Synergistic Effects of Freeze–Thaw and Osmoconvective Treatments on the Physicochemical Quality, Bioaccessibility, and Consumer Acceptability of Dehydrated Spondias tuberosa Arr. Câm. (Umbu) Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Harvesting and Processing
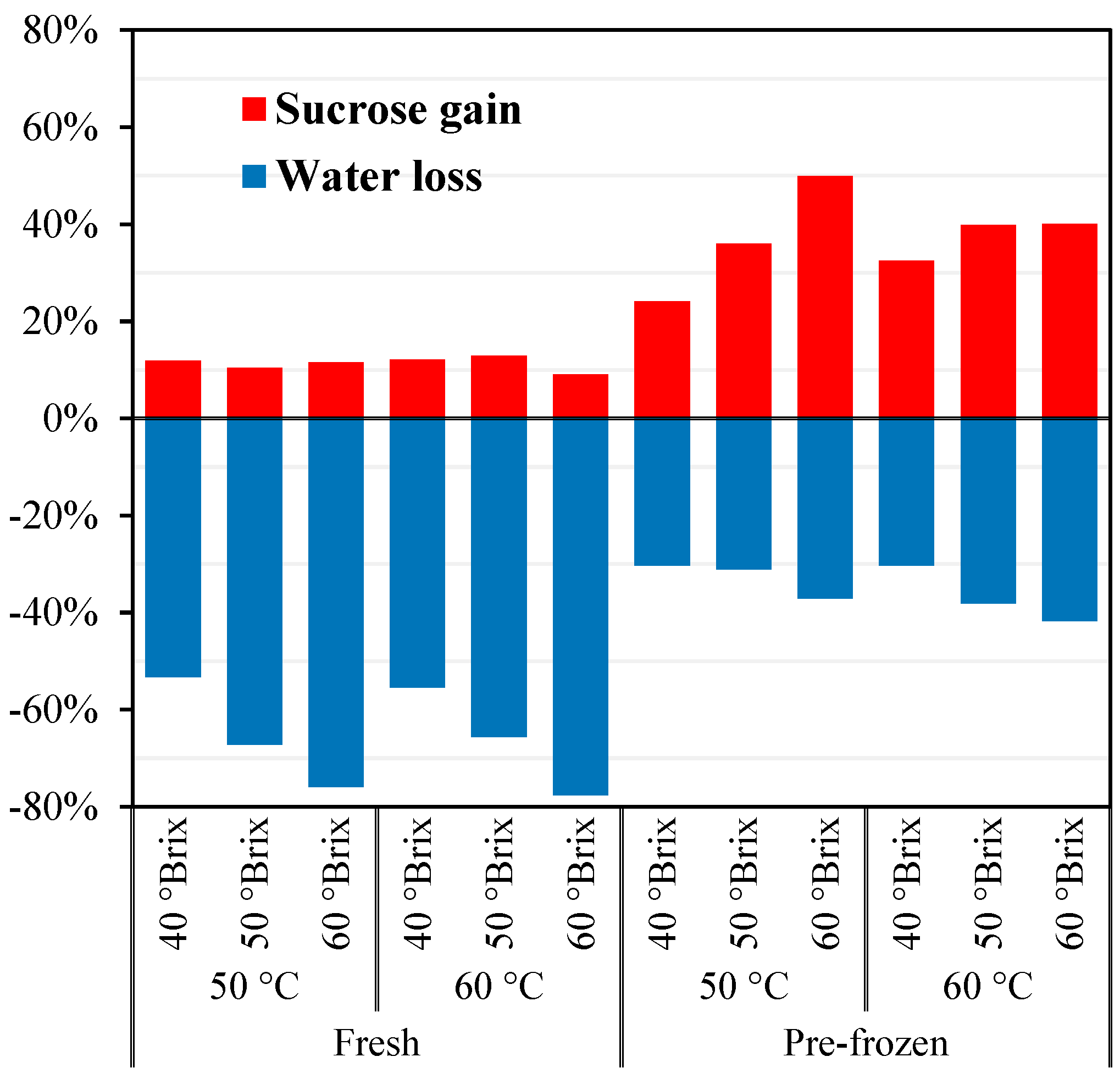
2.2. Description of Osmotic Treatments
2.3. Description of Convective Drying
2.4. Characterization of Dehydrated Umbu (Spondias Tuberosa Arr. Câm.) Slices
2.4.1. pH and Titratable Acidity
2.4.2. Soluble Solids and Ratio (SS/TA)
2.4.3. Sugars
2.4.4. Total Flavonoids and Anthocyanins
2.4.5. Chlorophylls and Carotenoids
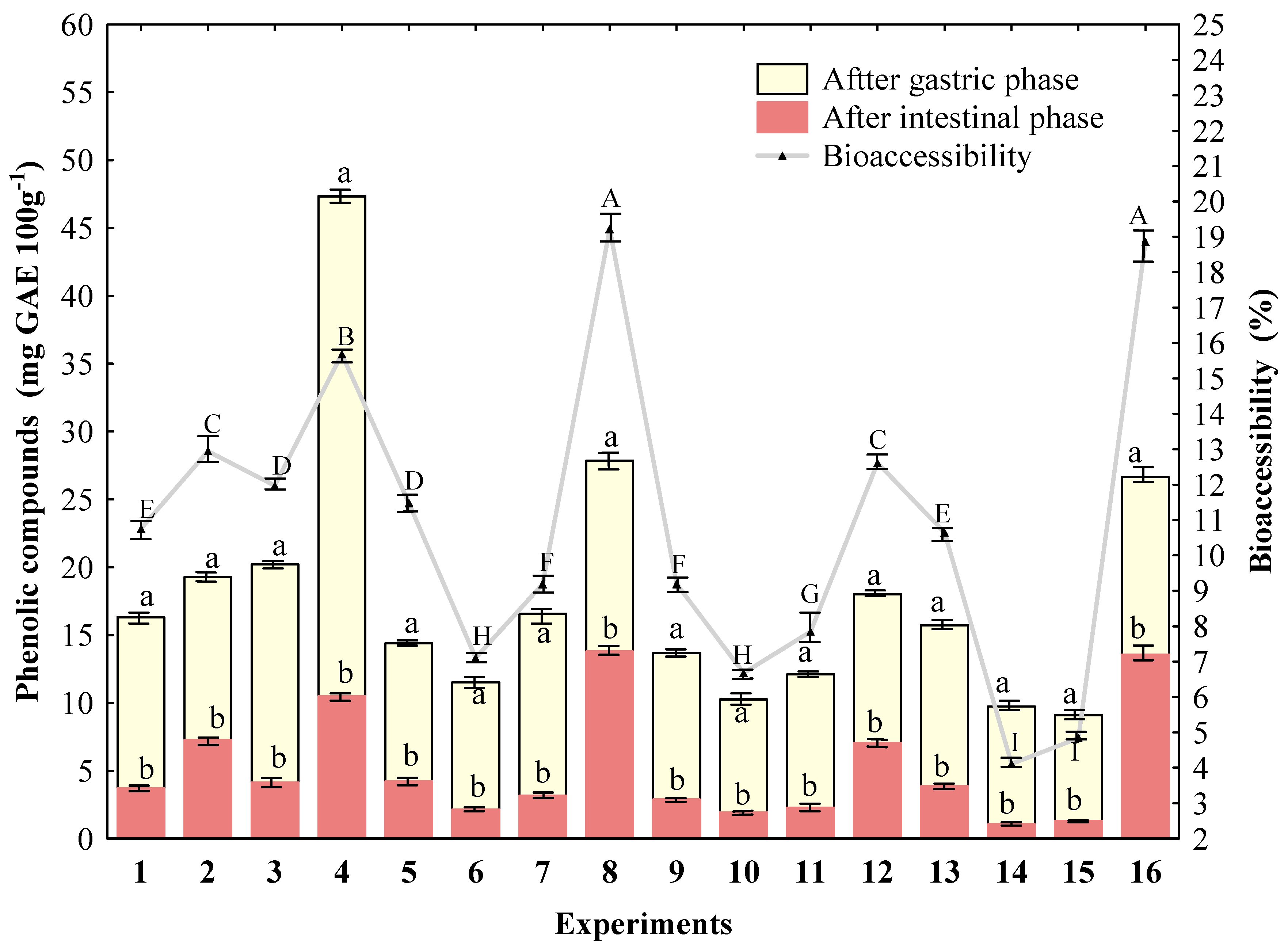
2.4.6. Phenolic Compounds
2.4.7. Bioaccessibility of Phenolic Compounds
- A = Phenolic content before in vitro gastrointestinal digestion;
- B = Phenolic content after the intestinal phase.
2.4.8. Antioxidant Activity
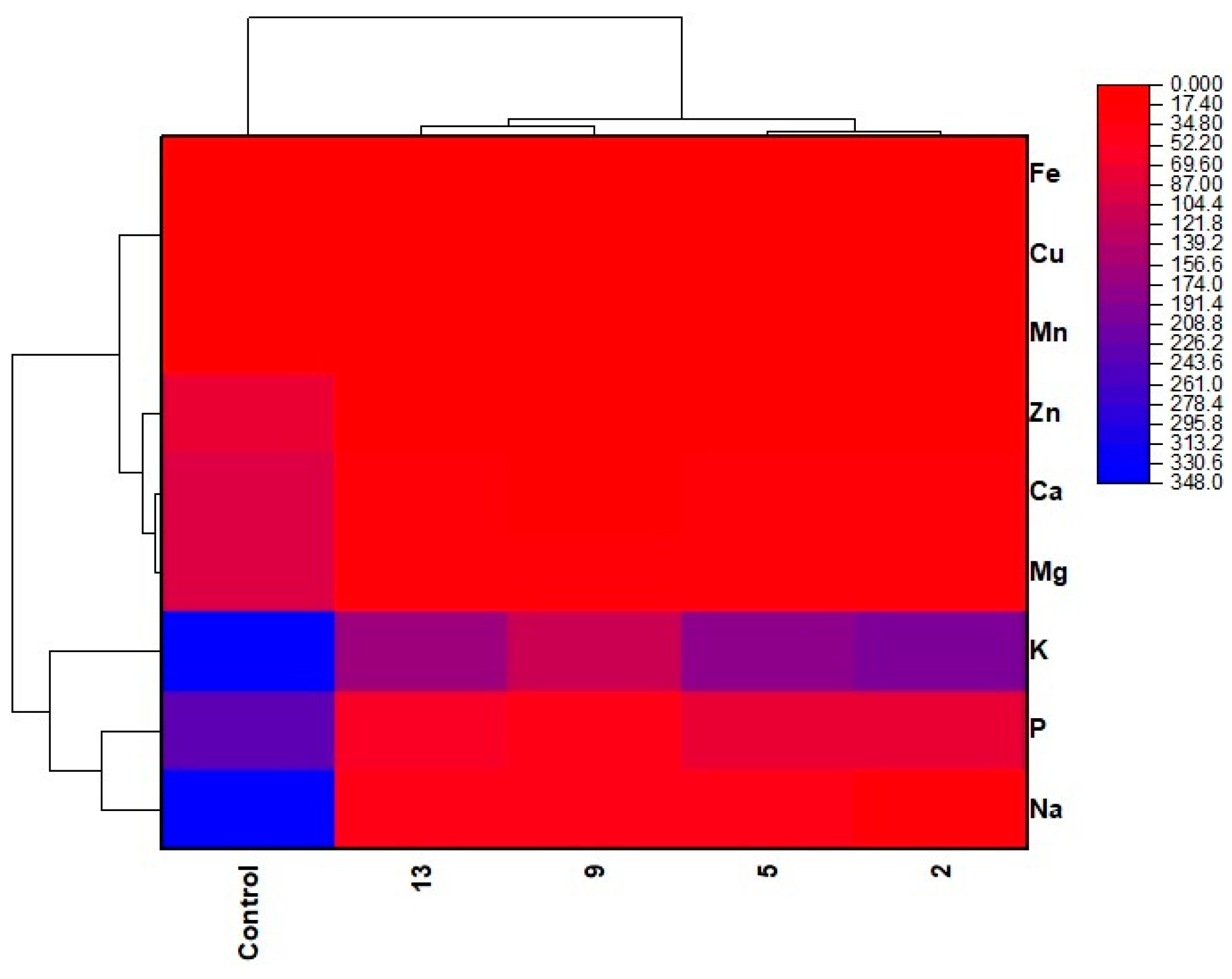
2.4.9. Mineral Profile
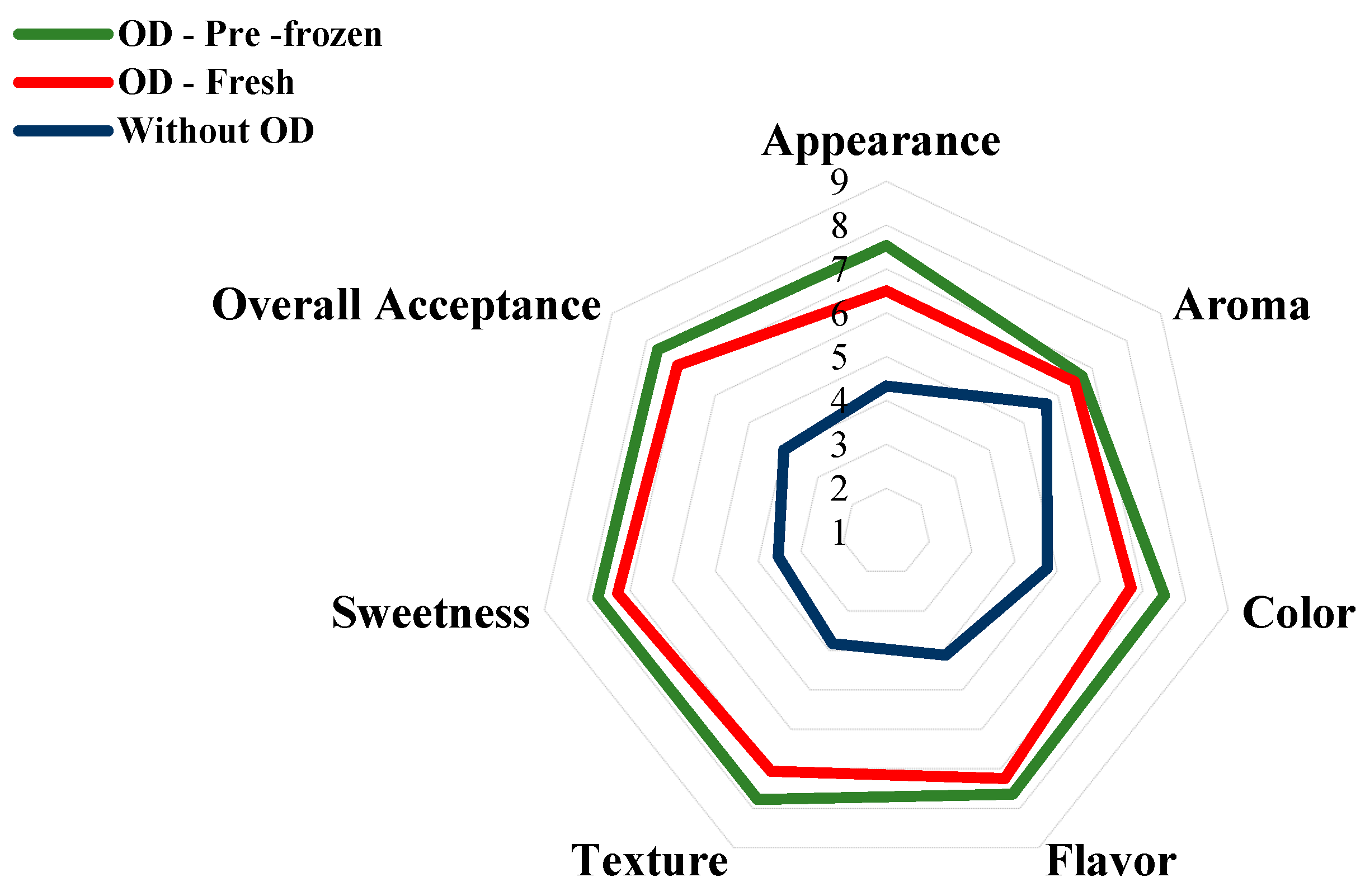
2.4.10. Microbiological, Sensory, and Purchase Intent Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Bioactive Compounds
3.3. In Vitro Digestibility
3.4. Antioxidant Capacity
3.5. Experiment Selection
3.6. Mineral Profile
3.7. Microbiological Analysis
3.8. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teodosio, A.E.M.M.; Araújo, R.H.C.R.; Santos, B.G.F.L.; Linné, J.A.; Medeiros, M.L.S.; Onias, E.A.; Morais, F.A.; Silva, S.M.; Lima, J.F. Effects of edible coating of Chlorella sp. containing pomegranate seed oil on quality of Spondias tuberosa fruit during cold storage. Food Chem. 2021, 338, 127916. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.C.; Silva, S.M.; Oliveira, V.R. Umbu—Spondias tuberosa. In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 427–433. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Ribeiro, L.D.O.; Viana, E.D.S.; Godoy, R.L.D.O.; Freitas, S.C.D.; Freitas, S.P.; Matta, V.M.D. Nutrients and bioactive compounds of pulp, peel and seed from umbu fruit. Ciência Rural 2019, 49, e20180806. [Google Scholar] [CrossRef]
- Rodrigues, N.L.; Souza, A.L.C.; Oliveira, C.C.; Carvalho, M.G.; Lima, B.R.; Akutsu, R.C.C.A.; Lana, V.S.; Raposo, A.; Saraiva, A.; Han, H.; et al. Nutritional and biological attributes of Spondias tuberosa (Umbu) fruit: An integrative review with a systematic approach. J. Food Compos. Anal. 2024, 130, 106196. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Fronza, P.; Franca, A.S.; Oliveira, L.S. Chemical Characterization and Bioaccessibility Assessment of Bioactive Compounds from Umbu (Spondias tuberosa A.) Fruit Peel and Pulp Flours. Foods 2021, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.O.; Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; Santos, A.L.S.; Moreira, D.L.; Amaral, B.S.; Jung, E.P.; Kunigami, C.N. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules 2022, 27, 410. [Google Scholar] [CrossRef]
- Mohamed, S.; Khan, M.I.; Ramlee, S.; Duah, N.; Mohamed, J.; Sulaiman, A.M.; Gan, S.H. Drying of fruits and vegetables: The need for technological innovations. Compr. Rev. Food Sci. Food Saf. 2024, 19, 573–603. [Google Scholar]
- Menon, A.; Stojceska, V.; Tassou, S.A. Systematic review on the recent advances of the energy efficiency improvements in non-conventional food drying technologies. Trends Food Sci. Technol. 2020, 100, 67–76. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Zou, B.; Yang, W. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT Food Sci. Technol. 2021, 138, 110653. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzinska, J.; Radziewska-Kubzdela, E.; Bieganska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa): The influence of ultrasound on process kinetics and product quality. Ultrason. Sonochemistry 2021, 71, 105377. [Google Scholar] [CrossRef]
- Wang, X.; Kapoor, R.; Feng, H. Exploring the effects of vacuum and ultrasound treatments on calcium fortification in osmotically dehydrated apple slices. LWT Food Sci. Technol. 2023, 187, 115386. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, J.; Bi, J.; Zhao, Y.; Li, X.; Wu, X.; Du, Q. Effect of ultrasound on mass transfer kinetics and phenolic compounds of apple cubes during osmotic dehydration. LWT Food Sci. Technol. 2021, 151, 112186. [Google Scholar] [CrossRef]
- Ferreira, J.P.D.L.; Castro, D.S.D.; Moreira, I.D.S.; Silva, W.P.D.; de Figueirêdo, R.M.; Queiroz, A.J.D.M. Convective drying kinetics os osmotically pretreated papaya cubes. Rev. Bras. De Eng. Agrícola E Ambiental 2020, 24, 200–208. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Du, Z.; Li, M.; Lv, W. Effects of osmotic dehydration pretreatment on the drying and characteristics of pineapple slices after microwave hot air rolling bed drying. Int. J. Food Eng. 2023, 19, 315–327. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S. The influence of ultrasound-assisted osmotic dehydration as a pre-treatment method on the quality of vacuum dried pineapple. Food Humanit. 2023, 1, 137–146. [Google Scholar] [CrossRef]
- Kriaa, K.; Nassar, A.F. Comparative study of pretreatment on microwave drying of Gala apples (Malus pumila): Effect of blanching, electric field and freezing. LWT Food Sci. Technol. 2022, 166, 113693. [Google Scholar] [CrossRef]
