Isolation, Identification, and Characterization of Probiotic Properties of Lactic Acid Bacterial Strains Isolated from Rose Blossom of Rosa damascena Mill
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.1.1. Lactic Acid Bacteria
2.1.2. Pathogenic Test Bacteria
2.2. Nutrient Media
2.3. Microbiological, Physiological, and Biochemical Methods
2.3.1. Isolation of Lactic Acid Bacteria
2.3.2. Determination of the Titratable Acidity
2.3.3. Determination of the Number of Viable Bacteria
2.3.4. Determination of the Biochemical Profile
2.3.5. Determination of the Antibiotic Susceptibility Profile
- Cell wall synthesis: penicillin, ampicillin, oxacillin, piperacillin, amoxicillin, bacitracin, and vancomycin;
- Protein synthesis: tetracycline, doxycycline, tobramycin, amikacin, gentamicin, erythromycin, chloramphenicol, and lincomycin;
- DNA synthesis and cell division: nalidixic acid, ciprofloxacin, novobiocin, and rifampin.
2.3.6. Determination of the Antibacterial Activity Against Pathogens—Agar Well Diffusion Method
2.4. Molecular Genetic Methods
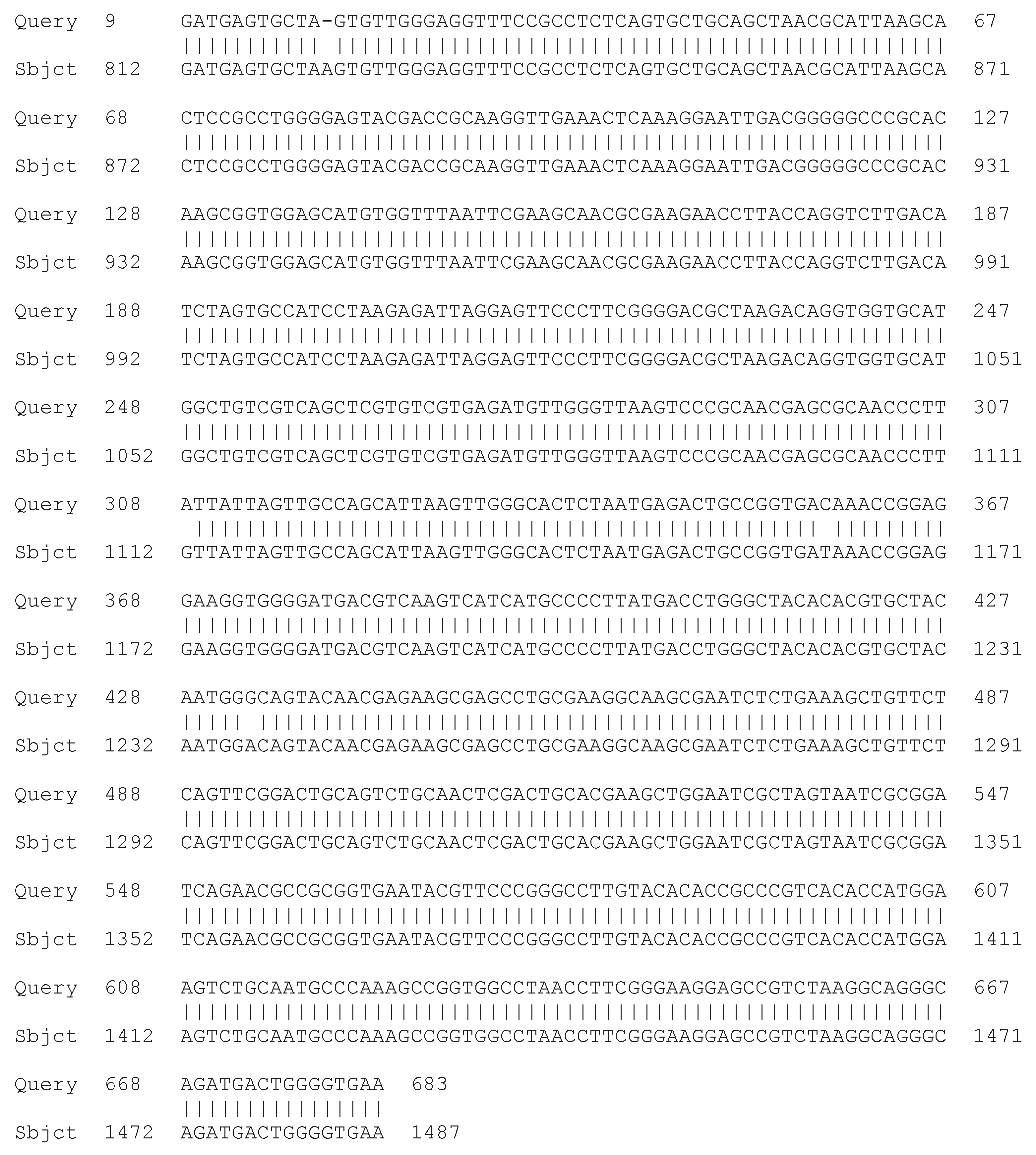
Sequencing of the 16S rRNA Gene
2.5. Batch Cultivation
2.6. Modeling of the Process Kinetics and Identification of the Model Parameters
2.7. Freeze-Drying
2.8. Microbiological Studies of the Lyophilized Concentrate
Microbiological Status of the Native and Lyophilized Samples
- According to BDS EN ISO 4833: 2004 [24].
- Indicators:
- Lactic acid bacteria, CFU/g [25];
- Total number of mesophilic aerobic and facultative anaerobic bacteria: CFU/g [26];
- E. coli in 0.1 g of the product [27];
- Pathogenic microorganisms, including Salmonella sp. in 25.0 g of the product [28];
- Coagulase-positive staphylococci in 1.0 g of the product [29];
- Sulfite-reducing clostridia in 0.1 g of the product [30];
- Microscopic mold spores, CFU/g [31];
- Yeast, CFU/g [31].
2.9. Processing of the Results
3. Results and Discussion
3.1. Isolation, Identification, and Selection of LAB Strains from the Rose Blossom of R. damascena
3.1.1. Phenotypic Characteristics of the Newly Isolated LAB Isolates
3.1.2. Physiological and Biochemical Characteristics of the Isolates
3.1.3. Molecular Taxonomic Characterization—Genotypic Characteristics of the LAB Isolates Studied—Sequencing of the 16S rRNA Gene
3.2. Characterization of the Probiotic Potential of the Newly Isolated Lactic Acid Bacteria
3.2.1. Antibacterial Activity of the Isolated LAB Strains Against Pathogenic Bacteria
3.2.2. Antibiotic Resistance of the Isolated LAB Strains
3.3. Batch Cultivation of Lp. plantarum 5/20 and Kinetic Modeling in MRS Broth Medium
3.4. Freeze-Drying
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, A.O.; Leveau, J.H.; Marco, M.L. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2020, 12, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Lingga, R.; Astuti, R.; Bahtera, N.; Adibrata, S.; Nurjannah, N.; Ahsaniyah, S.; Irawati, I. Effect of additional probiotic in cattle feed on cattle’s consumption and growth rate. Int. J. Sci. Technol. Manag. 2022, 3, 1418–1425. [Google Scholar] [CrossRef]
- Shivsharan, U.; Bhitre, M. Antimicrobial activity of lactic acid bacterial isolates. Int. Res. J. Pharm. 2013, 4, 197–201. [Google Scholar] [CrossRef]
- Nadirova, S.; Sinyavskiy, Y. Biotechnological approaches to the creation of new fermented dairy products. Eurasian J. Appl. Biotechnol. 2023, 4, 128–136. [Google Scholar] [CrossRef]
- Bayu, H.; Irwanto, R.; Dalimunthe, N.; Lingga, R. Isolation and identification of lactic acid bacteria from Channa sp. as potential probiotic. J. Pembelajaran Biol. Nukleus 2023, 9, 75–84. [Google Scholar] [CrossRef]
- Ar, I.; Laksmi, B.; Astawan, M.; Fujiyama, K.; Arief, I. Identification and probiotic characteristics of lactic acid bacteria isolated from Indonesian local beef. Asian J. Anim. Sci. 2014, 9, 25–36. [Google Scholar] [CrossRef]
- Pamungkaningtyas, F.; Mariyatun, M.; Kamil, R.; Setyawan, R.; Hasan, P.; Wiryohanjoyo, D.; Rahayu, E. Sensory evaluation of yogurt-like set and yogurt-like drink produced by indigenous probiotic strains for market test. Indones. Food Nutr. Prog. 2018, 15, 1. [Google Scholar] [CrossRef]
- Pundir, R.K.; Rana, S.; Kashyap, N.; Kaur, A. Probiotic potential of lactic acid bacteria isolated from food samples: An in vitro study. J. Appl. Pharm. Sci. 2013, 3, 85–93. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Xu, Q.; Li, Z.; Song, Y.; Zhou, S.; Luo, X. Bacterial diversity and lactic acid bacteria with high alcohol tolerance in the fermented grains of soy sauce aroma type baijiu in North China. Foods 2022, 11, 1794. [Google Scholar] [CrossRef]
- Aritonang, S.; Roza, E.; Rossi, E.; Purwati, E.; Husmaini, H. Isolation and identification of lactic acid bacteria from okara and evaluation of their potential as candidate probiotics. Pak. J. Nutr. 2017, 16, 618–628. [Google Scholar] [CrossRef][Green Version]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Krastanov, A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol. Biotechnol. Equip. 2014, 28, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Denkova, Z.; Zapryanova, P.; Denkova-Kostova, R.; Goranov, B.; Urshev, Z.; Gaytanska, Y.; Shopska, V. Isolation, identification and investigation of some properties of Lacticaseibacillus rhamnosus 1 for application in the composition of probiotics. BIO Web Conf. 2025, 170, 02006. [Google Scholar] [CrossRef]
- Paul, V.; Singh, A.; Pandey, R. Determination of titratable acidity (TA). In Laboratory Manual: Post-Harvest Physiology of Fruits and Flowers; Division of Plant Physiology, Indian Agricultural Research Institute: New Delhi, India, 2010; pp. 44–45. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. Available online: https://asm.org/protocols/kirby-bauer-disk-diffusion-susceptibility-test-pro (accessed on 27 April 2025).
