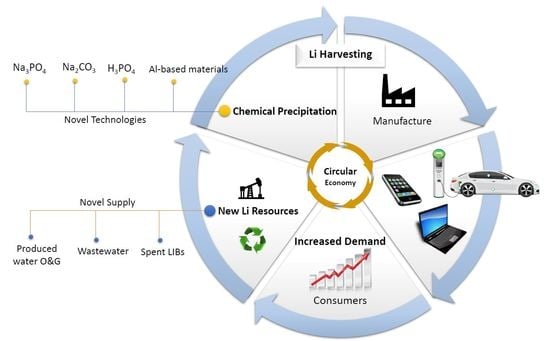
Lithium in a Sustainable Circular Economy: A Comprehensive Review
Abstract
1. Introduction
2. The Nature of Lithium
2.1. Sources of Lithium
2.1.1. Waste a New Source of Lithium
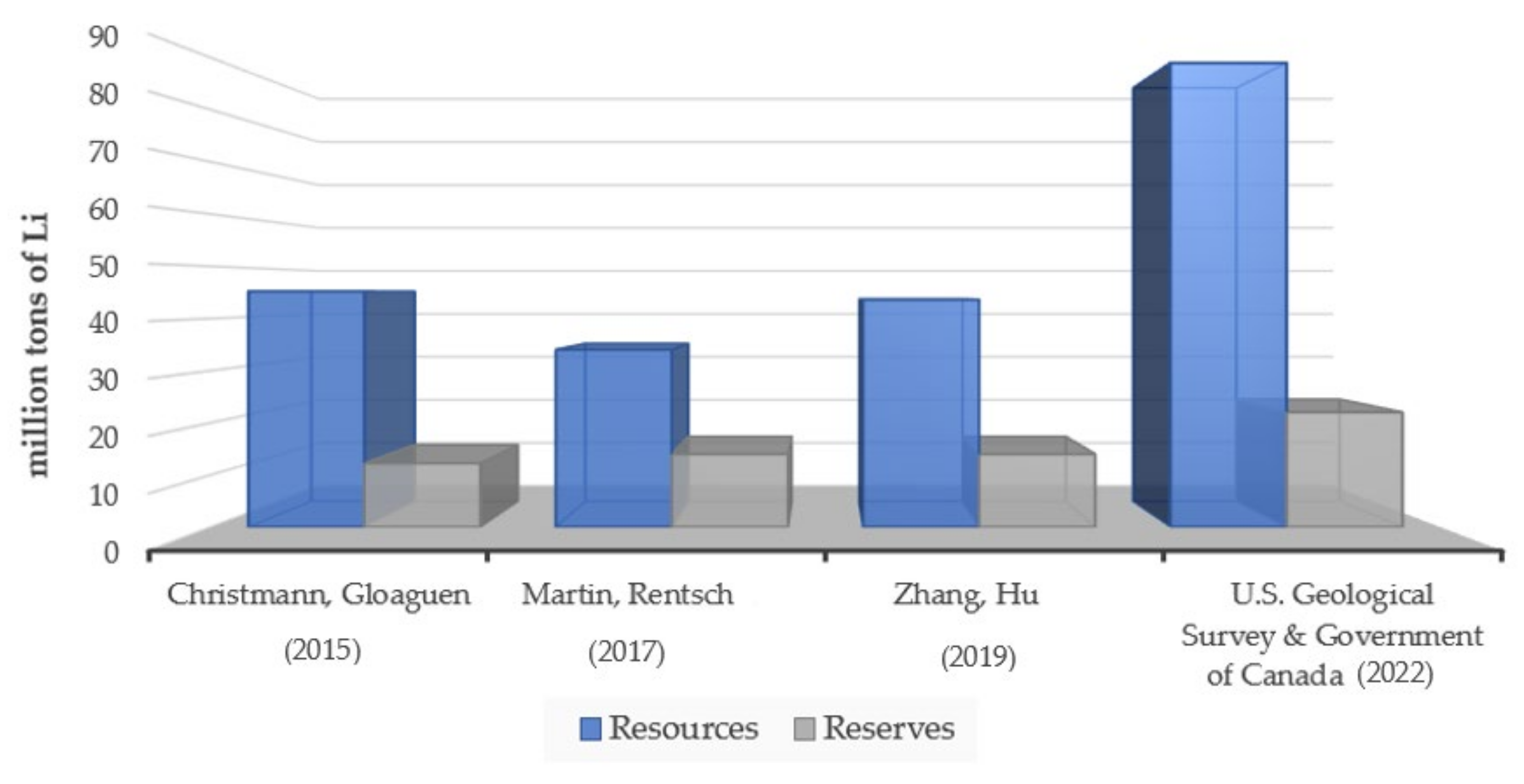
2.1.2. Global Availability
2.1.3. Environmental Challenges
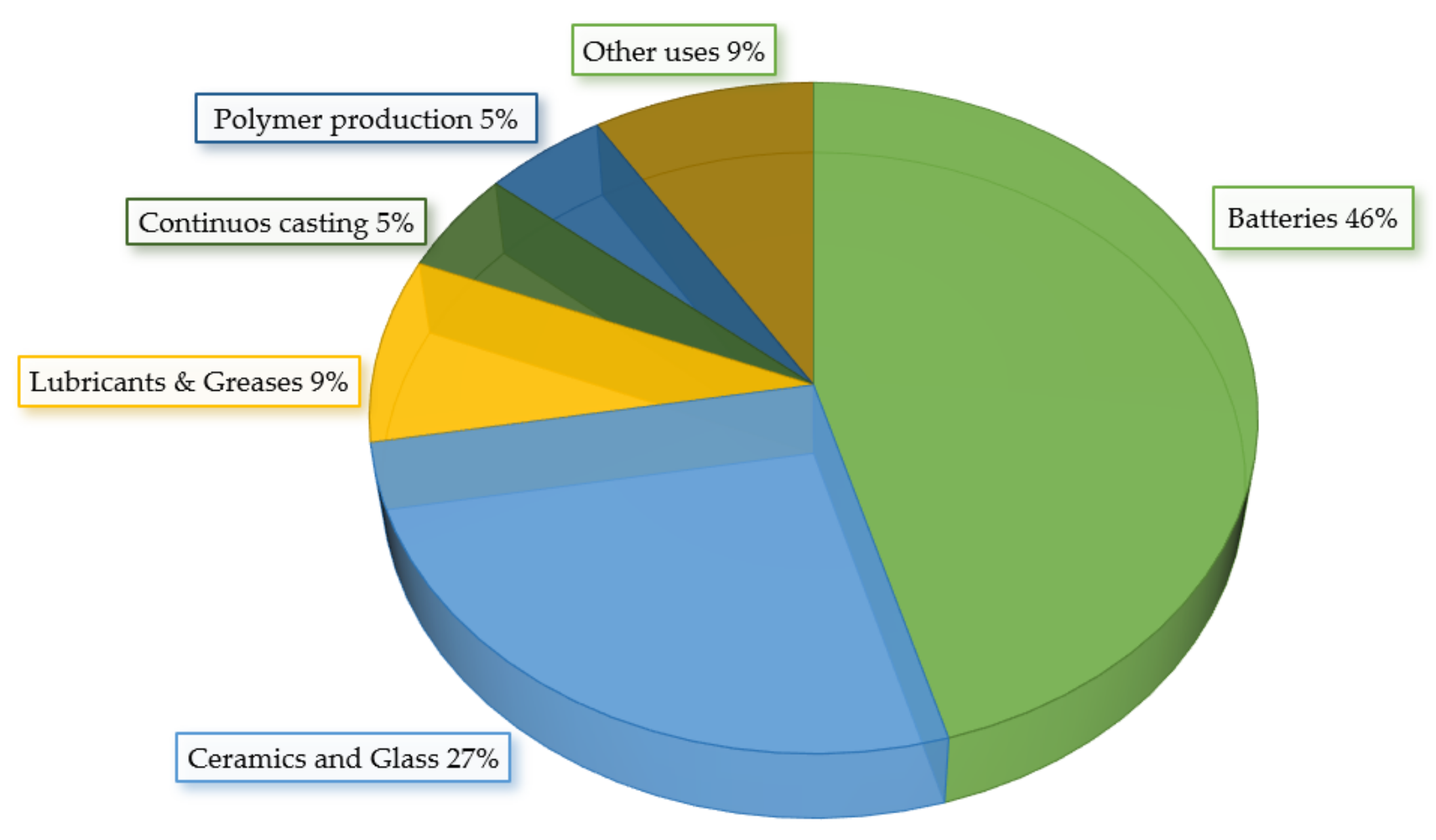
2.2. Lithium Applications
Electric Vehicles Market
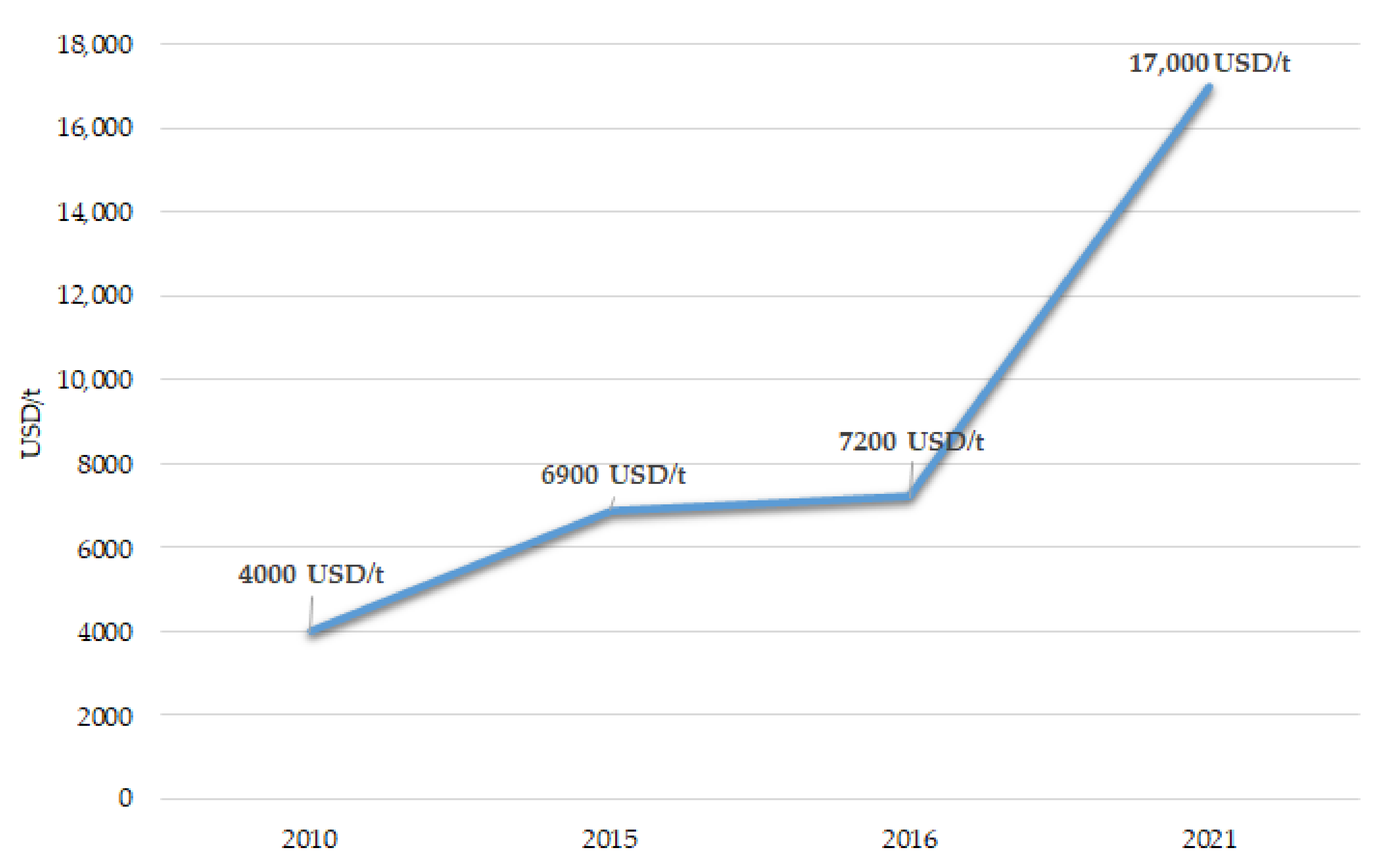
2.3. Lithium Demand and Economic Perspective
3. Lithium Recovery via Chemical Precipitation
3.1. Current Technology and Challenges
3.2. Current Advancements
3.2.1. Materials Used in the Recovery Process
3.2.2. Operating Conditions and Performance
Influence of pH on Lithium Precipitation
Influence of Temperature on Lithium Precipitation
Influence of Reaction Time on Lithium Precipitation
3.3. Challenges and Outlook
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, D.; Wang, G.; Gao, T.; Wen, B.; Dai, T. Potential impact of the end-of-life batteries recycling of electric vehicles on lithium demand in China: 2010–2050. Sci. Total Environ. 2021, 764, 142835. [Google Scholar] [CrossRef] [PubMed]
- Pavón, S.; Kahl, M.; Hippmann, S.; Bertau, M. Lithium recovery from production waste by thermal pre-treatment. Sustain. Chem. Pharm. 2022, 28, 100725. [Google Scholar] [CrossRef]
- Shankar Naik, S.; Lee, S.J.; Yu, Y.; Al-Mohaimeed, A.M.; Theerthagiri, J.; Choi, M.Y. Novel approach for the synthesis and recovery of lithium carbonate using a pulsed laser irradiation technique. Mater. Lett. 2022, 308, 131218. [Google Scholar] [CrossRef]
