Abstract
Lignin, one of the most abundant biopolymers on Earth, holds significant promise as a feedstock for applications such as resins, biofuels, foams, and carbon fibres. However, despite extensive research, lignin remains largely underutilised, with its primary use limited to combustion for energy. While lignin’s structural features are well documented, there is a lack of consistent data on its key physical properties such as density. This study addresses that gap by providing experimentally determined values for skeletal and bulk densities of lignins obtained through different extraction methods, including Kraft; soda pulping; and particularly the ionoSolv process, using ionic liquids such as N,N-dimethyl butyl ammonium hydrogen sulphate ([DMBA][HSO4]). The results reveal correlations between lignin chemical structure and density in ionoSolv-extracted lignins from Eucalyptus Red Grandis, suggesting opportunities to tune the extraction parameters for targeted material properties. The skeletal density of the lignin samples ranged from 1.3370 to 1.4598 g/cm3, while the bulk density varied more widely—from 0.0944 to 0.5302 g/cm3—reflecting significant differences in particle packing and porosity depending on the biomass source and extraction method. These findings contribute valuable data for process design and scale-up, advancing the commercial viability of lignin-based products.
1. Introduction
Lignin, an aromatic polymer formed of phenyl propane units, is the second most abundant polymer in nature after cellulose, and its valorisation is key for the successful development of future biorefineries. Lignin is found in lignocellulosic biomass embedding cellulose and hemicellulose in the form of lignin–carbohydrate complexes. It provides plants cell walls with strength, rigidity, and water stability [1,2]. Lignin produced industrially through processes such as Kraft or sulfite pumping represents a large portion of a waste stream in plant biomass processing. For instance, in 2015, nearly 100 million tons of lignin were produced, with annual lignin production expected to increase by 225 million tons per year in 2030 due to increased demand for biofuel production [3]. It is a complex and amorphous structure synthesised by plants from three main monolignols: p-coumaryl alcohol (H-type), coniferyl alcohol (G-type), and sinapyl alcohol (S-type) [4]. Incomplete and modified monolignols are also found in its structure. Lignin content and composition depend on the species, i.e., gymnosperm woods (softwoods) have high lignin content (25 to 35%), formed mainly by G-type units and low amounts of H units; angiosperm woods (hardwoods) contain 15 to 28% lignin, formed by G-type and S-type units and traces of H units; non-woody monocotyledon plants (grasses) have lower lignin contents, usually in the range of 9 to 20%, with the three types of G, S, and H units [4,5,6].
Before being analysed or monetised, lignin needs to be extracted and isolated from the original biomass matrix. It needs to be highlighted that the structure and properties of lignin fractions recovered after biomass processing are affected by the processes employed to extract it from the plants [7]. Therefore, the final structure and physicochemical properties of the recovered lignin depend not only on the species, but also on the isolation technique and the operational conditions applied. Traditionally, milled-wood lignin is considered to be the closest to native lignin, and commonly used as a rough model of native lignin when assessing the modifications to the lignin properties caused by other isolation processes [7]. E.g., the molecular weight (MW) of Kraft lignins is typically in the range of 1000 to 3000 Da, though it can go as high as 19,000 Da; lignins obtained by the sulfite method have MWs ranging from 1000 Da to 50,000 Da, depending on the feedstock; soda lignins, usually obtained from grasses, have MWs ranging from 1000 to 3000 Da, comparable to Kraft lignins; organosolv lignins also have low MWs (typically between 500 and 5000 Da) and low ash and carbohydrate contents [8].
Despite its importance, lignin is a major obstacle in the production of biofuels and other products from plant biomass (cellulose and hemicellulose fractions), as it is difficult to break down and extract and it also interacts negatively with enzymes and microorganisms [9]. However, research is underway to develop more efficient methods to remove lignin from plant biomass, which could help to make biofuels and other products more economically viable. Various approaches involve the use of ionic liquids—low-melting-point salts with a vast and tunable chemical space—offering diverse strategies for biomass fractionation [10]. The ionoSolv method employs protic ionic liquids such as N,N-dimethylbutylammonium hydrogen sulphate, [DMBA][HSO4], and monoethanolammonium acetate, [MEA][OAc], as cheaper alternatives to the more expensive aprotic ones, such as imidazolium-based ionic liquids [11,12,13]. The ionoSolv process can effectively remove lignin from the lignocellulosic biomass, with delignification rates of up to 90%, and produces lignins that can be tuned to be highly depolymerised or recondensed depending on the pretreatment conditions [14]. Furthermore, they can be recovered with targeted molecular weight and polydispersity using staged antisolvent addition during the precipitation step [15]. Both effects can be combined to produce lignins with controlled properties aimed at specific applications.
Currently, lignin is predominantly (approximately 98%) burned to generate heat and electricity in pulp and paper biorefineries [16]. Due to its high calorific heat, lignin has been mixed with coal and the mixture is used as fuel for the pulping boiler [17]. It is also known that the addition of lignin can increase the density and hydrophobicity of the composite materials, which could be beneficial for packaging or surface coating. Lignin can act like a glue by melting into the fibre structure and potentially cross-linking to the material’s functional groups such as hydroxyl [18]. However, in order to improve the overall economic feasibility of these refineries, alternative pathways for valorisation of lignin are necessary. The development of new valorisation pathways requires detailed physico-chemical characterisation of lignin [17,19]. While significant data on the structural characterisation of lignin can be found in the scientific literature, there is a lack of both density data and also comprehensive information on the relationship between its chemical structure and volumetric properties, which are essential for its use as fuel or a material, as previously mentioned. This study provides pioneering volumetric properties for several types of lignin —Kraft, soda, and ionoSolv—obtained from various lignocellulosic biomass feedstocks such as miscanthus, eucalyptus, and spruce, with a focus on ionoSolv lignins. Correlations between these volumetric properties and structural spectroscopic data (2D-HSQCT, 31P-NMR) are drawn and the obtained values are compared to literature data and ranges.
2. Materials and Methods
2.1. Lignins
Wheat straw soda lignin was purchased from Protobind 1000 (Rüschlikon, Switzerland). Kraft European softwood lignin was purchased from Södra (Växjö, Sweden). The Kraft lignin used was Lineo™ Prime, purchased from Stora Enso Oyj (Helsinki, Finland). Spruce softwood or mixed (M.) softwood lignin was obtained from the pretreatment of waste spruce softwood with [DMBA][HSO4], at a temperature of 145 °C for 30 min. Eucalyptus red grandis lignins were obtained by pretreatment of Eucalyptus red grandis with [DMBA][HSO4] at different times and temperatures [20]. Eucalyptus globulus lignins were obtained via hydrothermal pretreatment of the biomass and then subjected to treatment with [MEA][OAc] at 150 °C for 60 and 90 min [13].
To remove contaminants, lignin samples were washed by loading a 50 mL centrifuge tube with 2 g (dry basis) of the corresponding lignin sample and filled to capacity with deionised water. Samples were mixed by shaking at 2000 rpm for 30 min, followed by centrifugation for 45 min at 3000 rpm and removal of the supernatant by decantation. This was repeated four times. Finally, lignin samples were freeze-dried at <0.1 bar for 48 h. It is important to highlight the findings of this study are thus limited to freeze-dried lignins and may vary with other types of drying methods such as oven and vacuum. However, the skeletal density should not be affected by such changes in drying methods, only the bulk density may be affected. Additionally, the lignins generated in this study were not subjected to compositional analysis according to the NREL protocol, given that the goal was to relate their physico-chemical structure to density.
2.2. Skeletal Density
Skeletal density values for the lignin samples were measured by an AccuPyc II 1340 helium pycnometer (Micromeritics, Norcross, GA, USA). The freeze-dried samples were introduced in a calibrated volume of 1 cm3, which was then flushed 20 times with high-purity helium. Density measurements were performed 10 times and the average values and standard deviations were reported.
2.3. Bulk Density
The bulk density was measured by weighing the mass of lignin with an analytical balance with a precision of 0.1 mg in a pre-weighed glass vial. To determine the volume, the vial was taped against the table to remove empty spaces and to achieve an equal distribution of the powder, followed by marking the level on the vial. Lignin was removed and the container was filled with DI water to the same level to determine the mass of water. Finally, the volume was calculated using the density value of ρ = 0.9982 g/cm3. The determinations were performed in triplicate. The average values with the standard deviation are reported.