- Denkova-Kostova, R.; Goranov, B.; Tomova, T.; Yanakieva, V.; Blazheva, D.; Denkova, Z.; Kostov, G. Investigation of probiotic properties of Lactobacillus helveticus 2/20 isolated from rose blossom of Rosa damascena Mill. BIO Web Conf. 2023, 58, 02002. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Urshev, Z.; Doynova, D.; Prasev, I.; Denkova-Kostova, R.; Koleva, A.; Denkova, Z.; Goranov, B.; Kostov, G. Identification of lactic acid bacteria strains isolated from sourdoughs prepared with different flour types. Appl. Sci. 2024, 14, 2093. [Google Scholar] [CrossRef]
- Kostov, G.; Denkova, R.; Ivanova, P.; Denkova, I. Kinetics of batch fermentation in the cultivation of a probiotic strain of lactic acid bacteria. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 61–72. [Google Scholar] [CrossRef]
- Kostov, G.; Denkova, R.; Ivanova, P.; Denkova, I. Microbial growth of Lactobacillus delbrueckii ssp. bulgaricus B1 in a complex nutrient medium (MRS-broth). ECMS Proc. 2022, 16, 135–142. [Google Scholar] [CrossRef]
- Kostov, G.; Denkova-Kostova, R.; Ivanova, P.; Denkova, I. New approach to modelling the kinetics of the fermentation process in cultivation of lactic acid bacteria. In Proceedings of the 32nd European Conference on Modelling and Simulation, Wilhelmshaven, Germany, 22–26 May 2018; pp. 212–218. [Google Scholar] [CrossRef]
- Denkova, R.; Denkova, Z.; Yanakieva, V.; Georgieva, L. Highly active freeze-dried probiotic concentrates with long shelf life. Food Environ. Saf. 2013, 12, 225–232. Available online: https://fens.usv.ro/index.php/FENS/article/download/169/167 (accessed on 27 April 2025).
- Denkova, R.; Georgieva, L.; Denkova, Z. Freeze-dried sourdough starters. J. Food Packag. Sci. Tech. Technol. 2014, 4, 74–80. [Google Scholar]
- EN ISO 4833:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 °C. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2004.
- ISO 15214:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2002.
- EN ISO 4833-1:2013/A1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique; Amendment 1. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2022.
- ISO 16649-2:2014; Microbiology of the Food Chain—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl β-D-Glucuronide. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2014.
- EN ISO 6579-1:2017/A1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.; Amendment 1: Broader Range of Incubation Temperatures and Clarification of Testing of Samples with High Background Flora. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2020.
- EN ISO 6888-1:2021/A1:2023; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Staphylococcus aureus—Part 1: Technique Using Baird-Parker Agar Medium; Amendment 1. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2023.
- ISO 15213:2023; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Clostridium spp. —Colony-Count Technique. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2023.
- ISO 6611:2006; Milk and Milk Products—Enumeration of Colony-Forming Units of Yeasts and/or Moulds—Colony-Count Technique at 25 Degrees C. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2006.
- Majhenič, A.Č.; Lorbeg, P.M.; Treven, P. Enumeration and identification of mixed probiotic and lactic acid bacteria starter cultures. In Probiotic Dairy Products; CRC Press: Boca Raton, FL, USA, 2017; pp. 207–251. [Google Scholar]