- Siekierka, A.; Bryjak, M. Selective sorbents for recovery of lithium ions by hybrid capacitive deionization. Desalination 2021, 520, 115324. [Google Scholar] [CrossRef]
- Alessia, A.; Alessandro, B.; Maria, V.-G.; Carlos, V.-A.; Francesca, B. Challenges for sustainable lithium supply: A critical review. J. Clean. Prod. 2021, 300, 126954. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, L.; Li, X.; Luo, G.; Yu, Z.; Yin, J.; Fan, L.; Chao, Y.; Jiang, L.; Zhu, W. Construction of truncated-octahedral LiMn2O4 for battery-like electrochemical lithium recovery from brine. Green Energy Environ. 2022; in press. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Hatton, T.A.; Lienhard, J.H. Lithium Recovery from Oil and Gas Produced Water: A Need for a Growing Energy Industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Lee, J.; Chung, E. Lithium recovery by solvent extraction from simulated shale gas produced water—Impact of organic compounds. Appl. Geochem. 2020, 116, 104571. [Google Scholar] [CrossRef]
- Zante, G.; Trébouet, D.; Boltoeva, M. Solvent extraction of lithium from simulated shale gas produced water with a bifunctional ionic liquid. Appl. Geochem. 2020, 123, 104783. [Google Scholar] [CrossRef]
- Seip, A.; Safari, S.; Pickup, D.M.; Chadwick, A.V.; Ramos, S.; Velasco, C.A.; Cerrato, J.M.; Alessi, D.S. Lithium recovery from hydraulic fracturing flowback and produced water using a selective ion exchange sorbent. Chem. Eng. J. 2021, 426, 130713. [Google Scholar] [CrossRef]
