Dough Rheological Properties and Macronutrient Bioavailability of Cereal Products Fortified through Legume Proteins
Abstract
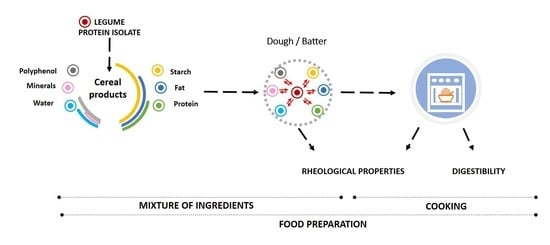
1. Introduction
2. Effect of the Legume Protein Isolate/Concentrate on the Rheological Properties of Raw Material
2.1. Dough Rheology
2.1.1. Bread
2.1.2. Pasta Noodle, Pasta and Spaghetti
2.2. Batter Rheology
Cake and Muffin Batter
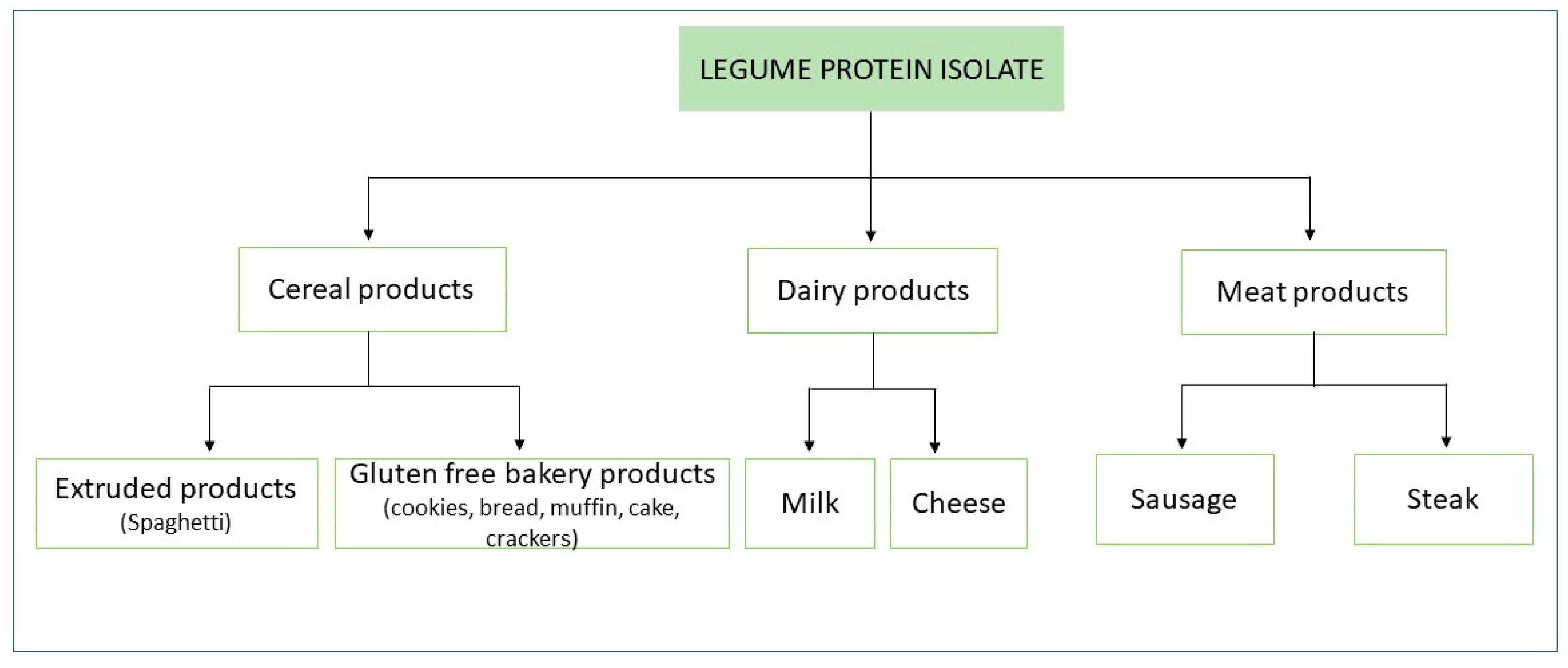
3. Effect of Legume Protein Isolate/Concentrate on Digestibility and Nutritional Quality of Cereal Foodstuff
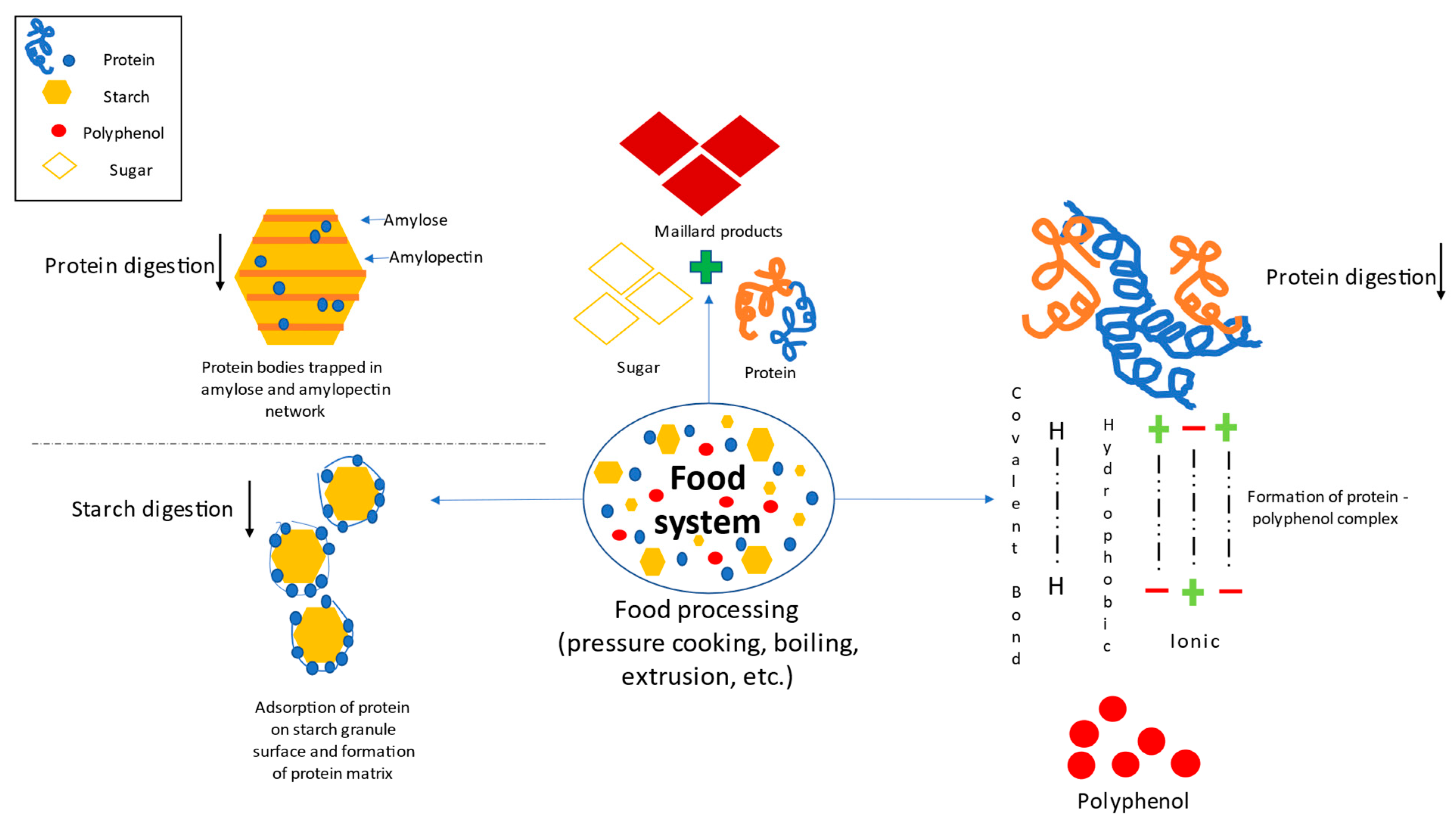
3.1. The Protein-Starch Type of Interaction and Their Relevance
3.1.1. Effect of Protein on Starch Digestibility
3.1.2. Effects of Starch on Proteins Digestibility
3.2. Effects of Protein Combinations on Their Digestibility
3.3. Effect of Polyphenols on Protein Digestibility
3.4. Effects of Protein Fortification on Lipid Digestibility
3.5. Effect of Protein Supplementation on Sugar Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos-Hernández, M.; Alfieri, F.; Gallo, V.; Miralles, B.; Masi, P.; Romano, A.; Ferranti, P.; Recio, I. Compared digestibility of plant protein isolates by using the INFOGEST digestion protocol. Food Res. Int. 2020, 137, 109708. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Singhal, A. Pulse Proteins: From Processing to Structure-Function Relationships; In IntechOpen: London, UK, 2016. [Google Scholar]
- Bianchini, A.; Stratton, J. Spoilage of Animal Products | Spoilage of Plant Products: Cereals and Cereal Flours. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 459–464. [Google Scholar]
- Jones, J.M.; Jones, C.I.M. cultural differences in processing and consumption. In Encyclopedia of Grain Science; Wrigley, C., Ed.; Elsevier: Oxford, UK, 2004; pp. 349–355. [Google Scholar]
- Hambræus, L. Protein and Amino Acids in Human Nutrition. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced properties of gluten-free cookies, cakes, and crackers: A review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Sahagun, M.; Gomez, M. Assessing Influence of Protein Source on Characteristics of Gluten-Free Breads Optimising their Hydration Level. Food Bioprocess Technol. 2018, 11, 1686–1694. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Neji, C.; Semwal, J.; Kamani, M.H.; Máthé, E.; Sipos, P. Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development. Processes 2022, 10, 2586. [Google Scholar] [CrossRef]
- Raymundo, A.; Torres, M.D.; Sousa, I. Special Issue: Rheology and Quality Research of Cereal-Based Food. Food 2021, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Subramanian, P.; Dar, B.N. Functionalization of legume proteins using high pressure processing: Effect on technofunctional properties and digestibility of legume proteins. LWT 2022, 158, 113106. [Google Scholar] [CrossRef]
