Sex Differences in MASLD After Age 50: Presentation, Diagnosis, and Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric Assessment
2.3. Laboratory Analysis
2.4. Non-Invasive Liver Disease Assessment (NILDAs)
- S0 (no steatosis): <248 dB/m
- S1 (mild): 248–267 dB/m
- S2 (moderate): 268–279 dB/m
- F0: <7.9 kPa
- F1 (significant): ≥7.9–9.4 kPa
- F2 (advanced): 9.5–12.4 kPa
- F3–4 (cirrhosis): ≥12.5 kPa
2.5. Cardiometabolic and Adipose Tissue Dysfunction Indices
2.6. Lifestyle and Other Variables
2.7. Ethics Statement
2.8. Statistical Analysis
3. Results
| Overall (n = 213) | Men (n = 73) | Women (n = 140) | p-Value | |
|---|---|---|---|---|
| Age (years) | 63.0 (62.7–64.6) | 63.0 (62.8–66.2) | 63.0 (61.9–64.2) | 0.10 |
| Diabetes (%) | 48.3 | 57.5 | 43.6 | 0.06 |
| Hypertension (%) | 76.5 | 76.7 | 76.4 | 0.97 |
| Dyslipidemia (%) | 63.4 | 63.0 | 63.6 | 0.88 |
| Antihyperlipidemic drugs (%) | 56.8 | 61.6 | 55.0 | 0.46 |
| Current smokers (%) | 11.3 | 16.4 | 8.6 | 0.001 |
| Ex-smokers (%) | 24.8 | 39.7 | 17.1 | 0.001 |
| Medas ≥ 9 (%) | 41.8 | 43.8 | 40.7 | 0.88 |
| Weight (kg) | 92.5 (92.9–97.7) | 102.0 (99.5–107.5) | 88.5 (88.1–93.7) | 0.001 |
| BMI (kg/m2) | 34.9 ± 5.5 | 33.8 ± 5.1 | 35.4 ± 5.6 | 0.051 |
| WC (cm) | 114.2 ± 12.7 | 117.1 ± 12.4 | 112.7 ± 12.6 | 0.001 |
| WC > 80 cm for women and WC > 94 cm for men (%) | 91.8 | 100 | 0.02 | |
| WHtR | 0.68 ± 0.07 | 0.64 ± 0.12 | 0.69 ± 0.07 | 0.001 |
| WHtR > 0.5 (%) | 98.6 | 95.9 | 100 | 0.02 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Srishord, M.; Mishra, A.; Younossi, Z.M. The Growing Burden of Disability Related to Nonalcoholic Fatty Liver Disease: Data from the Global Burden of Disease 2007–2017. Hepatol. Commun. 2020, 4, 1769–1780. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Newsome, P.N. NAFLD vs. MASLD (Metabolic Dysfunction–Associated Steatotic Liver Disease)—Why the Need for a Change of Nomenclature? J. Clin. Endocrinol. Metab. 2025, 110, e2407–e2410. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics 2022, 12, 2287. [Google Scholar] [CrossRef]
- Sun, D.-Q.; Zheng, K.I.; Xu, G.; Ma, H.-L.; Zhang, H.-Y.; Pan, X.-Y.; Zhu, P.-W.; Wang, X.-D.; Targher, G.; Byrne, C.D.; et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020, 40, 107–119. [Google Scholar] [CrossRef]
- Umehara, T. Nonalcoholic fatty liver disease with elevated alanine aminotransferase levels is negatively associated with bone mineral density: Cross-sectional study in U.S. adults. PLoS ONE 2018, 13, e0197900. [Google Scholar] [CrossRef]
- Noureddin, M.; Loomba, R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin. Liver Dis. 2012, 1, 104–107. [Google Scholar] [CrossRef]
- Crudele, L.; De Matteis, C.; Novielli, F.; Di Buduo, E.; Petruzzelli, S.; De Giorgi, A.; Antonica, G.; Berardi, E.; Moschetta, A. Fatty Liver Index (FLI) is the best score to predict MASLD with 50% lower cut-off value in women than in men. Biol. Sex. Differ. 2024, 15, 43. [Google Scholar] [CrossRef]