- Bazrgar Bajestani, M.; Moheb, A.; Dinari, M. Preparation of lithium ion-selective cation exchange membrane for lithium recovery from sodium contaminated lithium bromide solution by electrodialysis process. Desalination 2020, 486, 114476. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. Miner. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Razmjou, A.; Eshaghi, G.; Orooji, Y.; Hosseini, E.; Korayem, A.H.; Mohagheghian, F.; Boroumand, Y.; Noorbakhsh, A.; Asadnia, M.; Chen, V. Lithium ion-selective membrane with 2D subnanometer channels. Water Res. 2019, 159, 313–323. [Google Scholar] [CrossRef]
- Steven Kurniawan, Y.; Rao Sathuluri, R.; Ohto, K.; Iwasaki, W.; Kawakita, H.; Morisada, S.; Miyazaki, M. A rapid and efficient lithium-ion recovery from seawater with tripropyl-monoacetic acid calix[4]arene derivative employing droplet-based microreactor system. Sep. Purif. Technol. 2019, 211, 925–934. [Google Scholar] [CrossRef]
- Khalil, A.; Mohammed, S.; Hashaikeh, R.; Hilal, N. Lithium recovery from brine: Recent developments and challenges. Desalination 2022, 528, 115611. [Google Scholar] [CrossRef]
- Nishio, K. Primary Batteries—Nonaqueous Systems|Lithium–Manganese Dioxide. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 83–92. [Google Scholar]
- Choubey, P.K.; Kim, M.-S.; Srivastava, R.R.; Lee, J.-C.; Lee, J.-Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Wang, P.; Yu, T.; Du, X.; Hao, X.; Abudula, A.; Guan, G. Electrochemical technologies for lithium recovery from liquid resources: A review. Renew. Sustain. Energy Rev. 2022, 154, 111813. [Google Scholar] [CrossRef]