- Sarabhai, S.; Indrani, D.; Vijaykrishnaraj, M.; Milind; Arun Kumar, V.; Prabhasankar, P. Effect of protein concentrates, emulsifiers on textural and sensory characteristics of gluten free cookies and its immunochemical validation. J. Food Sci. Technol. 2015, 52, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Lupin Seed Protein Extract Can Efficiently Enrich the Physical Properties of Cookies Prepared with Alternative Flours. Foods 2020, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, S.W.; Foschia, M.; Arendt, E.K. Correlation analysis of protein quality characteristics with gluten-free bread properties. Food Funct. 2017, 8, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Wilderjans, E.; Pareyt, B.; Goesaert, H.; Brijs, K.; Delcour, J.A. The role of gluten in a pound cake system: A model approach based on gluten–starch blends. Food Chem. 2008, 110, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.; Kokini, J.L. Chapter 15—Measurement and Interpretation of Batter Rheological Properties. In Batters and Breadings in Food Processing, 2nd ed.; Elsevier Inc.: St. Paul, MN, USA, 2016; pp. 263–299. [Google Scholar]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules 2021, 11, 178. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33525520 (accessed on 30 December 2022). [CrossRef]
- Ionescu, A.; Aprodu, I.; Gurau, G.; Banu, I. Rheology of chickpea protein concentrate dispersions. Sci. Study Res. Chem. Chem. Eng. 2011, 12, 387–399. [Google Scholar]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Hossain Brishti, F.; Chay, S.Y.; Muhammad, K.; Rashedi Ismail-Fitry, M.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and rheological changes of texturized mung bean protein induced by feed moisture during extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- O′Flynn, T.D.; Hogan, S.A.; Daly, D.F.M.; O′Mahony, J.A.; McCarthy, N.A. Rheological and Solubility Properties of Soy Protein Isolate. Molecules 2021, 26, 3015. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Labropoulos, A.; Anestis, S. Rheological Properties of a Soy Protein Isolate and Concentrate: Effect of Gel Strength. In Proceedings of the 11th International Congress on Engineering and Food, NTUA, Athens, Greece, 22–25 May 2011; pp. 1103–1104. [Google Scholar]
- Zárate-Ramírez, L.S.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Linear viscoelasticity of carob protein isolate/locust bean gum blends. J. Food Eng. 2010, 100, 435–445. [Google Scholar] [CrossRef]
- Bengoechea, C.; Ortiz, S.E.M.; Guerrero, A.; Puppo, M.C. Effect of pH on the thermal gelation of carob protein isolate. J. Food Sci. Technol. 2017, 54, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.C.; Arêas, E.P.G.; Silva, M.A.; Arêas, J.A.G. Effects of Extrusion on the Emulsifying Properties of Rumen and Soy Protein. Food Biophys. 2010, 5, 94–102. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Rheological characterization of the thermal gelation of cowpea protein isolates: Effect of pretreatments with high hydrostatic pressure or calcium addition. LWT 2019, 115, 108472. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of salt-extracted pea protein induced by heat treatment. Food Res. Int. 2010, 43, 509–515. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Karwe, M.V.; Moraru, C.I. High pressure structuring of pea protein concentrates. J. Food Process Eng. 2019, 42, e13261. [Google Scholar] [CrossRef]