- Hernandez Roman, J.; Siddiqui, M.S. The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Endocrinol. Diabetes Metab. 2020, 3, e00127. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73. [Google Scholar] [CrossRef]
- He, Q.-J.; Li, Y.-F.; Zhao, L.-T.; Lin, C.-T.; Yu, C.-Y.; Wang, D. Recent advances in age-related metabolic dysfunction-associated steatotic liver disease. World J. Gastroenterol. 2024, 30, 652–662. [Google Scholar] [CrossRef]
- Yadav, A.K.; MacNeill, J.J.; Krylov, A.; Ashrafi, N.; Mimi, R.A.; Saxena, R.; Liu, S.; Graham, S.F.; Wan, J.; Morral, N. Sex- and age-associated factors drive the pathophysiology of MASLD. Hepatol. Commun. 2024, 8, e0523. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Liu, D.; Liang, Y.; Zhu, Z.; Dong, K.; Li, H.; Bao, Y.; Wu, J.; Hou, X.; et al. Sex- and age-specific associations between abdominal fat and non-alcoholic fatty liver disease: A prospective cohort study. J. Mol. Cell Biol. 2023, 15, mjad069. [Google Scholar] [CrossRef]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Booijink, R.; Ramachandran, P.; Bansal, R. Implications of innate immune sexual dimorphism for MASLD pathogenesis and treatment. Trends Pharmacol. Sci. 2024, 45, 614–627. [Google Scholar] [CrossRef]
- Milani, I.; Chinucci, M.; Leonetti, F.; Capoccia, D. MASLD: Prevalence, Mechanisms, and Sex-Based Therapies in Postmenopausal Women. Biomedicines 2025, 13, 855. [Google Scholar] [CrossRef]
- Le, M.H.; Le, D.M.; Baez, T.C.; Wu, Y.; Ito, T.; Lee, E.Y.; Lee, K.; Stave, C.D.; Henry, L.; Barnett, S.D.; et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J. Hepatol. 2023, 79, 287–295. [Google Scholar] [CrossRef]
- Liu, J.; Ayada, I.; Zhang, X.; Wang, L.; Li, Y.; Wen, T.; Ma, Z.; Bruno, M.J.; de Knegt, R.J.; Cao, W.; et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin. Gastroenterol. Hepatol. 2022, 20, e573–e582. [Google Scholar] [CrossRef]
- Eng, P.C.; Forlano, R.; Tan, T.; Manousou, P.; Dhillo, W.S.; Izzi-Engbeaya, C. Non-alcoholic fatty liver disease in women—Current knowledge and emerging concepts. JHEP Rep. 2023, 5, 100835. [Google Scholar] [CrossRef]
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Mahtab, M.A.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2022, 12, S19–S25. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women have Lower Risk of Nonalcoholic Fatty Liver Disease but Higher Risk of Progression vs. Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef]
- Tang, Z.; Pham, M.; Hao, Y.; Wang, F.; Patel, D.; Jean-Baptiste, L.; Fan, L.; Wang, W.; Wang, Y.; Cheng, F. Sex, Age, and BMI Modulate the Association of Physical Examinations and Blood Biochemistry Parameters and NAFLD: A Retrospective Study on 1994 Cases Observed at Shuguang Hospital, China. Biomed. Res. Int. 2019, 2019, 1246518. [Google Scholar] [CrossRef]
- Burnside, J.; Cinque, F.; Sebastiani, G.; Ramji, A.; Patel, K.; Swain, M.; Saeed, S. Sex differences in the prevalence and cardiometabolic risk profiles of steatotic liver disease: A Canadian Longitudinal Study on Aging analysis. Can. J. Public Health 2025. [Google Scholar] [CrossRef]
