.; Valdes Garcia, A.; Maestre Perez, S.E. Impact of UV-Light Irradiation on Sensory Properties, Volatile, Fatty Acid, and Tocopherol Composition of Peanuts (Arachis hypogaea L.). LWT-Food Sci. Technol. 2023, 173, 114247. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Bismara Regitano-d’Arce, M.A.; de Alencar, S.M.; Canniatti-Brazaca, S.G.; de Souza Vieira, T.M.; Shahidi, F. Chemical Changes and Oxidative Stability of Peanuts as Affected by the Dry-Blanching. J. Am. Oil Chem. Soc. 2016, 93, 1101–1109. [Google Scholar] [CrossRef]
- Reed, K.A.; Sims, C.A.; Gorbet, D.W.; O’Keefe, S.F. Storage Water Activity Affects Flavor Fade in High and Normal Oleic Peanuts. Food Res. Int. 2002, 35, 769–774. [Google Scholar] [CrossRef]
- Mexis, S.F.; Kontominas, M.G. Effect of γ-Irradiation on the Physicochemical and Sensory Properties of Cashew Nuts (Anacardium occidentale L.). LWT-Food Sci. Technol. 2009, 42, 1501–1507. [Google Scholar] [CrossRef]
- Sharma, G.K.; Semwal, A.D.; Mahesh, C.; Murthy, M.C.N.; Arya, S.S. Enhancement of the Shelf-Life of Deep Fat Fried Cashewnuts. LWT-Food Sci. Technol. 2000, 33, 173–177. [Google Scholar] [CrossRef]
- Leal, A.R.; Dionísio, A.P.; de Abreu, F.A.P.; de Oliveira, G.F.; da Araújo, I.M.S.; Magalhães, H.C.R.; Leite, A.B.; da Silva, E.K.M.; do Nascimento, R.F.; do Nascimento, H.O.; et al. Impact of Different Kernel Grades on Volatile Compounds Profile, Fatty Acids and Oxidative Quality of Cashew Nut Oil. Food Res. Int. 2023, 165, 112526. [Google Scholar] [CrossRef]
- Eleyinmi, A.F.; Bressler, D.C.; Amoo, I.A.; Sporns, P.; Oshodi, A.A. Chemical composition of bitter cola (Garcinia kola) seed and hulls. Pol. J. Food Nutr. Sci. 2006, 15, 395–400. [Google Scholar]
- Oyekanmi, A.M.; Adejoro, A.; Adeleke, B.B. The Fatty Acid Profile of Oils from Garcinia kola, Tetracarpodium Conopodium and Tectona Grandis. Int. J. Biochem. Res. Rev. 2019, 27, 1–5. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Xing, W.; Tang, C.; Feng, X.; Zhang, J. Simulating Fatty Acid Autoxidation and Exploring the Related Volatiles Formation Mechanism. LWT 2024, 214, 117083. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, K.; Xu, C.; Lai, C.; Liu, Y.; Cao, Y.; Zhao, L. Contribution of Lipid to the Formation of Characteristic Volatile Flavor of Peanut Oil. Food Chem. 2024, 442, 138496. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control Strategies of Pyrazines Generation from Maillard Reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Shahidi, F.; Oh, W.Y. Lipid-Derived Flavor and off-Flavor of Traditional and Functional Foods: An Overview. J. Food Bioact. 2020, 10, 20–31. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Kelebek, H.; Sonmezdag, A.S.; Rodríguez-Alcalá, L.M.; Fontecha, J.; Selli, S. Characterization of the Aroma-Active, Phenolic, and Lipid Profiles of the Pistachio (Pistacia vera L.) Nut as Affected by the Single and Double Roasting Process. J. Agric. Food Chem. 2015, 63, 7830–7839. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, H.; Stoner-Harris, T.; Adhikari, K.; Mishra, A.; Bock, C.H.; Kong, F. Changes in Chemical Characteristics and Modeling Sensory Parameters of Stored Pecan Nutmeats. J. Food Sci. 2023, 88, 1816–1834. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.R.; Ribeiro, Q.M.; Klein, B.; Duarte dos Santos, I.; Forgiarini, S.; Hamann, J.J.; Cichoski, A.J.; Fronza, D.; Brackmann, A.; Both, V.; et al. Effect of Low Oxygen on Quality Attributes of ‘Barton’ Pecan Nuts after Long-Term Storage at Different Temperatures. Sci. Hortic. 2020, 263, 109098. [Google Scholar] [CrossRef]