- Liu, H.; Kuo, M. Effect of microwave heating on the viscoelastic property and microstructure of soy protein isolate gel. J. Texture Stud. 2011, 42, 1–9. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Arnulphi, S.A.; León, A.E.; Añón, M.C. Effect of soybean addition on the rheological properties and breadmaking quality of wheat flour. J. Sci. Food Agric. 2005, 85, 1889–1896. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Tang, X. Effects of whey and soy protein addition on bread rheological property of wheat flour. J. Texture Stud. 2018, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- López, E.P. Influence of the addition of lupine protein isolate on the protein and technological characteristics of dough and fresh bread with added Brea Gum. Ciência E Tecnol. De Aliment. 2014, 34, 195–203. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Provatidou, E.; Tsotsiou, D.; Kiosseoglou, V. Dough rheology and baking performance of wheat flour–lupin protein isolate blends. Food Res. Int. 2010, 43, 1009–1016. [Google Scholar] [CrossRef]
- Marchais, L.D.; Foisy, M.; Mercier, S.; Villeneuve, S.; Mondor, M. Bread-making potential of pea protein isolate produced by a novel ultrafiltration/diafiltration process. Procedia Food Sci. 2011, 1, 1425–1430. [Google Scholar] [CrossRef]
- Belc, N.; Duta, D.E.; Culetu, A.; Stamatie, G.D. Type and Amount of Legume Protein Concentrate Influencing the Technological, Nutritional, and Sensorial Properties of Wheat Bread. Appl. Sci. 2021, 11, 436. [Google Scholar] [CrossRef]
- Campbell, L.; Euston, S.R.; Ahmed, M.A. Effect of addition of thermally modified cowpea protein on sensory acceptability and textural properties of wheat bread and sponge cake. Food Chem. 2016, 194, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, F.; Liu, K.; Lai, S.; Zhang, L.; Bu, G.; Gao, X.; Liu, S. Effects of Extruded Soy Protein on the Quality of Chinese Steamed Bread. J. Chem. 2016, 2016, 3691523. [Google Scholar] [CrossRef]
- Mariotti, M.; Lucisano, M.; Ambrogina Pagani, M.; Ng, P.K.W. The role of corn starch, amaranth flour, pea isolate, and Psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar] [CrossRef]
- Ziobro, R.; Witczak, T.; Juszczak, L.; Korus, J. Supplementation of gluten-free bread with non-gluten proteins. Effect on dough rheological properties and bread characteristic. Food Hydrocoll. 2013, 32, 213–220. [Google Scholar] [CrossRef]
- Marcoa, C.; Rosell, C.M. Effect of different protein isolates and transglutaminase on rice flour properties. J. Food Eng. 2008, 84, 132–139. [Google Scholar] [CrossRef]
- Ziobro, R.; Juszczak, L.; Witczak, M.; Korus, J. Non-gluten proteins as structure forming agents in gluten free bread. J. Food Sci. Technol. 2016, 53, 571–580. [Google Scholar] [CrossRef]
- Crockett, R.; Ie, P.; Vodovotz, Y. Effects of soy protein isolate and egg white solids on the physicochemical properties of gluten-free bread. Food Chem. 2011, 129, 84–91. [Google Scholar] [CrossRef]
- Assad Bustillos, M.; Jonchère, C.; Garnier, C.; Réguerre, A.L.; Della Valle, G. Rheological and microstructural characterization of batters and sponge cakes fortified with pea proteins. Food Hydrocoll. 2020, 101, 105553. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Chhikara, N.; Panghal, A. Effect of incorporation of germinated flour and protein isolate from chickpea on different quality characteristics of rice-based noodle. Cereal Chem. 2020, 97, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Xiong, H.; Zhu, T. Effect of modified soy protein isolate on dough rheological properties and noodle qualities. J. Food Process. Preserv. 2022, 46, e16371. [Google Scholar] [CrossRef]
