Development of High-Fibre, Ready-to-Bake Flour Mixtures from Purple Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Purple Wheat Flour Tests
2.2. Dough Tests
2.3. Bread Production
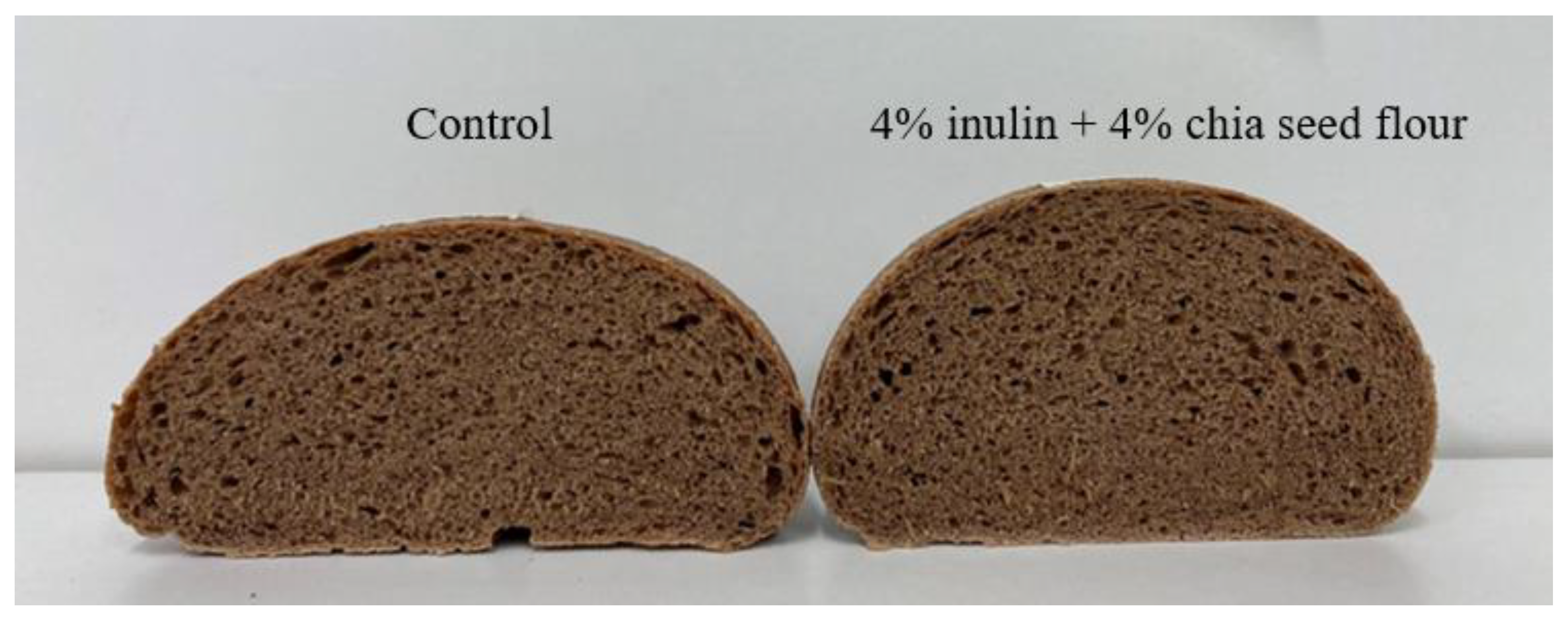
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saldivar, S.S. Cereals: Dietary Importance; Elsevier: Amsterdam, The Netherlands, 2016; pp. 703–711. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Continuing cereals research for sustainable health and well-being. Int. J. Agric. Sustain. 2021, 20, 693–704. [Google Scholar] [CrossRef]
- Anđelković, V.; Mesarović, J.; Srebrić, M.; Drinić, S.M. Pigmented maize-a potential source of β-carotene and α-tocopherol. J. Eng. Process. Manag. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Parizad, P.A.; Capraro, J.; Scarafoni, A.; Bonomi, F.; Blandino, M.; Marengo, M.; Iametti, S. The bio-functional properties of pigmented cereals may involve synergies among different bioactive species. Plant Foods Hum. Nutr. 2019, 74, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria× ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Paznocht, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and esterified carotenoids in pigmented wheat, tritordeum and barley grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef]