- Duan, H.; Gong, M.; Yuan, G.; Wang, Z. Sex Hormone: A Potential Target at Treating Female Metabolic Dysfunction-Associated Steatotic Liver Disease? J. Clin. Exp. Hepatol. 2025, 15, 102459. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Koukouliata, A.; Nena, E.; Koutlaki, N.; Liberis, V.; Constantinidis, T. Correlation of age at natural menopause with occupational status and other epidemiologic factors in women from Prefecture of Kavala, Greece. Hippokratia 2017, 21, 32–37. [Google Scholar]
- Namazi, M.; Sadeghi, R.; Behboodi Moghadam, Z. Social Determinants of Health in Menopause: An Integrative Review. Int. J. Womens Health 2019, 11, 637–647. [Google Scholar] [CrossRef]
- Gibson, S.; Ashwell, M. A simple cut-off for waist-to-height ratio (0·5) can act as an indicator for cardiometabolic risk: Recent data from adults in the Health Survey for England. Br. J. Nutr. 2020, 123, 681–690. [Google Scholar] [CrossRef]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2024, 80, e76–e77. [Google Scholar] [CrossRef]
- Ratziu, V.; Boursier, J.; AFEFGroup for the Study of Liver Fibrosis. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J. Hepatol. 2024, 80, e51–e52. [Google Scholar] [CrossRef]
- Song, S.J.; Lai, J.C.-T.; Wong, G.L.-H.; Wong, V.W.-S.; Yip, T.C.-F. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2024, 80, e54–e56. [Google Scholar] [CrossRef]
- Elsabaawy, M.; Naguib, M.; Abuamer, A.; Shaban, A. Comparative application of MAFLD and MASLD diagnostic criteria on NAFLD patients: Insights from a single-center cohort. Clin. Exp. Med. 2025, 25, 36. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Ozercan, A.M.; Ozkan, H. Vibration-controlled Transient Elastography in NAFLD: Review Study. Euroasian J. Hepatogastroenterol. 2022, 12, S41–S45. [Google Scholar] [CrossRef]
- Singh, S.; Muir, A.J.; Dieterich, D.T.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology 2017, 152, 1544–1577. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C. Visceral Adiposity Index: An Indicator of Adipose Tissue Dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, J.; Li, M.; Gao, Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index–related parameters. Front. Endocrinol. 2022, 13, 951689. [Google Scholar] [CrossRef]
- Malek, M.; Khamseh, M.E.; Chehrehgosha, H.; Nobarani, S.; Alaei-Shahmiri, F. Triglyceride glucose-waist to height ratio: A novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine 2021, 74, 538–545. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee: 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S27–S49. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Shephard, D.A. The 1975 Declaration of Helsinki and consent. Can. Med. Assoc. J. 1976, 115, 1191–1192. [Google Scholar]
- Loo, S.-Y.; Chan, W.-K. Emerging new standard for non-invasive assessment of liver disease mortality in non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2017, 6, 135–137. [Google Scholar] [CrossRef]