- Yang, K.-M.; Chao, L.K.; Wu, C.-S.; Ye, Z.-S.; Chen, H.-C. Headspace Solid-Phase Microextraction Analysis of Volatile Components in Peanut Oil. Molecules 2021, 26, 3306. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, S.; Pei, H.; Gao, K.; Gao, L.; Cao, X.; Sun, H.; Song, J. Effect of Hot Air Circulation Roasting Temperature on the Sensory Property and Odor Characteristics of Cashew Nut (Anacardium occidentale L.). J. Food Process Eng. 2024, 47, e14546. [Google Scholar] [CrossRef]
- Salahdeen, M.H.; Omoaghe, O.A.; Isehunwa, O.G.; Murtala, A.B.; Alada, A.A.R. Gas Chromatography Mass Spectrometry (GC-MS) Analysis of Ethanolic Extracts of Kolanut (Cola nitida) (Vent) and Its Toxicity Studies in Rats. J. Med. Plants Res. 2015, 9, 56–70. [Google Scholar] [CrossRef][Green Version]
- Hussain, R.A.; Owegby, A.G.; Waterman, P.G. Kolanone, a Novel Polyisoprenylated Benzophenone with Antimicrobial Properties from the Fruit of Garcinia kola. Planta Med. 1982, 44, 78–81. [Google Scholar] [CrossRef]
- Madubunyi, I.I. Antimicrobial Activities of the Constituents of Garcinia kola Seeds. Int. J. Pharmacogn. 1995, 33, 232–237. [Google Scholar] [CrossRef]
- Ajayi, T.O.; Moody, J.O.; Fukushi, Y.; Adeyemi, T.A.; Fakeye, T.O. Antimicrobial Activity of Garcinia kola (Heckel) Seed Extracts and Isolated Constituents Against Caries-Causing Microorganisms. Afr. J. Biomed. Res. 2014, 17, 165–171. [Google Scholar]
- Terashima, K.; Shimamura, T.; Tanabayashi, M.; Aqil, M.; Akinniyi, J.A.; Niwa, M. Constituents of the Seeds of Garcinia kola: Two New Antioxidants, Garcinoic Acid and Garcinal. Heterocycles 1997, 45, 1559–1566. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Salau, V.F.; Chukwuma, C.I.; Md Islam, S. Kolaviron: A Biflavonoid with Numerous Health Benefits. Curr. Pharm. Des. 2020, 27, 490–504. [Google Scholar] [CrossRef]
- Emmanuel, O.; Uche, M.E.; Dike, E.D.; Etumnu, L.R.; Ugbogu, O.C.; Ugbogu, E.A. A Review on Garcinia kola Heckel: Traditional Uses, Phytochemistry, Pharmacological Activities, and Toxicology. Biomarkers 2022, 27, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Srigley, C.T.; Mossoba, M.M. Food Safety: Innovative Analytical Tools for Safety Assessment. In Current Analytical Techniques for Food Lipids; Spizzirri, U.G., Cirillo, G., Eds.; United States Food and Drug Administration Publications: Silver Spring, MD, USA, 2017; Volume 7. [Google Scholar]
- Xie, C.; Ma, Z.F.; Li, F.; Zhang, H.; Kong, L.; Yang, Z.; Xie, W. Storage Quality of Walnut Oil Containing Lycopene during Accelerated Oxidation. J. Food Sci. Technol. 2018, 55, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, E.; Chen, R.; Zhao, C.; Farag, M.A. Lipidomics in Food Quality and Authentication: A Comprehensive Review of Novel Trends and Applications Using Chromatographic and Spectroscopic Techniques. Crit. Rev. Food Sci. Nutr. 2023, 64, 9058–9081. [Google Scholar] [CrossRef]
- Øvrebø, T. Identification and Quantification of Fatty Acids in Nut Oils by GC-MS; Norwegian University of Life Sciences: Ås, Norway, 2019. [Google Scholar]
- Houdkova, M.; Albarico, G.; Doskocil, I.; Tauchen, J.; Urbanova, K.; Tulin, E.E.; Kokoska, L. Vapors of Volatile Plant-Derived Products Significantly Affect the Results of Antimicrobial, Antioxidative and Cytotoxicity Microplate-Based Assays. Molecules 2020, 25, 6004. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, C.D.; Houdkova, M.; Urbanova, K.; Badarau, S.; Gurean, D.; Pamfil, D.; Kokoska, L. Chemical Composition of the Essential Oils of Aerial Parts of Aconitum, Anemone and Ranunculus (Ranunculaceae) Species from Romania. J. Essent. Oil Bear. Plants 2019, 22, 728–745. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.S.; Tomuta, I. Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; Domingues, M.R.M.; Alves, E. An Overview on Lipids in Nuts and Oily Fruits: Oil Content, Lipid Composition, Health Effects, Lipidomic Fingerprinting and New Biotechnological Applications of Their by-Products. Crit. Rev. Food Sci. Nutr. 2024, 64, 9132–9160. [Google Scholar] [CrossRef]