- Beleggia, R.; Ficco, D.B.; Nigro, F.M.; Giovanniello, V.; Colecchia, S.A.; Pecorella, I.; De Vita, P. Effect of sowing date on bioactive compounds and grain morphology of three pigmented cereal species. Agronomy 2021, 11, 591. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Singh, T.P.; Özogul, F. Retrospecting the concept and industrial significance of LAB bacteriocins. Food Biosci. 2022, 46, 101607. [Google Scholar] [CrossRef]
- Gupta, R.; Meghwal, M.; Prabhakar, P.K. Bioactive compounds of pigmented wheat (Triticum aestivum): Potential benefits in human health. Trends Food Sci. Technol. 2021, 110, 240–252. [Google Scholar] [CrossRef]
- Ficco, D.B.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; De Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumari, A.; Chunduri, V.; Kaur, S.; Banda, J.; Goyal, A.; Garg, M. Anthocyanin biofortified black, blue and purple wheat exhibited lower amino acid cooking losses than white wheat. LWT 2022, 154, 112802. [Google Scholar] [CrossRef]
- Milder, I.E.; Arts, I.C.; van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Beta, T. Flour and bread from black-, purple-, and blue-colored wheats. In Flour and Breads and their Fortification in Health and Disease, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 59–67. [Google Scholar] [CrossRef]
- Zeven, A.C. Wheats with purple and blue grains: A review. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super. Sanita 2007, 43, 369–374. [Google Scholar] [PubMed]