- Sterba, J.; Krzemień, A.; Riesgo Fernández, P.; Escanciano García-Miranda, C.; Fidalgo Valverde, G. Lithium mining: Accelerating the transition to sustainable energy. Resour. Policy 2019, 62, 416–426. [Google Scholar] [CrossRef]
- Oliazadeh, M.; Aghamirian, M.; Ali, S.; Legault, E.; Gibson, C. Flowsheet Development for Benefication of Lithium Minerals from Hard Rock Deposits. In Extraction; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Meshram, P.; Pandey, B.; Mankhand, T. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; de los Hoyos, C.R.; Declercq, J. Classification and Characteristics of Natural Lithium Resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Grew, E.S. The Minerals of Lithium. Elements 2020, 16, 235–240. [Google Scholar] [CrossRef]
- Kelly, J.C.; Wang, M.; Dai, Q.; Winjobi, O. Energy, greenhouse gas, water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Beneficiation of Lithium Ores. In Handbook of Flotation Reagents: Chemistry, Theory and Practice; Bulatovic, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 28; pp. 41–56. [Google Scholar]
- Sitando, O.; Crouse, P.L. Processing of a Zimbabwean petalite to obtain lithium carbonate. Int. J. Miner. Process. 2012, 102–103, 45–50. [Google Scholar] [CrossRef]
- Yelatontsev, D.; Mukhachev, A. Processing of lithium ores: Industrial technologies and case studies—A review. Hydrometallurgy 2021, 201, 105578. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Pistilli, M. Investing News Network. 2022. Available online: https://investingnews.com/how-to-invest-in-lithium/” (accessed on 26 October 2022).