- Singh, S.; Goswami, P.; Singh, R.; Heller, K.J. Application of molecular methods for identification of lactic acid bacteria. LWT—Food Sci. Technol. 2009, 42, 448–457. [Google Scholar] [CrossRef]
- Song, Y.L.; Kato, N.; Matsumiya, Y.; Liu, C.X.; Kato, H.; Watanabe, K. Identification of Lactobacillus species of human origin by a commercial kit, API50CHL. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai Shi 1999, 10, 77–82. [Google Scholar]
- Daliri, F.; Aboagye, A.A.; Daliri, E.B.M. Inactivation of Foodborne Pathogens by Lactic Acid Bacteria. J. Food Hyg. Saf. 2020, 35, 419–429. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef]
- Papagianni, M. Metabolic Engineering of Lactic Acid Bacteria for the Production of Industrially Important Compounds. Comput. Struct. Biotechnol. J. 2012, 3, e201210003. [Google Scholar] [CrossRef]
- Aryantini, N.P.D.; Yamasaki, E.; Kurazono, H.; Sujaya, I.N.; Urashima, T.; Fukuda, K. In Vitro Safety Assessments and Antimicrobial Activities of Lactobacillus rhamnosus Strains Isolated from a Fermented Mare’s Milk. Anim. Sci. J. 2017, 88, 517–525. [Google Scholar] [CrossRef]
- Dinev, T.; Beev, G.; Tzanova, M.; Denev, S.; Dermendzhieva, D.; Stoyanova, A. Antimicrobial Activity of Lactobacillus plantarum against Pathogenic and Food Spoilage Microorganisms: A Review. Bulg. J. Vet. Med. 2018, 21, 253–268. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Rivera-Espinoza, Y.; Hernández-Sánchez, H. Lactobacillus plantarum: An Overview with Emphasis in Biochemical and Healthy Properties. In Lactobacillus: Classification, Uses and Health Implications; Pérez Campos, A., Mena, A.L., Eds.; Nova Publishing: New York, NY, USA, 2012; pp. 1–31. [Google Scholar]
- Penderup Jensen, M.; Braun, A.; Vogensen, F.K.; Ardö, Y. Study of Antimicrobial Activity among Lactobacillus helveticus Strains Using Three Different Assays. Ann. Microbiol. 2009, 59, 187–190. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, F.; Jin, Z.; Xia, X. Ecological Succession and Functional Characteristics of Lactic Acid Bacteria in Traditional Fermented Foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 5841–5855. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-drying of lactic acid bacteria. In Methods in Molecular Biology; Spinger: New York, NY, USA, 2015; Volume 1257, pp. 477–488. [Google Scholar] [CrossRef]
- Tsvetkov, T.; Brankova, R. Viability of micrococci and lactobacilli upon freezing and freeze-drying in the presence of different cryoprotectants. Cryobiology 1983, 20, 318–323. [Google Scholar] [CrossRef]
- Montel Mendoza, G.; Pasteris, S.E.; Otero, M.C.; Nader-Macías, M.E.F. Survival and beneficial properties of lactic acid bacteria from raniculture subjected to freeze-drying and storage. Benef. Microbes 2014, 5, 381–390. [Google Scholar] [CrossRef]













| Strain | Cellular Morphology | Colonial Characteristics | ||
|---|---|---|---|---|
| Description | Visualization | Description | Visualization | |
| LAB 4/20 | Long, thickened rods with rounded ends, arranged singly, in pairs and in short chains |  | Snowflake-shaped colonies, serrated ends, 2–3 mm in diameter |  |
| LAB 5/20 | Fine, short rods with rounded ends, arranged in pairs and in short chains |  | Round colonies, with wavy ends, soft consistency, 2–3 mm in diameter | 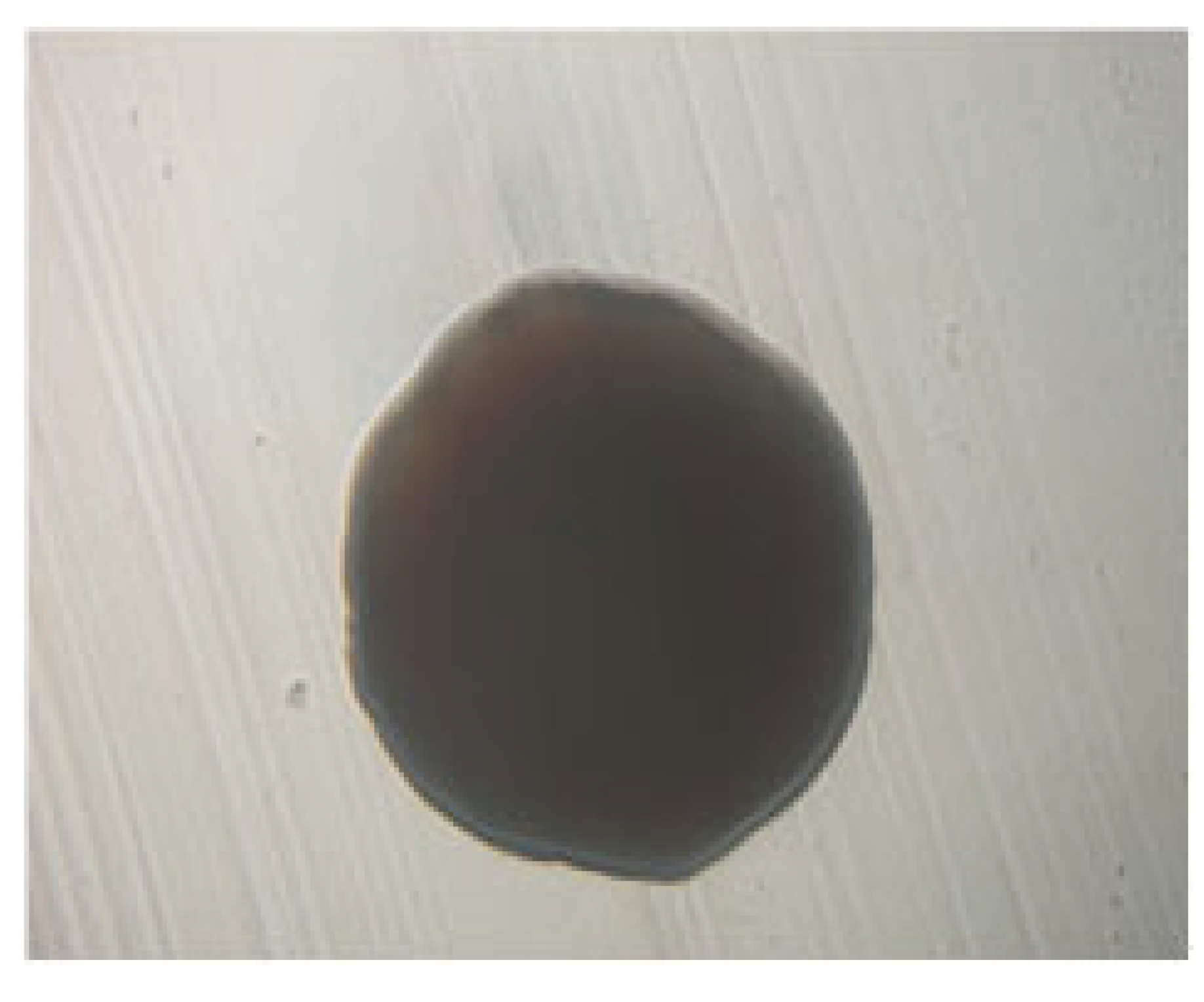 |
| LAB 6/20 | Long, thin rods with rounded ends, arranged singly and in long chains | 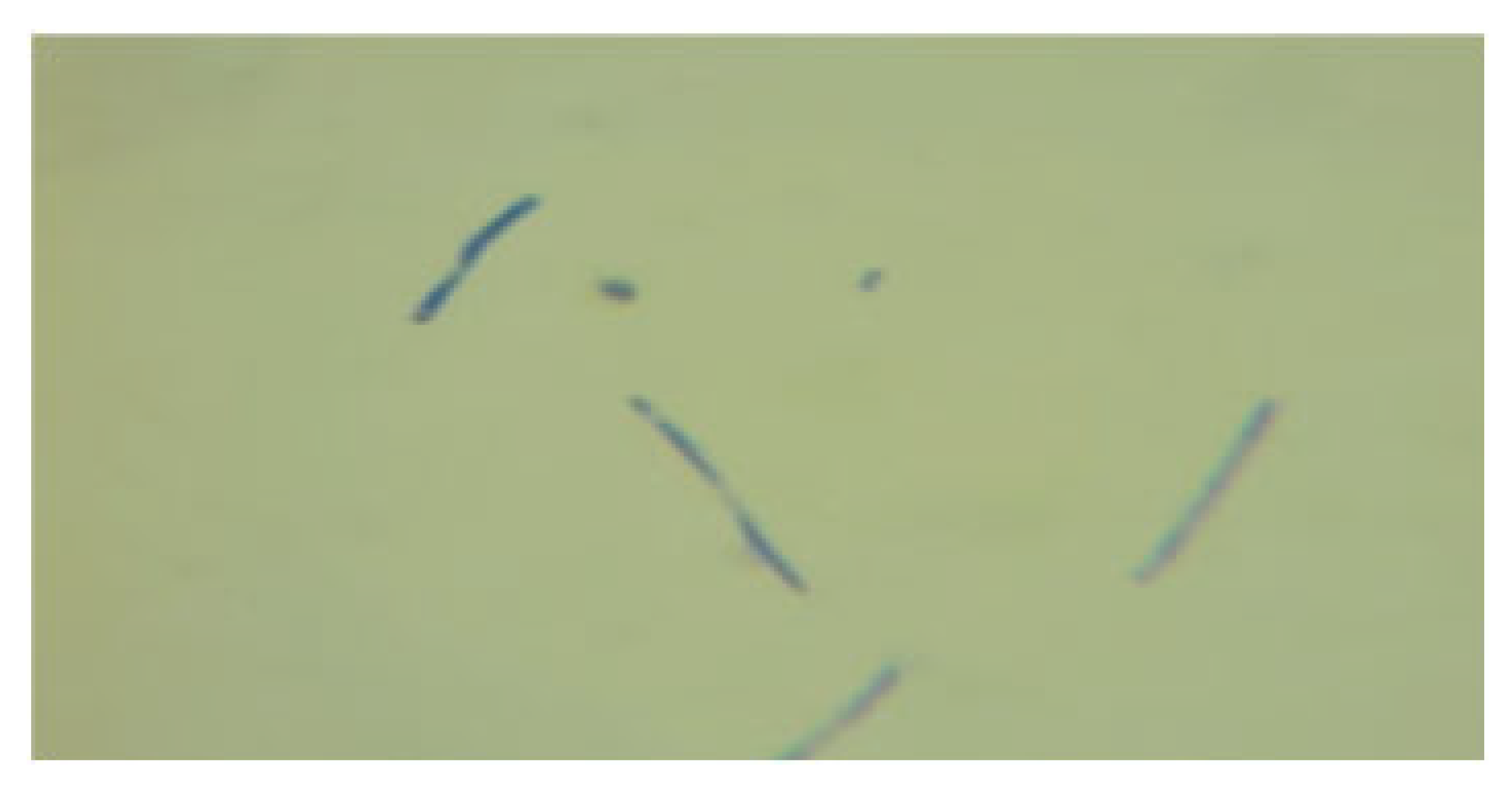 | Round colonies with wavy ends, soft consistency, 2–3 mm in diameter |  |