- Zheng, Q.; Li, B.; Li, H.; Li, Z. Utilization of blue-grained character in wheat breeding derived from Thinopyrum poticum. J. Genet. Genom. 2009, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-biofortified Colored Wheat Prevents High Fat Diet-induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, 1900999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, J.; Yue, Y.; Li, K.; Ren, G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Qiu, Y.; Liu, Q.; Borgen, A.; Apea-Bah, F.B. Purple Wheat (Triticum sp.) Seeds: Phenolic Composition and Antioxidant Properties. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier Inc.: London, UK, 2020; pp. 103–125. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of Phenolic Compounds and Expression Profiles of Phenolic Acid Biosynthesis-Related Genes in Developing Grains of White, Purple, and Red Wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Martinek, P.; Kotíková, Z.; Orsák, M.; Šulc, M. Genetics and chemistry of pigments in wheat grain—A review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Zivcak, M.; Abdelly, C.; Cerda, A.; Brestic, M. Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress. Molecules 2018, 23, 1518. [Google Scholar] [CrossRef] [PubMed]
- Uarrota, V.G.; Stefen, D.L.V.; Leolato, L.S.; Gindri, D.M.; Nerling, D. Revisiting Carotenoids and Their Role in Plant Stress Responses: From Biosynthesis to Plant Signaling Mechanisms During Stress. In Antioxidants and Antioxidant Enzymes in Higher Plants, 1st ed.; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 207–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Truzzi, F.; D’Amen, E.; Dinelli, G. Effect of Storage Conditions and Time on the Polyphenol Content of Wheat Flours. Processes 2021, 9, 248. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovanniello, V.; Del Nobile, M.A.; Padalino, L.; Lecce, L.; Borrelli, G.M.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gamel, T.H.; Wright, A.J.; Pickard, M.; Abdel-Aal, E.S.M. Characterization of anthocyanin-containing purple wheat prototype products as functional foods with potential health benefits. Cereal Chem. 2019, 97, 34–38. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Weegels, P.L. The future of bread in view of its contribution to nutrient intake as a starchy staple food. Plant Foods Hum. Nutr. 2019, 74, 1–9. [Google Scholar] [CrossRef]
- Rosell, C.M. Trends in science of doughs and bread quality. In Flour and Breads and their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2019; pp. 333–343. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Ilievska, N.; Pavlova, V.; Kirovska, V.; Ilievska, J.; Pavlovska, M. Nutritional and health benefits of inulin functional food and prebiotic. J. Hyg. Eng. Des. 2020, 45–48. [Google Scholar]
- Mohammadi, F.; Shiri, A.; Tahmouzi, S.; Mollakhalili-Meybodi, N.; Nematollahi, A. Application of inulin in bread: A review of technological properties and factors affecting its stability. Food Sci. Nutr. 2022, 00, 1–12. [Google Scholar] [CrossRef]
- Belorio, M.; Gómez, M. Psyllium: A useful functional ingredient in food systems. Crit. Rev. Food Sci. Nutr. 2021, 62, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Mikulec, A.; Pustkowiak, H. Sensory assessment and physicochemical properties of wheat bread supplemented with chia seeds. Pol. J. Food Nutr. Sci. 2020, 70, 387–397. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 44-15.02. Moisture—Air-Oven Methods; AACC International: Nairobi, Kenya, 2010. [Google Scholar]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 56-81.03 Determination of Falling Number; AACC International: Nairobi, Kenya, 2010. [Google Scholar]
- 4/1998. (XI. 11.) Regulation of EüM (Hungarian Ministry of Health) on the permissible level of microbiological contaminants in food. Available online: https://www.informea.org/en/legislation/decree-no-4-1998-ministry-health-allowable-limits-microbiological-contamination (accessed on 20 December 2022).
- AACC International. Approved Methods of Analysis, 11th ed.; Method 54-21. Farinograph Method for Flour; AACC International: Nairobi, Kenya, 2010. [Google Scholar]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 10-10.03; AACC International: Nairobi, Kenya, 2010. [Google Scholar]
- Codex Alimentarius Hungaricus Requirement No. 1-3/16-1 on bakery products 152/2009. (XI. 12.) decree, Ministry of agri-culture, Hungary. Available online: https://leap.unep.org/countries/hu/national-legislation/decree-no-152-2009-xi-12-fvm-ministry-agriculture-and-rural (accessed on 20 December 2022).