- Doxastakis, G.; Papageorgiou, M.; Mandalou, D.; Irakli, M.; Papalamprou, E.; D’Agostina, A.; Resta, D.; Boschin, G.; Arnoldi, A. Technological properties and non-enzymatic browning of white lupin protein enriched spaghetti. Food Chem. 2007, 101, 57–64. [Google Scholar] [CrossRef]
- Cutillo, S.; Farahnaky, A.; Marcotuli, I.; Gadaleta, A.; Sissons, M. In vitro starch digestion and technological properties of spaghetti fortified with lupin protein isolate. Int. J. Food Sci. Technol. 2021, 56, 3567–3577. [Google Scholar] [CrossRef]
- Matos, M.E.; Sanz, T.; Rosell, C.M. Establishing the function of proteins on the rheological and quality properties of rice based gluten free muffins. Food Hydrocoll. 2014, 35, 150–158. [Google Scholar] [CrossRef]
- Majzoobi, M.; Ghiasi, F.; Habibi, M.; Hedayati, S.; Farahnaky, A. Influence of Soy Protein Isolate on the Quality of Batter and Sponge Cake. J. Food Process. Preserv. 2014, 38, 1164–1170. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Wong, L.; Wismer, W.; Temelli, F.; Han, J.; Huang, W.; Eckhart, E.; Tian, Z.; Shi, K.; Sun, T.; et al. Quality characteristics of angel food cake and muffin using lentil protein as egg/milk replacer. Int. J. Food Sci. Technol. 2017, 52, 1604–1613. [Google Scholar] [CrossRef]
- Ronda, F.; Oliete, B.; Gómez, M.; Caballero, P.A.; Pando, V. Rheological study of layer cake batters made with soybean protein isolate and different starch sources. J. Food Eng. 2011, 102, 272–277. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N. Influence of kidney bean, field pea and amaranth protein isolates on the characteristics of starch-based gluten-free muffins. Int. J. Food Sci. Technol. 2014, 49, 2237–2244. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201400172270 (accessed on 30 December 2022). [CrossRef]
- Kulp, K. Batters and Breadings in Food Processing; Elsevier Science & Technology: Atlanta, GA, USA, 2011. [Google Scholar]
- Bala, M.; Arun Kumar, T.V.; Tushir, S.; Nanda, S.K.; Gupta, R.K. Quality protein maize based muffins: Influence of non-gluten proteins on batter and muffin characteristics. J. Food Sci. Technol. 2019, 56, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Kaur, A.; Kumar, S.; Singh, N. Cowpea protein isolates: Functional properties and application in gluten-free rice muffins. Food Sci. Technol. 2015, 63, 927–933. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; SRV, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Karim, Z. Polysaccharide. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Bhawani, S.A., Karim, Z., Jawaid, M., Eds.; Woodhead Publishing: Duxford, UK, 2021; pp. 1–14. [Google Scholar]
- Chima, B.; Mathews, P.; Morgan, S.; Johnson, S.; Van Buiten, C. Physicochemical Characterization of Interactions between Blueberry Polyphenols and Food Proteins from Dairy and Plant Sources. Foods 2022, 11, 2846. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Loud, D.E.; Tan, V.W.K.; Forde, C.G. Physical and sensory characterisation of noodles with added native and denatured pea protein isolate. Food Chem. 2019, 294, 152–159. [Google Scholar] [CrossRef]