- Uriarte, P.S.; Goicoechea, E.; Guillen, M.D. Volatile Components of Several Virgin and Refined Oils Differing in Their Botanical Origin. J. Sci. Food Agric. 2011, 91, 1871–1884. [Google Scholar] [CrossRef]
- Vas, G.; Vékey, K. Solid-Phase Microextraction: A Powerful Sample Preparation Tool Prior to Mass Spectrometric Analysis. J. Mass. Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas Chromatography–Olfactometry in Food Flavour Analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Farag, M.A. Headspace Analysis of Volatile Compounds in Leaves from the Juglandaceae (Walnut) Family. J. Essent. Oil Res. 2008, 20, 323–327. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Unterweger, H.; Buchbauer, G.; Krist, S. Characterization of Volatile Compounds and Triacylglycerol Profiles of Nut Oils Using SPME-GC-MS and MALDI-TOF-MS. Eur. J. Lipid Sci. Technol. 2009, 111, 170–182. [Google Scholar] [CrossRef]
- Ullrich, F.; Grosch, W. Identification of the Most Intense Volatile Flavour Compounds Formed during Autoxidation of Linoleic Acid. Z. Leb. Unters. Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Luque De Castro, M.D. Ultrasound-Assisted Soxhlet Extraction: An Expeditive Approach for Solid Sample Treatment: Application to the Extraction of Total Fat from Oleaginous Seeds. J. Chromatogr. A 2004, 1034, 237–242. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of Cold-Pressing and Soxhlet Extraction Systems for Bioactive Compounds, Antioxidant Properties, Polyphenols, Fatty Acids and Tocopherols in Eight Nut Oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction Methods of Fat from Food Samples and Preparation of Fatty Acid Methyl Esters for Gas Chromatography: A Review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Crowe, T.D.; White, P.J. Oxidative Stability of Walnut Oils Extracted with Supercritical Carbon Dioxide. J. Am. Oil Chem. Soc. 2003, 80, 575–578. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ghazani, S.M.; Corazza, M.L.; Marangoni, A.G.; Ribani, R.H. Assessment of Subcritical Propane, Supercritical CO2 and Soxhlet Extraction of Oil from Sapucaia (Lecythis pisonis) Nuts. J. Supercrit. Fluids 2018, 133, 122–132. [Google Scholar] [CrossRef]
- Crowe, T.D.; White, P.J. Oxidation, Flavor, and Texture of Walnuts Reduced in Fat Content by Supercritical Carbon Dioxide. J. Am. Oil Chem. Soc. 2003, 80, 569–574. [Google Scholar] [CrossRef]
- de Oliveira, A.L. Study of Extraction of Mesocarp and Almonds from Babaçu, from Brazil Nuts, Pequi Almonds and Sacha Inchi Seeds Using Supercritical CO2 and Pressurized Liquid; Virtual Library–FAPESP: São Paulo, SP, Brazil, 2016. [Google Scholar]
- Nur, N.H.M.; Zaiton, M.S.S.; Jamshed, M.S.; Zaidul, I.S.M.; Mokhlesur, M.R.; Juahir, H.; Tengku, M.A.; Wan, N.W.B.; Nurul, I.M.S. The Superiority of Supercritical Fluid Extraction Over Steam Distillation and Solvent Extraction Methods for the Extraction of Aroma from Salacca zalacca (Gaertn.) Voss. Orient. J. Chem. 2019, 35, 1669–1677. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of Supercritical CO2 in Lipid Extraction—A Review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and Applications in Food Samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Liu, T.; Li, Q.; Wang, D.; Zhang, Y. Analyzing the Effect of Baking on the Flavor of Defatted Tiger Nut Flour by E-Tongue, E-Nose and HS-SPME-GC-MS. Foods 2022, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation—A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Q.; Liang, Z.; Zhao, M.; Yu, X.; Yang, D.; Xu, X. The GC/MS Analysis of Volatile Components Extracted by Different Methods from Exocarpium Citri Grandis. J. Anal. Methods Chem. 2013, 2013, 918406. [Google Scholar] [CrossRef] [PubMed]