- Codex Alimentarius Hungaricus 2-201 Milling Products Directive. Available online: https://elelmiszerlanc.kormany.hu/download/e/64/b1000/2-201_2016-06-09.pdf (accessed on 20 December 2022).
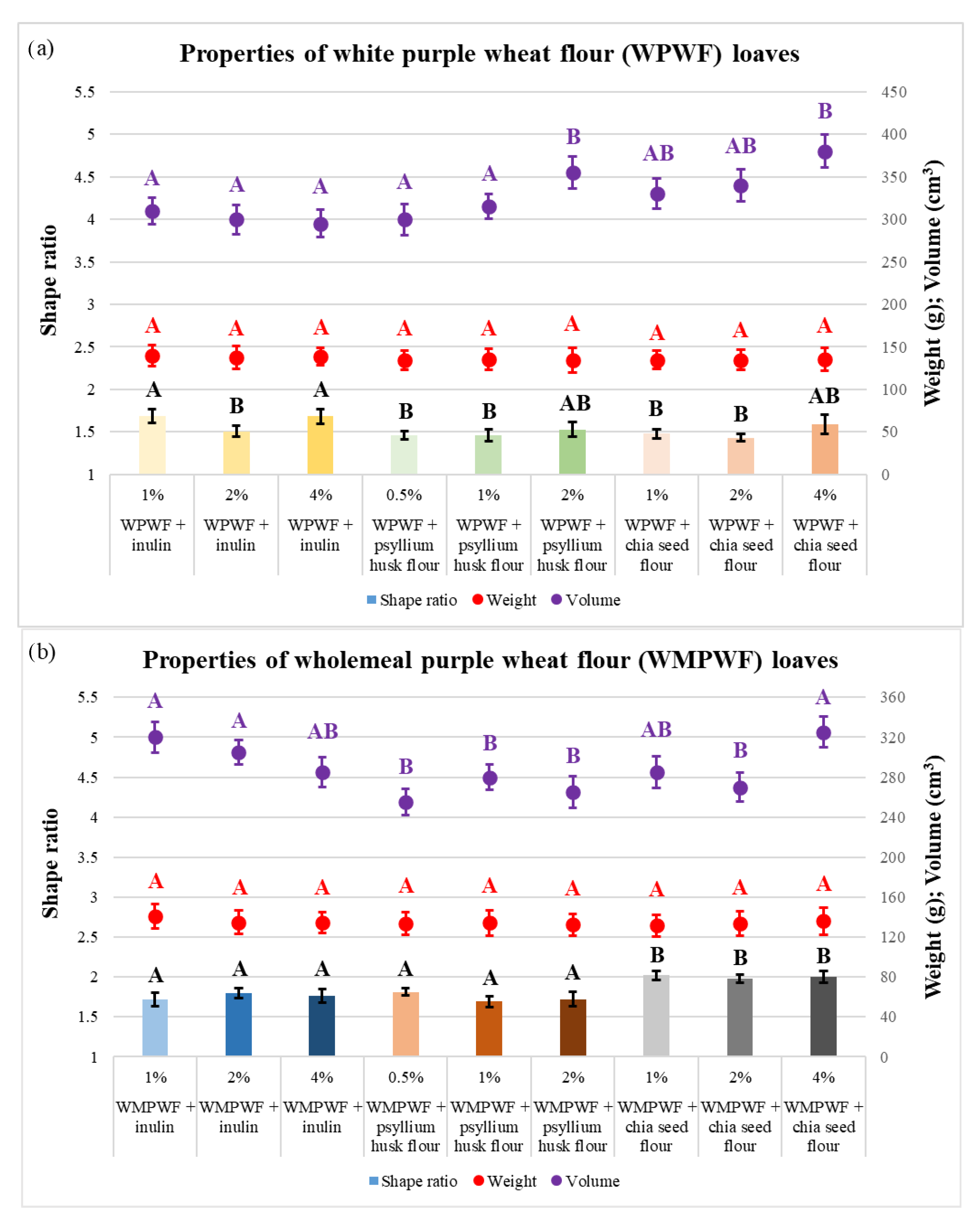
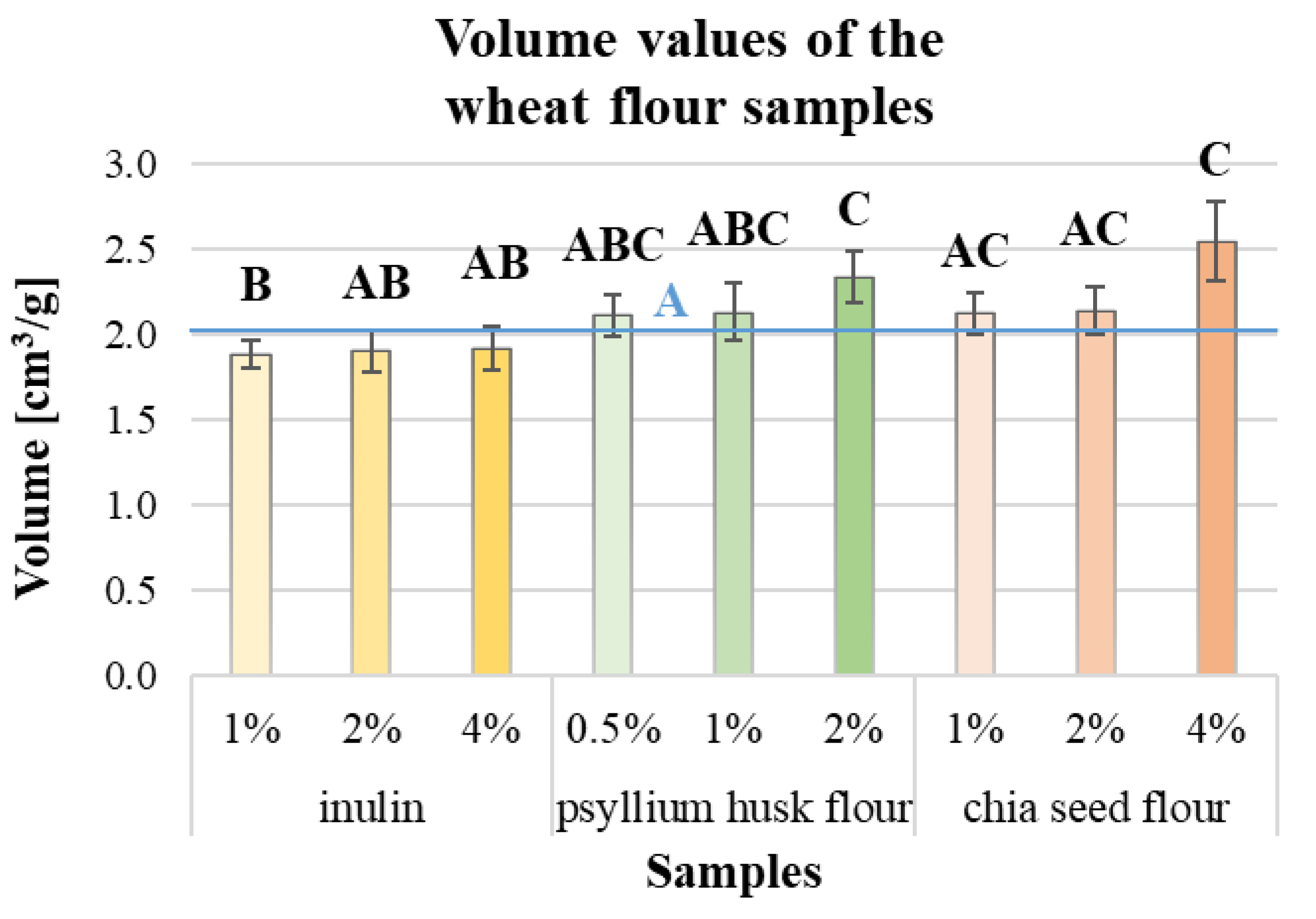
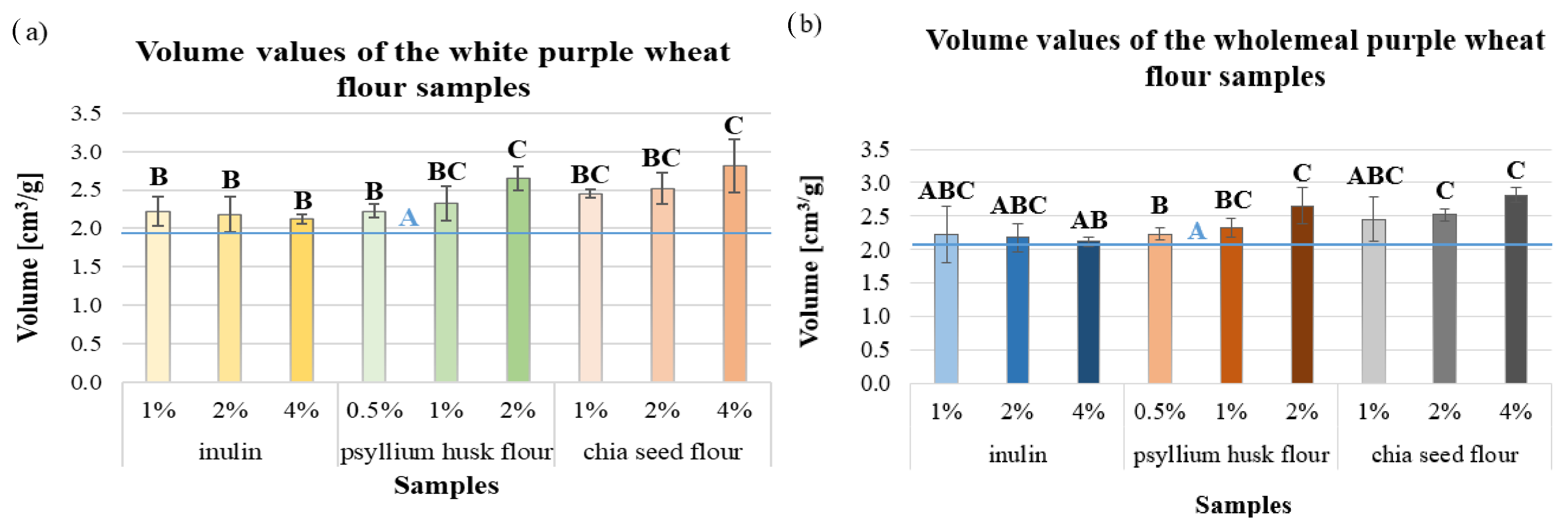
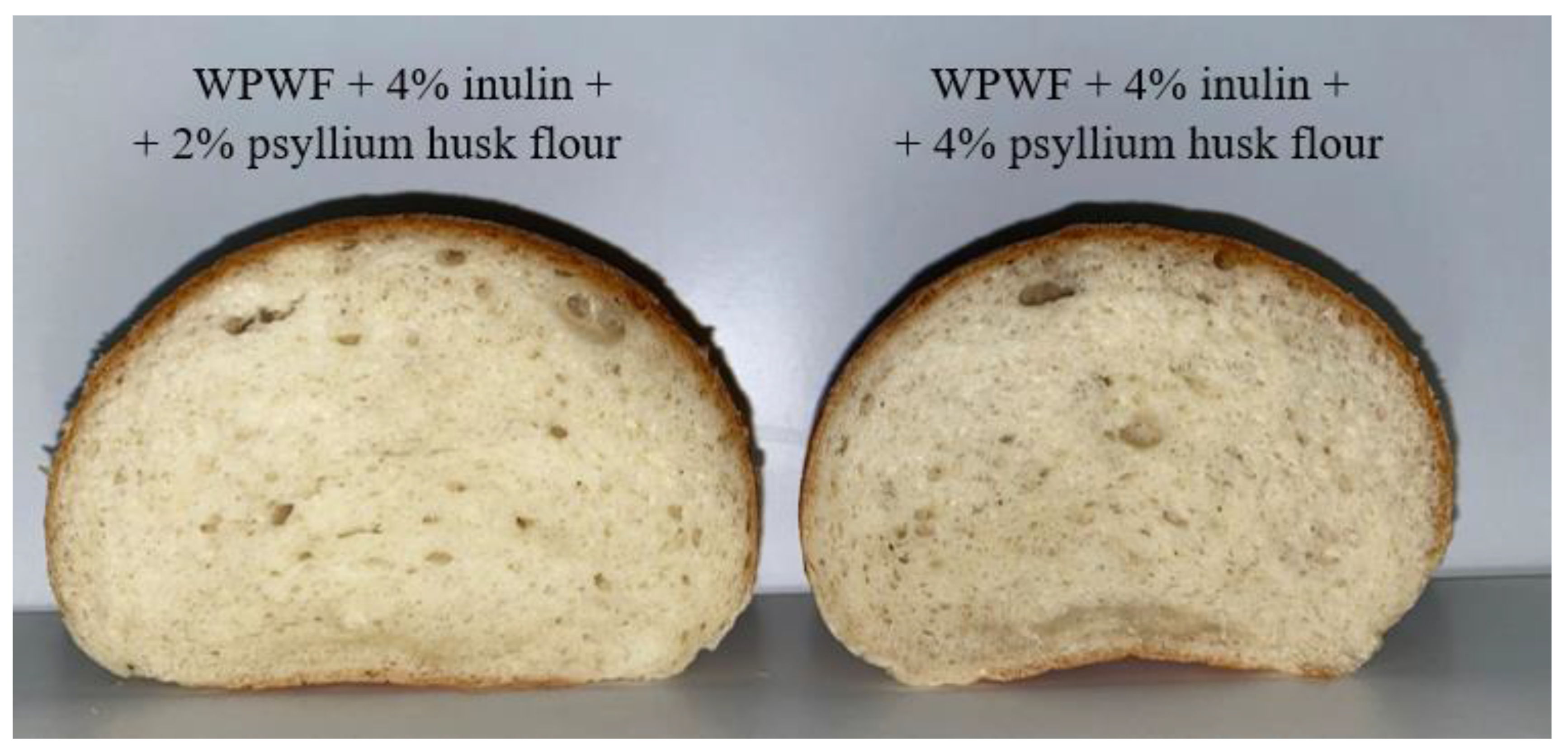

| Fluor Type | Moisture Content [w/w%] | Falling Number [s] | Salmonella [CFU/25 g] | Mould [CFU/g] | Total Phenol Content [µm/100 mL] |
|---|---|---|---|---|---|
| WPWF | 14.59 ± 0.15 A | 500 ± 13.4 A | 0 A | <10 A | 262.8 ± 20.4 A |
| WMPWF | 9.68 ± 0.11 B | 459 ± 5.7 B | 0 A | <10 A | 897.4 ± 27.9 B |
| Sample | The Amount of Water Added to 320PU [cm3] | ||
|---|---|---|---|
| WPWF | WMPWF | ||
| Control | 54.2 | 74.0 | |
| Inulin | 1.0% | 52.0 | 70.3 |
| 2.0% | 49.8 | 68.9 | |
| 4.0% | 47.4 | 66.5 | |
| Psyllium husk flour | 0.5% | 56.1 | 74.7 |
| 1.0% | 58.7 | 76.9 | |
| 2.0% | 62.6 | 83.0 | |
| Chia seed flour | 1.0% | 53.2 | 73.1 |
| 2.0% | 53.4 | 72.0 | |
| 4.0% | 53.2 | 72.1 | |
| Sample | Width [mm] | Height [mm] | Shape Ratio | Weight [g] | Volume [cm3] | Volume Values [cm3/g] |
|---|---|---|---|---|---|---|
| WPWF control | 96 | 64 | 1.50 | 135.7 | 350 | 2.58 |
| WMPWF control | 102 | 51 | 2.00 | 136.1 | 285 | 2.57 |
| WPWF 4% inulin + 0.5% psyllium husk flour | 96 | 67 | 1.43 | 139.6 | 420 | 3.01 |
| WPWF 4% inulin + 2% psyllium husk flour | 98 | 70 | 1.40 | 137.5 | 395 | 2.87 |