- Ryan, K.J.; Brewer, M.S. In situ examination of starch granule-soy protein and wheat protein interactions. Food Chem. 2007, 104, 619–629. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R.; Jaiswal, S.; Fabek, H.; Tulbek, M.; Anderson, G.H.; Chibbar, R.N. Nutritional Composition and In Vitro Starch Digestibility of Crackers Supplemented with Faba Bean Whole Flour, Starch Concentrate, Protein Concentrate and Protein Isolate. Foods 2022, 11, 645. [Google Scholar] [CrossRef]
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2017, 69, 19–27. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Zhang, B.; Fu, X.; Jane, J.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef]
- López-Barón, N.; Sagnelli, D.; Blennow, A.; Holse, M.; Gao, J.; Saaby, L.; Müllertz, A.; Jespersen, B.; Vasanthan, T. Hydrolysed pea proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2018, 79, 117–126. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R.; Jaiswal, S.; Chan, C.; Mollard, R.; Fabek, H.; Tulbek, M.; Jones, P.; Sanchez-Hernandez, D.; Anderson, G.H.; et al. Faba bean meal, starch or protein fortification of durum wheat pasta differentially influence noodle composition, starch structure and in vitro digestibility. Food Chem. 2021, 349, 129167. [Google Scholar] [CrossRef] [PubMed]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Oñate Narciso, J.; Brennan, C. Whey and Pea Protein Fortification of Rice Starches: Effects on Protein and Starch Digestibility and Starch Pasting Properties. Die Stärke 2018, 70, 1700315. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological properties of pea protein isolate-amylose/amylopectin mixtures and the application in the high-moisture extruded meat substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Brennan, M.; Darwish, A.M.G.; Brennan, C. Physicochemical, texture and sensorial evaluation of pasta enriched with chickpea flour and protein isolate. Ann. Agric. Sci. 2020, 65, 28–34. [Google Scholar] [CrossRef]
- Khalesi, M.; FitzGerald, R.J. In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends. Catalysts 2021, 11, 787. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic–Protein Interactions: Effects on Food Properties and Health Benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Udenigwe, C.C. Recent advances in protein–polyphenol interactions focusing on structural properties related to antioxidant activities. Curr. Opin. Food Sci. 2022, 45, 100840. [Google Scholar] [CrossRef]
- Laguna, L.; Picouet, P.; Guàrdia, M.D.; Renard, C.M.G.C.; Sarkar, A. In vitro gastrointestinal digestion of pea protein isolate as a function of pH, food matrices, autoclaving, high-pressure and re-heat treatments. Food Sci. Technol. 2017, 84, 511–519. [Google Scholar] [CrossRef]