| LAB 8/20 | Long, thin rods with rounded ends, arranged singly and in long chains | 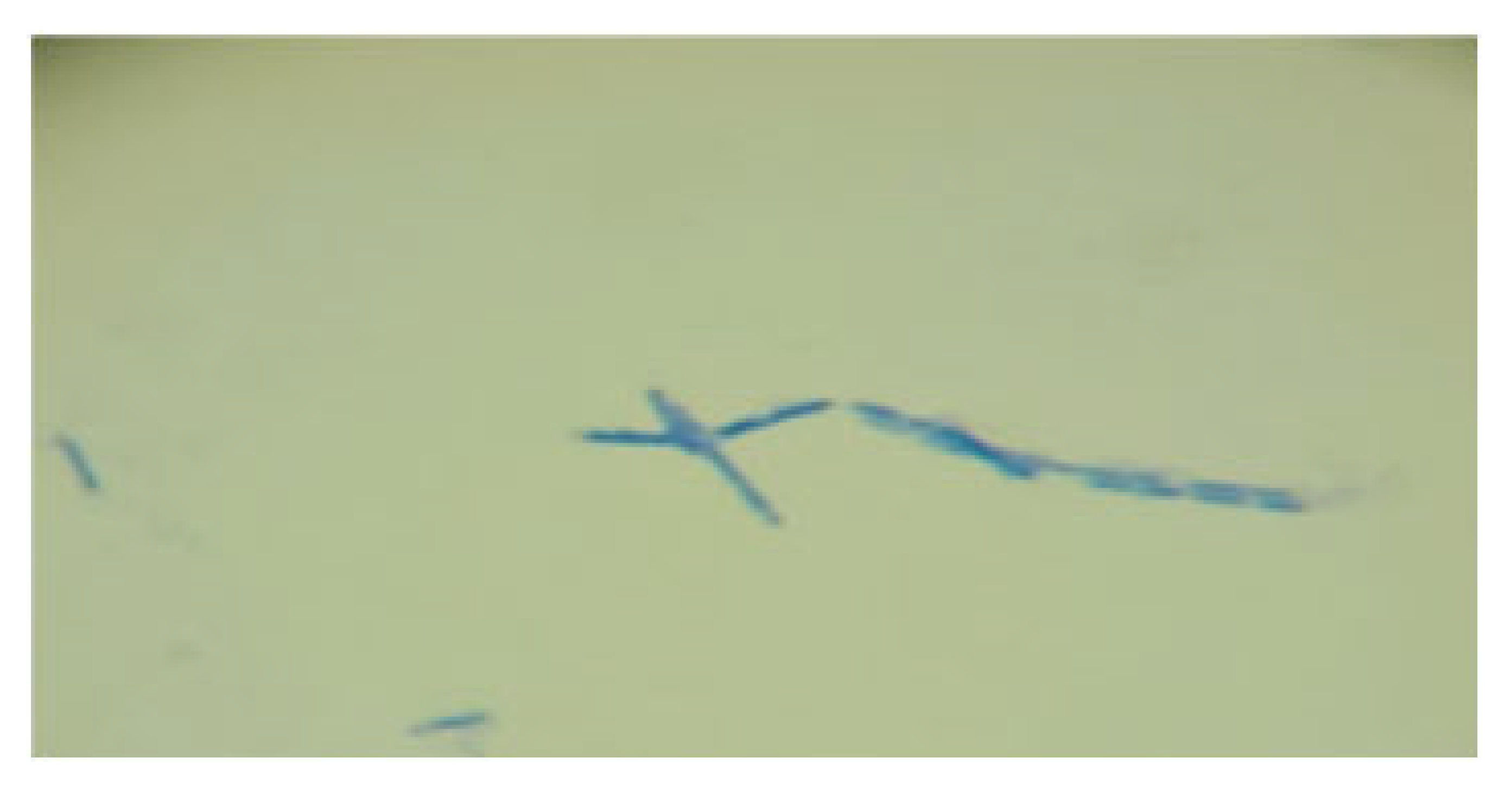 | Round colonies with wavy ends, soft consistency, 2–3 mm in diameter |  |
| LAB 9/20 | Short, thickened rods with rounded ends, arranged singly and in clusters | 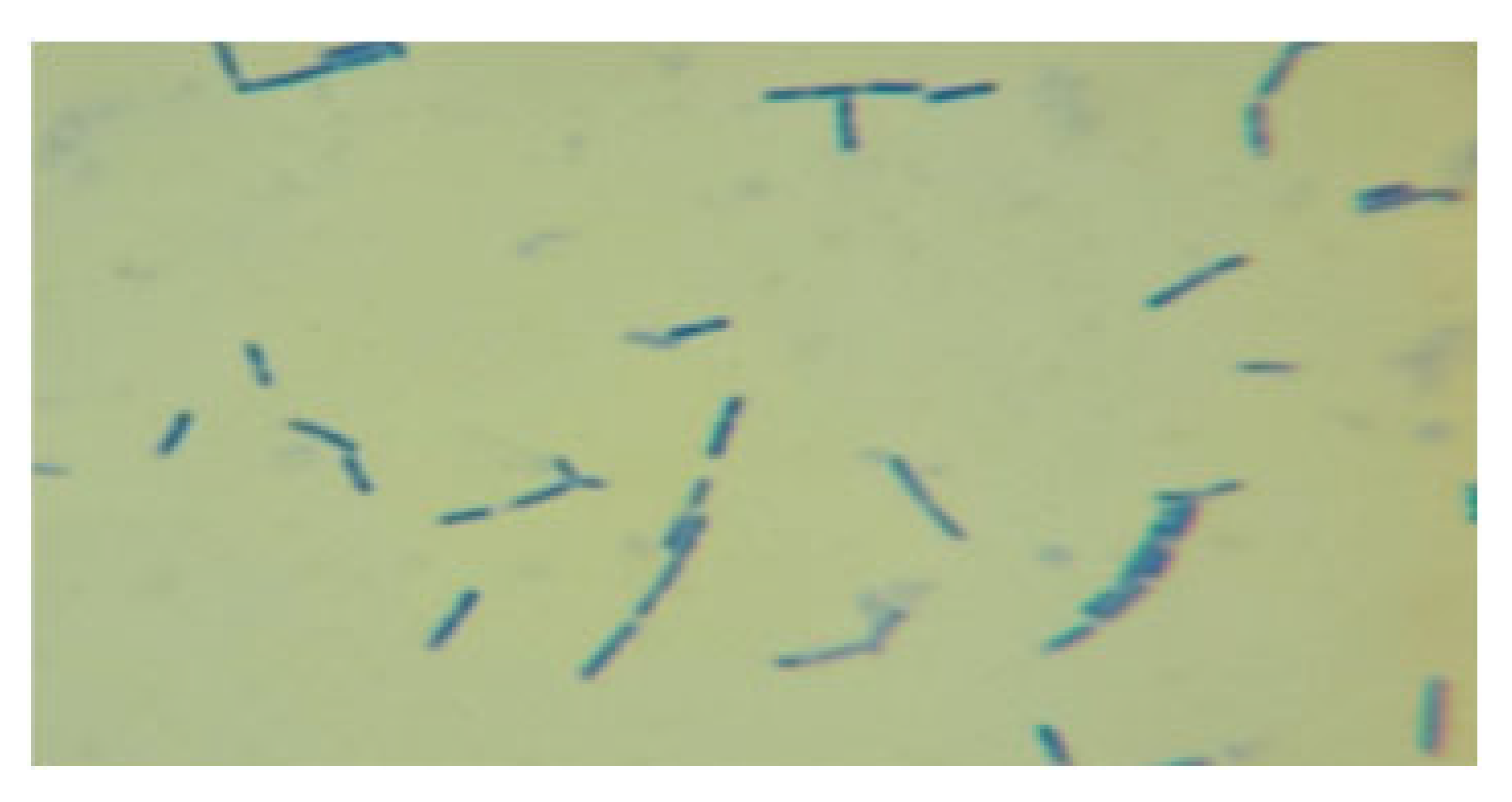 | Round colonies with wavy ends, soft consistency, 2–3 mm in diameter |  |
| LAB 10/20 | Long rods with rounded ends, arranged singly and in short chains |  | Snowflake-shaped colonies with wavy ends, soft consistency, 2–3 mm in diameter |  |
| LAB 12/20 | Long thin rods with rounded ends, arranged singly and in short chains |  | Snowflake-shaped colonies with serrated ends, 2–3 mm in diameter |  |
| LAB 13/20 | Short, thickened rods with rounded ends, arranged singly, in pairs, and in clusters | 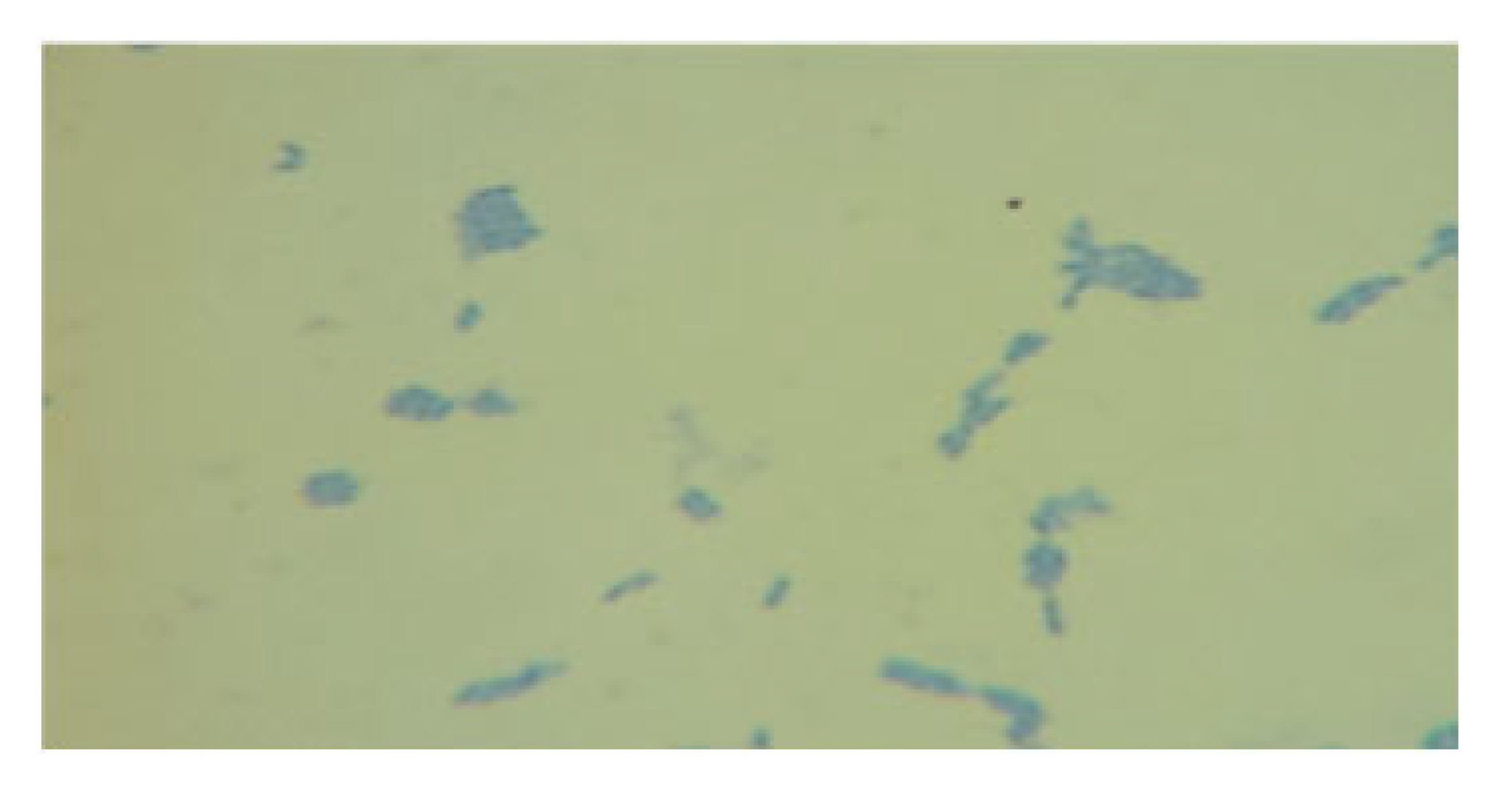 | Round colonies with even ends, soft consistency, 2–3 mm in diameter | 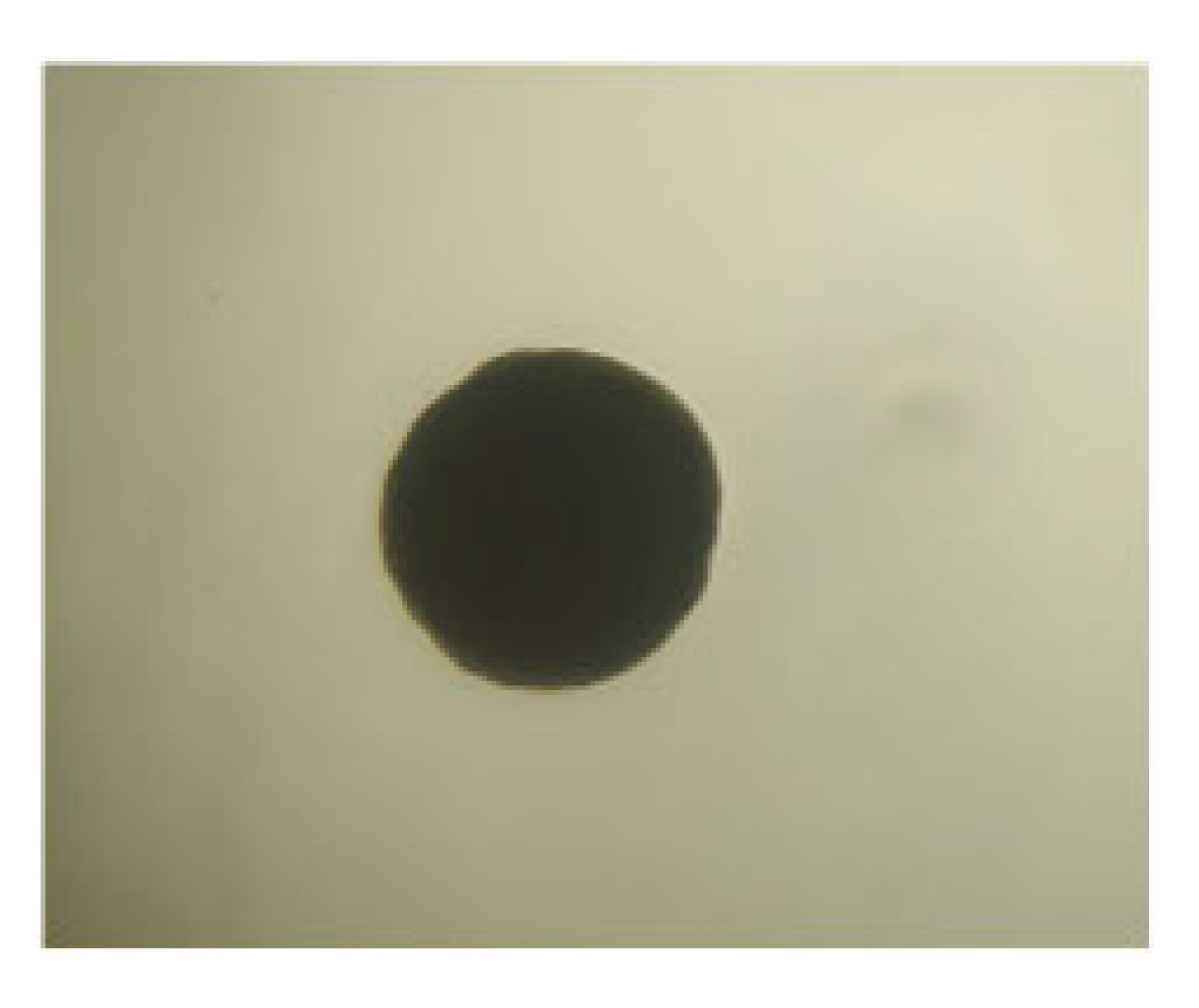 |
| LAB 16/20 | Cocci, arranged in pairs and in groups |  | Round colonies with even ends, soft consistency, 2–3 mm in diameter | 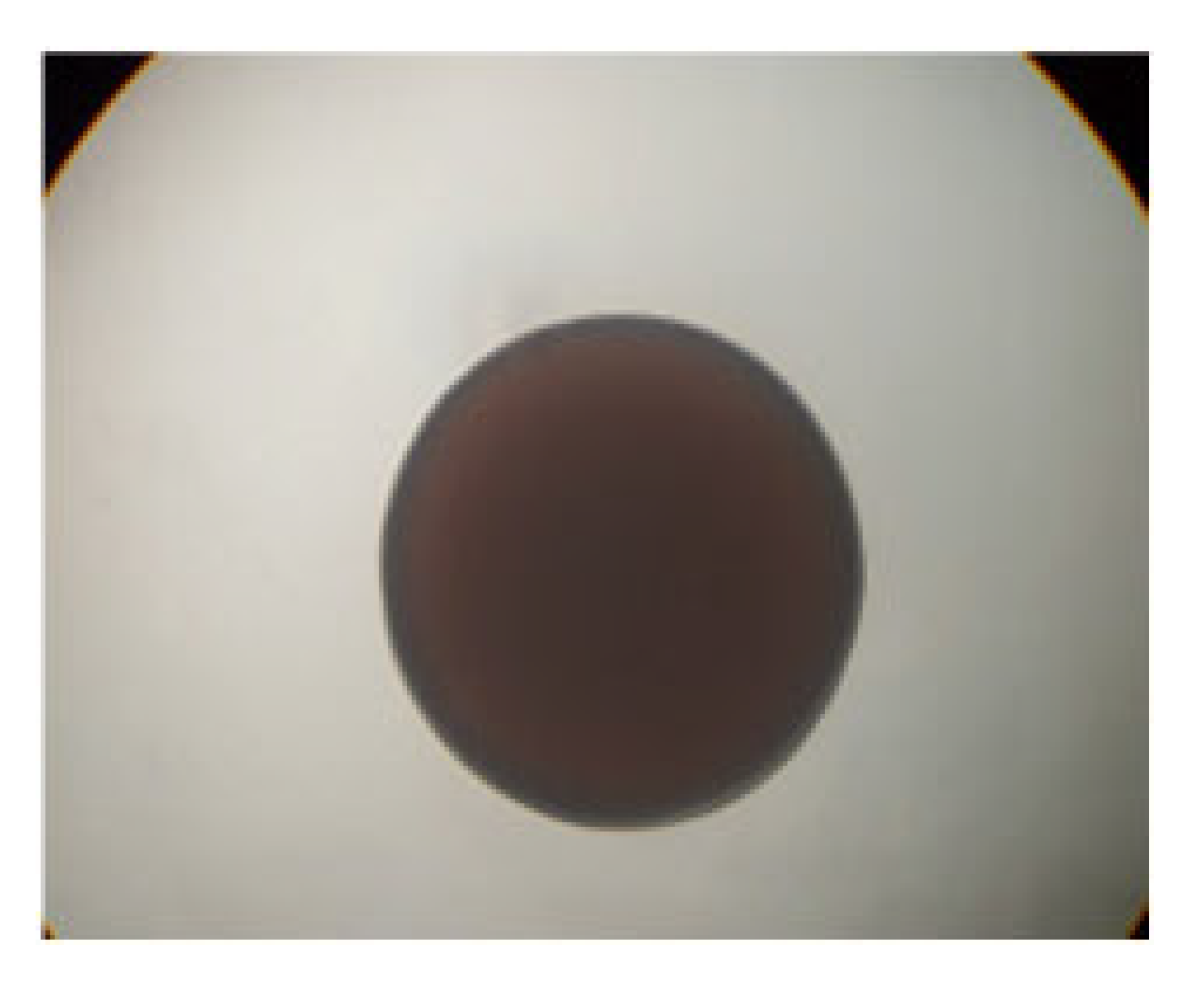 |
| LAB 19/20 | Very short rods with rounded ends, arranged singly and in groups |  | Round colonies with even ends, soft consistency, 2–3 mm in diameter |  |
| LAB 22/20 | Short rods with rounded, flat ends, arranged singly and in groups, soft consistency, 2–3 mm | 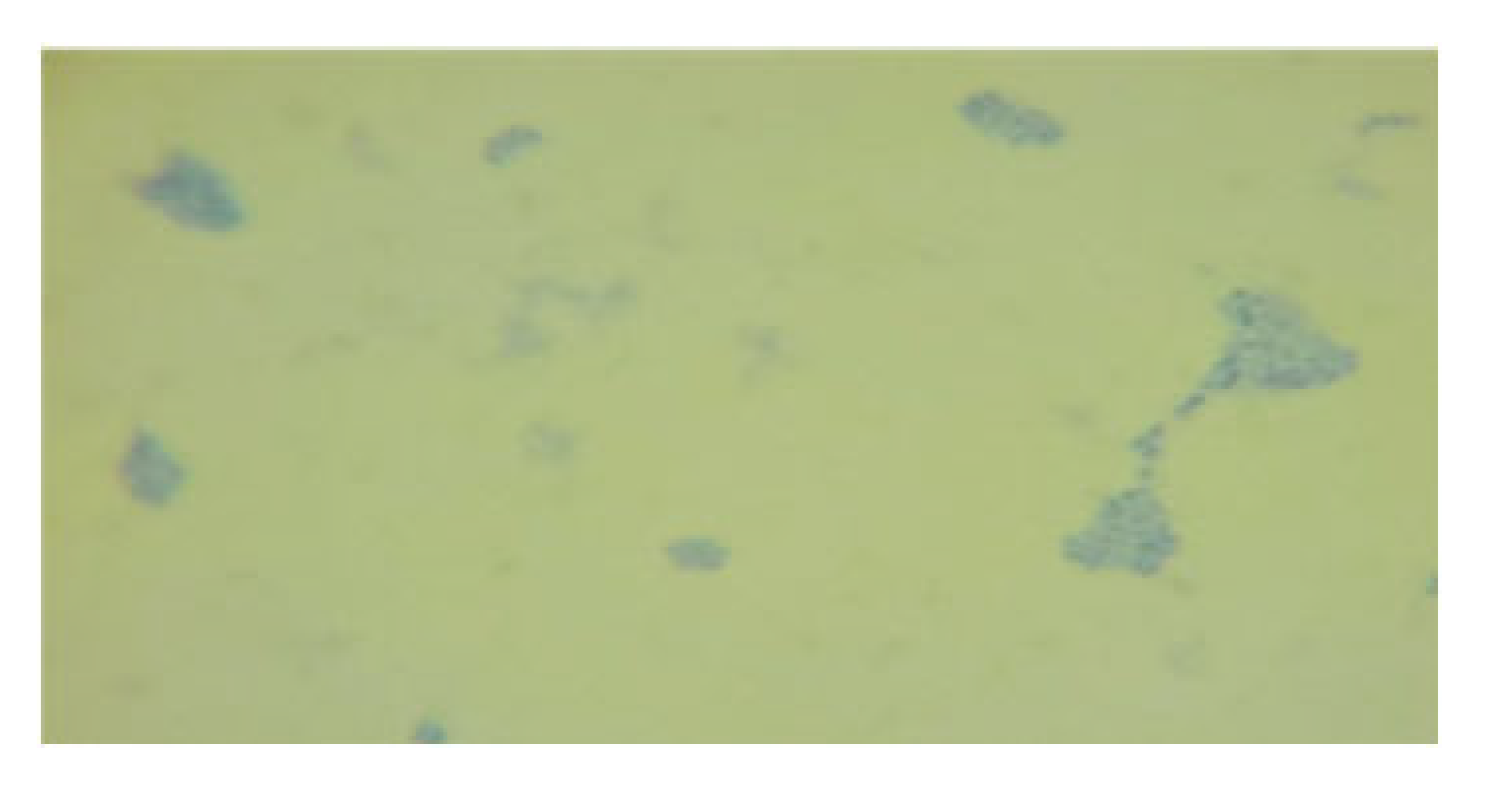 | Round colonies with even ends, soft consistency, 2–3 mm in diameter | 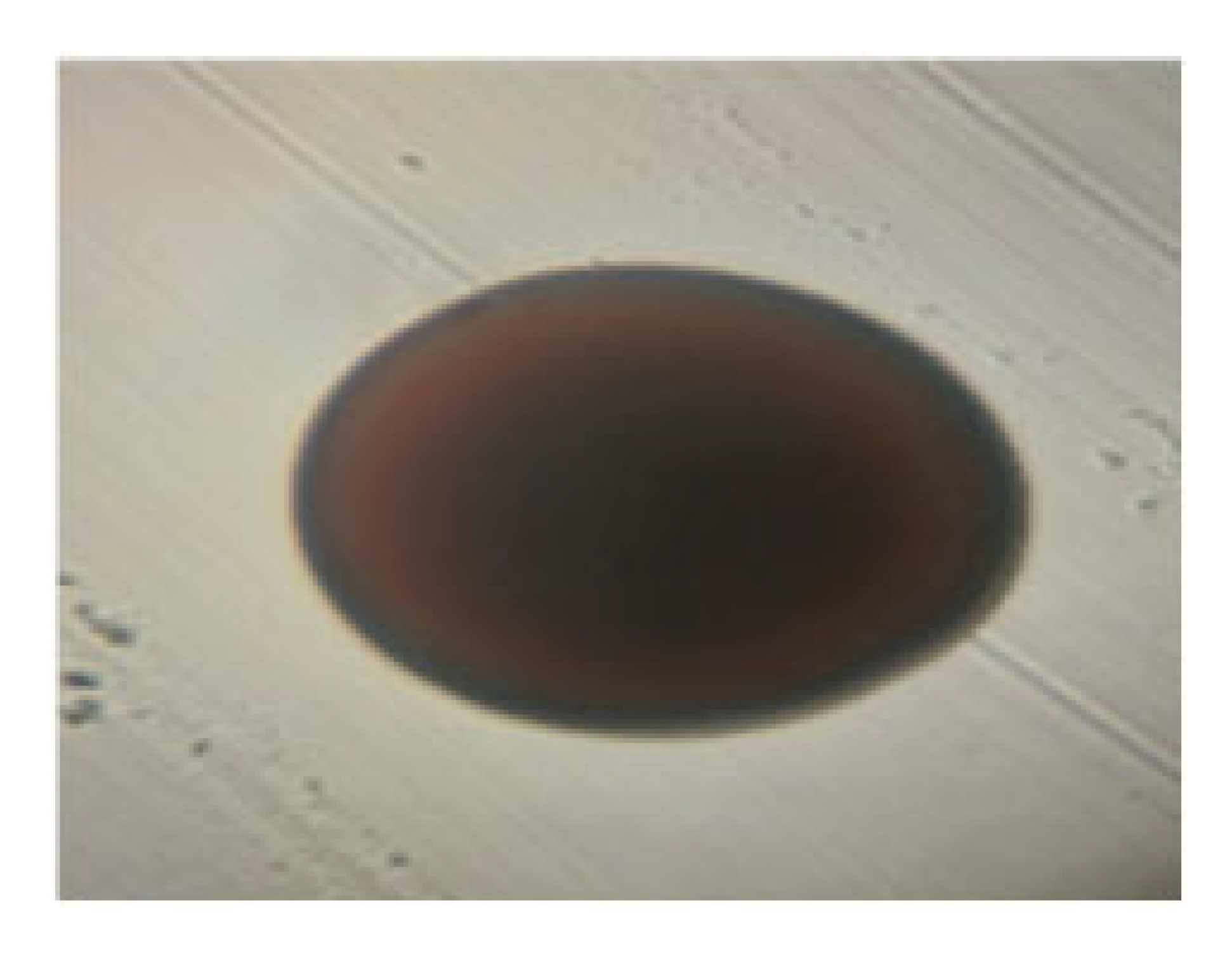 |
| (a) | |||||||
| # | Carbon source | LAB 4/20 | LAB 5/20 | LAB 6/20 | LAB 8/20 | LAB 9/20 | LAB 10/20 |
| 1 | Glycerol | - | - | - | - | - | - |
| 2 | Erythritol | - | - | - | - | - | - |
| 3 | D-arabinose | - | - | - | - | - | - |
| 4 | L-arabinose | - | + (90–100%) | - | - | - | + (90–100%) |
| 5 | Ribose | - | + (90–100%) | + (90–100%) | - | - | + (90–100%) |
| 6 | D-xylose | - | - | - | - | - | + (90–100%) |
| 7 | L-xylose | - | - | - | - | - | - |
| 8 | Adonitol | - | - | - | - | - | - |
| 9 | β-metil-D-xyloside | - | - | - | - | - | - |
| 10 | Galactose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 11 | D-glucose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 12 | D-fructose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 13 | D-mannose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 14 | L-sorbose | - | - | - | + (90–100%) | - | - |
| 15 | Rhamnose | - | - | - | + (90–100%) | - | - |
| 16 | Dulcitol | - | - | - | - | - | - |
| 17 | Inositol | - | - | - | - | - | - |
| 18 | Mannitol | - | + (90–100%) | + (90–100%) | + (90–100%) | - | + (90–100%) |
| 19 | Sorbitol | - | + (90–100%) | + (90–100%) | + (90–100%) | - | + (90–100%) |
| 20 | α-methyl-D-mannoside | - | + (90–100%) | + (90–100%) | - | - | + (90–100%) |
| 21 | α-methyl-D-glucoside | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 22 | N-acetyl-glucosamine | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 23 | Amygdalin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 24 | Arbutin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 25 | Esculin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 26 | Salicin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 27 | Cellobiose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 28 | Maltose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 29 | Lactose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 30 | Melibiose | - | + (90–100%) | + (90–100%) | + (90–100%) | - | + (90–100%) |