- Feng, G.; Targher, G.; Byrne, C.D.; Yilmaz, Y.; Wai-Sun Wong, V.; Adithya Lesmana, C.R.; Adams, L.A.; Boursier, J.; Papatheodoridis, G.; El-Kassas, M.; et al. Global burden of metabolic dysfunction-associated steatotic liver disease, 2010 to 2021. JHEP Rep. 2024, 7, 101271. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Goulis, D.G. Menopause and metabolic dysfunction-associated steatotic liver disease. Maturitas 2024, 186, 108024. [Google Scholar] [CrossRef]
- Ntikoudi, A.; Spyrou, A.; Evangelou, E.; Dokoutsidou, E.; Mastorakos, G. The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease. Healthcare 2024, 12, 1081. [Google Scholar] [CrossRef]
- Cherubini, A.; Torre, S.D.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024, 30, 1126–1136. [Google Scholar] [CrossRef]
- Zuo, Q.; Park, N.H.; Lee, J.K.; Santaliz-Casiano, A.; Madak-Erdogan, Z. Navigating nonalcoholic fatty liver disease (NAFLD): Exploring the roles of estrogens, pharmacological and medical interventions, and life style. Steroids 2024, 203, 109330. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Ninic, A. Predictive Values of Serum Uric Acid and Alanine-aminotransferase for Fatty Liver Index in Montenegrin Population. J. Med. Biochem. 2019, 38, 407–417. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lim, C.-W.; Lee, J.H.; Park, H.-B.; Suh, Y.; Cho, Y.-H.; Choi, T.-Y.; Hwang, E.-S.; Cho, D.-K.; Kim, H.-J. Gender-based differences in the relationship between fatty liver disease and atherosclerosis. Cardiovasc. J. Afr. 2016, 27, 281–286. [Google Scholar] [CrossRef][Green Version]
- Mera, J.R.; Dickson, B.; Feldman, M. Influence of Gender on the Ratio of Serum Aspartate Aminotransferase (AST) to Alanine Aminotransferase (ALT) in Patients with and Without Hyperbilirubinemia. Dig. Dis. Sci. 2008, 53, 799–802. [Google Scholar] [CrossRef]
- Ishibashi, E.; Eguchi, Y.; Eguchi, T.; Matsunobu, A.; Oza, N.; Nakashita, S.; Kitajima, Y.; Kuroki, S.; Ozaki, I.; Kawaguchi, Y.; et al. Waist circumference correlates with hepatic fat accumulation in male Japanese patients with non-alcoholic fatty liver disease, but not in females. J. Gastroenterol. Hepatol. 2008, 23, 908–913. [Google Scholar] [CrossRef]
- Oye-Somefun, A.; Blyuss, E.; Ardern, C.I. Trends in Serum AST-to-ALT Ratio Among U.S. Adults: Analysis of the U.S. National Health and Nutrition Examination Survey. Metab. Syndr. Relat. Disord. 2021, 19, 498–506. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The De Ritis Ratio: The Test of Time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Chen, J.; Li, J.; Qu, H.; Ning, T.; Xie, H.; Lu, G. A Mendelian randomization study: Years of education and nonalcoholic fatty liver disease. Medicine 2024, 103, e38761. [Google Scholar] [CrossRef] [PubMed]