- Vallespir, F.; Rodríguez, O.; Eim, V.S.; Rossello, C.; Simal, S. Freezing pretreatments on the intensification of the drying process of vegetables with different structures. J. Food Eng. 2018, 239, 83–91. [Google Scholar] [CrossRef]
- Vallespir, F.; Rodríguez, O.; Eim, V.S.; Rossello, C.; Simal, S. Effects of freezing treatments before convective drying on quality parameters: Vegetables with different microstructures. J. Food Eng. 2019, 249, 15–24. [Google Scholar] [CrossRef]
- Pavkov, I.; Radojcin, M.; Stamenkovic, Z.; Keselj, K.; Tylewicz, U.; Sipos, P.; Ponjican, O.; Sedlar, A. Effects of osmotic dehydration on the hot air drying of apricot halves: Drying kinetics, mass transfer, and shrinkage. Processes 2021, 9, 202. [Google Scholar] [CrossRef]
- Gomes, F.P.; Resende, O.; de Sousa, E.P. Food drying: A review on applications. Braz. J. Dev. 2022, 8, 12759–12777. [Google Scholar] [CrossRef]
- de Vilela Silva, E.T.; de Queiroz, A.J.M.; de Figueirêdo, R.M.F.; Moura, H.V.; dos Santos, F.S.; de França Silva, A.P.; Cavalcanti, C.F.; Gregório, M.G.; Galdino, P.O.; Gomes, J.P. Dynamic modelling of degradation kinetics of phenolic compounds, phenolic profiles, mineral content, and overall antioxidant capacity of germinated peanut flours. LWT 2023, 183, 114927. [Google Scholar] [CrossRef]
- Brazil, ANVISA—Agência Nacional de Vigilância Sanitária. Resolução-RDC n° 272, de 22 de Setembro de 2005. Regulamento Técnico Para Produtos de Vegetais, Produtos de Frutas e Cogumelos Comestíveis. 2022. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2005/rdc0272_22_09_2005.html (accessed on 25 December 2022).