| 31 | Sucrose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 32 | Trehalose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 33 | Inulin | - | - | + (90–100%) | - | - | - |
| 34 | Melezitose | - | + (90–100%) | + (90–100%) | + (90–100%) | - | + (90–100%) |
| 35 | D-raffinose | - | - | + (90–100%) | - | - | + (90–100%) |
| 36 | Starch | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | - |
| 37 | Glycogen | - | - | - | - | - | - |
| 38 | Xylitol | - | - | - | - | - | - |
| 39 | β-gentiobiose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 40 | D-turanose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) |
| 41 | D-lyxose | - | - | - | - | - | - |
| 42 | D-tagatose | - | - | - | - | - | - |
| 43 | D-fucose | - | - | - | - | - | - |
| 44 | L-fucose | - | - | - | - | - | - |
| 45 | D-arabitol | - | - | - | - | - | - |
| 46 | L-arabitol | - | - | - | - | - | - |
| 47 | Gluconate | - | + (90–100%) | + (90–100%) | - | - | + (90–100%) |
| 48 | 2-keto-gluconate | - | - | - | - | - | - |
| 49 | 5-keto-gluconate | - | - | - | - | - | - |
| (b) | |||||||
| # | Carbon source | LAB 12/20 | LAB 13/20 | LAB 16/20 | LAB 19/20 | LAB 22/20 | |
| 1 | Glycerol | - | - | - | - | - | |
| 2 | Erythritol | - | - | - | - | - | |
| 3 | D-arabinose | - | - | - | - | - | |
| 4 | L-arabinose | - | + (90–100%) | + (90–100%) | - | - | |
| 5 | Ribose | - | + (90–100%) | + (90–100%) | - | - | |
| 6 | D-xylose | - | - | + (90–100%) | - | - | |
| 7 | L-xylose | - | - | - | - | - | |
| 8 | Adonitol | - | - | - | - | - | |
| 9 | β-metil-D-xyloside | - | - | - | - | - | |
| 10 | Galactose | + (90–100%) | + (90–100%) | + (90–100%) | - | + (90–100%) | |
| 11 | D-glucose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 12 | D-fructose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 13 | D-mannose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 14 | L-sorbose | - | - | - | - | - | |
| 15 | Rhamnose | - | - | - | - | - | |
| 16 | Dulcitol | - | - | - | - | - | |
| 17 | Inositol | - | - | - | - | - | |
| 18 | Mannitol | - | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 19 | Sorbitol | - | + (90–100%) | - | + (90–100%) | + (90–100%) | |
| 20 | α-methyl-D-mannoside | - | + (90–100%) | + (90–100%) | - | - | |
| 21 | α-methyl-D-glucoside | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 22 | N-acetyl-glucosamine | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 23 | Amygdalin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 24 | Arbutin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 25 | Esculin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 26 | Salicin | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 27 | Cellobiose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 28 | Maltose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 29 | Lactose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 30 | Melibiose | - | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 31 | Sucrose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 32 | Trehalose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 33 | Inulin | - | - | + (90–100%) | - | - | |
| 34 | Melezitose | - | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 35 | D-raffinose | - | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 36 | Starch | + (90–100%) | - | - | - | - | |