2.4. Particle Size
The particle size of the lignin samples was measured with a MasterSizer 2000 (Malvern Instruments, UK) with a Hydro SM sample dispersion unit. Before each measurement, around 100 mL of fresh DI water was filled and recirculated between the dispersion unit and the measuring cell, and a background blank was measured. Then, suspensions containing the samples to be recorded were added sequentially to the apparatus and recirculated in the system until the obscuration surpassed the threshold value (acceptable range between 5 and 50%, with the ideal range being between 10 and 20%). The refractive index was set at 1.530. For each sample, 5 measurements were recorded and the average values for d(0.10), d(0.50), d(90), Dmin, Dmax, and peak values are reported.
2.5. Two-Dimensional-HSQC
Two-dimensional-HSQC-NMR spectra were recorded on a Bruker 600 MHz spectrometer (pulse sequence hsqcetgpsi, 2 spectral width of 10 ppm in F2 (1H) with 2048 data points and 160 ppm in F1 (13C) with 256 data points, 16 scans and 1 s interscan delay) according with Malaret et al. (2020) [20]. The spectra were analysed using the software MestReNova (Version 8.0.0, Mestrelab Research 2012). All spectra were obtained with reference to the DMSO-d6 signal at 2.50 ppm (1H) and 39.520 ppm (13C). For ether linkages, the C-Hα-signals were integrated. The integral sizes of S2,6′ and S2,6 were divided by two to account for the twice-as-large signal due to the symmetry in the S unit.
2.6. 31P-NMR
The protocol followed for phosphitylating the lignins was that of Pu et al. (2011) [21]. A 1.6:1 (v/v) pyridine-to-deuterated chloroform solvent solution (50 mL) was prepared in a round-bottomed flask. A mixed solution was then made by dissolving 100 mg of cyclohexanol and 90 mg of chromium acetylacetonate in 25 mL of the solvent. Lignin samples (20–25 mg) were weighed into 4 mL vials, each containing a stirring bar, followed by the addition of 400 µL of the solvent solution and 150 µL of the mixed solution. Samples were stirred at 400 rpm for 5 min to dissolve the lignin, after which 70 µL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (Sigma-Aldrich, St. Louis, MI, USA) was added to each sample for phosphitylation. The 31P31-NMR spectra were then recorded on a Bruker 600 MHz spectrometer.
3. Results
3.1. Density
Lignin extracted from biomass is often recovered as a powder. Two types of density can be associated with powders. Bulk (or envelope) density [ρbulk] is determined for porous materials when pore space spaces within the material and the inter-particle void volume are included in the volume measurement. It is an important parameter for fuel deliveries on a volume basis, and together with the net calorific value, it determines the energy density, which is an important parameter for the estimation of space requirements for transport and storage of solid fuels [22]. Skeletal density [ρ] is the ratio of the mass of solid material to the sum of the volumes of the solid material and closed (or blind) pores within the material [23]. The experimental values for different types of lignins extracted with different methods are shown in Table 1.

Table 1.
Experimental values for the skeletal and bulk density for different lignins.
From the values reported in Table 1, the measured values for the skeletal density of the different lignin samples at 25 °C range between 1.3370 and 1.4794 g/cm3, which correspond to values reported in the literature. For instance, the Databook of Adhesion Promoters reported the skeletal density of lignin at 20 °C to be in the range of 1.350–1.500 kg/m3 [24]. It has been noticed that the upper value of 1.5 kg/m3 has been used to model biomass conversion processes [25].
Some interesting trends for the skeletal density of the lignins can be observed: in general skeletal densities for hardwoods seem higher than for softwoods and grasses. The latter two have comparable overall values, with an apparent slightly higher value for softwood. The tested ionoSolv softwood lignin sample showed higher skeletal density than the Kraft softwood lignins, even though it was obtained under mild pretreatment conditions, using the [DMBA][HSO4] at 145 °C for 30 min, so a low degree of condensation and presence of lignin–carbohydrate complexes would be expected [14,20]. From the analysis of the ionoSolv hardwood lignins, obtained from Eucalyptus red grandis, also using [DMBA][HSO4], it was found that for the samples recovered after pretreatments at 150 °C, the skeletal density increased from 1.3708 g/cm3 for the sample recovered after 60 min to 1.4454 g/cm3 for the sample recovered after 90 min, which is regarded as the range of optimal pretreatment times for most applications at this temperature [14,20]. For longer pretreatments it was observed that the skeletal density started decreasing again. A possible explanation for this is that under higher-severity conditions, lignin oligomers dissolved in the pretreatment media can undergo side reactions with sugar degradation products, hence the recovered precipitate can be contaminated with humin moieties, in what is often referred to as pseudo-lignin [26]. For the lignin samples recovered after pretreatments at 120 °C, a trend of decreasing skeletal densities for pretreatment times longer than 120 min was also observed. However, this decrease was less pronounced, likely due to the milder pretreatment conditions, which were not as favourable for the formation of humins and pseudo-lignin in the pretreatment media. Interestingly, the lignins turned darker at higher severities, which could be an indication of these physico-chemical changes. The skeletal density of the samples treated with [MEA][OAc] shows the same tend, it increased with increasing pretreatment time. The experimental results suggest a difference in the density between hardwood and softwood lignins; however, a computational study to simulate the density of hardwood and softwood lignins resulted in the same skeletal density value of 1280 kg/m3 for both lignin types, which seems a very low value when compared to experimental data [27].
Kraft and soda lignins present moderate skeletal densities (~1.34–1.36 g/cm3) but relatively high bulk densities (~0.46–0.50 g/cm3), indicating compact, less porous particle structures. In contrast, ionoSolv lignins, particularly from eucalyptus, show consistently higher skeletal densities (up to 1.46 g/cm3), reflecting a dense molecular framework, yet they have significantly lower bulk densities (as low as 0.094 g/cm3), suggesting highly porous or loosely packed powders. Such a large difference between skeletal and bulk densities in ionoSolv lignins implies a high interparticle void volume and low packing density, likely due to differences in particle morphology and processing conditions. Overall, ionoSolv lignins demonstrate greater bulk porosity compared to Kraft and soda lignins, which could influence their behaviour in material applications such as composites or adsorbents. For instance, in a work by Barbosa et al. (2023) [28], they studied the addition of Kraft lignin in the pellets of a mixture of lignocellulosic residues (eucalyptus and corn) and they observed improvements in the physical and mechanical properties of the pellets, such as bulk density, mechanical durability, and fine content; such properties ultimately allow for the transportation of a greater amount of mass and energy, whilst also preserving the integrity of the biofuels. In a study performed by Elniski et al. (2023) [29], they examined the effect of lignin as an additive in pellets composed of willow. They employed two types of lignin: lignin recovered from the hot-water extraction of willow and commercial softwood Kraft lignin. They also observed a significant increase in the energy content, bulk density, and durability of lignin-added pellets. And, more importantly, there was also a significant reduction in carbon monoxide emissions from the willow pellets from hot-water-extraction lignin, showing how important is to consider the utilisation of lignin as an additive and how physico-chemical properties such as density need to be considered for such applications.