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 1982; pp. 280–282. [Google Scholar]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Waterhouse, A. Folin-ciocalteau micro method for total phenol in wine. Am. J. Enol. Vitic. 2006, 48, 3–5. [Google Scholar]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Saraiva, M.M.T.; De Alcântara Ribeiro, V.H.; De Sousa, F.M.; De Lima, T.L.B.; de Almeida Mota, M.M. Application of microwave-assisted freeze–thaw pretreatment in kiwi drying: Mass transfer, X-ray diffraction and bioaccessibility of phenolic compounds. J. Food Meas. Charact. 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; De Brito, E.S.; De Morais, S.M.; Sampaio, C.D.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre DPPH; Embrapa Agroindústria Tropical-Comunicado Técnico 128; Ministry of Agriculture and Livestock: Brasilia, Brazil, 2007. [Google Scholar]
- Rufino, M.D.S.M.; Alves, R.E.; De Brito, E.S.; De Morais, S.M.; Sampaio, C.D.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre ABTS°+; Embrapa Agroindústria Tropical-Comunicado Técnico 128; Ministry of Agriculture and Livestock: Brasilia, Brazil, 2007. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; De Brito, E.S.; De Morais, S.M.; Sampaio, C.D.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da Atividade Antioxidante Total em Frutas pelo Método de Redução do Ferro (FRAP); Embrapa Agroindústria Tropical—Comunicado técnico 125; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2006. [Google Scholar]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2001. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Barroso, A.J.R.; Almeida, F.A.C.; Castro, D.S.; Melo, B.A.; Moraes, J.S.; Gomes, J.P.; Silva, L.M.M.; Silva, W.P. Maltodextrin effect on the parameters of physicochemical quality of the umbu powder for characterization of the selected encapsulation. J. Food Agric. Environ. 2019, 17, 7–11. [Google Scholar]
- Araújo, E.J.S.; Santos, J.A.B.; Narain, N. Evaluation of the influence of different freeze-drying conditions on the physical chemical and sensory characteristics of the umbu powder. Braz. J. Dev. 2020, 9, 68815–68821. [Google Scholar] [CrossRef]
- Alfaro, L.; Siramard, S.; Chouljenko, A.; Sathivel, S. Effects of liquid nitrogen pretreatment on the osmotic dehydrated and quality of cryogenically frozen blueberries (Vaccinium angustifolium Ait.). Food Biosci. 2018, 22, 165–169. [Google Scholar] [CrossRef]
- Hossain, M.A.; Dey, P.; Joy, R.I. Effect of osmotic pretreatment and drying temperature on drying kinetics, antioxidant activity, and overall quality of taikor (Garcinia pedunculata Roxb.) slices. Saudi J. Biol. Sci. 2021, 28, 7269–7280. [Google Scholar] [CrossRef]
- Meena, L.; Gowda, N.N.; Sunil, C.K.; Rawson, A.; Janghu, S. Effect of ultrasonication on food bioactive compounds and their bio-accessibility: A review. J. Food Compos. Anal. 2024, 126, 105899. [Google Scholar] [CrossRef]
- Mazzeo, T.; Paciulli, M.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Ganino, T.; Pellegrini, N. Impact of the industrial freezing process on selected vegetables—Part II. Colour and bioactive compounds. Food Res. Int. 2015, 75, 89–97. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. A critical review on the stability of natural food pigments and stabilization techniques. Food Res. Int. 2024, 179, 114011. [Google Scholar] [CrossRef]