- Strauch, R.C.; Lila, M.A. Pea protein isolate characteristics modulate functional properties of pea protein–cranberry polyphenol particles. Food Sci. Nutr. 2021, 9, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Petzke, K.J.; Rawel, H.M.; Kroll, J. Reactions of chlorogenic acid and quercetin with a soy protein isolate—Influence on the in vivo food protein quality in rats. Mol. Nutr. Food Res. 2006, 50, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Sun, J.; Pei, M.; Zhang, G.; Li, C.; Li, C.; Ma, X.; He, S.; Liu, L. Impact of non-covalent bound polyphenols on conformational, functional properties and in vitro digestibility of pea protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lin, Y.; Xu, X.; Meng, L.; Dong, M. Effect of non-covalent and covalent complexation of (−)-epigallocatechin gallate with soybean protein isolate on protein structure and in vitro digestion characteristics. Food Chem. 2020, 309, 125718. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Pałecz, B.; Rachwał-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Belica, S.; Navarro-González, I.; Meseguer, J.M.V.; Pérez-Sánchez, H. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in β-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Kuhn, P.; Krueger, C.G.; Moskal, K.; Lila, M.A.; Raskin, I. Concord Grape Pomace Polyphenols Complexed to Soy Protein Isolate Are Stable and Hypoglycemic in Diabetic Mice. J. Agric. Food Chem. 2013, 61, 11428–11433. [Google Scholar] [CrossRef] [PubMed]
- Plundrich, N.J.; White, B.L.; Dean, L.L.; Davis, J.P.; Foegeding, E.A.; Lila, M.A. Stability and immunogenicity of hypoallergenic peanut protein-polyphenol complexes during in vitro pepsin digestion. Food Funct. 2015, 6, 2145–2154. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26007692 (accessed on 30 December 2022). [CrossRef]
- Guo, Y.; Bao, Y.; Sun, K.; Chang, C.; Liu, W. Effects of covalent interactions and gel characteristics on soy protein-tannic acid conjugates prepared under alkaline conditions. Food Hydrocoll. 2021, 112, 106293. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, C.; Ma, C.; He, S.; Brennan, C.S.; Yuan, Y. Interactions of grape seed procyanidins with soy protein isolate: Contributing antioxidant and stability properties. Food Sci. Technol. 2019, 115, 108465. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In Vitro Digestion of Lipids in Real Foods: Influence of Lipid Organization Within the Food Matrix and Interactions with Nonlipid Components. J. Food Sci. 2018, 83, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Effect of the presence of protein on lipolysis and lipid oxidation occurring during in vitro digestion of highly unsaturated oils. Food Chem. 2017, 235, 21–33. [Google Scholar] [CrossRef]
- Figarska, S.; Gustafsson, S.; Sundström, J.; Ärnlöv, J.; Mälarstig, A.; Elmståhl, S.; Fall, T.; Lind, L.; Ingelsson, E. Associations of Circulating Protein Levels With Lipid Fractions in the General Population. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2505–2518. [Google Scholar] [CrossRef]
- Lee, Y.; Siddiqui, W.J. (Eds.) Cholesterol Levels; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic effect of dietary pea proteins: Impact on genes regulating hepatic lipid metabolism. Mol. Nutr. Food Res. 2010, 54, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of white lupin seed, a naturally isoflavone-poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary lupin protein lowers triglyceride concentrations in liver and plasma in rats by reducing hepatic gene expression of sterol regulatory element-binding protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy Protein Intake, Cardiorenal Indices, and C-Reactive Protein