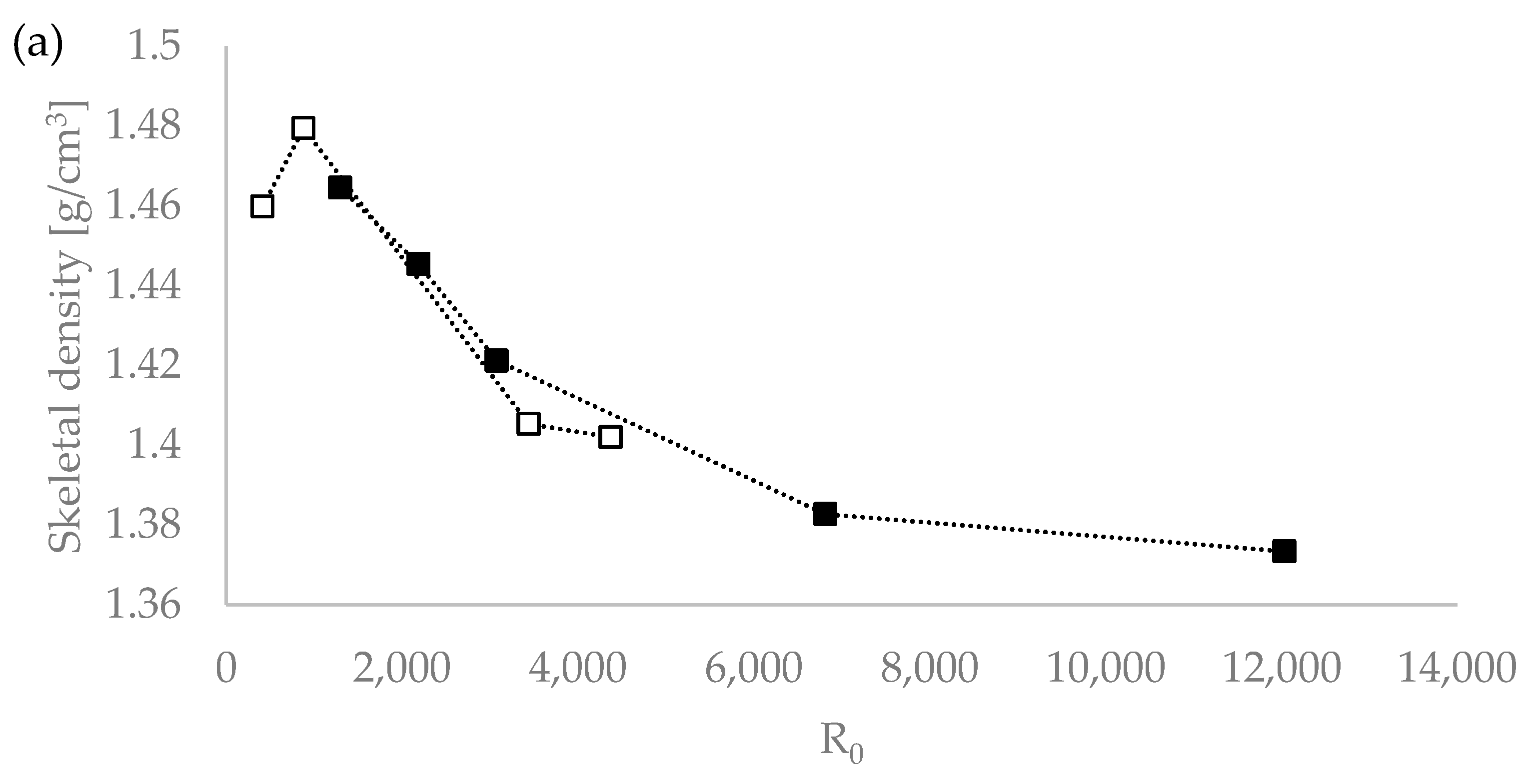
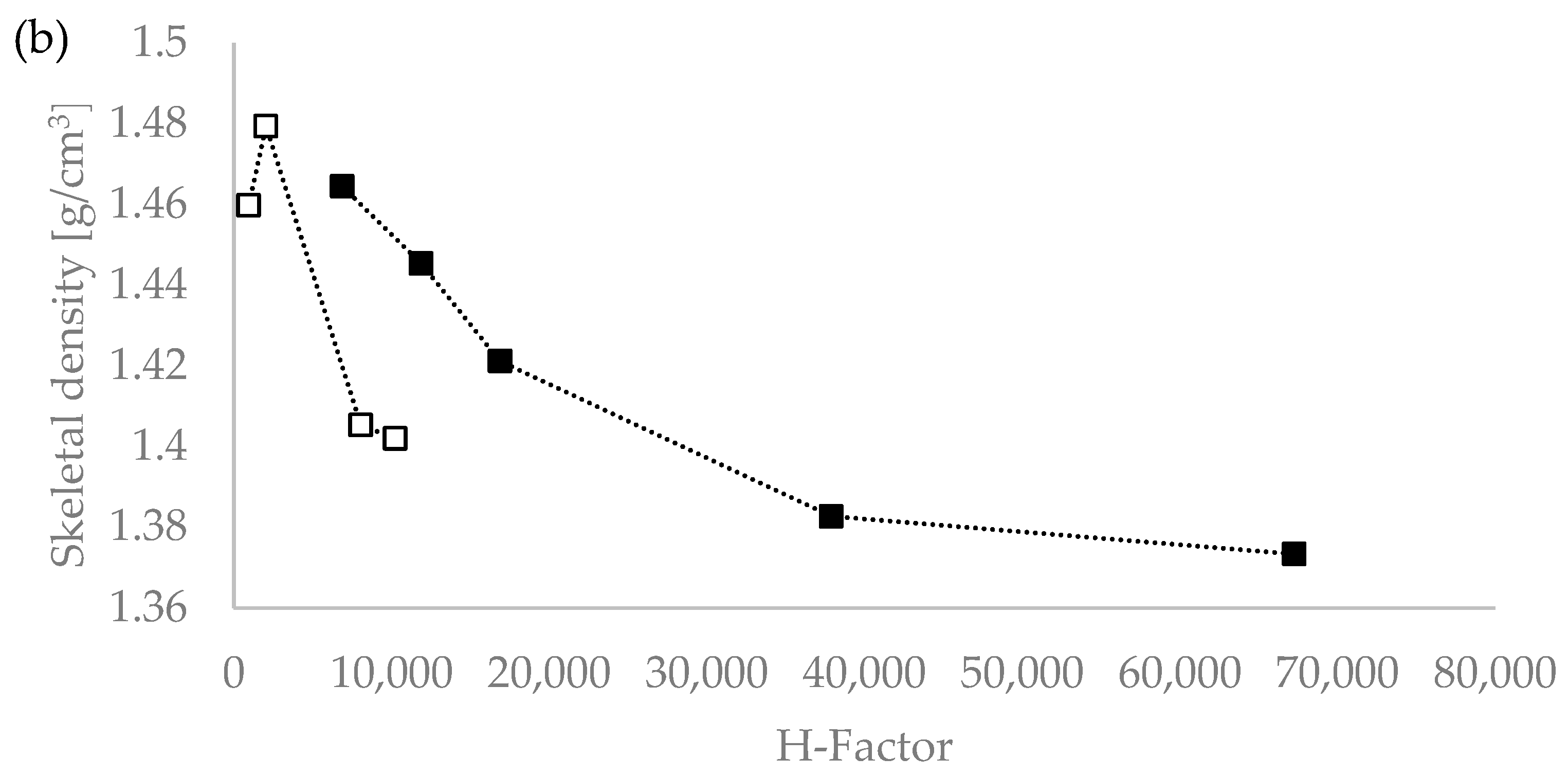
We tested the hypothesis of the influence of the severity of the extraction conditions on the density value by plotting the lignin density vs. the severity parameters R0 and the H-factor, with R0 being the severity factor developed by Chornet and Overend for the breakdown of hemicellulose [30,31,32]; and the H-factor equation having been developed by Rydholm to relate time and temperature for delignification in Kraft pulping [33]). These factors together account for the impact of acidity, time, and temperature on the severity of the pretreatment and can be used to compare biomass pretreatments performed under different temperatures (ESI). Figure 1 shows that the R0 severity factor correlates very well with the lignin density for the temperatures used (120 °C and 150 °C) [20]. This suggests that the severity factors can be used to adjust the extraction process conditions to achieve a lignin with a targeted density.
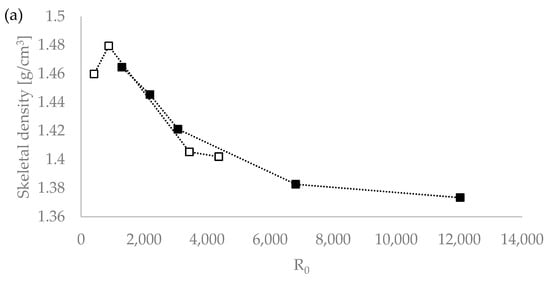
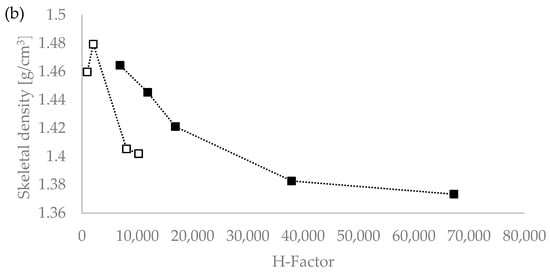
Figure 1.