| 37 | Glycogen | - | - | - | - | - | |
| 38 | Xylitol | - | - | - | - | - | |
| 39 | β-gentiobiose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | - | |
| 40 | D-turanose | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | + (90–100%) | |
| 41 | D-lyxose | - | - | - | - | - | |
| 42 | D-tagatose | - | - | - | - | - | |
| 43 | D-fucose | - | - | - | - | - | |
| 44 | L-fucose | - | - | - | - | - | |
| 45 | D-arabitol | - | - | - | - | - | |
| 46 | L-arabitol | - | - | - | - | - | |
| 47 | Gluconate | - | + (90–100%) | - | + (90–100%) | - | |
| 48 | 2-keto-gluconate | - | - | - | - | - | |
| 49 | 5-keto-gluconate | - | - | - | - | - | |
| Isolate | Species Affiliation | Reliability, % |
|---|---|---|
| LAB 4/20 | Lactobacillus crispatus | 65.3 |
| LAB 5/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 6/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 8/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 9/20 | Lactobacillus crispatus | 65.3 |
| LAB 10/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 12/20 | Lactobacillus crispatus | 65.3 |
| LAB 13/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 16/20 | Lactiplantibacillus plantarum | 94.1 |
| LAB 19/20 | Lactiplantibacillus plantarum | 99.9 |
| LAB 22/20 | Lactiplantibacillus plantarum | 99.9 |
| Isolate | Species Affiliation | Similarity, % |
|---|---|---|
| LAB4/20 | L. helveticus | 99% |
| LAB 5/20 | Lp. plantarum | 99% |
| LAB6/20 | L. helveticus | 99% |
| LAB8/20 | L. helveticus | 99% |
| LAB9/20 | L. helveticus | 99% |
| LAB10/20 | L. acidophilus | 99% |
| LAB12/20 | L. helveticus | 99% |
| LAB13/20 | Lp. plantarum | 99% |
| LAB16/20 | L. mesenteroides | 99% |
| LAB 19/20 | Lp. plantarum | 99% |
| LAB 22/20 | L. mesenteroides | 99% |
| (a) | |||||||
| dIZ, [mm] | E. coli ATCC 25922, 6 × 1011 CFU/cm3 | S. Abony NTCC 6017, 2 × 1011 CFU/cm3 | P. vulgarisDSM 13387, 1.5 × 1011 CFU/cm3 | L. monocytogenes ATCC 19115, 4 × 1011 CFU/cm3 | S. aureus ATCC 25093, 1.7 × 1011 CFU/cm3 | E. faecalis ATCC 29212, 4.2 × 1011 CFU/cm3 | |
| LAB strain | |||||||
| 4/20 | CL | 14.67 ± 0.47 | 15.50 ± 0.41 | 17.00 ± 0.00 | 12.67 ± 0.47 | 17.17 ± 0.24 | 16.67 ± 0.47 |
| BSS | 9.67 ± 0.47 | 12.33 ± 0.47 | 14.17 ± 0.24 | 11.17 ± 0.24 | 20.17 ± 0.24 | 13.33 ± 0.47 | |
| CFSN | 10.17 ± 0.24 | 12.00 ± 0.00 | 15.17 ± 0.24 | 10.50 ± 0.41 | 10.17 ± 0.24 | 11.33 ± 0.47 | |
| NCFSN | - | - | - | - | - | - | |
| 6/20 | CL | 11.33 ± 0.47 | 14.33 ± 0.47 | 18.33 ± 0.47 | 12.17 ± 0.24 | 15.00 ± 0.00 | 16.50 ± 0.41 |
| BSS | 8.00 ± 0.00 | 9.17 ± 0.24 | 14.00 ± 0.00 | 9.17 ± 0.24 | 16.17 ± 0.24 | 13.17 ± 0.24 | |
| CFSN | 9.50 ± 0.41 | 12.00 ± 0.00 | 15.67 ± 0.47 | 10.17 ± 0.24 | 13.67 ± 0.47 | 13.00 ± 0.00 | |
| NCFSN | - | - | - | - | - | - | |
| 8/20 | CL | 10.17 ± 0.24 | 13.17 ± 0.24 | 16.50 ± 0.41 | 11.17 ± 0.24 | 13.50 ± 0.41 | 16.00 ± 0.00 |
| BSS | 8.33 ± 0.47 | 8.17 ± 0.24 | 13.17 ± 0.24 | 8.00 ± 0.00 | 12.17 ± 0.24 | 12.17 ± 0.24 | |
| CFSN | 8.00 ± 0.00 | 10.00 ± 0.00 | 14.67 ± 0.47 | 10.50 ± 0.41 | 10.50 ± 0.41 | 13.17 ± 0.24 | |
| NCFSN | - | - | - | - | - | - | |
| 9/20 | CL | 11.67 ± 0.47 | 12.50 ± 0.41 | 18.67 ± 0.47 | 11.50 ± 0.41 | 16.00 ± 0.00 | 18.67 ± 0.47 |
| BSS | 8.17 ± 0.24 | 9.00 ± 0.00 | 12.17 ± 0.24 | 8.00 ± 0.00 | 10.17 ± 0.24 | 15.00 ± 0.00 | |
| CFSN | 9.50 ± 0.41 | 10.00 ± 0.00 | 15.17 ± 0.24 | 10.00 ± 0.00 | 10.67 ± 0.47 | 14.00 ± 0.00 | |
| NCFSN | - | - | - | - | - | - | |