| WPWF 4% inulin + 4% psyllium husk | 95 | 67 | 1.42 | 136.4 | 365 | 2.68 |
| WMPWF 70% + WPWF 30% | 99 | 59 | 1.68 | 136.1 | 340 | 2.50 |
| WMPWF 70% + WPWF 30% + 4% inulin + 4% chia seed flour | 94 | 60 | 1.57 | 138.5 | 335 | 2.42 |
| WMPWF 4% inulin + 4% chia seed flour | 95 | 57 | 1.67 | 140.6 | 345 | 2.45 |
| Mixture 1 (WPWF + 4% Inulin + 2% Psyllium Husk Flour) | Mixture 2 (WMPWF 70% + WPWF 30% + 4% Inulin + 4% Chia Seed Flour) | Mixture 3 (WMPWF + 4% Inulin + 4% Chia Seed Flour) | |
|---|---|---|---|
| Crude fibre content % (measured with Foss Fibertec 2010) | 1.24 | 1.98 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szőke-Trenyik, E.; Mihalkó, J.; Sipos, P.; Szabó, B.P. Development of High-Fibre, Ready-to-Bake Flour Mixtures from Purple Wheat. Processes 2023, 11, 389. https://doi.org/10.3390/pr11020389
Szőke-Trenyik E, Mihalkó J, Sipos P, Szabó BP. Development of High-Fibre, Ready-to-Bake Flour Mixtures from Purple Wheat. Processes. 2023; 11(2):389. https://doi.org/10.3390/pr11020389
Chicago/Turabian StyleSzőke-Trenyik, Eszter, József Mihalkó, Péter Sipos, and Balázs P. Szabó. 2023. "Development of High-Fibre, Ready-to-Bake Flour Mixtures from Purple Wheat" Processes 11, no. 2: 389. https://doi.org/10.3390/pr11020389
APA StyleSzőke-Trenyik, E., Mihalkó, J., Sipos, P., & Szabó, B. P. (2023). Development of High-Fibre, Ready-to-Bake Flour Mixtures from Purple Wheat. Processes, 11(2), 389. https://doi.org/10.3390/pr11020389