- Cabello, J. Lithium brine production, reserves, resources and exploration in Chile: An updated review. Ore Geol. Rev. 2021, 128, 103883. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, C.; Ha, G.-S.; Park, Y.-K.; Ali Khan, M.; Jang, M.; Kim, S.-H.; Amin, M.A.; Gacem, A.; Jeon, B.-H. Downstream recovery of Li and value-added metals (Ni, Co, and Mn) from leach liquor of spent lithium-ion batteries using a membrane-integrated hybrid system. Chem. Eng. J. 2022, 447, 137507. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yang, D.; Du, T.; Gong, H.; Luo, W.B. The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 578044. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J.; Soo, V. Opportunities to Improve Recycling of Automotive Lithium Ion Batteries. Procedia CIRP 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Rouquette, L.M.J.; Lemaître, T.; Vieceli, N.; Petranikova, M. Intensification of lithium carbonation in the thermal treatment of spent EV Li-ion batteries via waste utilization and selective recovery by water leaching. Resour. Conserv. Recycl. Adv. 2022, 17, 200125. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Bernardes, A.; Tenório, J. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Lain, M.J. Recycling of lithium ion cells and batteries. J. Power Sources 2001, 97–98, 736–738. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Guzolu, J.S.; Gharabaghi, M.; Mobin, M.; Alilo, H. Extraction of Li and Co from Li-ion Batteries by Chemical Methods. J. Inst. Eng. India Ser. D 2017, 98, 43–48. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, D.; Liu, N.; Hu, F.; Peng, C.; Zhou, X.; He, J.; Jie, Y.; Wang, H.; Wilson, B.P.; et al. Biomass-Assisted Reductive Leaching in H2SO4 Medium for the Recovery of Valuable Metals from Spent Mixed-Type Lithium-Ion Batteries. JOM 2019, 71, 4465–4472. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef]
- Liu, J.; Mak, T.Y.; Meng, Z.; Wang, X.; Cao, Y.; Lu, Z.; Suen, D.W.-S.; Lu, X.-Y.; Tang, Y. Efficient recovery of lithium as Li2CO3 and cobalt as Co3O4 from spent lithium-ion batteries after leaching with p-toluene sulfonic acid. Hydrometallurgy 2022, 216, 106012. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Lithium, Nickel, Cobalt, Manganese from Spent Lithium-Ion Batteries Using l-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef]
- Yadav, P.; Jie, C.J.; Tan, S.; Srinivasan, M. Recycling of cathode from spent lithium iron phosphate batteries. J. Hazard. Mater. 2020, 399, 123068. [Google Scholar] [CrossRef]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 7; pp. 233–267. [Google Scholar]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- He, Y.; Flynn, S.L.; Folkerts, E.J.; Zhang, Y.; Ruan, D.; Alessi, D.S.; Martin, J.W.; Goss, G.G. Chemical and toxicological characterizations of hydraulic fracturing flowback and produced water. Water Res. 2017, 114, 78–87. [Google Scholar] [CrossRef]
- Tian, L.; Liu, Y.; Tang, P.; Yang, Y.; Wang, X.; Chen, T.; Bai, Y.; Tiraferri, A.; Liu, B. Lithium extraction from shale gas flowback and produced water using H1.33Mn1.67O4 adsorbent. Resour. Conserv. Recycl. 2022, 185, 106476. [Google Scholar] [CrossRef]
- Christmann, P.; Gloaguen, E.; Labbé, J.-F.; Melleton, J.; Piantone, P. Global Lithium Resources and Sustainability Issues. In Lithium Process Chemistry; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 1; pp. 1–40. [Google Scholar]
- U.S. Geological Survey. Mineral commodity summaries 2022. In Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2022; pp. 100–101. [Google Scholar]
- Canada, G.O. Lithium Facts. Available online: https://www.nrcan.gc.ca/our-natural-resources/minerals-mining/minerals-metals-facts/lithium-facts/24009 (accessed on 10 March 2022).
- Garcés, I.; Álvarez, G. Water mining and extractivism of the Salar de Atacama, Chile. WIT Trans. Ecol. Environ. 2020, 245, 189–199. [Google Scholar]
- Chordia, M.; Wickerts, S.; Nordelöf, A.; Arvidsson, R. Life cycle environmental impacts of current and future battery-grade lithium supply from brine and spodumene. Resour. Conserv. Recycl. 2022, 187, 106634. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.; Galli, C. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Stamp, A.; Lang, D.; Wäger, P. Environmental impacts of a transition toward e-mobility: The present and future role of lithium carbonate production. J. Clean. Prod. 2012, 23, 104–112. [Google Scholar] [CrossRef]
- Wanger, T.C. The Lithium future—Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Ejeian, M.; Grant, A.; Shon, H.K.; Razmjou, A. Is lithium brine water? Desalination 2021, 518, 115169. [Google Scholar] [CrossRef]
- Baspineiro, C.F.; Franco, J.; Flexer, V. Performance of a double-slope solar still for the concentration of lithium rich brines with concomitant fresh water recovery. Sci. Total Environ. 2021, 791, 148192. [Google Scholar] [CrossRef]
- Feo, G.D.; Gisi, S.D. Using MCDA and GIS for hazardous waste landfill siting considering land scarcity for waste disposal. Waste Manag. 2014, 34, 2225–2238. [Google Scholar] [CrossRef]
- Morse, I. Water or Mineral? In Chile, a Debate Over Lithium Brine. Available online: https://undark.org/2020/12/21/chile-debate-over-lithium-brine/ (accessed on 21 December 2020).