Skeletal density values of Eucalyptus red grandis lignin extracted with [DMBA][HSO4] as a function of pretreatment severity using the severity parameters R0 (a) and H-factor (b). White squares (□) for 120 °C and the filled squares (▪) for 150 °C series. Severity values taken from Malaret et al. (2020) [20].
3.2. Density and Chemical Structure Correlations
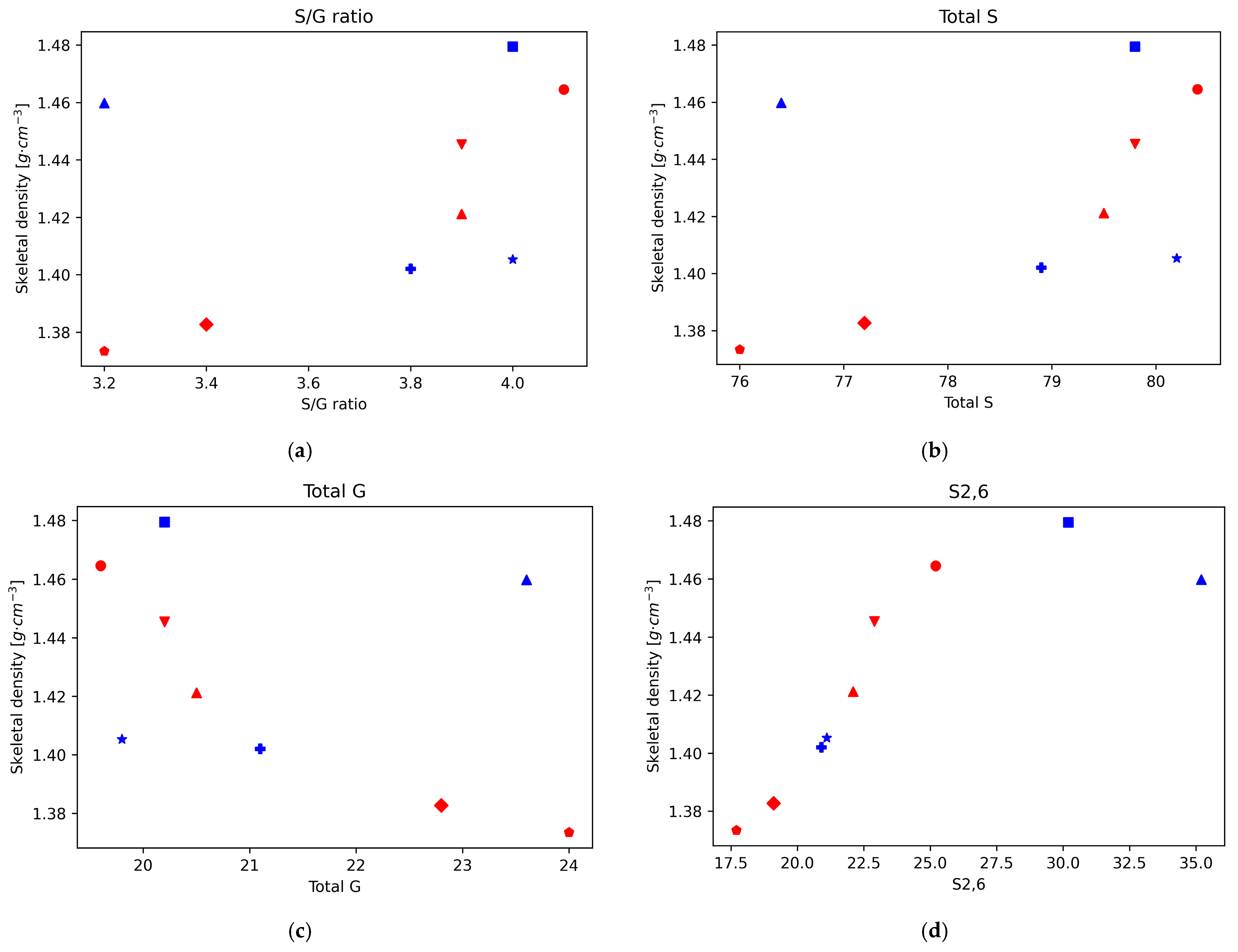
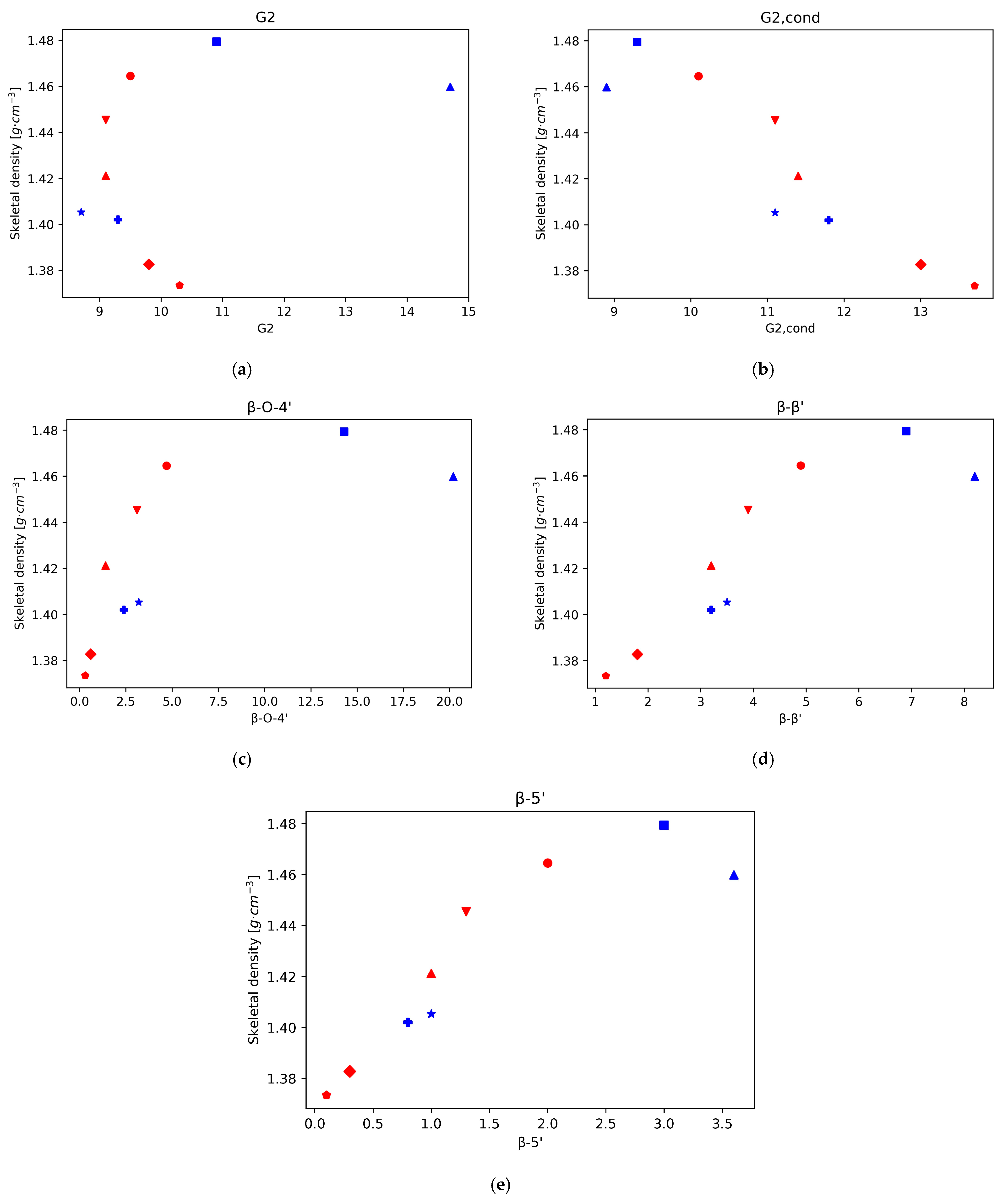
In a previous work, the chemical structure of eucalyptus lignin fractions obtained by the ionoSolv process at 120 °C and 150 °C at various time points was investigated by 2D HSQC-NMR spectroscopy, focusing on the subunit and ether linkage content and the degree of C-C bond condensation [20]. Here, we analyse the correlations of the structural features of the recovered ionoSolv lignins, such as the degree of condensation and the abundance of certain bonds and subunits, with the skeletal density. For this we plot the density values of those eucalyptus lignins as a function of the HSQC integral value, normalised to 100 S + G units, for the different investigated lignin bonds and subunits (Figure 2). It should be noted that, for eucalyptus, the main subunit is the syringyl type, followed by the guaiacyl type, with only residual traces of p-hydroxyphenyl units [20].