- Netiwaranon, S.; Mason, R.; D’Arcy, B.R. Volatile Constituents in Macadamia Nuts. In Proceedings of the 33rd Annual Convention of Australian Institute of Food Science & Technology, Brisbane, Australia, 20–23 August 2000; Australian Institute of Food Science & Technology: Sydney, Australia, 2000; p. 26. [Google Scholar]
- Zhang, Z.; Xie, X.; Jia, H.; Le, W.; Xiang, P. Effect of Freeze-Thaw Treatment on the Yield and Quality of Tiger Nut Oil. Food Chem.-X 2024, 23, 101733. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Recent Developments of Stir Bar Sorptive Extraction for Food Applications: Extension to Polar Solutes. J. Agric. Food Chem. 2018, 66, 7249–7255. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Zhang, Q.; Zang, L.; Chen, B.; Hu, B. Stir Bar Sorptive Extraction and Its Application. J. Chromatogr. A 2021, 1637, 461810. [Google Scholar] [CrossRef] [PubMed]
- Phatanayindee, S.; Borompichaichartkul, C.; Srzednicki, G.; Craske, J.; Wootton, M. Changes of Chemical and Physical Quality Attributes of Macadamia Nuts during Hybrid Drying and Processing. Dry. Technol. 2012, 30, 1870–1880. [Google Scholar] [CrossRef]
- Gong, Y.; Kerrihard, A.L.; Pegg, R.B. Characterization of the Volatile Compounds in Raw and Roasted Georgia Pecans by HS-SPME-GC-MS. J. Food Sci. 2018, 83, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Pintea, A.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Pop, E.A.; Opriță, V.A.; Giuffrida, D.; Cacciola, F.; Bartolomeo, G.; Mondello, L. Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach. Antioxidants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, D.; Cao, Y.; Duan, X.; Sun, H. Widely Targeted Metabolomic Approach Reveals Dynamic Changes in Non-Volatile and Volatile Metabolites of Peanuts during Roasting. Food Chem. 2023, 412, 135577. [Google Scholar] [CrossRef]
- Doǧan, H.H. Evaluation of Phenolic Compounds, Antioxidant Activities and Fatty Acid Composition of Amanita ovoidea (Bull.) Link. in Turkey. J. Food Compos. Anal. 2013, 31, 87–93. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q.; Huang, F. Unraveling of the Aroma-Active Compounds in Virgin Camellia Oil (Camellia oleifera Abel) Using Gas Chromatography-Mass Spectrometry-Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; Cisternino, E.; Rosario Dambruoso, P.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, F.; Gasperi, F.; Yeretzian, C.; Märk, T.D. PTR-MS Monitoring of VOCs and BVOCs in Food Science and Technology. TrAC-Trends Anal. Chem. 2011, 30, 968–977. [Google Scholar] [CrossRef]
- Zardin, E.; Tyapkova, O.; Buettner, A.; Beauchamp, J. Performance Assessment of Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry (PTR-TOF-MS) for Analysis of Isobaric Compounds in Food-Flavour Applications. LWT-Food Sci. Technol. 2014, 56, 153–160. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.A.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Kantartzis, K.; Celebi, N.; Staiger, H.; Machann, J.; Schick, F.; Cegan, A.; Elcnerova, M.; Schleicher, E.; Fritsche, A.; et al. Circulating Palmitoleate Strongly and Independently Predicts Insulin Sensitivity in Humans. Diabetes Care 2010, 33, 405–407. [Google Scholar] [CrossRef]
- Yang, Z.H.; Takeo, J.; Katayama, M. Oral Administration of Omega-7 Palmitoleic Acid Induces Satiety and the Release of Appetite-Related Hormones in Male Rats. Appetite 2013, 65, 1–7. [Google Scholar] [CrossRef]