- Yu, F.; Wang, Y.; Zhang, L. Effect of spodumene leaching with sodium hydroxide on its flotation. Physicochem. Probl. Miner. Process. 2015, 51, 745–754. [Google Scholar]
- Sverdrup, H.U. Modelling global extraction, supply, price and depletion of the extractable geological resources with the LITHIUM model. Resour. Conserv. Recycl. 2016, 114, 112–129. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.d.C.; Rodriguez, M.H. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Prior, T.; Wäger, P.A.; Stamp, A.; Widmer, R.; Giurco, D. Sustainable governance of scarce metals: The case of lithium. Sci. Total Environ. 2013, 461–462, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Breeze, P. Power System Energy Storage Technologies. In Power Generation Technologies, 3rd ed.; Breeze, P., Ed.; Newnes: Oxford, UK, 2019; Chapter 10; pp. 219–249. [Google Scholar]
- Shen, K.; Xu, X.; Tang, Y. Recent progress of magnetic field application in lithium-based batteries. Nano Energy 2022, 92, 106703. [Google Scholar] [CrossRef]
- Bandini, G.; Caposciutti, G.; Marracci, M.; Buffi, A.; Tellini, B. Characterization of lithium-batteries for high power applications. J. Energy Storage 2022, 50, 104607. [Google Scholar] [CrossRef]
- Gutsch, M.; Leker, J. Global warming potential of lithium-ion battery energy storage systems: A review. J. Energy Storage 2022, 52, 105030. [Google Scholar] [CrossRef]
- Das, D.; Abarajitha, R.; Kay, P.; Ramamurthy, V.; Goycoolea, F.M.; Das, N. Selective recovery of lithium from spent coin cell cathode leachates using ion imprinted blended chitosan microfibers: Pilot scale studies provide insights on scalability. J. Hazard. Mater. 2022, 431, 128535. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Lithium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2011; pp. 116–125. [Google Scholar]
- Gao, K.; Calabrese, J.R. The mechanisms of action of lithium in bipolar disorder. In Neurobiology of Bipolar Disorder; Quevedo, J., Carvalho, A.F., Vieta, E., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 31; pp. 357–364. [Google Scholar]
- Callery, S. NASA’s Scientific Visualization Studio; NASA: Washington, DC, USA, 2022. [Google Scholar]
- Lau, L.C.; Lee, K.; Mohamed, A. Global warming mitigation and renewable energy policy development from the Kyoto Protocol to the Copenhagen Accord—A comment. Renew. Sustain. Energy Rev. 2012, 16, 5280–5284. [Google Scholar] [CrossRef]
- E.P.A. U.S. Fast Facts on Transportation Greenhouse Gas Emissions. Available online: https://www.epa.gov/greenvehicles/fast-facts-transportation-greenhouse-gas-emissions (accessed on 20 May 2022).
- Manzetti, S.; Mariasiu, F. Electric vehicle battery technologies: From present state to future systems. Renew. Sustain. Energy Rev. 2015, 51, 1004–1012. [Google Scholar] [CrossRef]
- Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative life cycle assessment of Li-Sulphur and Li-ion batteries for electric vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086. [Google Scholar] [CrossRef]
- Renault. The Electric Car: How Does Its Lithium-Ion Battery Work? Available online: https://www.renaultgroup.com/en/news-on-air/news/the-electric-car-how-does-its-lithium-ion-battery-work/ (accessed on 18 October 2019).
- Office of Energy Efficiency & Renewable Energy. How Does a Lithium-Ion Battery Work? Available online: https://www.energy.gov/eere/articles/how-does-lithium-ion-battery-work (accessed on 14 September 2017).
- Li, Y. Electrochemical Ion Insertion: Mechanisms and Applications in Energy Storage and Computing. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2018. [Google Scholar]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-Art and Energy Management System of Lithium-Ion Batteries in Electric Vehicle Applications: Issues and Recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Iskandar Radzi, Z.; Helmy Arifin, K.; Zieauddin Kufian, M.; Balakrishnan, V.; Rohani Sheikh Raihan, S.; Abd Rahim, N.; Subramaniam, R. Review of spinel LiMn2O4 cathode materials under high cut-off voltage in lithium-ion batteries: Challenges and strategies. J. Electroanal. Chem. 2022, 920, 116623. [Google Scholar] [CrossRef]
- Jeevanantham, B.; Shobana, M.K. Enhanced cathode materials for advanced lithium-ion batteries using nickel-rich and lithium/manganese-rich LiNixMnyCozO2. J. Energy Storage 2022, 54, 105353. [Google Scholar]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Shang, R.; Cheng, C.; Cheng, Y.; Xing, J.; Wei, Z.; Zhao, Y. Recent advances in lithium-ion battery separators with reversible/irreversible thermal shutdown capability. Energy Storage Mater. 2021, 43, 143–157. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Samadiy Murodjon, X.Y.; Li, M.; Duo, J.; Deng, T. Lithium Recovery from Brines Including Seawater, Salt Lake Brine, Underground Water and Geothermal Water. In Thermodynamics and Energy Engineering; InTech Open: London, UK, 2020; pp. 1–39. [Google Scholar] [CrossRef]
- Treadgold, T. Lithium Price Tipped To Rise After Warning of ‘Perpetual Deficit’. Available online: https://www.forbes.com/sites/timtreadgold/2021/07/02/lithium-price-tipped-to-rise-after-warning-of-perpetual-deficit/?sh=178e8dcc4ab7 (accessed on 2 July 2021).