| 12/20 | CL | 14.00 ± 0.00 | 15.00 ± 0.00 | 18.00 ± 0.82 | 14.67 ± 0.47 | 16.17 ± 0.24 | 18.17 ± 0.24 |
| BSS | 10.00 ± 0.00 | 10.00 ± 0.00 | 15.17 ± 0.24 | 9.17 ± 0.24 | 12.33 ± 0.47 | 13.17 ± 0.24 | |
| CFSN | 10.17 ± 0.24 | 13.50 ± 0.41 | 15.67 ± 0.47 | 12.00 ± 0.00 | 13.17 ± 0.24 | 13.00 ± 0.00 | |
| NCFSN | - | - | - | - | - | 8.00 ± 0.00 | |
| (b) | |||||||
| dIZ, [mm] | E. coli ATCC 25922, 6 × 1011 CFU/cm3 | S. Abony NTCC 6017, 2 × 1011 CFU/cm3 | P. vulgarisDSM 13387, 1.5 × 1011 CFU/cm3 | L. monocytogenes ATCC 19115, 4 × 1011 CFU/cm3 | S. aureus ATCC 25093, 1.7 × 1011 CFU/cm3 | E. faecalis ATCC 29212, 4.2 × 1011 CFU/cm3 | |
| LAB strain | |||||||
| 5/20 | CL | 13.33 ± 0.47 | 17.00 ± 0.00 | 20.00 ± 0.00 | 13.67 ± 0.47 | 19.67 ± 0.47 | 18.50 ± 0.41 |
| BSS | 9.17 ± 0.24 | 10.00 ± 0.00 | 16.17 ± 0.24 | 9.17 ± 0.24 | 17.33 ± 0.47 | 14.67 ± 0.47 | |
| CFSN | 11.33 ± 0.47 | 13.17 ± 0.24 | 17.67 ± 0.47 | 11.17 ± 0.24 | 14.67 ± 0.47 | 13.50 ± 0.41 | |
| NCFSN | - | - | 9.17 ± 0.24 | 8.17 ± 0.24 | - | - | |
| 13/20 | CL | 10.17 ± 0.24 | 14.50 ± 0.41 | 17.17 ± 0.24 | 11.17 ± 0.24 | 19.50 ± 0.41 | 17.67 ± 0.47 |
| BSS | 8.00 ± 0.00 | 10.17 ± 0.24 | 15.00 ± 0.00 | 9.00 ± 0.00 | 17.17 ± 0.24 | 14.17 ± 0.24 | |
| CFSN | 9.00 ± 0.00 | 13.17 ± 0.24 | 15.17 ± 0.24 | 10.50 ± 0.41 | 13.00 ± 0.00 | 13.00 ± 0.00 | |
| NCFSN | - | - | 8.00 ± 0.00 | - | - | - | |
| 19/20 | CL | 9.67 ± 0.47 | 10.67 ± 0.47 | 11.00 ± 0.00 | 10.17 ± 0.24 | 10.17 ± 0.24 | 10.17 ± 0.24 |
| BSS | 8.00 ± 0.00 | 8.17 ± 0.24 | 8.00 ± 0.00 | 8.00 ± 0.00 | 9.17 ± 0.24 | 8.67 ± 0.47 | |
| CFSN | 8.50 ± 0.41 | 9.00 ± 0.00 | 9.00 ± 0.00 | 8.67 ± 0.47 | 8.00 ± 0.00 | 9.17 ± 0.24 | |
| NCFSN | - | - | - | - | - | - | |
| 10/20 | CL | 9.00 ± 0.00 | 10.17 ± 0.24 | 9.67 ± 0.47 | 10.17 ± 0.24 | 8.00 ± 0.00 | 8.17 ± 0.24 |
| BSS | 8.17 ± 0.24 | 8.17 ± 0.24 | 8.17 ± 0.24 | 8.17 ± 0.24 | 8.17 ± 0.24 | 8.17 ± 0.24 | |
| CFSN | 9.00 ± 0.00 | 9.17 ± 0.24 | 9.17 ± 0.24 | 9.17 ± 0.24 | 8.00 ± 0.00 | 8.00 ± 0.00 | |
| NCFSN | - | - | - | - | - | - | |
| (a) | |||||||||
| # | Mechanism of action | Antibiotic | Concentration | 4/20 | 6/20 | 8/20 | 9/20 | 12/20 | |
| 1 | Inhibitor of cell wall synthesis | Penicillin | P | 10 E/disc | R | S | S | R | R |
| 2 | Bacitracin | Cm | 0.07 E/disc | R | S | S | SR | SR | |
| 3 | Piperacillin | P | 100 µg/disc | S | R | S | S | SR | |
| 4 | Ampicillin | A | 10 µg/disc | R | R | S | R | R | |
| 5 | Oxacillin | O | 1 µg/disc | R | S | R | R | R | |
| 6 | Amoxicillin | Ax | 25 µg/disc | S | S | S | R | SR | |
| 7 | Vancomycin | V | 30 µg/disc | R | S | S | R | R | |
| 8 | Inhibitor of protein synthesis | Tetracycline | T | 30 µg/disc | R | R | S | R | S |
| 9 | Doxycycline | D | 30 µg/disc | R | SR | SR | SR | S | |
| 10 | Gentamicin | G | 10 µg/disc | SR | R | S | SR | SR | |
| 11 | Tobramycin | Tb | 10 µg/disc | R | S | S | R | R | |
| 12 | Amikacin | Am | 30 µg/disc | R | R | SR | R | SR | |
| 13 | Lincomycin | L | 15 µg/disc | R | R | SR | R | SR | |
| 14 | Chloramphenicol | C | 30 µg/disc | S | SR | S | S | S | |
| 15 | Erythromycin | E | 15 µg/disc | SR | S | S | R | S | |
| 16 | Inhibitor of DNA synthesis and/or of cell division | Novobiocin | Nb | 5 µg/disc | S | R | S | SR | R |
| 17 | Nalidixic acid | Nx | 30 µg/disc | R | R | SR | R | R | |
| 18 | Rifampin | R | 5 µg/disc | SR | S | S | SR | S | |
| 19 | Ciprofloxacin | Cp | 5 µg/disc | R | R | SR | SR | SR | |
| (b) | |||||||||
| # | Mechanism of action | Antibiotic | Concentration | 5/20 | 13/20 | 19/20 | 10/20 | ||