- Pernoud, L.E.; Gardiner, P.A.; Fraser, S.D.; Dillon-Rossiter, K.; Dean, M.M.; Schaumberg, M.A. A systematic review and meta-analysis investigating differences in chronic inflammation and adiposity before and after menopause. Maturitas 2024, 190, 108119. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Fan, Y.; Luo, M.; Zhang, D.; Xie, Z.; Huang, F.; Wang, Y.; Liu, G.; Wang, Y.; Lin, S.; et al. General and Central Obesity Are Associated with Increased Severity of the VMS and Sexual Symptoms of Menopause Among Chinese Women: A Longitudinal Study. Front. Endocrinol. 2022, 13, 814872. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kim, J.U.; Lee, S. Association of menopausal status with body composition and anthropometric indices in Korean women. PLoS ONE 2024, 19, e0298212. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, W.; Lou, Y.; Yan, Q.; Zhang, Y.; Qi, F.; Xiang, L.; Lv, T.; Fang, Z.; Yu, J.; et al. Sex- and reproductive status-specific relationships between body composition and non-alcoholic fatty liver disease. BMC Gastroenterol. 2023, 23, 364. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeon, S.; Lee, H.S.; Kwon, Y.-J. Gender Differences in the Risk for Incident Non-Alcoholic Fatty Liver Disease According to the Transition of Abdominal Obesity Status: A 16-Year Cohort Study. Nutrients 2023, 15, 2880. [Google Scholar] [CrossRef]
- Ismaiel, A.; Hosiny, B.E.; Ismaiel, M.; Leucuta, D.-C.; Popa, S.-L.; Catana, C.S.; Dumitrascu, D.L. Waist to height ratio in nonalcoholic fatty liver disease—Systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102160. [Google Scholar] [CrossRef]
- Camhi, S.M.; Bray, G.A.; Bouchard, C.; Greenway, F.L.; Johnson, W.D.; Newton, R.L.; Ravussin, E.; Ryan, D.H.; Smith, S.R.; Katzmarzyk, P.T. The Relationship of Waist Circumference and BMI to Visceral, Subcutaneous, and Total Body Fat: Sex and Race Differences. Obesity 2011, 19, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Kullberg, J.; Von Below, C.; Lönn, L.; Lind, L.; Ahlström, H.; Johansson, L. Practical approach for estimation of subcutaneous and visceral adipose tissue. Clin. Physiol. Funct. Imaging 2007, 27, 148–153. [Google Scholar] [CrossRef]
- De Vincentis, A.; Tavaglione, F.; Spagnuolo, R.; Pujia, R.; Tuccinardi, D.; Mascianà, G.; Picardi, A.; Antonelli Incalzi, R.; Valenti, L.; Romeo, S.; et al. Metabolic and genetic determinants for progression to severe liver disease in subjects with obesity from the UK Biobank. Int. J. Obes. 2022, 46, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Lathief, S.; Charoenngam, N.; Pal, L. Aging and Adiposity—Focus on Biological Females at Midlife and Beyond. Int. J. Mol. Sci. 2024, 25, 2972. [Google Scholar] [CrossRef]
- Lovejoy, J.; Champagne, C.; de Jonge, L.; Xie, H.; Smith, S. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Buday, B.; Pach, P.F.; Literati-Nagy, B.; Vitai, M.; Kovacs, G.; Vecsei, Z.; Koranyi, L.; Lengyel, C. Sex influenced association of directly measured insulin sensitivity and serum transaminase levels: Why alanine aminotransferase only predicts cardiovascular risk in men? Cardiovasc. Diabetol. 2015, 14, 55. [Google Scholar] [CrossRef]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and Subcutaneous Adipose Tissue Volumes Are Cross-Sectionally Related to Markers of Inflammation and Oxidative Stress. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef]