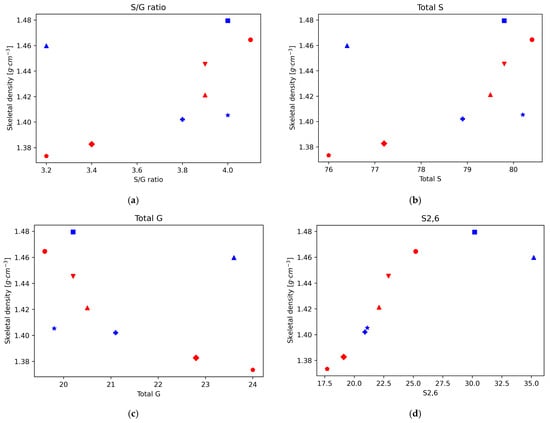
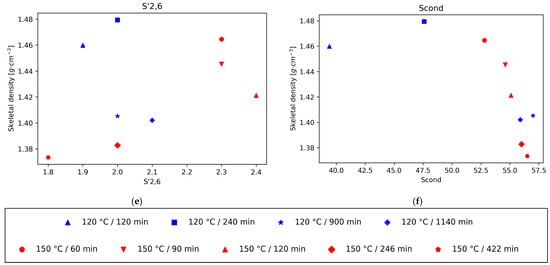
Figure 2.
Skeletal density for Eucalyptus red grandis as a function of the integral area obtained by 2D-HSQC-NMR. (a) S/G ratio. (b) Total S. (c) Total G. (d) S2,6. (e) S’2,6. (f) Scond.
In our previous work, it was found that the S/G ratio in the lignins precipitated from the ionoSolv pretreatment of eucalyptus increased during the first 15 h of pretreatment at 120 °C, and decreased after that point. Also, that for pretreatment at 150 °C the S/G ratio in the precipitated lignin decreased over the whole time range. This seems consistent with recent findings suggesting that ionic liquid pretreatment under mild conditions gives preferential guaiacyl unit degradation, whereas for harsher conditions (higher temperatures and/or longer pretreatment times) the degradation occurs preferentially on the S units by an acidic mechanism [34]. The plot of the variation of density vs. S/G ratio shows a direct correlation, with higher densities for higher S/G ratios for the lignins recovered from the pretreatment at 150 °C, where the degradation mechanism follows the acidic mechanism with preference for the S units.
For the lignins recovered from the 120 °C pretreatment, the degradation mechanism seems to shift and the lignin where the S degradation is predominant (1140 min) is close to the trend observed for the 150 °C lignins, which follow the same mechanism, but for the lignins obtained under the milder conditions the trend is not clear and both mechanisms might be playing a role. In brief, it seems that the skeletal density of eucalyptus ionoSolv lignins follows a clear trend when the S degradation mechanism is predominant (S subunits are predominant in eucalyptus), and higher degradation of S units leads to lower skeletal densities. This is further supported by the plots of density vs. total S and total G units. In both cases, for the lignins recovered under conditions where the acidic mechanism is predominant, there is a correlation with the density that matches that of the S/G ratio (positive for the case of total S content—higher densities at higher S content— and negative for the case of total G content—lower densities at higher G content). On the other hand, under the mildest conditions, when the ionic liquid mechanism that degrades G units preferentially is predominant, those trends are not followed.
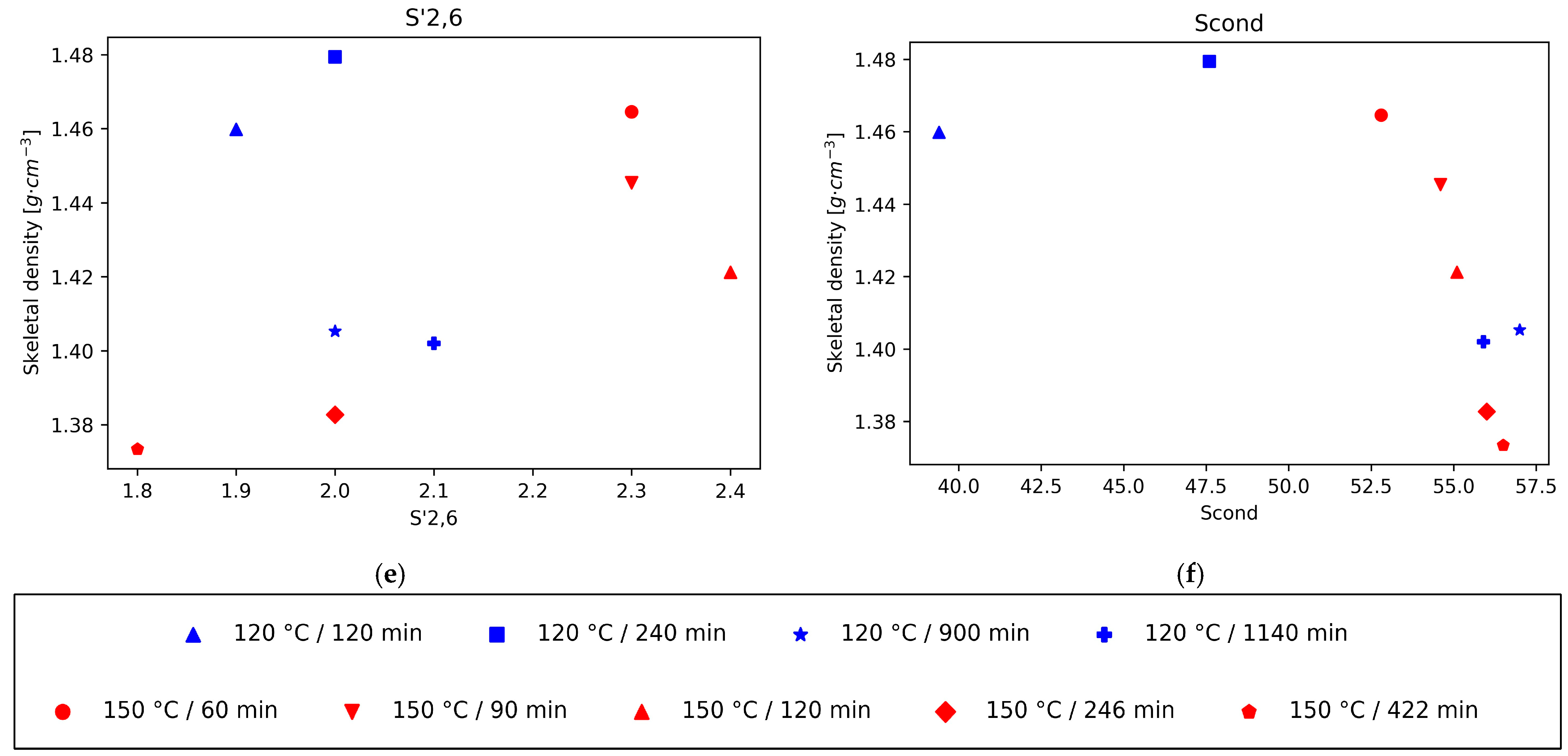
The decrease in the intensity of the normalised integrals S2,6 and S’2,6 and the increase in intensity in the normalised integral of Scond correspond to the condensation of S units, which increases at longer pretreatment times and higher temperatures. Likewise, the variation in the intensity of the different G signals with respect to the total amount of G provides information on the condensation of G units; when this happens, G2 shifts to G2cond. Our previous work showed that the S aromatic carbons have higher reactivity than the G aromatic ones, and the condensation is quicker for S units than for G units, with a quicker shift in S signals to Scond than in the case of G signals to Gcond. Furthermore, in the case of G units, condensation happens mostly on position 6, but also some condensation can happen in position 5 at high temperatures and long pretreatment times. This is consistent with the trends found when plotting the density of the different lignins as a function of the different subunit intensities.