in Type 2 Diabetes With Nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. American Heart Association Nutrition Committee Soy Protein, Isoflavones, and Cardiovascular Health: An American Heart Association Science Advisory for Professionals From the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: Lentil, pea, and faba bean proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Renzone, G.; Arena, S.; Scaloni, A. Cross-linking reactions in food proteins and proteomic approaches for their detection. Mass Spectrom. Rev. 2022, 41, 861–898. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Villanea, S.; Bruininx, E.M.A.M.; Butré, C.I.; van der Poel, A.F.B. Processing temperature and sugar type affect the rate and the extent of proteolysis of a model soy protein isolate system. Anim Feed Sci Technol 2020, 269, 114680. [Google Scholar] [CrossRef]
- Semenova, M.G.; Antipova, A.S.; Belyakova, L.E. Food protein interactions in sugar solutions. Curr. Opin. Colloid Interface Sci. 2002, 7, 438–444. [Google Scholar] [CrossRef]
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Long, X.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Sun, T. Effect of d-galactose on physicochemical and functional properties of soy protein isolate during Maillard reaction. Food Hydrocoll. 2022, 133, 107914. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Gokmen, V.; Serpen, A.; Morales, F.J. Determination of Furosine in Thermally Processed Foods by Hydrophilic Interaction Liquid Chromatography. J. AOAC Int. 2009, 92, 1460–1463. [Google Scholar] [CrossRef]
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; Barać, M.; Gökmen, V. Effects of isolation, enzymatic hydrolysis, heating, hydratation and Maillard reaction on the antioxidant capacity of cereal and legume proteins. Food Res. Int. 2012, 49, 1–6. [Google Scholar] [CrossRef]
- Sharma, J.K.; Sihmar, M.; Santal, A.R.; Prager, L.; Carbonero, F.; Singh, N.P. Barley Melanoidins: Key Dietary Compounds With Potential Health Benefits. Front. Nutr. 2021, 8, 708194. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Zhang, S.; Pan, S. A novel method to prepare protein-polysaccharide conjugates with high grafting and low browning: Application in encapsulating curcumin. LWT 2021, 145, 111349. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neji, C.; Semwal, J.; Máthé, E.; Sipos, P. Dough Rheological Properties and Macronutrient Bioavailability of Cereal Products Fortified through Legume Proteins. Processes 2023, 11, 417. https://doi.org/10.3390/pr11020417
Neji C, Semwal J, Máthé E, Sipos P. Dough Rheological Properties and Macronutrient Bioavailability of Cereal Products Fortified through Legume Proteins. Processes. 2023; 11(2):417. https://doi.org/10.3390/pr11020417
Chicago/Turabian StyleNeji, Chaima, Jyoti Semwal, Endre Máthé, and Péter Sipos. 2023. "Dough Rheological Properties and Macronutrient Bioavailability of Cereal Products Fortified through Legume Proteins" Processes 11, no. 2: 417. https://doi.org/10.3390/pr11020417
APA StyleNeji, C., Semwal, J., Máthé, E., & Sipos, P. (2023). Dough Rheological Properties and Macronutrient Bioavailability of Cereal Products Fortified through Legume Proteins. Processes, 11(2), 417. https://doi.org/10.3390/pr11020417