- Khera, A.; Vega, G.L.; Das, S.R.; Ayers, C.; McGuire, D.K.; Grundy, S.M.; de Lemos, J.A. Sex Differences in the Relationship between C-Reactive Protein and Body Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Thorand, B.; Baumert, J.; Döring, A.; Herder, C.; Kolb, H.; Rathmann, W.; Giani, G.; Koenig, W. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis 2006, 184, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Lattanzio, M.S.; Minetti, S.; Ansaldo, A.M.; Ferrara, D.; Molina-Molina, E.; Belfiore, A.; Elia, E.; Pugliese, S.; Palmieri, V.O.; et al. Circulating CRP Levels Are Associated with Epicardial and Visceral Fat Depots in Women with Metabolic Syndrome Criteria. Int. J. Mol. Sci. 2019, 20, 5981. [Google Scholar] [CrossRef]
- Kang, S.; Kyung, C.; Park, J.S.; Kim, S.; Lee, S.-P.; Kim, M.K.; Kim, H.K.; Kim, K.R.; Jeon, T.J.; Ahn, C.W. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body Fat: An 18 F-FDG-PET/CT study. Cardiovasc. Diabetol. 2014, 13, 70. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Tada, T.; Akita, T.; Kondo, R.; Suzuki, Y.; Imajo, K.; Kokubu, S.; Abe, T.; Kuroda, H.; Hirooka, M.; et al. Diagnostic performance of attenuation imaging versus controlled attenuation parameter for hepatic steatosis with MRI-based proton density fat fraction as the reference standard: A prospective multicenter study. J. Gastroenterol. 2025, 60, 727–737. [Google Scholar] [CrossRef]
- Wu, J.; Tian, S.; Li, H.; Xu, Z.; Li, S.; Chen, Y.; Liang, X.; Xiao, J.; Song, J.; She, R.; et al. Population-specific cut-off points of fatty liver index: A study based on the National Health and Nutrition Examination Survey data. BMC Gastroenterol. 2022, 22, 265. [Google Scholar] [CrossRef]
- Chen, L.-W.; Huang, P.-R.; Chien, C.-H.; Lin, C.-L.; Chien, R.-N. A community-based study on the application of fatty liver index in screening subjects with nonalcoholic fatty liver disease. J. Formos. Med. Assoc. 2020, 119, 173–181. [Google Scholar] [CrossRef]
- Dehnavi, Z.; Razmpour, F.; Belghaisi Naseri, M.; Nematy, M.; Alamdaran, S.A.; Vatanparast, H.A.; Azimi Nezhad, M.; Abbasi, B.; Ganji, A. Fatty Liver Index (FLI) in Predicting Non-alcoholic Fatty Liver Disease (NAFLD). Hepat. Mon. 2018, 18, e63227. [Google Scholar] [CrossRef]
- Noureddin, M.; Truong, E.; Mayo, R.; Martínez-Arranz, I.; Mincholé, I.; Banales, J.M.; Arrese, M.; Cusi, K.; Arias-Loste, M.T.; Bruha, R.; et al. Serum identification of at-risk MASH: The metabolomics-advanced steatohepatitis fibrosis score (MASEF). Hepatology 2024, 79, 135. [Google Scholar] [CrossRef] [PubMed]