- Hu, W.; Fitzgerald, M.; Topp, B.; Alam, M.; O’Hare, T.J. A Review of Biological Functions, Health Benefits, and Possible de Novo Biosynthetic Pathway of Palmitoleic Acid in Macadamia Nuts. J. Funct. Foods 2019, 62, 103520. [Google Scholar] [CrossRef]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of Protocatechuic Acid and Its Alkyl Esters, Ethyl and Heptyl Protocatechuates, to Counteract UVB-Induced Oxidative Injuries and Photoaging in Fibroblasts L929 Cell Line. J. Photochem. Photobiol. B 2020, 203, 111771. [Google Scholar] [CrossRef]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its Biologic Targets and Functional Activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut Consumption and Incidence of Cardiovascular Diseases and Cardiovascular Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee Bravatti, M.A.; Johnson, E.J.; Raman, G. Daily Almond Consumption in Cardiovascular Disease Prevention via LDL-C Change in the U.S. Population: A Cost-Effectiveness Analysis. BMC Public Health 2020, 20, 558. [Google Scholar] [CrossRef] [PubMed]
- Houston, L.; Probst, Y.C.; Chandra Singh, M.; Neale, E.P. Tree Nut and Peanut Consumption and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1029–1049. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Cioccoloni, G.; Bernardini, S.; Abenavoli, L.; Aiello, V.; Marchetti, M.; Cammarano, A.; Alipourfard, I.; Ceravolo, I.; Gratteri, S. A Hazelnut-Enriched Diet Modulates Oxidative Stress and Inflammation Gene Expression without Weight Gain. Oxidative Med. Cell Longev. 2019, 2019, 4683723. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. A Comprehensive Review of Almond Clinical Trials on Weight Measures, Metabolic Health Biomarkers and Outcomes, and the Gut Microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef]
- Freisling, H.; Noh, H.; Slimani, N.; Chajès, V.; May, A.M.; Peeters, P.H.; Weiderpass, E.; Cross, A.J.; Skeie, G.; Jenab, M.; et al. Nut Intake and 5-Year Changes in Body Weight and Obesity Risk in Adults: Results from the EPIC-PANACEA Study. Eur. J. Nutr. 2018, 57, 2399–2408. [Google Scholar] [CrossRef]
- Guarneiri, L.L.; Cooper, J.A. Intake of Nuts or Nut Products Does Not Lead to Weight Gain, Independent of Dietary Substitution Instructions: A Systematic Review and Meta-Analysis of Randomized Trials. Adv. Nutr. 2021, 12, 384–401. [Google Scholar] [CrossRef]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L.; Popa, D.S.; Ferreira, I.C.F.R. Benefits of Tree Nut Consumption on Aging and Age-Related Diseases: Mechanisms of Actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Effect of 12 Weeks High Oleic Peanut Consumption on Cardio-Metabolic Risk Factors and Body Composition. Nutrients 2015, 7, 7381–7398. [Google Scholar] [CrossRef]
- Mah, E.; Schulz, J.A.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew Consumption Reduces Total and LDL Cholesterol: A Randomized, Crossover, Controlled-Feeding Trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef] [PubMed]
- de Vilela, D.L.S.; da Silva, A.; Pelissari Kravchychyn, A.C.; Bressan, J.; Hermsdorff, H.H.M. Effect of Nuts Combined with Energy Restriction on the Obesity Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2024, 13, 3008. [Google Scholar] [CrossRef]
- Jung, H.; Chen, C.Y.O.; Blumberg, J.B.; Kwak, H.K. The Effect of Almonds on Vitamin E Status and Cardiovascular Risk Factors in Korean Adults: A Randomized Clinical Trial. Eur. J. Nutr. 2018, 57, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effect of Almond Supplementation on Glycemia and Cardiovascular Risk Factors in Asian Indians in North India with Type 2 Diabetes Mellitus: A 24-Week Study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef]