- Gualberto, N.C.; Oliveira, C.S.; Nogueira, J.P.; Jesus, M.S.; Araújo, H.C.S.; Rajan, M.; Neta, M.T.S.L.; Narain, M. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Food Res. Int. 2021, 147, 1–13. [Google Scholar]
- Pasquet, P.L.; Julien-David, D.; Zhao, M.; Villain-Gambier, M.; Trébouet, D. Stability and preservation of phenolic compounds and related antioxidant capacity from agro-food matrix: Effect of pH and atmosphere. Food Biosci. 2024, 57, 103586. [Google Scholar] [CrossRef]
- Bozkir, H.; Ergun, A.R. Effect of sonication and osmotic dehydration applications on the hot air drying kinetics and quality of persimmon. LWT Food Sci. Technol. 2020, 131, 109704. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjiha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F. Natural pigments: Anthocyanins, carotenoids, chlorophylls, and betalains as colorants in food products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Ávila, S.; Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C.W.I. The association between chromaticity, phenolics, carotenoids, and in vitro antioxidant activity of frozen fruit pulp in Brazil: An application of chemometrics. J. Food Sci. 2014, 79, 510–516. [Google Scholar] [CrossRef]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegria, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Costa, A.L.R.; França, A.S.; Oliveira, L.S. Chemical characterization and the bioaccessibility of bioactive compounds from seriguela (Spondias purpurea L.) pulp and by-products flour. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100404. [Google Scholar] [CrossRef]
- Xue, T.T.; Ruan, K.H.; Xu, H.B.; Liu, H.B.; Tang, Z.S.; Yang, Y.G.; Duan, J.A.; Sun, X.X.; Wang, M.; Song, Z.X. Effect of different drying methods on the drying characteristics, chemical properties and antioxidant capacity of Ziziphus jujuba var. Spinosa fruit. LWT Food Sci. Technol. 2024, 196, 115873. [Google Scholar] [CrossRef]
- Rahman, N.; Xin, T.B.; Kamilah, H.; Ariffin, F. Effects of osmotic dehydration treatment on volatile compound (Myristicin) content and antioxidants property of nutmeg (Myristica fragrans) pericarp. J. Food Sci. Technol. 2018, 55, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nudar, J.; Roy, M.; Ahmed, S. Combined osmotic pretreatment and hot air drying: Evaluation of drying kinetics and quality parameters of adajamir (Citrus assamensis). Heliyon 2023, 9, e19545. [Google Scholar] [CrossRef]
- Hossain, M.A.; Talukder, S.; Zaman, A.U.; Sarkar, A.; Yasin, M.; Biswas, R. Effective drying processes for Taikor (Garcinia pedunculata Roxb.) fruit by ultrasound-assisted osmotic pretreatment: Analysis of quality and kinetic models. Ultrason. Sonochemistry 2024, 103, 106784. [Google Scholar] [CrossRef]


| Experiments | Fruit | Temperature (°C) | Osmotic Solution (°Brix) | Drying Time (min) | Dehydrated Umbu |
|---|---|---|---|---|---|
| 1 | Fresh | 50 | 40 | 170 |  |
| 2 | 50 | 170 |  | ||
| 3 | 60 | 130 |  | ||
| 4 | - | 450 |  | ||
| 5 | 60 | 40 | 170 |  | |
| 6 | 50 | 130 |  | ||
| 7 | 60 | 150 |  | ||
| 8 | - | 300 |  | ||
| 9 | Pre-frozen | 50 | 40 | 240 |  |
| 10 | 50 | 210 |  | ||
| 11 | 60 | 210 |  | ||
| 12 | - | 210 |  | ||
| 13 | 60 | 40 | 130 |  | |
| 14 | 50 | 130 |  | ||
| 15 | 60 | 210 |  | ||
| 16 | - | 170 |  |
| Experiments | pH | Titratable Acidity (% Citric Acid) | Soluble Solids (°Brix) | Ratio |
|---|---|---|---|---|
| 1 | 3.01 ± 0.04 c | 1.56 ± 0.02 e | 23.66 ± 0.58 f | 15.18 ± 0.42 g |
| 2 | 2.95 ± 0.03 c | 1.52 ± 0.05 e | 25.50 ± 0.50 e | 16.76 ± 0.80 g |