| 1 | Inhibitor of cell wall synthesis | Penicillin | P | 10 E/disc | R | R | R | R | |
| 2 | Bacitracin | Cm | 0.07 E/disc | R | SR | SR | SR | ||
| 3 | Piperacillin | P | 100 µg/disc | R | SR | S | S | ||
| 4 | Ampicillin | A | 10 µg/disc | R | R | R | R | ||
| 5 | Oxacillin | O | 1 µg/disc | R | R | R | R | ||
| 6 | Amoxicillin | Ax | 25 µg/disc | S | SR | R | R | ||
| 7 | Vancomycin | V | 30 µg/disc | R | R | R | R | ||
| 8 | Inhibitor of protein synthesis | Tetracycline | T | 30 µg/disc | SR | S | R | R | |
| 9 | Doxycycline | D | 30 µg/disc | S | S | SR | SR | ||
| 10 | Gentamicin | G | 10 µg/disc | SR | SR | SR | SR | ||
| 11 | Tobramycin | Tb | 10 µg/disc | R | R | R | R | ||
| 12 | Amikacin | Am | 30 µg/disc | R | SR | R | R | ||
| 13 | Lincomycin | L | 15 µg/disc | R | SR | R | R | ||
| 14 | Chloramphenicol | C | 30 µg/disc | S | S | S | S | ||
| 15 | Erythromycin | E | 15 µg/disc | R | S | R | R | ||
| 16 | Inhibitor of DNA synthesis and/or of cell division | Novobiocin | Nb | 5 µg/disc | SR | R | SR | SR | |
| 17 | Nalidixic acid | Nx | 30 µg/disc | R | R | R | R | ||
| 18 | Rifampin | R | 5 µg/disc | SR | S | SR | SR | ||
| 19 | Ciprofloxacin | Cp | 5 µg/disc | R | SR | SR | SR | ||
| Batch Cultivation of Lp. plantarum 5/20 | ||||
|---|---|---|---|---|
| µ, h−1 | β, cm3/(CFU.h) | Xkp, LogN | R2 | Error |
| 0.130 | 0.0095 | 13.63 | 0.9995 | 0.778 |
| Type of Pathogenic Microorganisms Tested | According to BDS | Analysis Result |
|---|---|---|
| Total number of mesophilic aerobic and facultative anaerobic bacteria | No more than 800 | 180 |
| E. coli in 0.l g of the product | None should be detected | Not detected |
| Sulfite-reducing clostridia in 0.l g of the product | None should be detected | Not detected |
| Salmonella sp. in 25.0 g of the product | None should be detected | Not detected |
| Coagulase-positive staphylococci in 1.0 g of the product | None should be detected | Not detected |
| Spores of microscopic molds, CFU/g | No more than 100 | Not detected |
| Yeasts, CFU/g | No more than 100 | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denkova, Z.; Zapryanova, P.; Gaytanska, Y.; Goranov, B.; Shopska, V.; Denkova-Kostova, R.; Kostov, G. Isolation, Identification, and Characterization of Probiotic Properties of Lactic Acid Bacterial Strains Isolated from Rose Blossom of Rosa damascena Mill. Processes 2025, 13, 1644. https://doi.org/10.3390/pr13061644
Denkova Z, Zapryanova P, Gaytanska Y, Goranov B, Shopska V, Denkova-Kostova R, Kostov G. Isolation, Identification, and Characterization of Probiotic Properties of Lactic Acid Bacterial Strains Isolated from Rose Blossom of Rosa damascena Mill. Processes. 2025; 13(6):1644. https://doi.org/10.3390/pr13061644
Chicago/Turabian StyleDenkova, Zapryana, Polina Zapryanova, Yordanka Gaytanska, Bogdan Goranov, Vesela Shopska, Rositsa Denkova-Kostova, and Georgi Kostov. 2025. "Isolation, Identification, and Characterization of Probiotic Properties of Lactic Acid Bacterial Strains Isolated from Rose Blossom of Rosa damascena Mill" Processes 13, no. 6: 1644. https://doi.org/10.3390/pr13061644
APA StyleDenkova, Z., Zapryanova, P., Gaytanska, Y., Goranov, B., Shopska, V., Denkova-Kostova, R., & Kostov, G. (2025). Isolation, Identification, and Characterization of Probiotic Properties of Lactic Acid Bacterial Strains Isolated from Rose Blossom of Rosa damascena Mill. Processes, 13(6), 1644. https://doi.org/10.3390/pr13061644