- Imran, T.F.; Kim, E.; Buring, J.E.; Lee, I.M.; Gaziano, J.M.; Djousse, L. Nut Consumption, Risk of Cardiovascular Mortality, and Potential Mediating Mechanisms: The Women’s Health Study. J. Clin. Lipidol. 2021, 15, 266–274. [Google Scholar] [CrossRef]
- Tindall, A.M.; Johnston, E.A.; Kris-Etherton, P.M.; Petersen, K.S. The Effect of Nuts on Markers of Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Liu, J.F.; Li, S.C.; Huang, C.L.; Hsirh, A.T.; Weng, S.F.; Chang, M.L.; Li, H.T.; Mohn, E.; Chen, C.Y.O. Almonds Ameliorate Glycemic Control in Chinese Patients with Better Controlled Type 2 Diabetes: A Randomized, Crossover, Controlled Feeding Trial. Nutr. Metab. 2017, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, S.; Daneshzad, E.; Lotfi, K.; Azadbakht, L. The Effects of Almond Consumption on Inflammatory Biomarkers in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2022, 13, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Y.; Ojo, O.; Wang, L.L.; Wang, Q.; Jiang, Q.; Shao, X.Y.; Wang, X.H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef]
- Nogueira, M.; Luísa, A.; Afonso, F.; Al-Shammari, S.K.; Al-Nouri, D.M.; Arzoo, S.; Al-Harbi, L.N. Effect of Different Nuts Oil Consumption on Morphological Features and Some Biomarkers of Inflammation in Adjuvant-Induced Arthritis (AIA) Rat Model. Appl. Sci. 2023, 13, 3318. [Google Scholar] [CrossRef]
- Hong, M.Y.; Groven, S.; Marx, A.; Rasmussen, C.; Beidler, J. Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats. Molecules 2018, 23, 3126. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.P.N.; Cominetti, C.; Simões Filho, A.; Naves, M.M.V. Baru Almond Improves Lipid Profile in Mildly Hypercholesterolemic Subjects: A Randomized, Controlled, Crossover Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Maranhão, P.A.; Kraemer-Aguiar, L.G.; De Oliveira, C.L.; Kuschnir, M.C.C.; Vieira, Y.R.; Souza, M.G.C.; Koury, J.C.; Bouskela, E. Brazil Nuts Intake Improves Lipid Profile, Oxidative Stress and Microvascular Function in Obese Adolescents: A Randomized Controlled Trial. Nutr. Metab. 2011, 8, 32. [Google Scholar] [CrossRef]
- Li, N.; Jia, X.; Chen, C.Y.O.; Blumberg, J.B.; Song, Y.; Zhang, W.; Zhang, X.; Ma, G.; Chen, J. Almond Consumption Reduces Oxidative DNA Damage and Lipid Peroxidation in Male Smokers. J. Nutr. 2007, 137, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Liu, Y.H.; Chen, C.M.; Chang, W.H.; Chen, C.Y.O. The Effect of Almonds on Inflammation and Oxidative Stress in Chinese Patients with Type 2 Diabetes Mellitus: A Randomized Crossover Controlled Feeding Trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of Pistachio Nuts on Body Composition, Metabolic, Inflammatory and Oxidative Stress Parameters in Asian Indians with Metabolic Syndrome: A 24-Wk, Randomized Control Trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, N.A.; Paskakulis, L.C.; Garcias, R.C.; Botelho, F.F.R.; Toledo, G.Q.; Cury, M.F.R.; Carvalho, N.Z.; Mendes, G.E.F.; Iembo, T.; Bizotto, T.S.G.; et al. Prior Intake of Brazil Nuts Attenuates Renal Injury Induced by Ischemia and Reperfusion. J. Bras. Nefrol. 2018, 40, 10–17. [Google Scholar] [CrossRef]
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.G.; Gheldiu, A.M.; Mates, L.; et al. Antioxidant Effects of Walnut (Juglans regia L.) Kernel and Walnut Septum Extract in a D-Galactose-Induced Aging Model and in Naturally Aged Rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative Effects and Mechanism Study of Bioactive Peptides from Defatted Walnut (Juglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).