| 3 | 3.03 ± 0.07 c | 1.44 ± 0.17 e | 24.50 ± 0.50 f | 17.18 ± 2.44 g |
| 4 | 2.49 ± 0.03 f | 12.46 ± 0.28 a | 20.05 ± 0.50 g | 1.65 ± 0.08 h |
| 5 | 3.19 ± 0.12 b | 1.01 ± 0.07 f | 23.33 ± 0.58 f | 23.24 ± 2.34 f |
| 6 | 3.21 ± 0.03 b | 0.79 ± 0.04 f | 24.50 ± 0.50 f | 30.88 ± 1.39 e |
| 7 | 3.38 ± 0.11 a | 0.62 ± 0.06 f | 32.00 ± 1.00 c | 52.15 ± 5.39 b |
| 8 | 2.83 ± 0.14 d | 9.79 ± 0.71 b | 17.00 ± 1.00 g | 1.75 ± 0.23 h |
| 9 | 3.50 ± 0.04 a | 0.49 ± 0.03 f | 26.50 ± 0.50 e | 53.95 ± 3.70 b |
| 10 | 3.44 ± 0.03 a | 0.71 ± 0.01 f | 27.50 ± 0.50 d | 38.66 ± 1.26 d |
| 11 | 3.18 ± 0.10 b | 0.56 ± 0.03 f | 32.50 ± 0.50 c | 58.27 ± 2.89 a |
| 12 | 2.60 ± 0.01 e | 7.64 ± 0.29 d | 14.83 ± 0.29 i | 1.94 ± 0.11 h |
| 13 | 3.15 ± 0.03 b | 0.57 ± 0.06 f | 36.00 ± 1.00 a | 63.03 ± 7.53 a |
| 14 | 3.10 ± 0.01 b | 0.75 ± 0.06 f | 33.50 ± 0.50 b | 45.00 ± 2.88 c |
| 15 | 3.05 ± 0.02 c | 0.85 ± 0.05 f | 34.00 ± 1.00 b | 40.03 ± 3.55 d |
| 16 | 2.42 ± 0.02 f | 8.43 ± 0.10 c | 15.00 ± 1.00 i | 1.78 ± 0.10 h |
| Experiments | Reducing Sugar | Non-Reducing Sugars | Total Sugar |
|---|---|---|---|
| 1 | 5.67 ± 0.05 g | 23.05 ± 1.02 b | 28.72 ± 1.07 c |
| 2 | 6.07 ± 0.35 e | 22.40 ± 0.58 c | 28.47 ± 0.34 c |
| 3 | 6.34 ± 0.15 d | 23.03 ± 1.18 b | 29.38 ± 1.34 c |
| 4 | 3.82 ± 0.05 j | 20.37 ± 0.27 d | 24.19 ± 0.32 e |
| 5 | 6.11 ± 0.08 e | 22.26 ± 0.24 c | 28.37 ± 0.31 c |
| 6 | 5.92 ± 0.02 f | 24.32 ± 0.20 a | 30.25 ± 0.19 b |
| 7 | 5.23 ± 0.06 h | 23.36 ± 0.14 b | 28.58 ± 0.89 c |
| 8 | 4.42 ± 0.06 i | 19.64 ± 0.29 d | 24.06 ± 0.35 e |
| 9 | 6.86 ± 0.03 b | 22.13 ± 0.16 c | 28.99 ± 0.19 c |
| 10 | 7.26 ± 0.04 a | 21.91 ± 1.11 c | 29.17 ± 1.08 c |
| 11 | 7.23 ± 0.07 a | 22.83 ± 0.20 b | 30.06 ± 0.27 b |
| 12 | 4.28 ± 0.12 i | 21.05 ± 0.60 d | 25.34 ± 0.72 d |
| 13 | 6.64 ± 0.08 c | 23.35 ± 0.28 b | 29.99 ± 0.37 b |
| 14 | 6.66 ± 0.06 c | 24.71 ± 0.30 a | 31.36 ± 0.36 a |
| 15 | 6.93 ± 0.03 b | 24.88 ± 0.13 a | 31.81 ± 0.17 a |
| 16 | 4.51 ± 0.02 i | 19.99 ± 0.73 d | 24.50 ± 0.71 e |
| Experiments | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids | Phenolic Compound | Flavonoids | Anthocyanins |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 ± 0.01 f | 0.48 ± 0.02 c | 0.78 ± 0.02 e | 0.20 ± 0.02 d | 43.79 ± 2.14 f | 3.94 ± 0.10 d | 0.62 ± 0.01 d |
| 2 | 0.26 ± 0.01 g | 0.36 ± 0.05 d | 0.62 ± 0.01 f | 0.15 ± 0.01 e | 71.95 ± 1.90 d | 3.53 ± 0.10 e | 0.47 ± 0.01 f |
| 3 | 0.26 ± 0.01 g | 0.30 ± 0.01 e | 0.56 ± 0.01 g | 0.14 ± 0.02 e | 44.30 ± 1.61 f | 3.93 ± 0.06 d | 0.50 ± 0.01 f |
| 4 | 0.53 ± 0.01 c | 0.93 ± 0.02 a | 1.46 ± 0.02 c | 0.33 ± 0.02 a | 85.80 ± 1.89 c | 7.59 ± 0.17 b | 1.03 ± 0.02 b |
| 5 | 0.34 ± 0.01 e | 0.27 ± 0.01 f | 0.61 ± 0.01 f | 0.12 ± 0.01 g | 46.46 ± 1.00 e | 3.80 ± 0.06 d | 0.47 ± 0.01 f |
| 6 | 0.23 ± 0.01 h | 0.29 ± 0.00 f | 0.52 ± 0.01 g | 0.10 ± 0.02 h | 38.56 ± 0.68 g | 3.61 ± 0.05 e | 0.58 ± 0.01 e |
| 7 | 0.20 ± 0.02 i | 0.28 ± 0.01 f | 0.48 ± 0.03 h | 0.11 ± 0.03 g | 42.46 ± 0.94 f | 3.39 ± 0.03 f | 0.43 ± 0.05 g |
| 8 | 0.84 ± 0.05 a | 0.95 ± 0.01 a | 1.79 ± 0.06 a | 0.28 ± 0.01 c | 91.19 ± 1.48 b | 7.07 ± 0.18 c | 0.86 ± 0.02 c |
| 9 | 0.25 ± 0.00 g | 0.31 ± 0.00 e | 0.56 ± 0.01 g | 0.09 ± 0.01 h | 41.56 ± 0.59 f | 2.89 ± 0.06 g | 0.40 ± 0.01 h |
| 10 | 0.21 ± 0.00 h | 0.34 ± 0.01 d | 0.55 ± 0.00 g | 0.10 ± 0.01 h | 38.19 ± 0.72 g | 2.56 ± 0.11 h | 0.45 ± 0.01 g |
| 11 | 0.18 ± 0.03 i | 0.22 ± 0.01 g | 0.40 ± 0.01 i | 0.13 ± 0.02 f | 38.43 ± 1.20 g | 2.30 ± 0.10 i | 0.28 ± 0.01 j |