There is a strong positive correlation of the density values with the normalised integral values of the subunit S2,6, and the lignin linkages β-5′ and β-β’, while a negative correlation is observed with the subunits Scond and G2,cond (Figure 2). For the normalised integral values of the subunit S2,6 a strong positive correlation with the density values was found, while a negative correlation was observed with Scond. Therefore, the representations of lignin density vs. the intensity of S2,6 and Scond suggests, in both cases, that the density of lignin decreases with increasing condensation levels of S units. Furthermore, for the lignins obtained after pretreatment at 150 °C, the relationship between the S’2,6 signal intensity and the density also follows a positive correlation, as in the case of S2,6, which further supports the idea of lower densities at higher condensation levels. For the lignins obtained at 120 °C this trend is not followed but, interestingly, the density roughly follows a decreasing correlation with the pretreatment time. This could be related to the signal intensity of the integral normalised for 100 S + G units, which is much lower for S’2,6 (around 2%, while the intensities of S2,6 and Scond range between 17.7 and 37.9 and 31.4 and 57, respectively), in particular for the 120 °C lignins. The accuracy errors in the signal intensity measurements could be the culprit for the lack of trend in this case. A similar trend of lower density for higher condensation degree can be observed from the plot of density vs. G2cond graph. Finally, there is a correlation between lignin density and the normalised values of the subunits G2, G5, G6, and S’2,6.
Also, in accordance with that reported in literature, the main interunit linkage is β-O-4′, followed by resinol (β-β’) and phenylcoumaran (β-5′) [13]. Since the main subunit in eucalyptus lignin is the S unit, the 5 position is blocked, hence the lower proportion of phenilcoumaran structures. The intensity of the signals for all these interunit linkages decreases with time, in particular the β-O-4′ signals, since C-O bonds are more labile than C-C bonds (Figure 3). The cleavage of these linkages is related to depolymerisation of lignin. Again, when plotting the density of the isolated lignins vs. the signal intensity of the three types of linkages, β-O-4′, β- β’, and β-5′, a strong correlation can be seen for those lignins in which the acidity of the medium is the main driving force (lignins isolated from pretreatment at 120 °C for 900 min or more, and all the lignins isolated after pretreatments at 150 °C). This trend is particularly strong for the β-O-4′ signal. This result suggests that a higher degree of depolymerisation tends to rapidly lower the lignin density. It is worth noting, however, that this trend is not followed by the lignin fractions recovered at milder conditions (120 °C for less than 240 min), where the higher intensity of interunit linkages is obtained and densities, although high, are a bit lower than those observed just before the acidic mechanism starts dominating the chemistry of the medium. For the other bonds, G2, G5, and S2,6, total G, total S, and the S/G ratio, there is not a strong correlation between the skeletal density and the integral of the intensities.
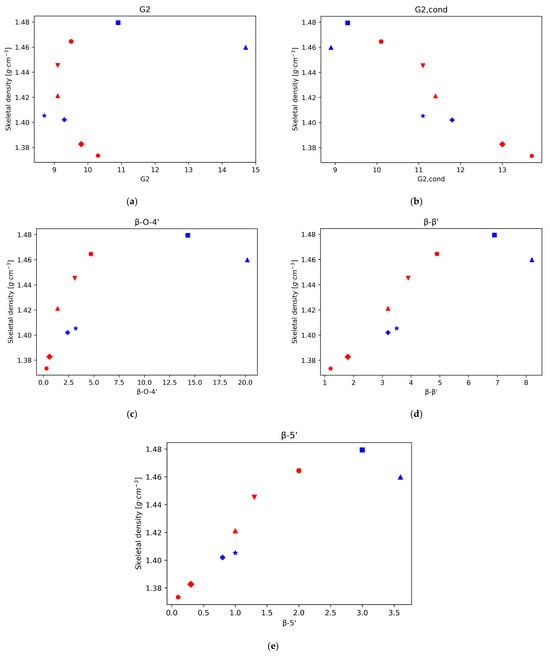

Figure 3.
Skeletal density for Eucalyptus red grandis as a function of the integral area obtained by 2D-HSQC-NMR. (a) G2. (b) G2,cond. (c) β-O-4′ (d) β-β’ (e) β-5′.
3.3. 31P-NMR
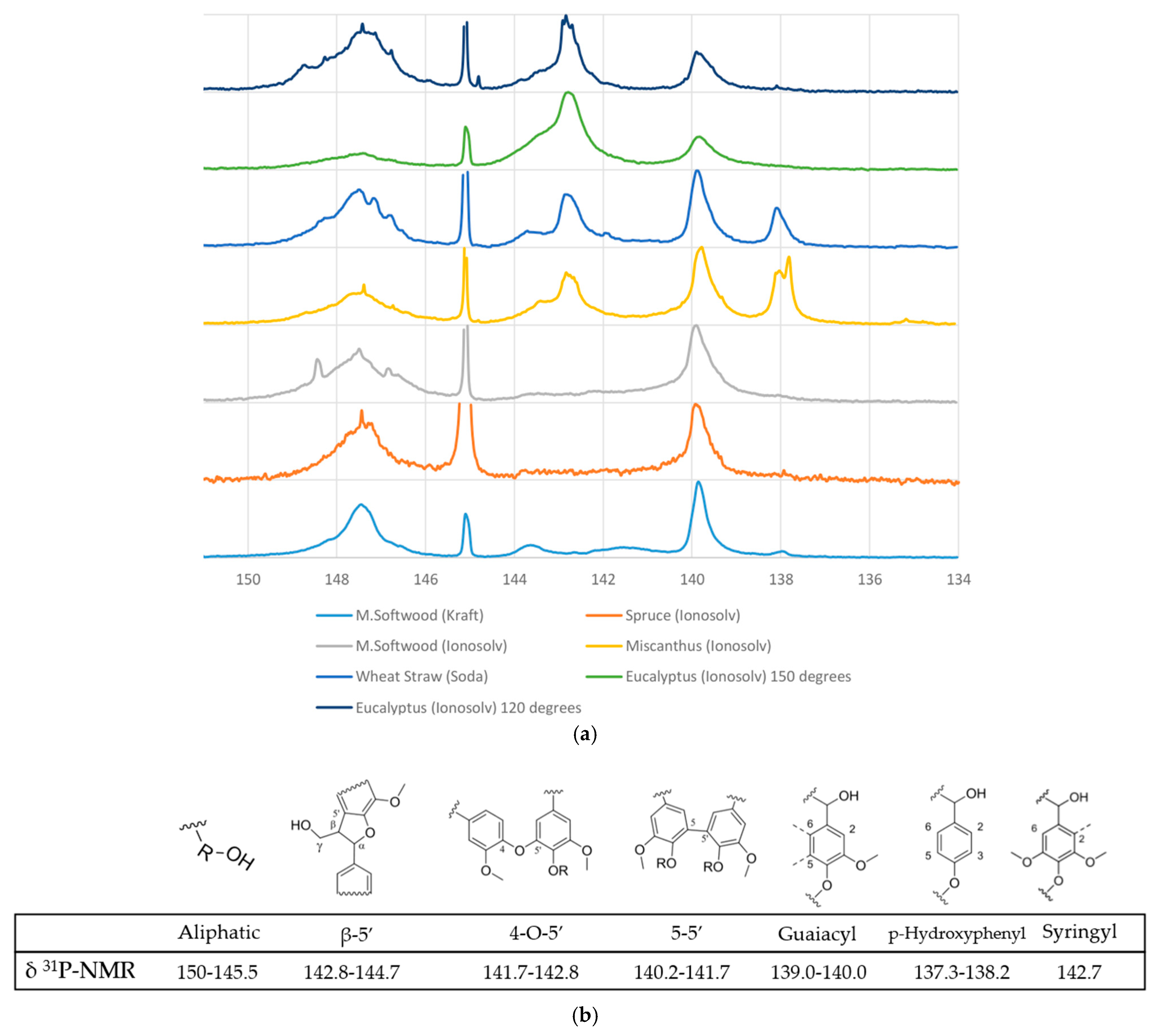
One of the most crucial and interesting functionalities of lignin is the hydroxyl group content, which characterises the polymer’s reactivity and behaviour. The quantitative 31P-NMR analysis results can be seen in Figure 4 and Table 2. The technique enables the quantification of aliphatic and aromatic hydroxyl groups, condensed hydroxyl units, and carboxylic acids, in mmol/g.