- Gangireddy, V.G.R.; Pilkerton, C.; Xiang, J.; Tinajero, R.; Ashcraft, A.M. Hepatic Fibrosis and Steatosis in Metabolic Syndrome. J. Obes. Metab. Syndr. 2022, 31, 61–69. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Webb, M.; Assy, N.; Blendis, L.; Yeshua, H.; Leshno, M.; Ratziu, V.; Halpern, Z.; Oren, R.; Santo, E. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J. Gastroenterol. 2013, 19, 57–64. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.L.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L.A. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef]
- Earle, N.J.; Doughty, R.N.; Devlin, G.; White, H.; Riddell, C.; Choi, Y.; Kerr, A.J.; Poppe, K.K. Sex differences in outcomes after acute coronary syndrome vary with age: A New Zealand national study. European Heart Journal. Acute Cardiovasc. Care 2024, 13, 284–292. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Jutras, G.; Flemming, J.A. Global Epidemiology of Cirrhosis in Women. Off. J. Am. Coll. Gastroenterol. ACG 2025, 120, 518. [Google Scholar] [CrossRef]
- Paklar, N.; Mijic, M.; Filipec-Kanizaj, T. The Outcomes of Liver Transplantation in Severe Metabolic Dysfunction-Associated Steatotic Liver Disease Patients. Biomedicines 2023, 11, 3096. [Google Scholar] [CrossRef]
- Gauci, S.; Cartledge, S.; Redfern, J.; Gallagher, R.; Huxley, R.; Lee, C.M.Y.; Vassallo, A.; O’Neil, A. Biology, Bias, or Both? The Contribution of Sex and Gender to the Disparity in Cardiovascular Outcomes Between Women and Men. Curr. Atheroscler. Rep. 2022, 24, 701–708. [Google Scholar] [CrossRef]
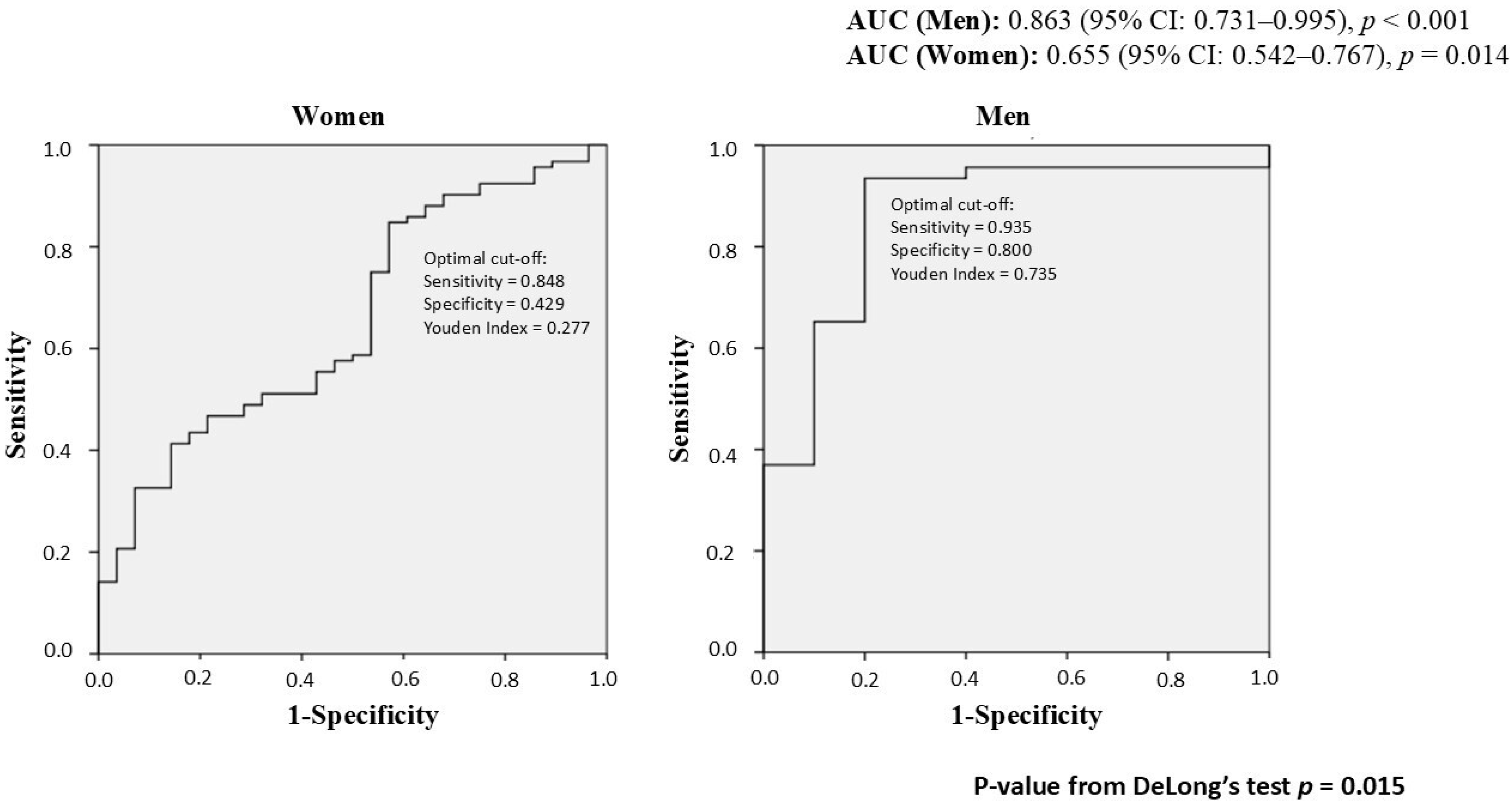
| Overall | Men | Women | p-Value | |
|---|---|---|---|---|
| SBP (mmHg) | 130.0 (129.1–133.5) | 130.0 (126.3–134.4) | 131.2 (129.2–134.4) | 0.67 |
| DBP (mmHg) | 80.0 (78.5–81.4) | 80.0 (78.2–82.8) | 80.0 (77.7–81.6) | 0.87 |
| FBG (mg/dL) | 99.0 (100.6–109.0) | 104.0 (104.9–118.8) | 96.0 (95.8–106.5) | 0.001 |
| HbA1c (%) | 6.0 (5.9–6.8) | 6.2 (6.0–6.7) | 5.9 (5.8–7.0) | 0.17 |
| HbA1c (mmol/mol) | 42.0 (42.9–46.3) | 44.0 (42.9–52.2) | 41.0 (41.1–44.8) | 0.15 |
| Total cholesterol (mg/dL) | 175.1 ± 41.3 | 162.5 ± 38.8 | 181.3 ± 41.1 | 0.001 |
| LDL cholesterol (mg/dL) | 98.5 ± 36.8 | 88.6 ± 34.3 | 105.6 ± 36.9 | 0.02 |
| HDL cholesterol (mg/dL) | 50.0 (50.7–55.1) | 47.0 (45.5–56.7) | 52.0 (51.9–55.9) | 0.001 |
| TG (mg/dL) | 122.5 ± 49.5 | 124.9 ± 60.4 | 119.7 ± 42.9 | 0.52 |
| CRP (mg/dL) | 0.30 (0.4–0.6) | 0.30 (0.2–0.6) | 0.35 (0.4–0.7) | 0.001 |
| Creatinine (mg/dL) | 0.85 ± 0.2 | 1.01 ± 0.19 | 0.77 ± 0.18 | 0.001 |
| ALT (U/L) | 21.0 (22.7–27.1) | 25.5 (24.4–32.4) | 19.0 (20.6–25.7) | 0.001 |
| AST (U/L) | 21.0 (21.7–24.7) | 22.0 (22.2–27.9) | 21.0 (20.6–23.6) | 0.29 |
| GGT (U/L) | 24.0 (21.8–32.2) | 28.5 (28.9–45.5) | 22.0 (21.8–32.2) | 0.02 |
| Overall | Men | Women | p-Value | |
|---|---|---|---|---|
| FLI | 88.1 (77.7–83.5) | 92.5 (75.9–86.9) | 86.0 (76.7–83.6) | 0.31 |
| FIB-4 | 1.45 (1.4–1.6) | 1.43 (1.43–1.71) | 1.45 (1.40–1.52) | 0.75 |
| AST/ALT | 1.0 (1.0–1.1) | 0.9 (0.8–1.0) | 1.1 (1.0–1.1) | 0.001 |
| AST/ALT > 0.8 (%) | 66.7 | 57.5 | 71.4 | 0.001 |
| ALT ≥ 33 U/L for men and ≥25 U/L n (%) | 24.6 | 30.7 | 0.06 | |
| AST ≥ 30 U/L for men and ≥26 U/L n (%) | 19.2 | 24.3 | 0.09 | |
| TyG-WHtR | 5.9 ± 0.9 | 5.6 ± 1.0 | 6.0 ± 0.8 | 0.02 |