- Garside, M. Statista. 2022. Available online: https://www.statista.com/ (accessed on 28 October 2022).
- Wu, Y.-C.; Kontou, E. Designing electric vehicle incentives to meet emission reduction targets. Transp. Res. Part D Transp. Environ. 2022, 107, 103320. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Luko-Sulato, K.; Yabuki, L.N.M.; Santos, L.M.; da Silva, Y.J.A.B.; da Silva, Y.J.A.B. Rare earth elements and radionuclides. In Emerging Freshwater Pollutants; Dalu, T., Tavengwa, N.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 17; pp. 309–329. [Google Scholar]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.; Zhao, Z. Separating lithium and magnesium in brine by aluminum-based materials. Hydrometallurgy 2018, 176, 73–77. [Google Scholar] [CrossRef]
- Celso Quintero, J.M.D.; Fierro, F.; Thennis, T.; Zhang, Y.; Videla, Á.; Rojas, R. Development of a co-precipitation process for the preparation of magnesium hydroxide containing lithium carbonate from Li-enriched brines. Hydrometallurgy 2020, 198, 105515. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Cao, H.; Zheng, X.; Van Gerven, T.; Hu, Y.; Sun, Z. Lithium carbonate recovery from lithium-containing solution by ultrasound assisted precipitation. Ultrason. Sonochem. 2019, 52, 484–492. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L. Thermodynamic study on recovery of lithium using phosphate precipitation method. Hydrometallurgy 2018, 178, 283–286. [Google Scholar] [CrossRef]
- Alsabbagh, A.; Aljarrah, S.; Almahasneh, M. Lithium enrichment optimization from Dead Sea end brine by chemical precipitation technique. Miner. Eng. 2021, 170, 107038. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Liang, J.; Yan, H.; Cai, Z. Study on the Synergistic Extraction of Lithium from Spent Lithium Cobalt Oxide Batteries by Molten Salt Electrolysis and Two-Step Precipitation Method. Crystals 2021, 11, 1163. [Google Scholar] [CrossRef]
- Shin, D.J.; Joo, S.-H.; Lee, D.; Shin, S.M. Precipitation of lithium phosphate from lithium solution by using sodium phosphate. Can. J. Chem. Eng. 2021, 100, 3760–3767. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of lepidolite and recovery of lithium hydroxide from purified alkaline pressure leach liquor by phosphate precipitation and lime addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, J.-M.; Lee, J.B.; Cho, H.-J.; Kim, Y.H.; Ryu, T. Preparation of lithium carbonate from waste lithium solution through precipitation and wet conversion methods. Hydrometallurgy 2022, 210, 105863. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhao, Z.-W.; Liu, X.-H.; Chen, X.-Y.; Zhong, M.-L. Extraction of lithium from salt lake brine by aluminum-based alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 3484–3489. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 2012, 117, 64–70. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Wang, R.; Gui, W.; Liu, G.; Yang, Y. Systemic and Direct Production of Battery-Grade Lithium Carbonate from a Saline Lake. Ind. Eng. Chem. Res. 2014, 53, 16502–16507. [Google Scholar] [CrossRef]
- Wu, S.; Tao, W.; Zheng, Y.; Yang, Y.; Yu, J.; Cui, J.; Lu, Y.; Shi, Z.; Wang, Z. Novel process for the extraction of lithium carbonate from spent lithium-containing aluminum electrolytes by leaching with aluminum nitrate and nitric acid. Hydrometallurgy 2020, 198, 105505. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.; Li, J.; He, L.; Zhao, Z. Enriching lithium and separating lithium to magnesium from sulfate type salt lake brine. Hydrometallurgy 2020, 192, 105247. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; He, L.; Zhao, Z. Facet engineered Li3PO4 for lithium recovery from brines. Desalination 2021, 514, 115186. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Xu, W.; Xiong, J.; He, L. A closed-loop process for selective lithium recovery from brines via electrochemical and precipitation. Desalination 2021, 519, 115302. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Zhao, C.; He, M.; Cao, H.; Zheng, X.; Gao, W.; Sun, Y.; Zhao, H.; Liu, D.; Zhang, Y.; Sun, Z. Investigation of solution chemistry to enable efficient lithium recovery from low-concentration lithium-containing wastewater. Front. Chem. Sci. Eng. 2020, 14, 639–650. [Google Scholar] [CrossRef]