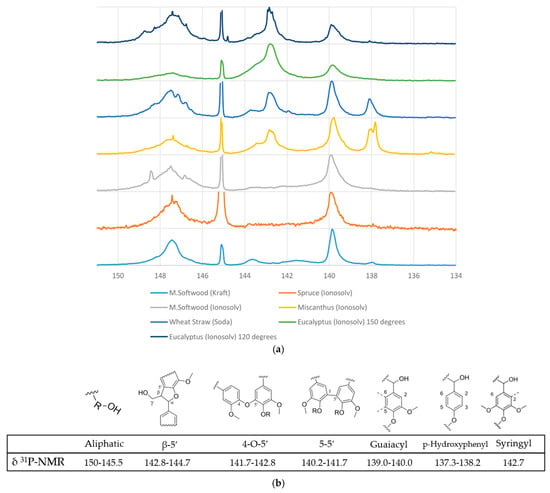
Figure 4.
(a) Stacked 31P-NMR spectra for ionoSolv, Kraft, and soda lignins. (b) Hydroxyl subunits and linkages within lignin analysed by 31P-NMR [35].

Table 2.
Lignin hydroxyl group molar content via 31P-NMR.
The 31P-NMR analysis reveals distinct differences in hydroxyl group composition among lignins derived from various biomass sources and treatments, especially regarding pretreatment severity. No correlations are observed between the skeletal density and any of the hydroxyl group moieties quantified by 31P-NMR (ESI). Aliphatic hydroxyl content is generally higher in lignins processed with the ionoSolv method, particularly at lower temperatures, with eucalyptus ionoSolv showing the highest value (224.40 mmol/g), suggesting milder depolymerisation. In contrast, spruce ionoSolv exhibited the lowest value for aliphatic hydroxyls (121.71 mmol/g), which may indicate condensation or degradation. The aliphatic hydroxyl content decreased with increased pretreatment severity, as can be seen by the eucalyptus ionoSolv from 120 °C to 150 °C (224 to 132 mmol/g). As reported by Brandt et al. (2015) [36], it is observed that aliphatic hydroxyl content decreases with length of pretreatment; in this case, the increase in temperature of pretreatment. This could be due to the loss of Cα hydroxyl groups during β-O-4 ether breakage and during condensation. The broad peak around the 5-substituted area may be due to contributions from phenolic ends caused by the cleavage of β-O-4 linkages, which was also noted by Brandt et al. (2015) [36].
Condensed linkages such as β-5′, 4-O-5′, and 5-5′ are prominent in M. softwood Kraft lignin, indicating extensive condensation during the Kraft process, while soda lignin from wheat straw shows moderate condensation. IonoSolv-treated lignins generally exhibit reduced condensation, except for eucalyptus ionoSolv, which shows an exceptionally high 5–5′ signal (396.63 mmol/g), likely due to overlap with syringyl groups. Aromatic hydroxyl distributions reflect biomass origin: softwoods (e.g., M. softwood, spruce) are rich in guaiacyl units, hardwoods (e.g., eucalyptus) show syringyl dominance, and grasses (e.g., wheat straw, miscanthus) contain a mix of syringyl, guaiacyl, and para-hydroxyphenyl units. These findings illustrate how both biomass type and processing method significantly influence the structural features of extracted lignin.
3.4. Particle Size
The particle size analysis of some of the lignin samples probed in this study can be seen in Table 3, together with data from other types of lignin from the literature. There were no clear trends observed for the particle size range and either the pretreatment type or condition employed. In fact, lignin particle size depends on several factors including the production method, lignin type, solvent type, stirring rate, and drying method [37]. Experimental values are reported here as they can provide ranges to design solid–liquid separation and purification units and other transformation processes for the production of lignin nanoparticles, or the use of lignin as an additive in cement, pellets, etc. [8,38].

Table 3.
Comparison between bulk densities and particle size of several types of lignin.
4. Conclusions
This study demonstrates that there are clear relationships between the structure of lignin and its skeletal density. Hardwood-derived lignins tend to exhibit higher skeletal densities compared to those from softwoods and grasses. Notably, the ionoSolv-extracted hardwood lignin displayed a greater skeletal density than the softwood lignins obtained via the Kraft process.
A general trend was observed in the ionoSolv process, where increasing pretreatment severity corresponded to a reduction in skeletal density. Among the various structural features, the β-β′ and β-5′ linkages—associated with the degree of condensation—showed the strongest correlations with skeletal density. These findings align with broader trends in lignin structure and pretreatment conditions. No significant correlations were found between density and the hydroxyl group content determined by 31P-NMR, or with particle size. Nevertheless, the density and particle size values reported here provide valuable baseline data for early-stage process modelling and scale-up efforts when detailed property data are unavailable.
To build a more comprehensive understanding of lignin density and its influencing factors, further investigation is necessary. This includes examining the impact of drying methods, conducting detailed compositional analyses, and expanding the dataset to include lignins derived from a wider variety of biomass sources and fractionation methods. Such efforts—combined with the insights into the relationship between process severity factors, lignin structure, and skeletal density demonstrated in this work—could further improve our understanding of lignin’s chemical transformations. This is essential for developing robust strategies to tailor lignin density for specific applications and to enhance the commercial viability of lignin-based materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13061848/s1, Figure S1. Relationship between skeletal density and (a) aliphatic hydroxyl groups, (b) p-hydroxyphenyl groups, (c) β-5’, (d) guaiacyl groups, (e) 5-5’, and (f) 4-O-5’.
Author Contributions
Conceptualisation, F.M.; methodology, F.M.; formal analysis, F.M., P.Y.S.N. and P.V.B.; resources, J.H.; data curation, C.H., F.M. and P.Y.S.N.; writing—original draft preparation, F.M.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
I was funded by the SuperGen Project while doing the experiments I did for this paper: Pedro Verdía Barbará would like to thank the EPSRC for its financial support via the “SuperGen Bioenergy Hub” project (EP/S000771/1).