| VAI | 1.82 (1.8–2.1) | 1.38 (1.4–2.1) | 1.97 (1.9–2.3) | 0.001 |
| VAI > 1.93 (%) | 41.8 | 30.1 | 47.8 | 0.046 |
| Overall | Men | Women | p-Value | |
|---|---|---|---|---|
| CAP (dB/m) | 283.0 ± 48.9 | 294.5 ± 50.9 | 277.4 ± 46.9 | 0.02 |
| CAP S0 (%) | 22.5 (48/213) | 16.4 (12/73) | 25.7 (36/140) | 0.29 |
| CAP S1 (%) | 13.6 (29/213) | 17.8 (13/73) | 11.4 (16/140) | 0.29 |
| CAP S2 (%) | 11.7 (25/213) | 10.9 (8/73) | 12.1 (17/140) | 0.82 |
| CAP S3 (%) | 53.0 (113/213) | 57.5 (42/73) | 50.7 (71/140) | 0.32 |
| LSM (kPa) | 5.8 (5.9–6.5) | 5.9 (5.9–6.9) | 5.8 (5.7–6.4) | 0.38 |
| LSM F0 (%) | 77.5 (165/213) | 79.4 (58/73) | 76.4 (107/140) | 0.60 |
| LSM F1 (%) | 13.6 (29/213) | 8.2 (6/73) | 16.5 (23/140) | 0.07 |
| LSM F2 (%) | 7.5 (16/213) | 9.6 (7/73) | 6.5 (9/140) | 0.58 |
| LSM F3–F4 (%) | 1.4 (3/213) | 2.7 (2/73) | 0.7 (1/140) | 0.55 |
| Beta Coefficient | Sig. | Exp(B) | 95% CI per EXP(B) | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Women | FLI | 0.026 | 0.018 | 1.026 | 1.004 | 1.049 |
| Constant | −0.845 | 0.331 | 0.430 | |||
| Men | FLI | 0.064 | 0.001 | 1.066 | 1.026 | 1.107 |
| Constant | −3.220 | 0.022 | 0.040 | |||
| Men | Women | |
|---|---|---|
| Sensitivity (%) | 93.5 (CI 95%: 86.4–99.6%) | 90.2 (CI 95%: 82.6–95.3%) |
| Specificity (%) | 70.0 (CI 95%: 41.6–98.4%) | 28.6 (CI 95%: 13.2–48.7%) |
| Positive Predictive Value (%) | 93.5 (CI 95%: 86.4–99.6%) | 80.6 (CI 95%: 71.9–87.6%) |
| Negative Predictive Value (%) | 70.0 (CI 95%: 41.6–98.4%) | 47.1 (CI 95%: 23.0–72.2%) |
| Overall Accuracy (%) | 89.3 (CI 95%: 81.2–97.4%) | 75.8 (CI 95%: 67.1–83.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, I.; Parrotta, M.E.; Colangeli, L.; Chinucci, M.; Palleschi, S.; Rossi, B.; Sbraccia, P.; Mantovani, A.; Leonetti, F.; Guglielmi, V.; et al. Sex Differences in MASLD After Age 50: Presentation, Diagnosis, and Clinical Implications. Biomedicines 2025, 13, 2292. https://doi.org/10.3390/biomedicines13092292
Milani I, Parrotta ME, Colangeli L, Chinucci M, Palleschi S, Rossi B, Sbraccia P, Mantovani A, Leonetti F, Guglielmi V, et al. Sex Differences in MASLD After Age 50: Presentation, Diagnosis, and Clinical Implications. Biomedicines. 2025; 13(9):2292. https://doi.org/10.3390/biomedicines13092292
Chicago/Turabian StyleMilani, Ilaria, Maria Eugenia Parrotta, Luca Colangeli, Marianna Chinucci, Simonetta Palleschi, Barbara Rossi, Paolo Sbraccia, Alessandro Mantovani, Frida Leonetti, Valeria Guglielmi, and et al. 2025. "Sex Differences in MASLD After Age 50: Presentation, Diagnosis, and Clinical Implications" Biomedicines 13, no. 9: 2292. https://doi.org/10.3390/biomedicines13092292
APA StyleMilani, I., Parrotta, M. E., Colangeli, L., Chinucci, M., Palleschi, S., Rossi, B., Sbraccia, P., Mantovani, A., Leonetti, F., Guglielmi, V., & Capoccia, D. (2025). Sex Differences in MASLD After Age 50: Presentation, Diagnosis, and Clinical Implications. Biomedicines, 13(9), 2292. https://doi.org/10.3390/biomedicines13092292