| 12 | 0.44 ± 0.02 d | 0.91 ± 0.06 b | 1.35 ± 0.06 d | 0.30 ± 0.04 b | 72.74 ± 1.58 d | 8.00 ± 0.07 a | 1.08 ± 0.01 a |
| 13 | 0.17 ± 0.01 i | 0.28 ± 0.02 f | 0.45 ± 0.03 h | 0.12 ± 0.02 g | 47.16 ± 1.23 e | 2.13 ± 0.06 i | 0.24 ± 0.01 k |
| 14 | 0.15 ± 0.04 j | 0.25 ± 0.02 g | 0.39 ± 0.01 i | 0.10 ± 0.03 h | 33.92 ± 1.06 h | 2.57 ± 0.12 h | 0.33 ± 0.01 j |
| 15 | 0.13 ± 0.01 j | 0.25 ± 0.00 g | 0.38 ± 0.01 i | 0.10 ± 0.01 h | 34.31 ± 0.17 h | 3.32 ± 0.14 f | 0.42 ± 0.01 g |
| 16 | 0.72 ± 0.02 b | 0.88 ± 0.03 b | 1.60 ± 0.02 b | 0.30 ± 0.03 b | 93.88 ± 1.80 a | 8.16 ± 0.31 a | 1.06 ± 0.04 a |
| Experiments | DPPH (µM Trolox g−1) | ABTS (µM Trolox g−1) | FRAP (µM FeSO4 g−1) |
|---|---|---|---|
| 1 | 14.47 ± 0.62 g | 10.27 ± 0.98 e | 4.10 ± 0.09 f |
| 2 | 19.73 ± 0.50 d | 16.45 ± 0.49 c | 7.30 ± 0.29 d |
| 3 | 16.32 ± 0.32 f | 12.36 ± 0.39 d | 4.31 ± 0.22 f |
| 4 | 21.90 ± 0.35 c | 17.77 ± 0.87 b | 8.47 ± 0.39 c |
| 5 | 16.20 ± 0.18 f | 12.84 ± 0.30 d | 5.03 ± 0.14 e |
| 6 | 12.01 ± 0.16 i | 7.26 ± 0.32 g | 3.75 ± 0.47 f |
| 7 | 14.30 ± 0.21 g | 10.14 ± 0.79 e | 4.18 ± 0.13 f |
| 8 | 27.58 ± 0.74 a | 21.85 ± 1.29 a | 12.65 ± 0.53 a |
| 9 | 13.37 ± 0.45 h | 9.14 ± 0.77 f | 3.84 ± 0.25 f |
| 10 | 11.16 ± 0.66 j | 7.03 ± 0.10 g | 3.00 ± 0.17 g |
| 11 | 12.92 ± 0.13 h | 7.63 ± 0.55 g | 3.56 ± 0.34 f |
| 12 | 18.38 ± 0.26 e | 15.67 ± 0.43 c | 6.96 ± 0.24 d |
| 13 | 15.81 ± 0.40 f | 11.21 ± 0.13 e | 4.56 ± 0.60 e |
| 14 | 10.17 ± 0.11 k | 6.37 ± 0.34 g | 2.12 ± 0.14 h |
| 15 | 10.48 ± 0.36 k | 6.67 ± 0.27 g | 2.35 ± 0.18 h |
| 16 | 26.16 ± 0.95 b | 20.95 ± 0.37 a | 11.80 ± 0.66 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, M.M.T.; da Silva, W.P.; Santos, N.C.; Silva, A.P.d.F.; Costa, C.C.; Junior, N.d.M.A.; Silva, E.T.d.V.; Moura, H.V.; Andrade, F.S. Synergistic Effects of Freeze–Thaw and Osmoconvective Treatments on the Physicochemical Quality, Bioaccessibility, and Consumer Acceptability of Dehydrated Spondias tuberosa Arr. Câm. (Umbu) Slices. Processes 2025, 13, 1518. https://doi.org/10.3390/pr13051518
Saraiva MMT, da Silva WP, Santos NC, Silva APdF, Costa CC, Junior NdMA, Silva ETdV, Moura HV, Andrade FS. Synergistic Effects of Freeze–Thaw and Osmoconvective Treatments on the Physicochemical Quality, Bioaccessibility, and Consumer Acceptability of Dehydrated Spondias tuberosa Arr. Câm. (Umbu) Slices. Processes. 2025; 13(5):1518. https://doi.org/10.3390/pr13051518
Chicago/Turabian StyleSaraiva, Maria Monique Tavares, Wilton Pereira da Silva, Newton Carlos Santos, Aline Priscila de França Silva, Caciana Cavalcanti Costa, Nailton de Macedo Albuquerque Junior, Eugênia Telis de Vilela Silva, Henrique Valentim Moura, and Fabrícia Santos Andrade. 2025. "Synergistic Effects of Freeze–Thaw and Osmoconvective Treatments on the Physicochemical Quality, Bioaccessibility, and Consumer Acceptability of Dehydrated Spondias tuberosa Arr. Câm. (Umbu) Slices" Processes 13, no. 5: 1518. https://doi.org/10.3390/pr13051518
APA StyleSaraiva, M. M. T., da Silva, W. P., Santos, N. C., Silva, A. P. d. F., Costa, C. C., Junior, N. d. M. A., Silva, E. T. d. V., Moura, H. V., & Andrade, F. S. (2025). Synergistic Effects of Freeze–Thaw and Osmoconvective Treatments on the Physicochemical Quality, Bioaccessibility, and Consumer Acceptability of Dehydrated Spondias tuberosa Arr. Câm. (Umbu) Slices. Processes, 13(5), 1518. https://doi.org/10.3390/pr13051518