Data Availability Statement
All data used in this study are provided either in the main manuscript or in the Electronic Supplementary Information.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT (version 4o) for the purposes of text editing. No GenAI tools were used for analysis and interpretation of data. The authors have reviewed and edited the output and take full responsibility for the content of this publication. The authors would like to acknowledge funding and support from the UKRI (EPSRC and BBSRC) Supergen Bioenergy Impact Hub 2023 (EP/Y016300/1), Supergen Bioenergy Hub (EP/S000771/1) and the Bio-derived and Bio-inspired Advanced Materials for Sustainable Industries (VALUED) programme (EP/W031019/1) and the United Kingdom Department for Science, Innovation, and Technology (DSIT) Royal Academy of Engineering Chair in Emerging Technologies (CiET-2223-135).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 31P-NMR | Phosphorus 31 nuclear magnetic resonance |
| 2D-HSQC | Two-dimensional heteronuclear quantum coherence |
| ρ | Density |
| Da | Daltons |
| DI | De-ionized |
| M. | Mixed |
| MW | Molecular weight |
| [MEA][OAc] | Monoethanol ammonium acetate |
| [DMBA][HSO4] | N,N-Dimethyl butylammonium hydrogen sulphate |
| R0 | Severity factor |
| rpm | Rotations per minute |
| Std Dev | Standard deviation |
| 31P-NMR | Phosphorus 31 nuclear magnetic resonance |
References
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Weigand, L.; Mostame, S.; Brandt-Talbot, A.; Welton, T.; Hallett, J.P. Effect of pretreatment severity on the cellulose and lignin isolated from Salix using ionoSolv pretreatment. Faraday Discuss. 2017, 202, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Ragauskas, A. (Eds.) Opportunities and challenges of lignin utilization. In Lignin Utilization Strategies: From Processing to Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1377, pp. 1–12. [Google Scholar] [CrossRef]
- Chambon, C.L.; Fitriyanti, V.; Verdía, P.; Yang, S.M.; Hérou, S.; Titirici, M.-M.; Brandt-Talbot, A.; Fennell, P.S.; Hallett, J.P. Fractionation by Sequential Antisolvent Precipitation of Grass, Softwood, and Hardwood Lignins Isolated Using Low-Cost Ionic Liquids and Water. ACS Sustain. Chem. Eng. 2020, 8, 3751–3761. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume XXV, pp. 233–253. [Google Scholar]
- Voitkevich, O.V.; Kabo, G.J.; Blokhin, A.V.; Paulechka, Y.U.; Shishonok, M.V. Thermodynamic properties of plant biomass components. Heat capacity, combustion energy, and gasification equilibria of lignin. J. Chem. Eng. Data 2012, 57, 1903–1909. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Philippi, F.; Malaret, F.; Welton, T. A review on machine learning algorithms for the ionic liquid chemical space. Chem. Sci. 2021, 12, 6820–6843. [Google Scholar] [CrossRef]
- Nakasu, P.Y.; Barbará, P.V.; Firth, A.E.; Hallett, J.P. Pretreatment of biomass with protic ionic liquids. Trends Chem. 2022, 4, 175–178. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Clarke, C.J.; Rabelo, S.C.; Costa, A.C.; Brandt-Talbot, A.; Hallett, J.P. Interplay of acid–base ratio and recycling on the pretreatment performance of the protic ionic liquid monoethanolammonium acetate. ACS Sustain. Chem. Eng. 2020, 8, 7952–7961. [Google Scholar] [CrossRef]
- Ovejero-Pérez, A.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Effect of autohydrolysis and ionosolv treatments on eucalyptus fractionation and recovered lignin properties. RSC Adv. 2023, 13, 10338–10348. [Google Scholar] [CrossRef] [PubMed]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Chambon, C.L.; Chen, M.; Fennell, P.S.; Hallett, J.P. Efficient Fractionation of Lignin- and Ash-Rich Agricultural Residues Following Treatment With a Low-Cost Protic Ionic Liquid. Front. Chem. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Opedal, M.T. Green materials from added-lignin thermoformed pulps. Ind. Crop. Prod. 2022, 185. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Malaret, F.; Gschwend, F.J.V.; Lopes, J.M.; Tu, W.-C.; Hallett, J.P. Eucalyptus red grandis pretreatment with protic ionic liquids: Effect of severity and influence of sub/super-critical CO2 atmosphere on pretreatment performance. RSC Adv. 2020, 10, 16050–16060. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- ISO 17828:2025; Solid Biofuels—Determination of Bulk Density, 2nd ed. International Organization for Standardization: London, UK, 2025. Available online: https://www.iso.org/standard/84596.html (accessed on 30 April 2025).
- ASTM D3766-08(2018); Standard Terminology Relating to Catalysts and Catalysis. ASTM International: West Conshohocken, PA, USA, 2024. Available online: https://store.astm.org/d3766-08r18.html (accessed on 30 April 2025).
- Wypych, A. (Ed.) Front Matter. In Databook of Adhesion Promoters; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Michelsen, F.A.; Foss, B.A. A comprehensive mechanistic model of a continuous Kamyr digester. Appl. Math. Model. 1996, 20, 523–533. [Google Scholar] [CrossRef]
- Chen, L.; Xin, J.; Ni, L.; Dong, H.; Yan, D.; Lu, X.; Zhang, S. Conversion of lignin model compounds under mild conditions in pseudo-homogeneous systems. Green Chem. 2016, 18, 2341–2352. [Google Scholar] [CrossRef]
- Zhang, L.; LeBoeuf, E.J. A molecular dynamics study of natural organic matter: 1. Lignin, kerogen and soot. Org. Geochem. 2009, 40, 1132–1142. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Vaz, S.; Colodette, J.L.; de Siqueira, H.F.; da Silva, C.M.S.; Cândido, W.L. Effects of kraft lignin and corn residue on the production of eucalyptus pellets. BioEnergy Res. 2023, 16, 484–493. [Google Scholar] [CrossRef]
- Elniski, A.; Dongre, P.; Bujanovic, B.M. Lignin Use in Enhancing the Properties of Willow Pellets. Forests 2023, 14, 2041. [Google Scholar] [CrossRef]
- D’Amour, R. Modèle Réactionnel de La Dépolymérisation Hydrolytique de La Cellulose En Cellulose Microcristalline; Université de Sherbrooke: Sherbrooke, QC, Canada, 2001. [Google Scholar]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Joseph, P.; Opedal, M.T.; Moe, S.T. The O-factor: Using the H-factor concept to predict the outcome of organosolv pretreatment. Biomass Conv. Bioref. 2023, 13, 6727–6736. [Google Scholar] [CrossRef]
- Wyman, C.E.; Yang, B. Combined Severity Factor for Predicting Sugar Recovery in Acid-Catalyzed Pretreatment Followed by Enzymatic Hydrolysis. In Hydrothermal Processing in Biorefineries; Ruiz, H.A., Hedegaard Thomsen, M., Trajano, H.L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 161–180. [Google Scholar] [CrossRef]
- Ovejero-Pérez, A.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Acidic depolymerization vs ionic liquid solubilization in lignin extraction from eucalyptus wood using the protic ionic liquid 1-methylimidazolium chloride. Int. J. Biol. Macromol. 2020, 157, 461–469. [Google Scholar] [CrossRef]
- Stücker, A.; Podschun, J.; Saake, B.; Lehnen, R. A novel quantitative31P NMR spectroscopic analysis of hydroxyl groups in lignosulfonic acids. Anal. Methods 2018, 10, 3481–3488. [Google Scholar] [CrossRef]
- Brandt, A.; Chen, L.; van Dongen, B.E.; Welton, T.; Hallett, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef]
- Posoknistakul, P.; Tangkrakul, C.; Chaosuanphae, P.; Deepentham, S.; Techasawong, W.; Phonphirunrot, N.; Bairak, S.; Sakdaronnarong, C.; Laosiripojana, N. Fabrication and Characterization of Lignin Particles and Their Ultraviolet Protection Ability in PVA Composite Film. ACS Omega 2020, 5, 20976–20982. [Google Scholar] [CrossRef]
- Tsvetkov, M.V.; Salganskii, E.A. Lignin: Applications and Ways of Utilization (Review). Russ. J. Appl. Chem. 2018, 91, 1129–1136. [Google Scholar] [CrossRef]
- Boschetti, W.T.N.; Lopes, A.D.C.P.; Ribeiro, R.A.; Reyes, R.Q.; Carneiro, A.d.C.O. Kraft lignin as an additive in pine and eucalyptus particle composition for briquette production. Rev. Arvore 2019, 43. [Google Scholar] [CrossRef]
- Setter, C.; Costa, K.L.S.; de Oliveira, T.J.P.; Mendes, R.F. The effects of kraft lignin on the physicomechanical quality of briquettes produced with sugarcane bagasse and on the characteristics of the bio-oil obtained via slow pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar] [CrossRef]
- Findorák, R.; Legemza, J.; Fröhlichová, M.; Fabriciová, G.; Džupková, M. New utilization of specific biomass: Lignin in the iron ore sintering process. Metals 2020, 10, 1170. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Alvarez, R.; Santos, V.; Parajó, J.C. Production of hemicellulosic sugars from Pinus pinaster wood by sequential steps of aqueous extraction and acid hydrolysis. Wood Sci. Technol. 2012, 46, 271–285. [Google Scholar] [CrossRef]
- Li, J.; Galebach, P.H.; Johnson, J.K.; Fredriksen, T.; Wittrig, A.; Bai, X.; Yang, H.; Huber, G.W. Supercritical methanol depolymerization and hydrodeoxygenation of pyrolytic lignin over reduced copper porous metal oxides. Green Chem. 2020, 22, 8403–8413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).