- Song, Y.-J. Recovery of Lithium as Li3PO4 from Waste Water in a LIB Recycling Process. Korean J. Met. Mater. 2018, 56, 755–762. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Marti, E.J. A review of the application of cerium and lanthanum in phosphorus removal during wastewater treatment: Characteristics, mechanism, and recovery. Chemosphere 2022, 309, 136462. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Fischer, D.; Risso, L.; Koury, D.; Marti, E.J. Application of cerium and lanthanum coagulants in wastewater treatment—A comparative assessment to magnesium, aluminum, and iron coagulants. Chem. Eng. J. 2021, 426, 131268. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Banu, H.; Meenakshi, S. Removal of phosphate and nitrate ions from aqueous solution using La3+ incorporated chitosan biopolymeric matrix membrane. Int. J. Biol. Macromol. 2019, 124, 492–504. [Google Scholar] [CrossRef]
- Han, K.N. Characteristics of Precipitation of Rare Earth Elements with Various Precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef]
| Reagents/Precipitant | Dosage | pH | T (°C) | Time (hours) | Efficiency (Li Recovery) | Reference |
|---|---|---|---|---|---|---|
| Sodium carbonate (Na2CO3) | Stoichiometric amount of solid Na2CO3 | 12 | 80 | 3 | 72.9% | [110] |
| Lime milk, NaOH, oxalic acid and carbonate | - | 4.6 | 85 | - | 84% | [111] |
| Al−Ca alloy | Al/Li mole ratio of 3.5:1 | - | 70 | 3 | 94.6% | [109] |
| Aluminum powder and NaCl | - | 6 | 80 | 3 | 78.3% | [99] |
| Tri-sodium phosphate (Na3PO4·12H2O) | 1:1 theoretical amount | 11–13 | 25 | 5 | 96.5% | [102] |
| Tri-sodium phosphate (Na3PO4) | PO43−/Li+ molar ratio of 1.3:3 | - | 65 | 2 | 65% | [106] |
| Sodium carbonate (Na2CO3) | Na/Li molar ratio of 1 | - | 80 | 0.6 | 82.62% | [101] |
| Sodium carbonate (Na2CO3) | CO3−2/Li+ molar ratio of 1.1:2 | 7–8 | 95 | - | 46.5% | [112] |
| Al/Na2SO4 composite | Al/Li mole ratio of 3:1 | - | 70 | 3 | 89.2% | [113] |
| NaOH and Na2CO3 solution | - | - | 80 | 1.5 | 85% | [100] |
| Aluminum chloride (AlCl3·6H2O) | 30–40 g/L | 6.6–7.2 | 25 | 3 | 90% | [93] |
| Al/Na2SO4 composite | Al/Li mole ratio of 3:1 | - | 70 | 3 | 89.2% | [113] |
| Tri-sodium phosphate (Na3PO4) | Li/Na3PO4 mole ratio of 1 | 9.5 | 90 | 5 | 95.4% | [105] |
| Tri-sodium phosphate (Na3PO4) | 5 g/L | 6.3 | 40 | 0.5 | 40% | [103] |
| Facet engineered Li3PO4 crystal and sodium phosphate dodecahydrate (Na3PO4⸱12H2O) | 40 g/L(S/L ratio) seed + 3:1 theoretical Li+/PO43− molar ratio of Na3PO4⸱12H2O | 10–12 | 30 | 0.5 | 51.62% | [114] |
| Tri-sodium phosphate dodecahydrate (Na3PO4⸱12H2O) | Li/P mole ratio of 3:1 | 10–10.3 | 30 | 2 | 88.49% | [115] |
| Tri-sodium phosphate (Na3PO4) | 1.2:1 theoretical amount | 8 | 70 | 1.5 | 88.44% | [104] |
| Activated Al-Ca and Al-Fe alloys | 30–40 g/L | - | 70 | 1 | 94.6% | [15] |
| Phosphoric acid H3PO4 | Li/P mole ratio of 3:1.6 | 12.5 | 90 | 2 | 83% | [107] |
| Phosphoric acid H3PO4 | Li+/PO43− mole ratio of 3 | 12.4–13.5 | 25 | 24 | 81% | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, L.V.; Ho, Y.-C.; Myo Thant, M.M.; Han, D.S.; Lim, J.W. Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes 2023, 11, 418. https://doi.org/10.3390/pr11020418
Garcia LV, Ho Y-C, Myo Thant MM, Han DS, Lim JW. Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes. 2023; 11(2):418. https://doi.org/10.3390/pr11020418
Chicago/Turabian StyleGarcia, Laura Vega, Yeek-Chia Ho, Maung Maung Myo Thant, Dong Suk Han, and Jun Wei Lim. 2023. "Lithium in a Sustainable Circular Economy: A Comprehensive Review" Processes 11, no. 2: 418. https://doi.org/10.3390/pr11020418
APA StyleGarcia, L. V., Ho, Y.-C., Myo Thant, M. M., Han, D. S., & Lim, J. W. (2023). Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes, 11(2), 418. https://doi.org/10.3390/pr11020418